Garlicnin B1, an Active Cyclic Sulfide from Garlic, Exhibits Potent Anti-Inflammatory and Anti-Tumor Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction and Purification of Garlicnin B1
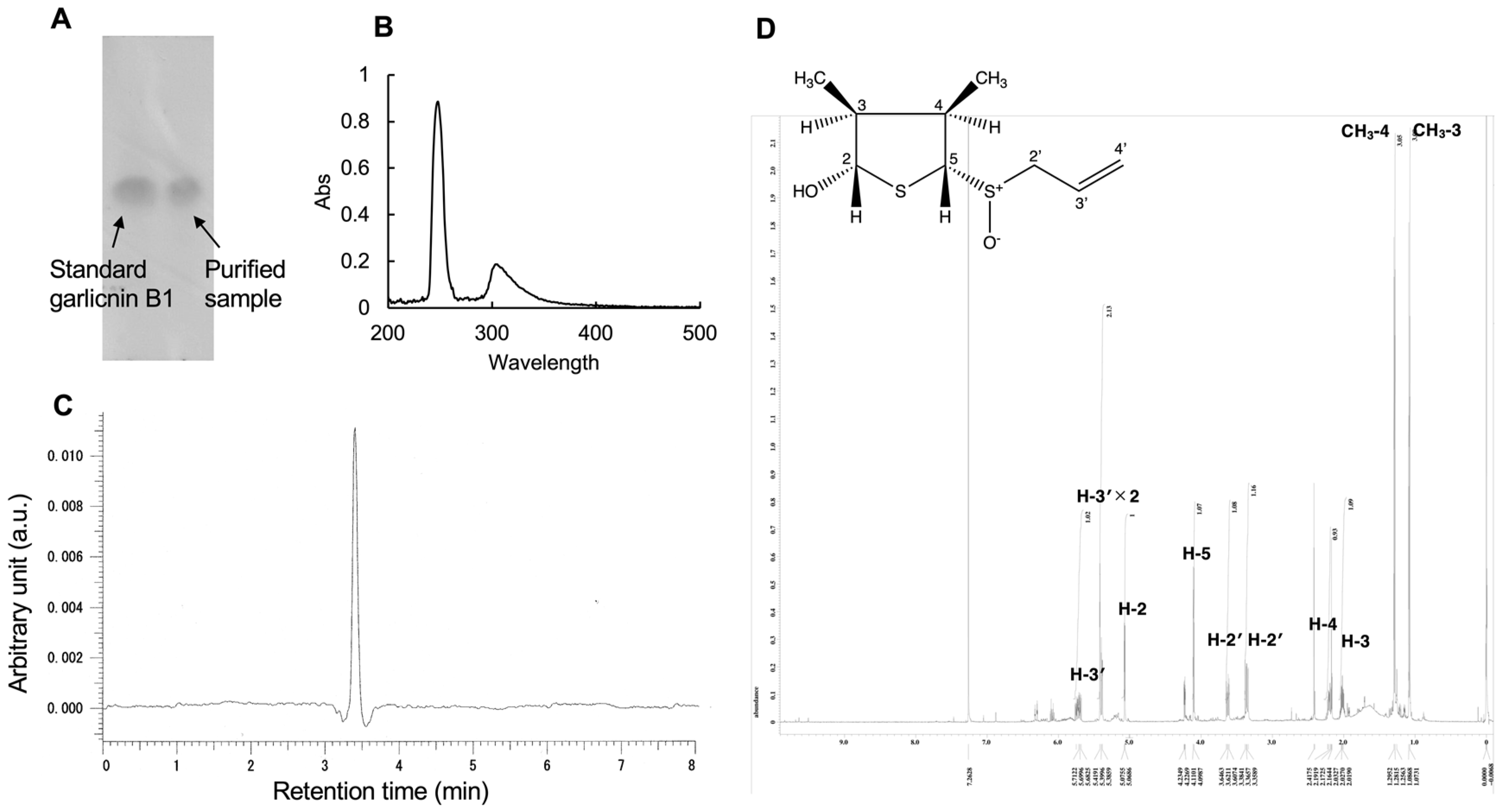
2.3. UV–Visible Spectrophotometry
2.4. Nuclear Magnetic Resonance (NMR)
2.5. High-Performance Liquid Chromatography
2.6. Animals
2.7. In Vivo Anti-Inflammatory Effect of Garlicnin B1
2.8. In Vivo Anti-Tumor Effect of Garlicnin B1
2.9. In Vitro Cytotoxicity of Garlicnin B1
2.10. Evaluation of the Anti-Oxidative Effect of Garlicnin B1 on C26 Cancer Cells
2.11. Statistical Analyses
3. Results
3.1. Purification of Garlicnin B1
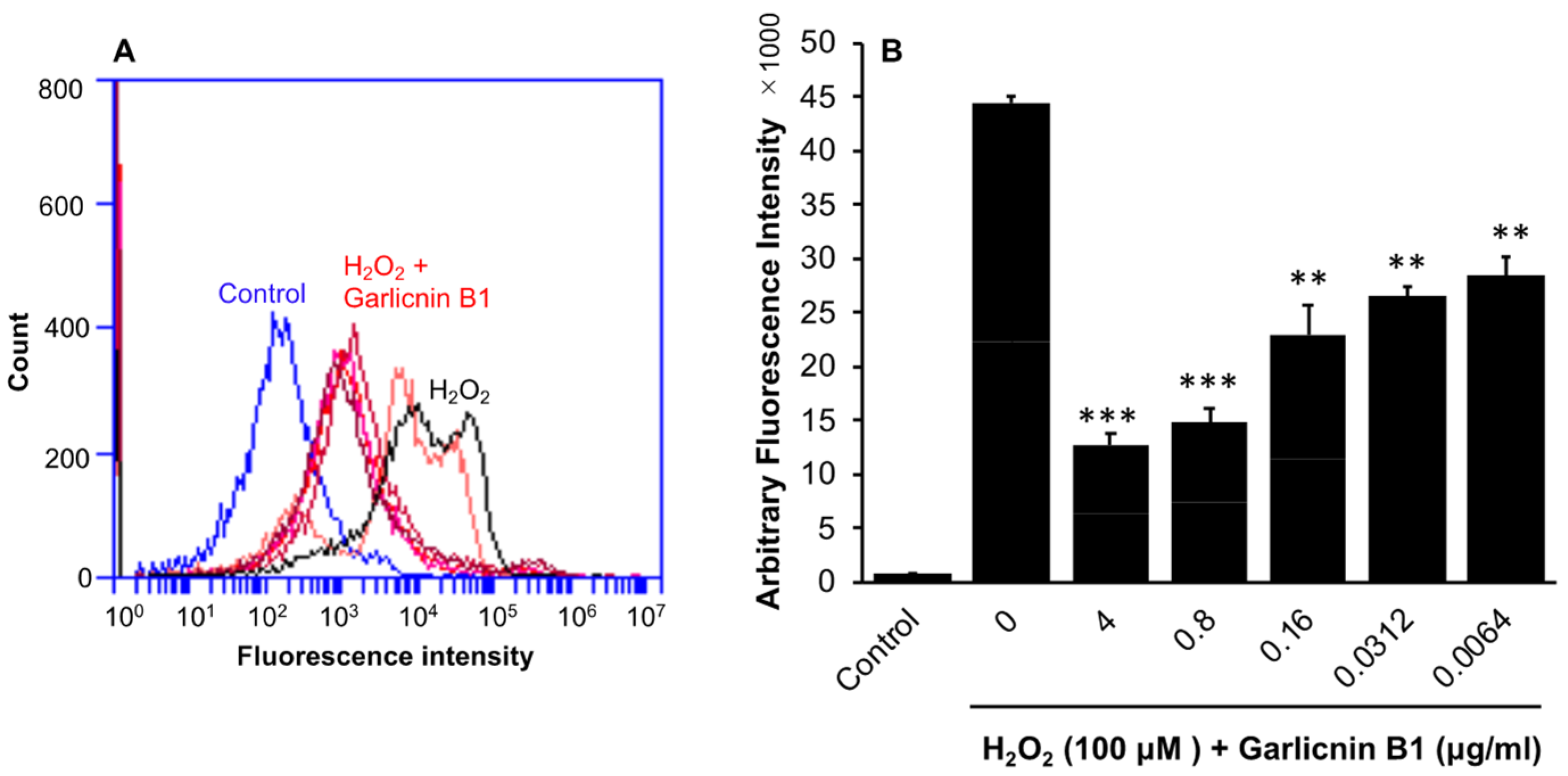
3.2. Suppression of Intracellular ROS Triggered by H2O2 Treatment by Garlicnin B1
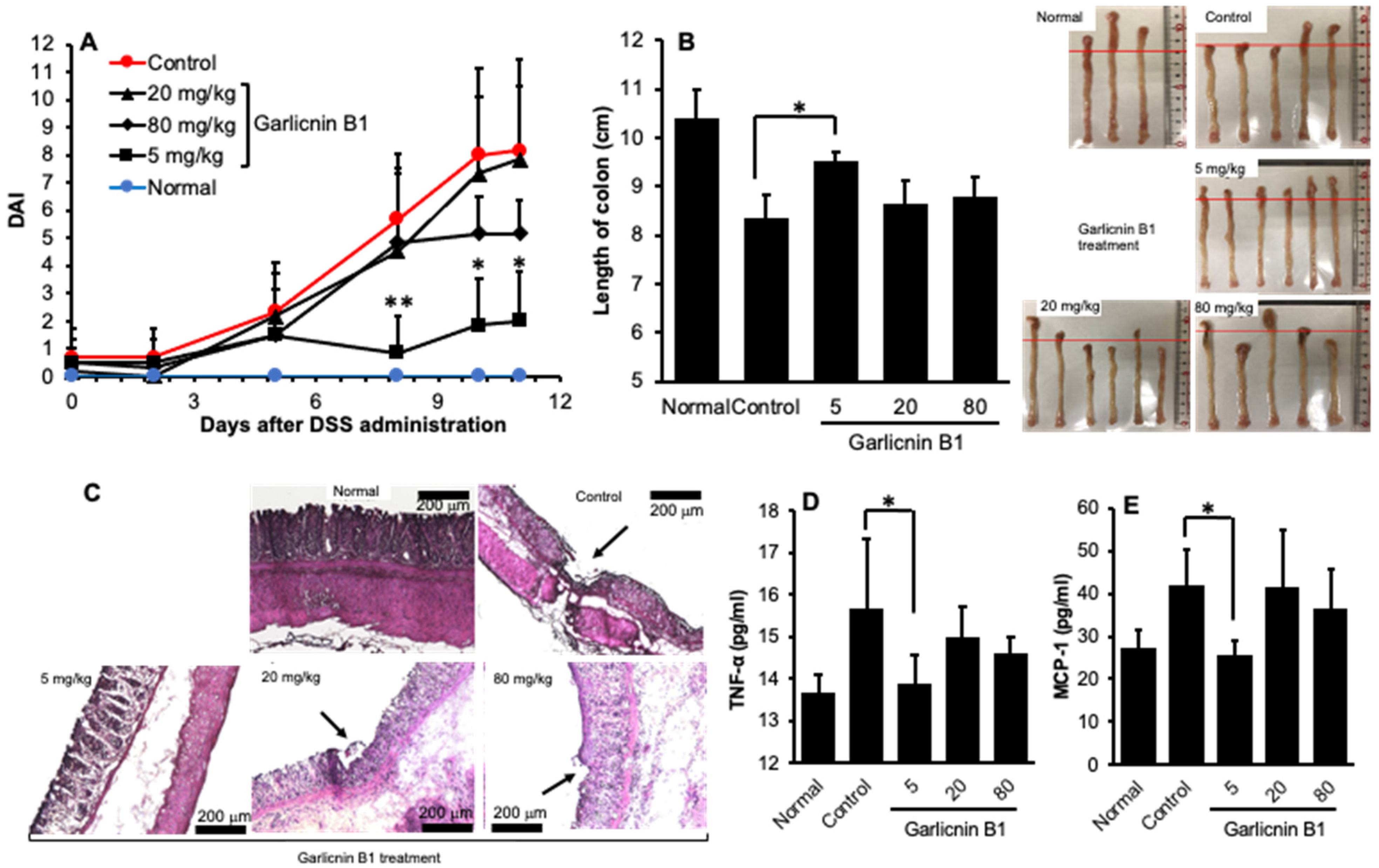
3.3. Garlicnin B1 Exerts Remarkable Anti-Inflammatory Effect against DSS-Induced Mouse Colitis
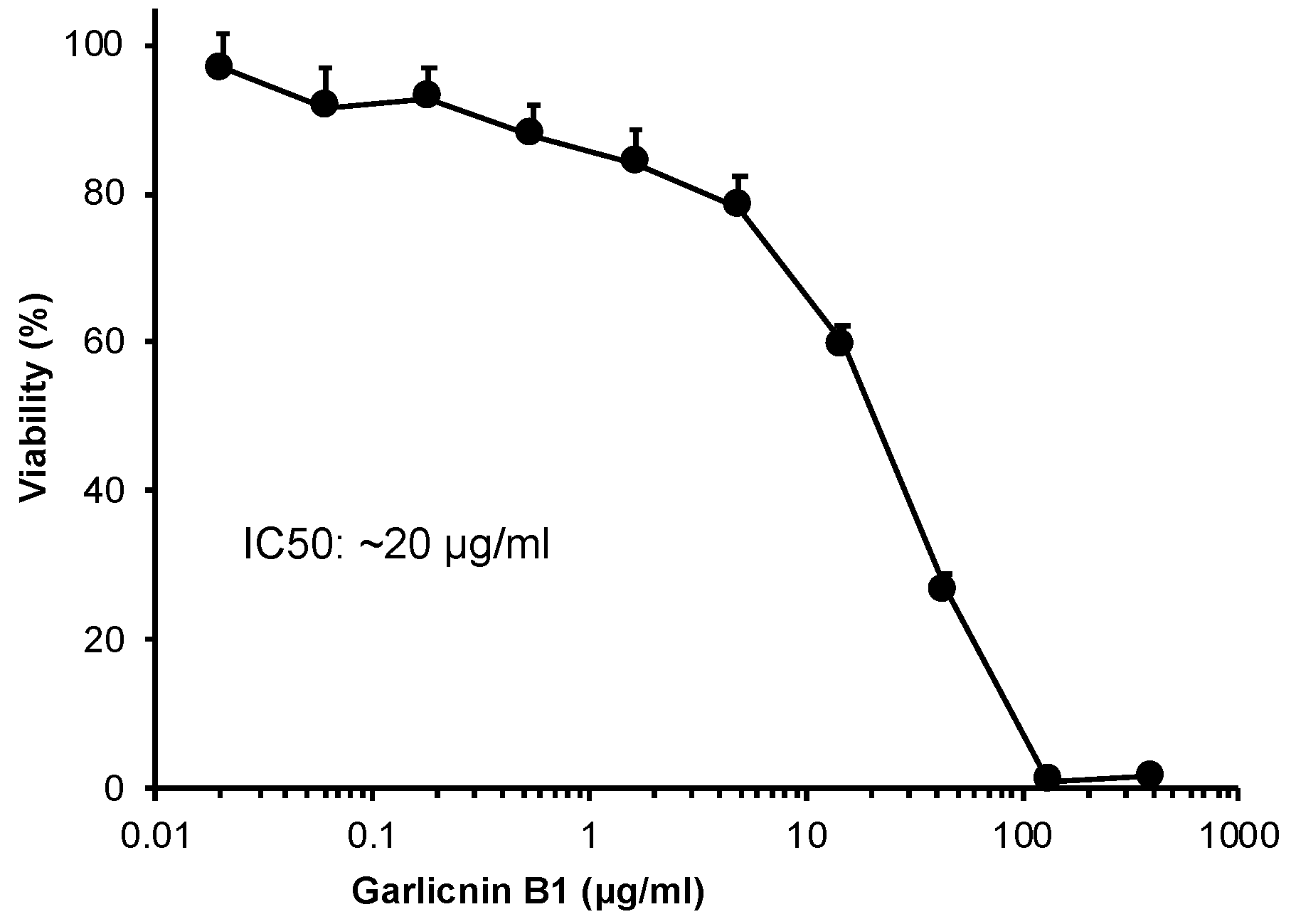
3.4. In Vitro Cytotoxicity of Garlicnin B1
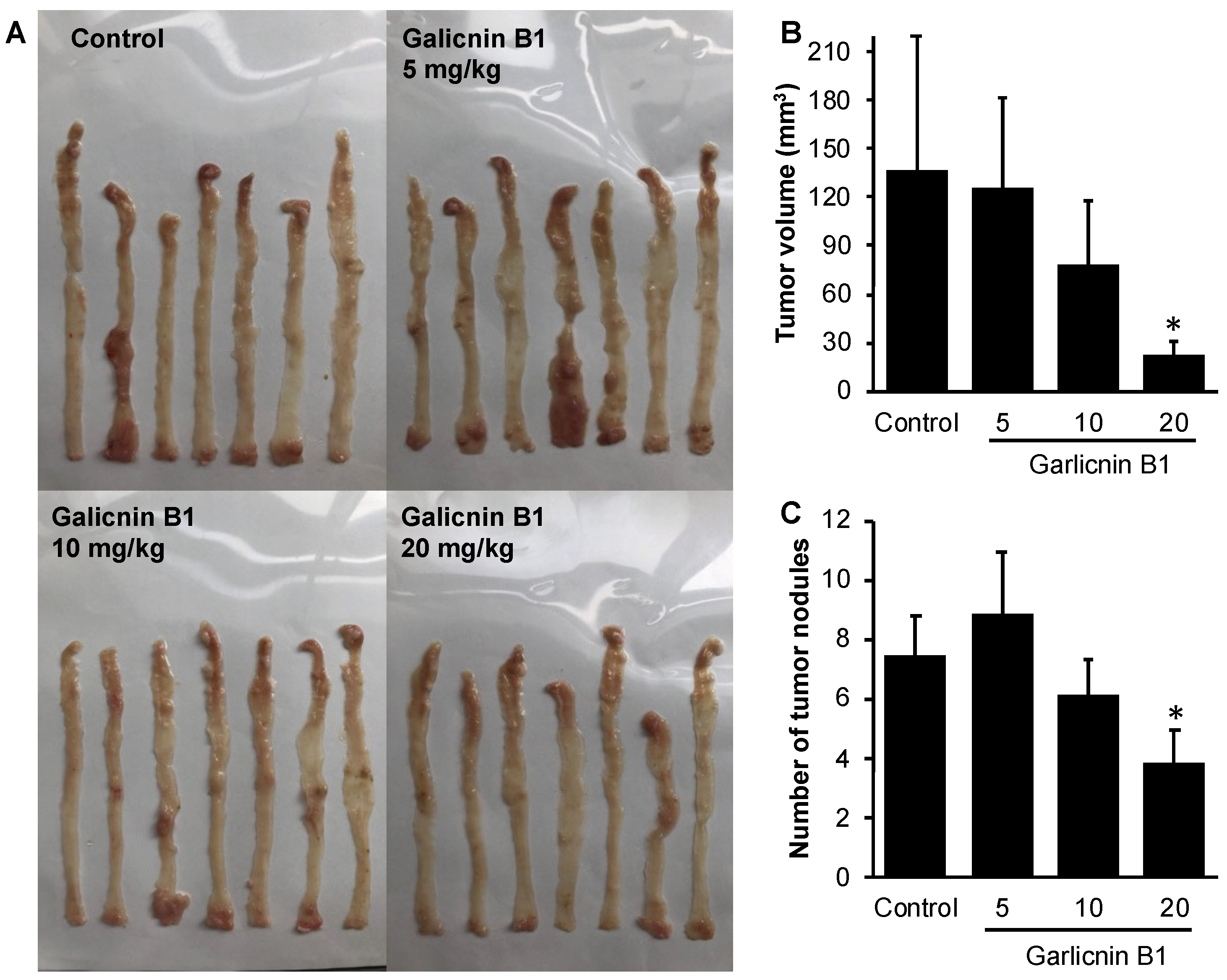
3.5. Garlicnin B1 Exhibits Potent Anti-Cancer Effect in AOM/DSS-Induced Colon Cancer Model
3.6. Garlicnin B1 Exhibits Potent Cancer Therapeutic Effect in S180 Solid Tumor Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dzobo, K. The role of natural products as sources of therapeutic agents for innovative drug discovery. In Comprehensive Pharmacology, 1st ed.; Kenakin, T., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 408–422. