Protective Effect of Lonicera japonica on PM2.5-Induced Pulmonary Damage in BALB/c Mice via the TGF-β and NF-κB Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. Physiological Compound Analysis
2.4. Evaluation of Pulmonary Protective Effect
2.4.1. Cell Culture and Treatment
2.4.2. Cell Viability
2.4.3. Reactive Oxygen Species (ROS) Contents
2.5. Animal Experimental Design
2.6. Determination of T Cells by Flow Cytometry
2.7. Serum Immunoglobulins (Ig) by ELISA
2.8. Pulmonary Antioxidant System
2.8.1. SOD Contents
2.8.2. Reduced Glutathione (GSH) Contents
2.8.3. Malondialdehyde (MDA) Contents
2.9. Pulmonary Mitochondrial Function
2.9.1. Mitochondrial Isolation
2.9.2. Mitochondrial ROS Contents
2.9.3. Mitochondrial Membrane Potential (MMP)
2.9.4. ATP Contents
2.10. Western Blot
2.11. Statistical Anaylsis
3. Results
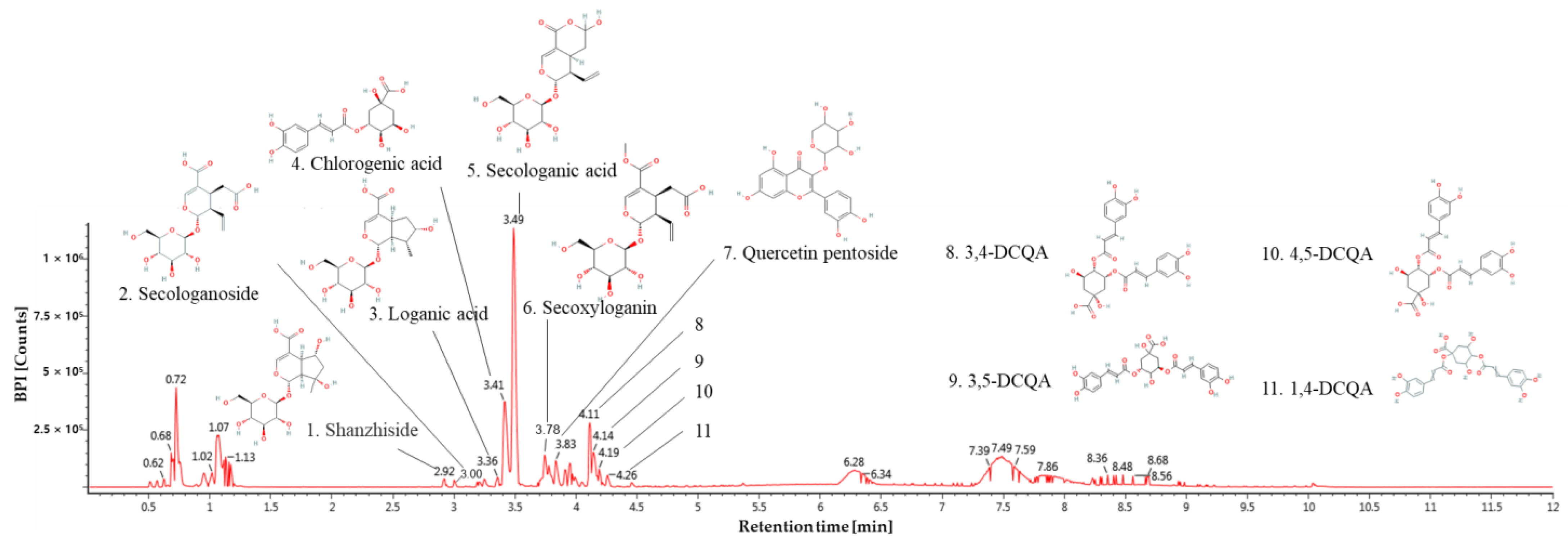
3.1. Physiological Compound of Lonicera japonica
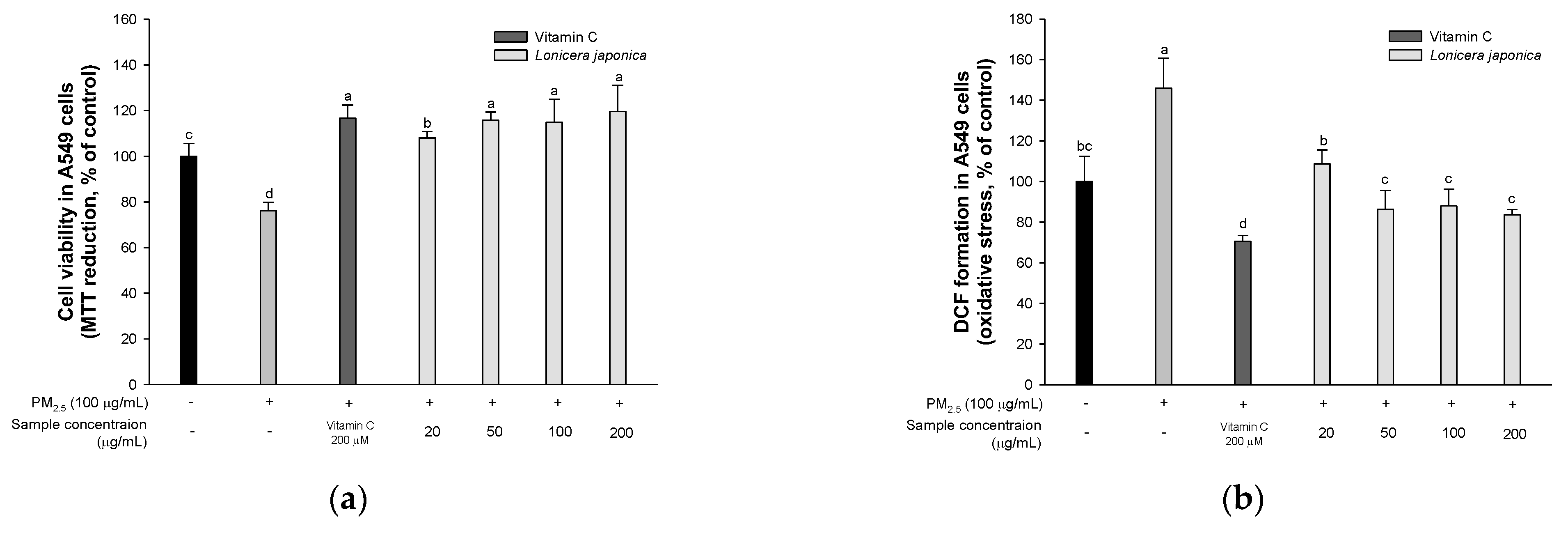
3.2. Protective Effect of A549 Cells
3.2.1. Cell Viability
3.2.2. ROS Production
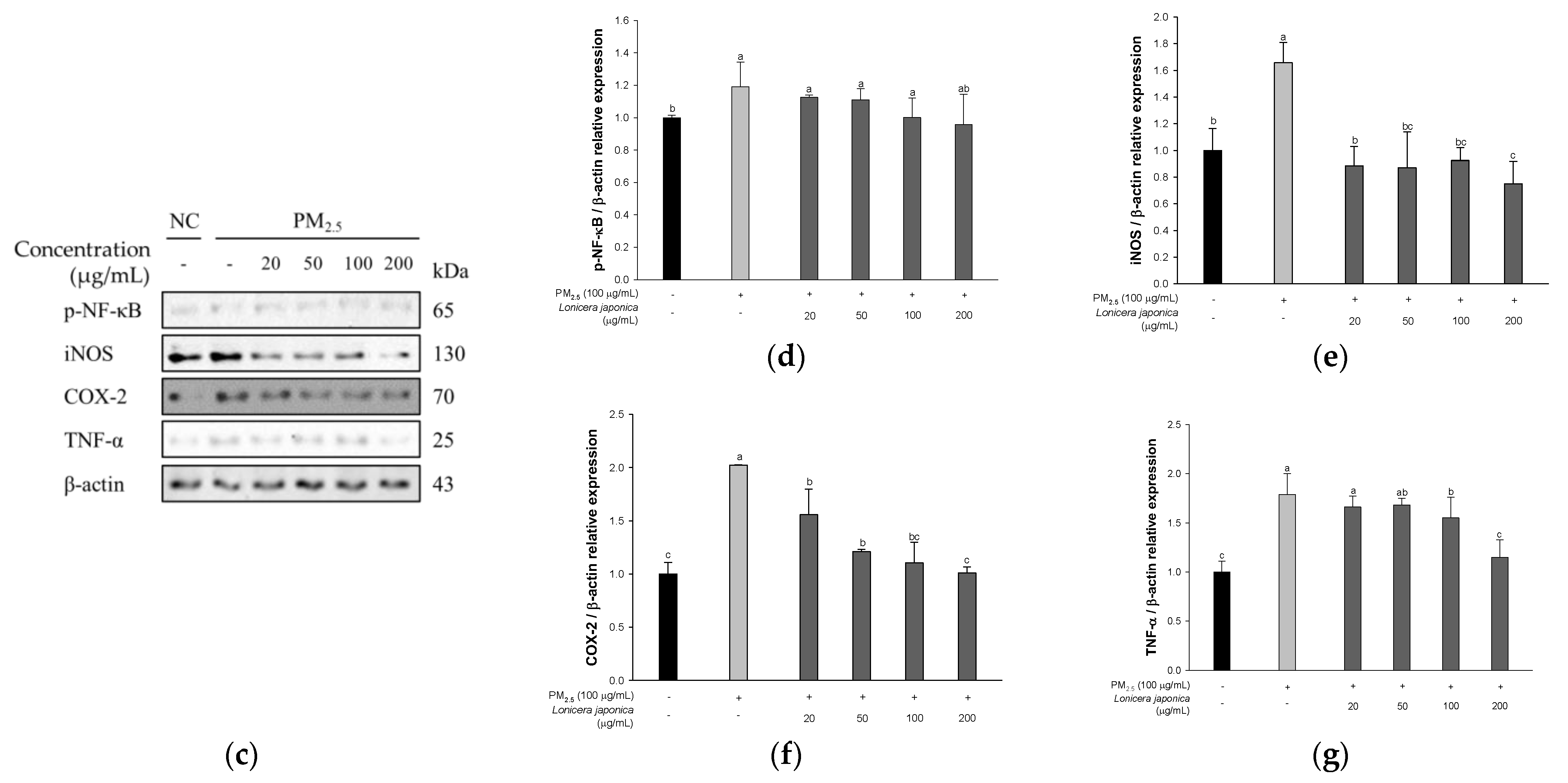
3.2.3. Protein Expression of Inflammation in A549 Cells
3.3. Serum Inflammatory Cytokines
3.3.1. T Cells
3.3.2. Immunoglobulins
3.4. Antioxidant System in Lung Tissue
3.4.1. SOD Activities
3.4.2. Reduced GSH Contents
3.4.3. MDA Levels
3.5. Mitochondrial Function
3.5.1. Mitochondrial ROS Contents
3.5.2. Mitochondrial Membrane Potential (MMP)
3.5.3. Mitochondrial ATP Contents
3.6. Protein Expression of Inflammatory Pathway
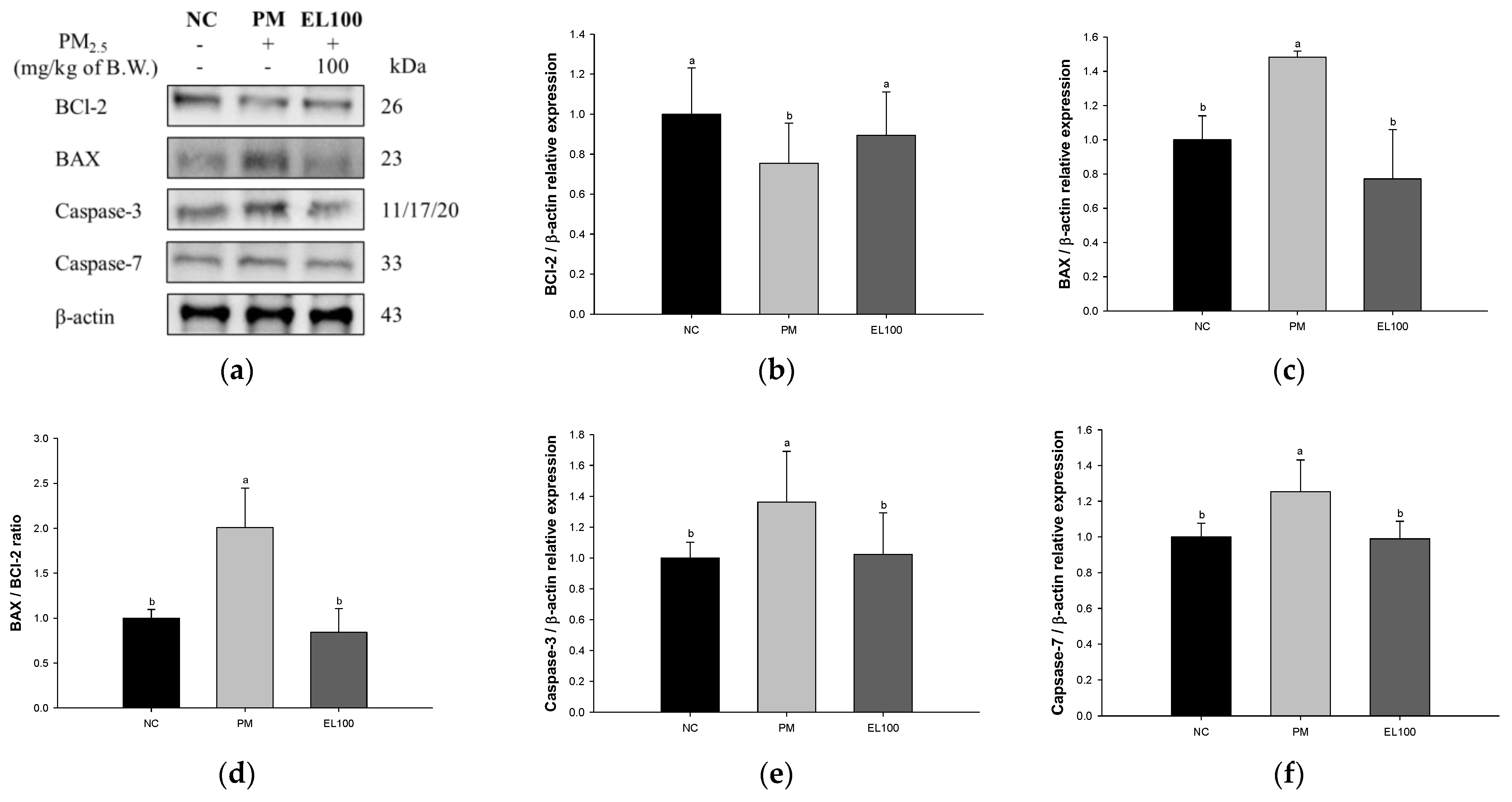
3.7. Protein Expression of Apoptosis
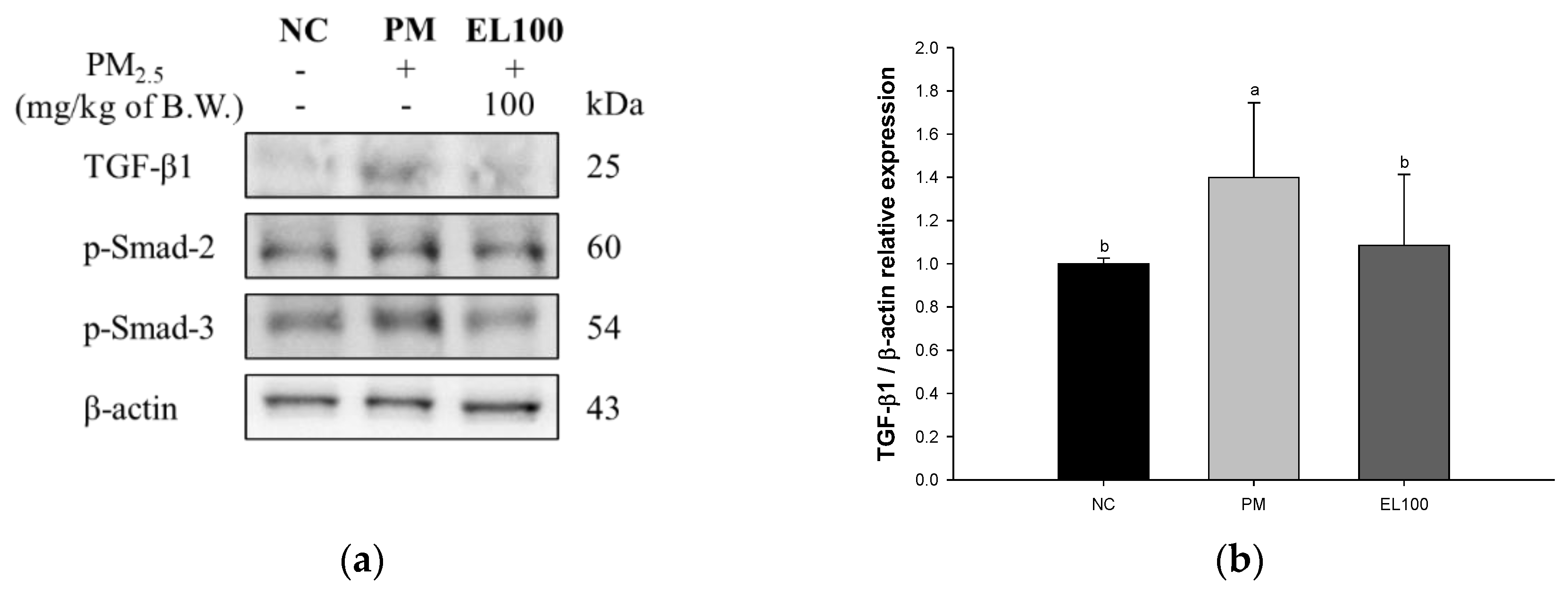
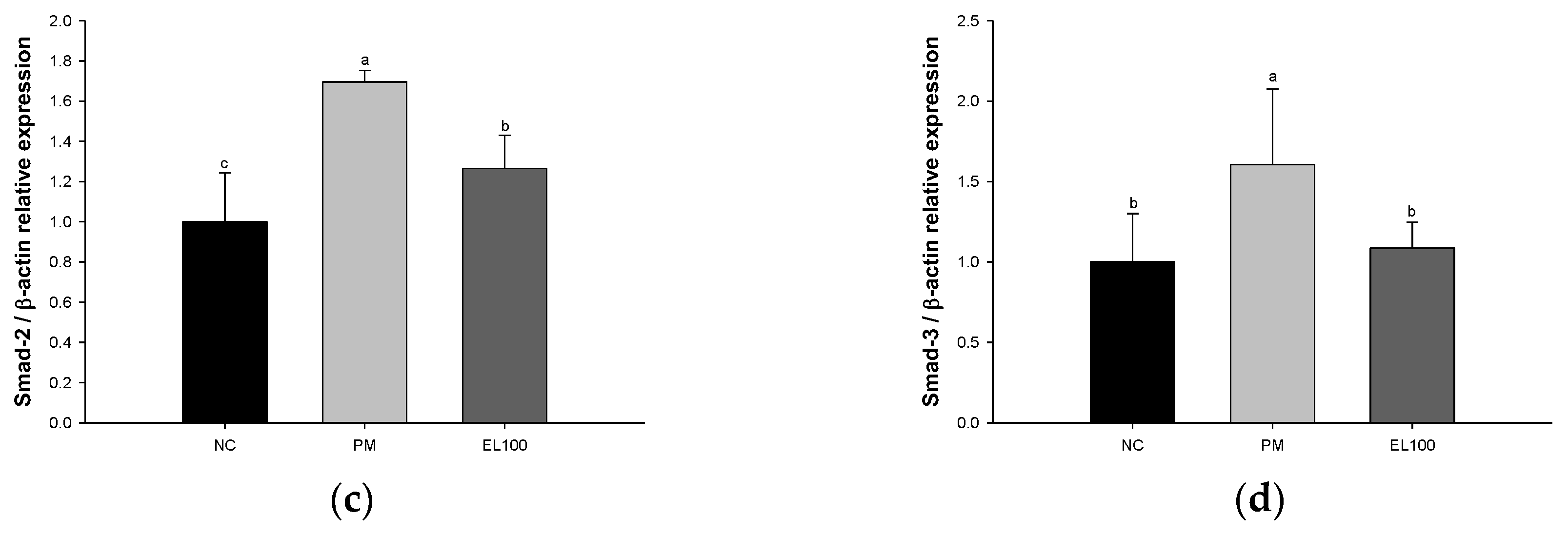
3.8. Protein Expression of Pulmonary Fibrosis
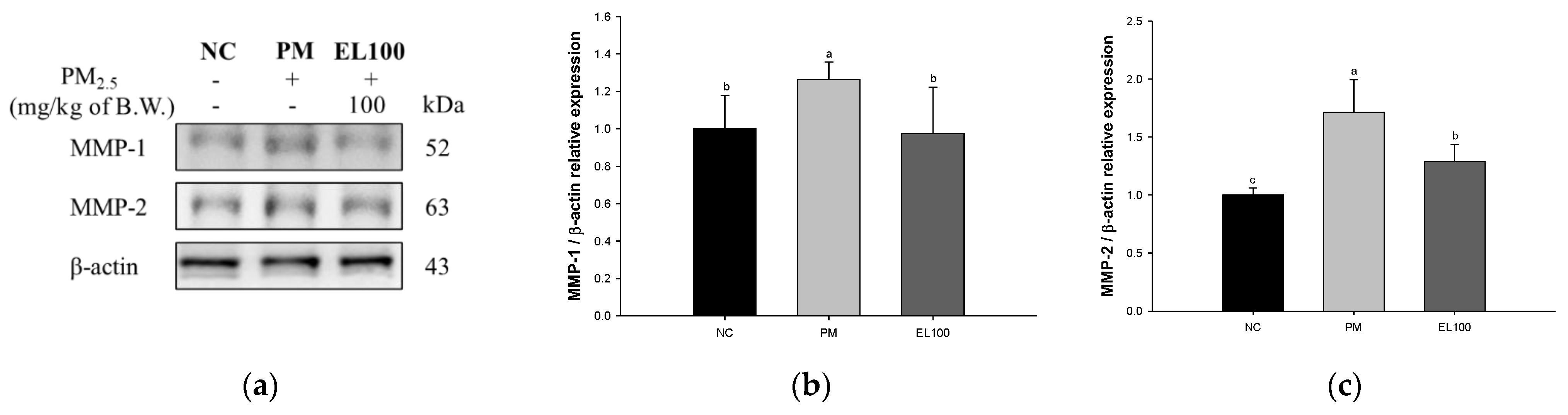
3.9. Protein Expression of Matrix Metalloproteinases (MMPs)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ling, S.H.; van Eeden, S.F. Particulate matter air pollution exposure: Role in the development and exacerbation of chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2009, 4, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Kermani, M.; Rahmatinia, T.; Oskoei, V.; Norzaee, S.; Shahsavani, A.; Farzadkia, M.; Kazemi, M.H. Potential cytotoxicity of trace elements and polycyclic aromatic hydrocarbons bounded to particulate matter: A review on in vitro studies on human lung epithelial cells. Environ. Sci. Pollut. Res. 2021, 28, 55888–55904. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.Y.; Huang, D.Y.; Zhang, H.J.; Wang, S.; Chen, X.F. Exposure to particulate matter 2.5 (PM2.5) induced macrophage-dependent inflammation, characterized by increased Th1/Th17 cytokine secretion and cytotoxicity. Int. Immunopharmacol. 2017, 50, 139–145. [Google Scholar] [CrossRef]
- Mukherjee, A.; Agrawal, S.B.; Agrawal, M. Responses of tropical tree species to urban air pollutants: ROS/RNS formation and scavenging. Sci. Total Environ. 2020, 710, 136363. [Google Scholar] [CrossRef] [PubMed]
- Della Guardia, L.; Shin, A.C. The role of adipose tissue dysfunction in PM2.5-induced vascular pathology. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, 971–972. [Google Scholar] [CrossRef]
- Zhu, R.X.; Nie, X.H.; Chen, Y.H.; Chen, J.; Wu, S.W.; Zhao, L.H. Relationship between particulate matter (PM2.5) and hospitalizations and mortality of chronic obstructive pulmonary disease patients: A meta-analysis. Am. J. Med. Sci. 2020, 359, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.W.; Carey, I.M.; Kent, A.J.; Van Staa, T.P.; Anderson, H.R.; Cook, D.G. Long-term exposure to outdoor air pollution and the incidence of chronic obstructive pulmonary disease in a national English cohort. Occup. Environ. Med. 2015, 72, 42–48. [Google Scholar] [CrossRef]
- Wang, W.; Kang, P.M. Oxidative stress and antioxidant treatments in cardiovascular diseases. Antioxidants 2020, 9, 1292. [Google Scholar] [CrossRef]
- Wyzga, R.E.; Rohr, A.C. Long-term particulate matter exposure: Attributing health effects to individual PM components. J. Air Waste Manag. Assoc. 2015, 65, 523–543. [Google Scholar] [CrossRef]
- Li, Y.; Xie, L.; Liu, K.; Li, X.; Xie, F. Bioactive components and beneficial bioactivities of flowers, stems, leaves of Lonicera japonica Thunberg: A review. Biochem. Syst. Ecol. 2023, 106, 104570. [Google Scholar] [CrossRef]
- Rupasinghe, H.V.; Arumuggam, N.; Amararathna, M.; De Silva, A.B.K.H. The potential health benefits of haskap (Lonicera caerulea L.): Role of cyanidin-3-O-glucoside. J. Funct. Foods 2018, 44, 24–39. [Google Scholar] [CrossRef]
- Xiong, J.; Li, S.; Wang, W.; Hong, Y.; Tang, K.; Luo, Q. Screening and identification of the antibacterial bioactive compounds from Lonicera japonica Thunb. leaves. Food Chem. 2013, 138, 327–333. [Google Scholar] [CrossRef]
- Tzeng, T.F.; Tzeng, Y.C.; Cheng, Y.J.; Liou, S.S.; Liu, I.M. The ethanol extract from Lonicera japonica Thunb. regresses nonalcoholic steatohepatitis in a methionine-and choline-deficient diet-fed animal model. Nutrients 2015, 7, 8670–8684. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, P.; Huang, X.; Wu, X. Validation of the protective effects of Lonicera japonica polysaccharide on lipopolysaccharide-induced learning and memory impairments via regulation of autophagy based on network pharmacology. Ann. Palliat. Med. 2021, 10, 1089–1100. [Google Scholar] [CrossRef]