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Ruan, J.; Zhuang, X.; Zhang, X.; Li, Z. Phytochemicals of garlic: Promising candidates for cancer therapy. Biomed. Pharmacother. 2020, 123, 109730. [Google Scholar] [CrossRef]
- Rauf, A.; Abu-Izneid, T.; Thiruvengadam, M.; Imran, M.; Olatunde, A.; Shariati, M.I.; Bawazeer, S.; Naz, S.; Shirooie, S.; Sanches-Silva, A.; et al. Garlic (Allium sativum L.): Its chemistry, nutritional composition, toxicity, and anticancer properties. Curr. Top. Med. Chem. 2022, 22, 957–972. [Google Scholar] [CrossRef] [PubMed]
- Nicastro, H.L.; Ross, S.A.; Milner, J.A. Garlic and onions: Their cancer prevention properties. Cancer Prev. Res. 2015, 8, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Stoll, A.; Seebeck, E. Chemical investigations on alliin, the specific principle of garlic. Adv. Enzymol. Relat. Subj. Biochem. 1951, 11, 377–400. [Google Scholar] [CrossRef] [PubMed]
- Cavallito, C.J.; Bailey, J.H. Preliminary note on the inactivation of antibiotics. Science 1944, 100, 390. [Google Scholar] [CrossRef] [PubMed]
- Milner, J.A. Preclinical perspectives on garlic and cancer. J. Nutr. 2006, 136, 827S–831S. [Google Scholar] [CrossRef] [PubMed]
- Block, E. The chemistry of garlic and onions. Sci. Am. 1985, 252, 114–119. [Google Scholar] [CrossRef]
- Fenwick, G.R.; Hanley, A.B. The genus allium—Part 3. Crit. Rev. Food Sci. Nutr. 1985, 23, 1–73. [Google Scholar] [CrossRef]