- Zhou, X.; Lu, Q.; Kang, X.; Tian, G.; Ming, D.; Yang, J. Protective role of a new polysaccharide extracted from Lonicera japonica Thunb in mice with ulcerative colitis induced by dextran sulphate sodium. BioMed Res. Int. 2021, 2021, 8878633. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D.; Kim, Y.; Lee, G.; Kim, S.J.; Jung, S.; Jung, H.; Bae, H. Lipopolysaccharide induced lung inflammation is inhibited by Lonicera japonica. Mol. Cell. Toxicol. 2011, 7, 87–93. [Google Scholar] [CrossRef]
- Lee, H.; Lee, G.; Yoon, M.S.; Hong, M.; Shin, M.; Bae, H. Effect of fermented Lonicera japonica on LPS-induced acute lung inflammation. Orient. Pharm. Exp. Med. 2011, 11, 287–292. [Google Scholar] [CrossRef]
- Hong, S.-H.; Kwon, J.-T.; Shin, J.-Y.; Kim, J.-E.; Minai-Tehrani, A.; Yu, K.-N.; Lee, S.; Park, S.-J.; Chang, S.-H.; Jiang, H.-L.; et al. Therapeutic effect of Broussonetia papyrifera and Lonicera japonica in ovalbumin-induced murine asthma model. Nat. Prod. Commun. 2013, 8, 1609–1614. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Deng, L.; Bartlett, M.; Ren, Y.; Lu, J.; Chen, Q.; Pan, Y.; Wang, H.; Guo, X.; Liu, C. A novel herbal extract blend product prevents particulate matters-induced inflammation by improving gut microbiota and maintaining the integrity of the intestinal barrier. Nutrients 2022, 14, 2010. [Google Scholar] [CrossRef]
- Kim, H.Y.; Yoon, J.J.; Kim, D.S.; Kang, D.G.; Lee, H.S. Yg-1 extract improves acute pulmonary inflammation by inducing bronchodilation and inhibiting inflammatory cytokines. Nutrients 2021, 13, 3414. [Google Scholar] [CrossRef]
- Kim, J.M.; Park, S.K.; Kang, J.Y.; Park, S.B.; Yoo, S.K.; Han, H.J.; Kim, C.W.; Lee, U.; Kim, S.H.; Heo, H.J. Ethyl acetate fraction from persimmon (Diospyros kaki) ameliorates cerebral neuronal loss and cognitive deficit via the JNK/Akt pathway in TMT-induced mice. Int. J. Mol. Sci. 2018, 19, 1499. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Park, S.K.; Kang, J.Y.; Park, S.B.; Yoo, S.K.; Han, H.J.; Cho, K.H.; Kim, J.C.; Heo, H.J. Green tea seed oil suppressed Aβ1–42-induced behavioral and cognitive deficit via the Aβ-related Akt pathway. Int. J. Mol. Sci. 2019, 20, 1865. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Lee, U.; Kang, J.Y.; Park, S.K.; Kim, J.C.; Heo, H.J. Matcha improves metabolic imbalance-induced cognitive dysfunction. Oxidative Med. Cell. Longev. 2020, 2020, 8882763. [Google Scholar] [CrossRef]
- Kim, J.M.; Park, S.K.; Guo, T.J.; Kang, J.Y.; Ha, J.S.; Lee, D.S.; Lee, U.; Heo, H.J. Anti-amnesic effect of Dendropanax morbifera via JNK signaling pathway on cognitive dysfunction in high-fat diet-induced diabetic mice. Behav. Brain Res. 2016, 312, 39–54. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Huang, X.; Zhao, F.L.; Tang, Y.L.; Yin, L. Study on the chemical markers of Caulis Lonicerae japonicae for quality control by HPLC-QTOF/MS/MS and chromatographic fingerprints combined with chemometrics methods. Anal. Methods 2015, 7, 2064–2076. [Google Scholar] [CrossRef]