- Gao, S.; Nohara, T.; Ikeda, T.; Zhou, J.; Yokomizo, K. Novel acyclic sulfide, gralicin L-5, from garlic. Curr. Top. Phytochem. 2021, 17, 29–34. [Google Scholar]
- Nohara, T.; EI-Asar, M.; Ikeda, T.; Gao, S.; Yokomizo, K. Determination of absolute configuration of the most abundant garlic sulfide, garlicnin B1. Curr. Top. Phytochem. 2021, 17, 55–61. [Google Scholar]
- Tsuboki, J.; Fujiwara, Y.; Horlad, H.; Shiraishi, D.; Nohara, T.; Tayama, S.; Motohara, T.; Saito, Y.; Ikeda, T.; Takaishi, K.; et al. Onionin A inhibits ovarian cancer progression by suppressing cancer cell proliferation and the protumour function of macrophages. Sci. Rep. 2016, 6, 29588. [Google Scholar] [CrossRef]
- Fujiwaran, Y.; Horlad, H.; Shiraishi, D.; Tsuboki, J.; Kudo, R.; Ikeda, T.; Nohara, T.; Takeya, M.; Komohara, Y. Onionin A, a sulfur-containing compound isolated from onions, impairs tumor development and lung metastasis by inhibiting the protumoral and immunosuppressive functions of myeloid cells. Mol. Nutr. Food Res. 2016, 60, 2467–2480. [Google Scholar] [CrossRef] [PubMed]
- Nohara, T.; Fujiwara, Y.; El-Aasr, M.; Ikeda, T.; Ono, M.; Nakano, D.; Kinjo, J. Antitumor allium sulfides. Chem. Pharm. Bull. 2017, 65, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Dale, J.A.; Mosher, H.S. Nuclear magnetic resonance enantiomer regents. Configurational correlations via nuclear magnetic resonance chemical shifts of diastereomeric mandelate, O-methylmandelate, and.alpha.-methoxy-.alpha.-trifluoromethylphenylacetate (MTPA) esters. J. Am. Chem. Soc. 1973, 95, 512–519. [Google Scholar] [CrossRef]