- Jiang, M.; Han, Y.Q.; Zhou, M.G.; Zhao, H.Z.; Xiao, X.; Hou, Y.Y.; Luo, G.A. The screening research of anti-inflammatory bioactive markers from different flowering phases of Flos Lonicerae Japonicae. PLoS ONE 2014, 9, e96214. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Chen, W.; Zhou, T. A sensitive LC-MS/MS method for simultaneous assay of three iridiods in Gardenia jasminoides Ellis. J. Pharm. Pract. 2019, 6, 19–22. [Google Scholar]
- Xing, Y.F.; Xu, Y.H.; Shi, M.H.; Lian, Y.X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, 69–74. [Google Scholar]
- Xiong, R.; Jiang, W.; Li, N.; Liu, B.; He, R.; Wang, B.; Geng, Q. PM2.5-induced lung injury is attenuated in macrophage-specific NLRP3 deficient mice. Ecotoxicol. Environ. Safe 2021, 221, 112433. [Google Scholar] [CrossRef]
- Zhu, X.; Ji, X.; Shou, Y.; Huang, Y.; Hu, Y.; Wang, H. Recent advances in understanding the mechanisms of PM2. 5-mediated neurodegenerative diseases. Toxicol. Lett. 2020, 329, 31–37. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, D.; Qin, L.; Yang, X.; Gao, C. Comparative investigation for hypoglycemic effects of polysaccharides from four substitutes of Lonicera japonica in Chinese medicine. Int. J. Biol. Macromol. 2018, 109, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Jun, C.H.; Song, C.H. Inhibitory effect of Lonicera japonica Thunb. flower buds against glutamate-induced cytotoxicity in HT22 hippocampal neurons. Korean J. Acupunct. 2021, 38, 32–42. [Google Scholar] [CrossRef]
- Bang, B.W.; Park, D.; Kwon, K.S.; Lee, D.H.; Jang, M.J.; Park, S.K.; Kim, J.Y. BST-104, a water extract of Lonicera japonica, has a gastroprotective effect via antioxidant and anti-inflammatory activities. J. Med. Food 2019, 22, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, S.; Chen, L.; Zhang, L.; Meng, F.; Sha, S.; Ai, C.; Tai, J. Chlorogenic acid ameliorates lead-induced renal damage in mice. Biol. Trace Elem. Res. 2019, 189, 109–117. [Google Scholar] [CrossRef]
- Bardestani, A.; Ebrahimpour, S.; Esmaeili, A.; Esmaeili, A. Quercetin attenuates neurotoxicity induced by iron oxide nanoparticles. J. Nanobiotechnol. 2021, 19, 327. [Google Scholar] [CrossRef]
- Guo, Z.; Hong, Z.; Dong, W.; Deng, C.; Zhao, R.; Xu, J.; Zhuang, G.; Zhang, R. PM2.5-induced oxidative stress and mitochondrial damage in the nasal mucosa of rats. Int. J. Environ. Res. Public Health 2017, 14, 134. [Google Scholar] [CrossRef]
- Ning, X.; Ji, X.; Li, G.; Sang, N. Ambient PM2.5 causes lung injuries and coupled energy metabolic disorder. Ecotoxicol. Environ. Safe 2019, 170, 620–626. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, D.; Wu, W.; Huo, X. PM2.5 exposure inducing ATP alteration links with NLRP3 inflammasome activation. Environ. Sci. Pollut. Res. 2022, 29, 24445–24456. [Google Scholar] [CrossRef]
- Li, R.; Kou, X.; Geng, H.; Xie, J.; Yang, Z.; Zhang, Y.; Dong, C. Effect of ambient PM2.5 on lung mitochondrial damage and fusion/fission gene expression in rats. Chem. Res. Toxicol. 2015, 28, 408–418. [Google Scholar] [CrossRef]
- Ma, C.J.; Weon, J.B.; Lee, B.; Ahn, J.H.; Yang, H.J.; Yun, B.-R.; Lee, H.Y. Neuroprotective activity of the methanolic extract of Lonicera japonica in glutamate-injured primary rat cortical cells. Pharmacogn. Mag. 2011, 7, 284–288. [Google Scholar] [CrossRef]
- Kwon, S.H.; Hong, S.I.; Jung, Y.H.; Kim, M.J.; Kim, S.Y.; Kim, H.C.; Lee, S.Y.; Jang, C.G. Lonicera japonica THUNB. protects 6-hydroxydopamine-induced neurotoxicity by inhibiting activation of MAPKs, PI3K/Akt, and NF-κB in SH-SY5Y cells. Food Chem. Toxicol. 2012, 50, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; He, J.; Cheng, Y.; Xu, J.; Zhang, W. Biologically active secoiridoids: A comprehensive update. Med. Res. Rev. 2023, 2023, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.Y.; Park, S.K.; Guo, T.J.; Ha, J.S.; Lee, D.S.; Kim, J.M.; Lee, U.; Kim, D.O.; Heo, H.J. Reversal of trimethyltin-induced learning and memory deficits by 3, 5-dicaffeoylquinic acid. Oxidative Med. Cell. Longev. 2016, 2016, 6981595. [Google Scholar] [CrossRef] [PubMed]
- Sigaud, S.; Goldsmith, C.A.W.; Zhou, H.; Yang, Z.; Fedulov, A.; Imrich, A.; Kobzik, L. Air pollution particles diminish bacterial clearance in the primed lungs of mice. Toxicol. Appl. Pharmacol. 2007, 223, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, Y.; Yu, Z.; Ding, H.; Ma, Z. The influence of PM2.5 on lung injury and cytokines in mice. Exp. Ther. Med. 2019, 18, 2503–2511. [Google Scholar] [CrossRef]
- He, M.; Ichinose, T.; Yoshida, S.; Nishikawa, M.; Mori, I.; Yanagisawa, R.; Takano, H.; Inoue, K.; Sun, G.; Shibamoto, T. Urban particulate matter in Beijing, China, enhances allergen-induced murine lung eosinophilia. Inhal. Toxicol. 2010, 22, 709–718. [Google Scholar] [CrossRef]
- Kim, J.M.; Kang, J.Y.; Park, S.K.; Moon, J.H.; Kim, M.J.; Lee, H.L.; Jeong, H.R.; Kim, J.C.; Heo, H.J. Powdered green tea (matcha) attenuates the cognitive dysfunction via the regulation of systemic inflammation in chronic PM2.5-exposed BALB/c mice. Antioxidants 2021, 10, 1932. [Google Scholar] [CrossRef]
- Park, J.; Kim, Y.; Lee, C.; Kim, Y.T. 3,5-Dicaffeoylquinic acid attenuates microglial activation-mediated inflammatory pain by enhancing autophagy through the suppression of MCP3/JAK2/STAT3 signaling. Biomed. Pharmacother. 2022, 153, 113549. [Google Scholar] [CrossRef]
- Hong, S.; Joo, T.; Jhoo, J.W. Antioxidant and anti-inflammatory activities of 3,5-dicaffeoylquinic acid isolated from Ligularia fischeri leaves. Food Sci. Biotechnol. 2015, 24, 257–263. [Google Scholar] [CrossRef]