- Sullivan, G.R.; Dale, J.A.; Mosher, H.S. Correlation of configuration and fluorine-19 chemical shifts of.alpha.-methoxy-.alpha.-trifluoromethylphenyl acetate derivatives. J. Org. Chem. 1973, 38, 2143–2147. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Beshbishy, A.M.; Wasef, L.G.; Elewa, Y.H.A.; Al-Sagan, A.A.; El-Hack, M.E.A.; Taha, A.E.; Abd-Elhakim, Y.M.; Devkota, H.P. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): A review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef]
- Zhang, T.; Tsutsuki, H.; Ono, K.; Akaike, T.; Sawa, T. Antioxidative and anti-inflammatory actions of reactive cysteine persulfides. J. Clin. Biochem. Nutr. 2021, 68, 5–8. [Google Scholar] [CrossRef]
- Wirtz, S.; Neufert, C.; Weigmann, B.; Neurath, M.F. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2007, 2, 541–546. [Google Scholar] [CrossRef]
- Yin, H.; Fang, J.; Liao, L.; Nakamura, H.; Maeda, H. Styrene-maleic acid copolymer-encapsulated CORM2, a water-soluble carbon monoxide (CO) donor with a constant CO-releasing property, exhibits therapeutic potential for inflammatory bowel disease. J. Control. Release 2014, 187, 14–21. [Google Scholar] [CrossRef]
- Islam, W.; Fang, J.; Imamura, T.; Etrych, T.; Subr, V.; Ulbrich, K.; Maeda, H. Augmentation of the enhanced permeability and retention effect with nitric oxide-generating agents improves the therapeutic effects of nanomedicines. Mol. Cancer Ther. 2018, 17, 2643–2653. [Google Scholar] [CrossRef]