- Jang, G.; Lee, S.; Hong, J.; Park, B.; Kim, D.; Kim, C. Anti-inflammatory effect of 4,5-dicaffeoylquinic acid on RAW264.7 cells and a rat model of inflammation. Nutrients 2021, 13, 3537. [Google Scholar] [CrossRef]
- Guo, X.; Yu, X.; Zheng, B.; Zhang, L.; Zhang, F.; Zhang, Y.; Li, J.; Pu, G.; Zhang, L.; Wu, H. Network pharmacology-based identification of potential targets of Lonicerae japonicae flos acting on anti-inflammatory effects. BioMed Res. Int. 2021, 2021, 5507003. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.C.; Jin, M.; Kim, S.H.; Kim, M.H.; Namgung, U.; Yeo, Y. Effects of inhalable microparticle of flower of Lonicera japonica in a mouse model of COPD. J. Ethnopharmacol. 2014, 151, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Speyer, C.L.; Neff, T.A.; Warner, R.L.; Guo, R.F.; Sarma, J.V.; Riedemann, N.C.; Murphy, M.E.; Murphy, H.S.; Ward, P.A. Regulatory effects of iNOS on acute lung inflammatory responses in mice. Am. J. Pathol. 2003, 163, 2319–2328. [Google Scholar] [CrossRef] [PubMed]
- Olson, T.S.; Ley, K. Chemokines and chemokine receptors in leukocyte trafficking. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R7–R28. [Google Scholar] [CrossRef]
- Weinberger, B.; Laskin, D.L.; Heck, D.E.; Laskin, J.D. The toxicology of inhaled nitric oxide. Toxicol. Sci. 2001, 59, 5–16. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, Y.; Zhang, Y.; Fu, Y.; Yang, Z.; Fan, Y.; Sun, Z.; Zhao, M.; Zhu, L.; Dai, B.; et al. EGFR inhibitors regulate Ca2+ concentration and apoptosis after PM2.5 exposure based on a lung-mimic microfluidic system. Sci. Total Environ. 2021, 761, 143200. [Google Scholar] [CrossRef]
- Foo, J.; Bellot, G.; Pervaiz, S.; Alonso, S. Mitochondria-mediated oxidative stress during viral infection. Trends Microbiol. 2022, 30, 679–692. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, W.; Zhang, F.; Roepstorff, P.; Yang, F.; Lu, Z.; Ding, W. TMT-based quantitative proteomics analysis reveals airborne PM2.5-induced pulmonary fibrosis. Int. J. Environ. Res. Public Health 2019, 16, 98. [Google Scholar] [CrossRef]
- Xiao, L.; Liang, S.; Ge, L.; Wan, H.; Wu, W.; Fei, J.; Wu, S.; Zhou, B.; Zeng, X. 4,5-di-O-caffeoylquinic acid methyl ester isolated from Lonicera japonica Thunb. targets the Keap1/Nrf2 pathway to attenuate H2O2-induced liver oxidative damage in HepG2 cells. Phytomedicine 2020, 70, 153219. [Google Scholar] [CrossRef]
- Han, D.; Chen, W.; Gu, X.; Shan, R.; Zou, J.; Liu, G.; Shahid, M.; Gao, J.; Han, B. Cytoprotective effect of chlorogenic acid against hydrogen peroxide-induced oxidative stress in MC3T3-E1 cells through PI3K/Akt-mediated Nrf2/HO-1 signaling pathway. Oncotarget 2017, 8, 14680–14692. [Google Scholar] [CrossRef]
- Saito, A.; Horie, M.; Nagase, T. TGF-β signaling in lung health and disease. Int. J. Mol. Sci. 2018, 19, 2460. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, K.A.; Skoda, M.; Koczurkiewicz, P.; Sanak, M.; Czyż, J.; Michalik, M. Apigenin inhibits TGF-β1 induced fibroblast-to-myofibroblast transition in human lung fibroblast populations. Pharmacol. Rep. 2013, 65, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Yu, H.; Hao, J.J.; Peng, Y.; Yin, T.T.; Qiu, Y.N. PM2.5 promotes Drp1-mediated mitophagy to induce hepatic stellate cell activation and hepatic fibrosis via regulating miR-411. Exp. Cell Res. 2021, 407, 112828. [Google Scholar] [CrossRef]
- Ma, K.; Li, C.; Xu, J.; Ren, F.; Xu, X.; Liu, C.; Niu, B.; Li, F. LncRNA Gm16410 regulates PM2.5-induced lung endothelial-mesenchymal transition via the TGF-β1/Smad3/p-Smad3 pathway. Ecotoxicol. Environ. Saf. 2020, 205, 111327. [Google Scholar] [CrossRef]
- Liu, S.; Chen, S.; Zeng, J. TGF-β signaling: A complex role in tumorigenesis. Mol. Med. Rep. 2018, 17, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Li, Y.; Kou, X.; Xue, Z. Mechanisms of biochanin a alleviating PM2.5 organic extracts-induced EMT of A549 cells through the PI3K/Akt pathway. J. Nat. Prod. 2022, 85, 2290–2301. [Google Scholar] [CrossRef]
- Wang, Y.C.; Dong, J.; Nie, J.; Zhu, J.X.; Wang, H.; Chen, Q.; Chen, J.Y.; Xia, J.M.; Shuai, W. Amelioration of bleomycin-induced pulmonary fibrosis by chlorogenic acid through endoplasmic reticulum stress inhibition. Apoptosis 2017, 22, 1147–1156. [Google Scholar] [CrossRef]
- Ganesan, S.; Faris, A.N.; Comstock, A.T.; Chattoraj, S.S.; Chattoraj, A.; Burgess, J.R.; Curtis, J.L.; Martinez, F.J.; Zick, S.; Hershenson, M.B.; et al. Quercetin prevents progression of disease in elastase/LPS-exposed mice by negatively regulating MMP expression. Respir. Res. 2010, 11, 131. [Google Scholar] [CrossRef]
- Ouchi, H.; Fujita, M.; Ikegame, S.; Ye, Q.; Inoshima, I.; Harada, E.; Kuwano, K.; Nakanishi, Y. The role of collagenases in experimental pulmonary fibrosis. Pulm. Pharmacol. Ther. 2008, 21, 401–408. [Google Scholar] [CrossRef]
- Chuliá-Peris, L.; Carreres-Rey, C.; Gabasa, M.; Alcaraz, J.; Carretero, J.; Pereda, J. Matrix metalloproteinases and their inhibitors in pulmonary fibrosis: EMMPRIN/CD147 comes into play. Int. J. Mol. Sci. 2022, 23, 6894. [Google Scholar] [CrossRef]





| Antibody | Catalog | Concentration | Manufacturer |
|---|---|---|---|
| TLR-4 | sc-52962 | 1:1000 | Santa Cruz Biotech (Dallas, TX, USA) |
| p-JNK | sc-6254 | 1:1000 | Santa Cruz Biotech (Dallas, TX, USA) |
| p-NF-κB | 3033 | 1:1000 | Cell Signaling Tech (Danvers, MA, USA) |
| p-IκB-α | #sc-8404 | 1:1000 | Santa Cruz Biotech (Dallas, TX, USA) |
| COX-2 | sc-376861 | 1:1000 | Santa Cruz Biotech (Dallas, TX, USA) |