- Fang, J.; Gao, S.; Islam, R.; Teramoto, Y.; Maeda, H. Extracts of Phellinus linteus, bamboo (Sasa senanensis) leaf and chaga mushroom (Inonotus obliquus) exhibit antitumor activity through activating innate immunity. Nutrients 2020, 12, 2279. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, H.; Khaturia, S.; Saquib, M.; Khatik, N.; Khandelwal, A.R.; Meena, R.; Sharma, K. Oxygen- and sulphur-containing heterocyclic compounds as potential anticancer agents. Appl. Biochem. Biotechnol. 2022, 194, 6438–6467. [Google Scholar] [CrossRef]
- Keri, R.S.; Chand, K.; Budagumpi, S.; Balappa Somappa, S.; Patil, S.A.; Nagaraja, B.M. An overview of benzo[b]thiophene-based medicinal chemistry. Eur. J. Med. Chem. 2017, 138, 1002–1033. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, A.; Parker, R.D.; Abdollahi, M. Oxidative stress and pathogenesis of inflammatory bowel disease: An epiphenomenon or the cause? Dig. Dis. Sci. 2007, 52, 2015–2021. [Google Scholar] [CrossRef]
- Pavlick, K.P.; Laroux, F.S.; Fuseler, J.; Wolf, R.E.; Gray, L.; Hoffman, J.; Grisham, M.B. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic. Biol. Med. 2002, 33, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Baldwin, L.A. U-shaped dose-responses in biology, toxicology, and public health. Annu. Rev. Public Health 2001, 22, 15–33. [Google Scholar] [CrossRef]