| iNOS | sc-7271 | 1:1000 | Santa Cruz Biotech (Dallas, TX, USA) |
| TNF-α | sc-393887 | 1:1000 | Santa Cruz Biotech (Dallas, TX, USA) |
| IL-1β | sc-4592 | 1:1000 | Santa Cruz Biotech (Dallas, TX, USA) |
| BCl-2 | sc-509 | 1:1000 | Santa Cruz Biotech (Dallas, TX, USA) |
| BAX | sc-7480 | 1:1000 | Santa Cruz Biotech (Dallas, TX, USA) |
| Caspase-3 | CSB-PA05689A0Rb | 1:1000 | Cusabio (Wuhan, China) |
| TGF-β1 | sc-130348 | 1:1000 | Santa Cruz Biotech (Dallas, TX, USA) |
| p-Smad-2 | #3108 | 1:1000 | Cell Signaling Tech (Danvers, MA, USA) |
| p-Smad-3 | sc-517575 | 1:1000 | Santa Cruz Biotech (Dallas, TX, USA) |
| MMP-1 | sc-21731 | 1:1000 | Santa Cruz Biotech (Dallas, TX, USA) |
| MMP-2 | sc-13595 | 1:1000 | Santa Cruz Biotech (Dallas, TX, USA) |
| β-actin | 66009-1-Ig | 1:1000 | Proteintech (Rosemont, IL, USA) |
| No. | Retention Time (min) | Parent Ion (m/z) | MSE Fragment (m/z) | Compound |
|---|---|---|---|---|
| 1 | 2.92 | 391 | 149,167 | Shanzhiside |
| 2 | 3.00 | 389 | 165,183,345 | Secologanoside |
| 3 | 3.36 | 375 | 99,116,151,195,341 | Loganic acid |
| 4 | 3.41 | 353 | 191 | Chlorogenic acid |
| 5 | 3.49 | 373 | 149,167,179,193 | Secologanic acid |
| 6 | 3.78 | 403 | 121,165,223,371 | Secoxyloganin |
| 7 | 3.83 | 433 | 271,300,301 | Quercetin pentoside |
| 8 | 4.11 | 515 | 133,135,179,191,353 | 3,4-O-DCQA * |
| 9 | 4.14 | 515 | 116,179,191,353 | 3,5-O-DCQA |
| 10 | 4.19 | 515 | 135,173,179,191,353 | 4,5-O-DCQA |
| 11 | 4.26 | 515 | 161,173,191,353 | 1,4-O-DCQA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.L.; Kim, J.M.; Go, M.J.; Kim, T.Y.; Joo, S.G.; Kim, J.H.; Lee, H.S.; Kim, H.-J.; Heo, H.J. Protective Effect of Lonicera japonica on PM2.5-Induced Pulmonary Damage in BALB/c Mice via the TGF-β and NF-κB Pathway. Antioxidants 2023, 12, 968. https://doi.org/10.3390/antiox12040968
Lee HL, Kim JM, Go MJ, Kim TY, Joo SG, Kim JH, Lee HS, Kim H-J, Heo HJ. Protective Effect of Lonicera japonica on PM2.5-Induced Pulmonary Damage in BALB/c Mice via the TGF-β and NF-κB Pathway. Antioxidants. 2023; 12(4):968. https://doi.org/10.3390/antiox12040968
Chicago/Turabian StyleLee, Hyo Lim, Jong Min Kim, Min Ji Go, Tae Yoon Kim, Seung Gyum Joo, Ju Hui Kim, Han Su Lee, Hyun-Jin Kim, and Ho Jin Heo. 2023. "Protective Effect of Lonicera japonica on PM2.5-Induced Pulmonary Damage in BALB/c Mice via the TGF-β and NF-κB Pathway" Antioxidants 12, no. 4: 968. https://doi.org/10.3390/antiox12040968
APA StyleLee, H. L., Kim, J. M., Go, M. J., Kim, T. Y., Joo, S. G., Kim, J. H., Lee, H. S., Kim, H.-J., & Heo, H. J. (2023). Protective Effect of Lonicera japonica on PM2.5-Induced Pulmonary Damage in BALB/c Mice via the TGF-β and NF-κB Pathway. Antioxidants, 12(4), 968. https://doi.org/10.3390/antiox12040968