- Seki, T.; Kida, K.; Maeda, H. Immunostimulation-Mediated Anti-tumor Activity of Bamboo (Sasa senanensis) Leaf Extracts Obtained Under ‘Vigorous’ Condition. Evid. Based Complement Altern. Med. 2010, 7, 447–457. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the anti- tumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Maeda, H. The link between infection and cancer: Tumor vasculature, free radicals, and drug delivery to tumors via the EPR effect. Cancer. Sci. 2013, 104, 779–789. [Google Scholar] [CrossRef]
- Fang, J.; Islam, W.; Maeda, H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug. Deliv. Rev. 2020, 157, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zhang, C.; Hu, W.; Guo, C.; Xia, Z.; Hu, W.; Qin, M.; Jiang, W.; Lv, J.; Xu, D.; et al. Nano-designed carbon monoxide donor SMA/CORM2 exhibits protective effect against acetaminophen induced liver injury through macrophage reprograming and promoting liver regeneration. J. Control. Release 2021, 331, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhang, C.; Xia, Z.; Song, B.; Hu, W.; Cui, Y.; Xue, Y.; Xia, M.; Xu, D.; Zhang, S.; et al. Nano-designed CO donor ameliorates bleomycin-induced pulmonary fibrosis via macrophage manipulation. J. Control. Release 2022, 341, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, Y.; Zhang, J.; Liu, B.; Li, G.; Xin, S.; Xu, K. Diallyl disulfide attenuates nonalcoholic steatohepatitis by suppressing key regulators of lipid metabolism, lipid peroxidation and inflammation in mice. Mol. Med. Rep. 2019, 20, 1363–1372. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, C.; Guo, C.; Song, B.; Hu, W.; Cui, Y.; Xue, Y.; Xia, M.; Xu, D.; Zhang, S.; et al. Nanoformulation of a carbon monoxide releasing molecule protects against cyclosporin A-induced nephrotoxicity and renal fibrosis via the suppression of the NLRP3 inflammasome mediated TGF-β/Smad pathway. Acta Biomater. 2022, 144, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.K.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Therapeutic regulation of the NLRP3 inflammasome in chronic inflammatory diseases. Arch. Pharm. Res. 2021, 44, 16–35. [Google Scholar]
- Bauer, C.; Duewell, P.; Mayer, C.; Lehr, H.A.; Fitzgerald, K.A.; Dauer, M.; Tschopp, J.; Endres, S.; Latz, E.; Schnurr, M. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 2010, 59, 1192–1199. [Google Scholar] [CrossRef]
- Fernández-Bedmar, Z.; Demyda-Peyrás, S.; Merinas-Amo, T.; Del Río-Celestino, M. Nutraceutic potential of two allium species and their distinctive organosulfur compounds: A multi-assay evaluation. Foods 2019, 8, 222. [Google Scholar] [CrossRef]
- Chang, H.S.; Yamato, O.; Yamasaki, M.; Ko, M.; Maede, Y. Growth inhibitory effect of alk(en)yl thiosulfates derived from onion and garlic in human immortalized and tumor cell lines. Cancer Lett. 2005, 223, 47–55. [Google Scholar] [CrossRef]
- Mellado-García, P.; Maisanaba, S.; Puerto, M.; Prieto, A.I.; Marcos, R.; Pichardo, S.; Cameán, A.M. In vitro toxicological assessment of an organosulfur compound from Allium extract: Cytotoxicity, mutagenicity and genotoxicity studies. Food Chem. Toxicol. 2017, 99, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Osipova, V.; Gracheva, Y.; Polovinkina, M.; Burmistrova, D.; Berberova, N. Antioxidant activity and cytotoxicity of aromatic oligosulfides. Molecules 2022, 27, 3961. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Yang, K.; Nohara, T.; Ikeda, T.; Zhou, J.-R.; Yokomizo, K.; Fang, J. Garlicnin B1, an Active Cyclic Sulfide from Garlic, Exhibits Potent Anti-Inflammatory and Anti-Tumor Activities. Antioxidants 2023, 12, 869. https://doi.org/10.3390/antiox12040869
Gao S, Yang K, Nohara T, Ikeda T, Zhou J-R, Yokomizo K, Fang J. Garlicnin B1, an Active Cyclic Sulfide from Garlic, Exhibits Potent Anti-Inflammatory and Anti-Tumor Activities. Antioxidants. 2023; 12(4):869. https://doi.org/10.3390/antiox12040869
Chicago/Turabian StyleGao, Shanghui, Kai Yang, Toshihiro Nohara, Tsuyoshi Ikeda, Jian-Rong Zhou, Kazumi Yokomizo, and Jun Fang. 2023. "Garlicnin B1, an Active Cyclic Sulfide from Garlic, Exhibits Potent Anti-Inflammatory and Anti-Tumor Activities" Antioxidants 12, no. 4: 869. https://doi.org/10.3390/antiox12040869
APA StyleGao, S., Yang, K., Nohara, T., Ikeda, T., Zhou, J.-R., Yokomizo, K., & Fang, J. (2023). Garlicnin B1, an Active Cyclic Sulfide from Garlic, Exhibits Potent Anti-Inflammatory and Anti-Tumor Activities. Antioxidants, 12(4), 869. https://doi.org/10.3390/antiox12040869






