Salvianolic Acid A Protects against Acetaminophen-Induced Hepatotoxicity via Regulation of the miR-485-3p/SIRT1 Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals and Treatments
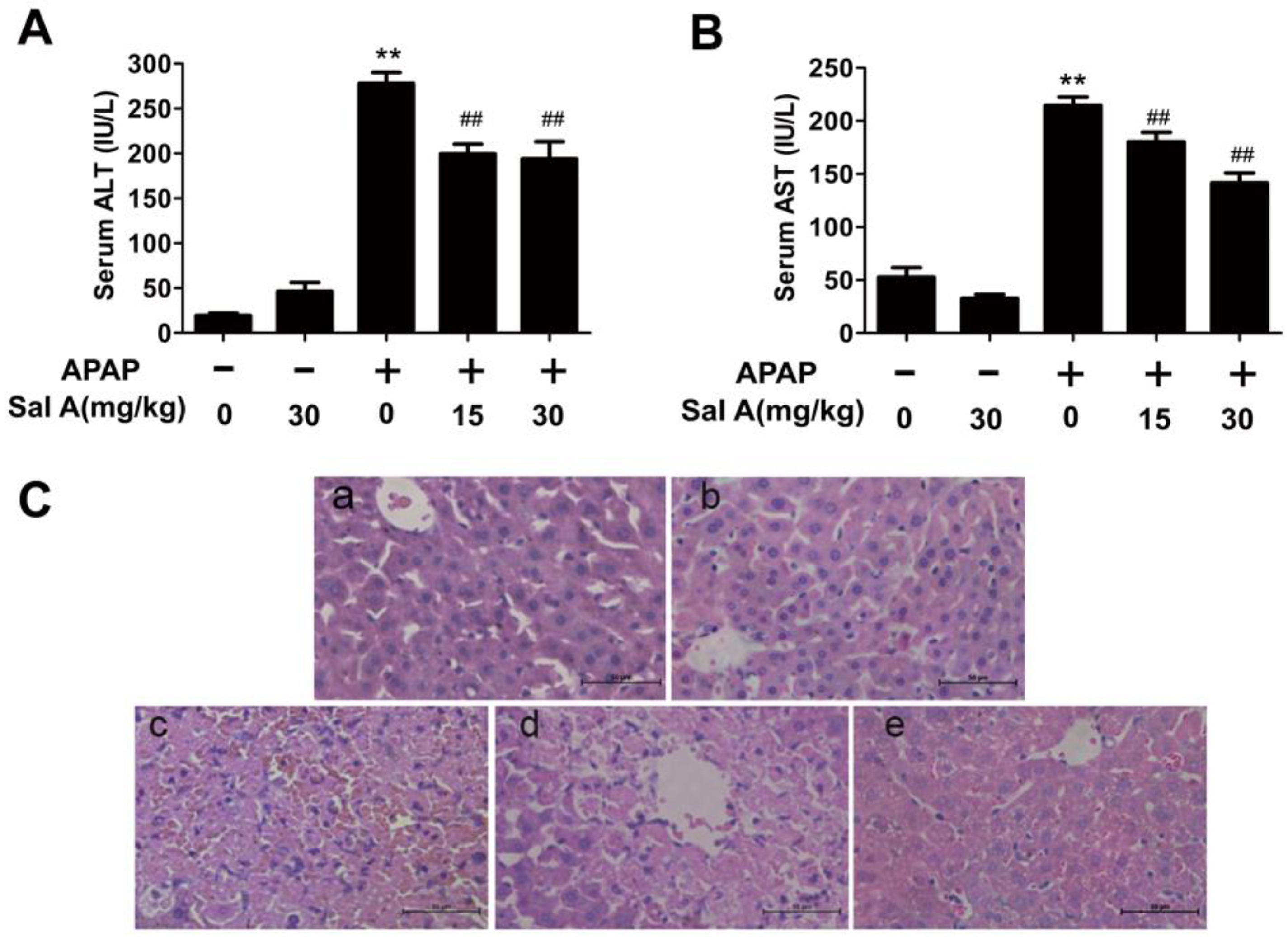
2.3. Analysis of Serum ALT/AST Activities
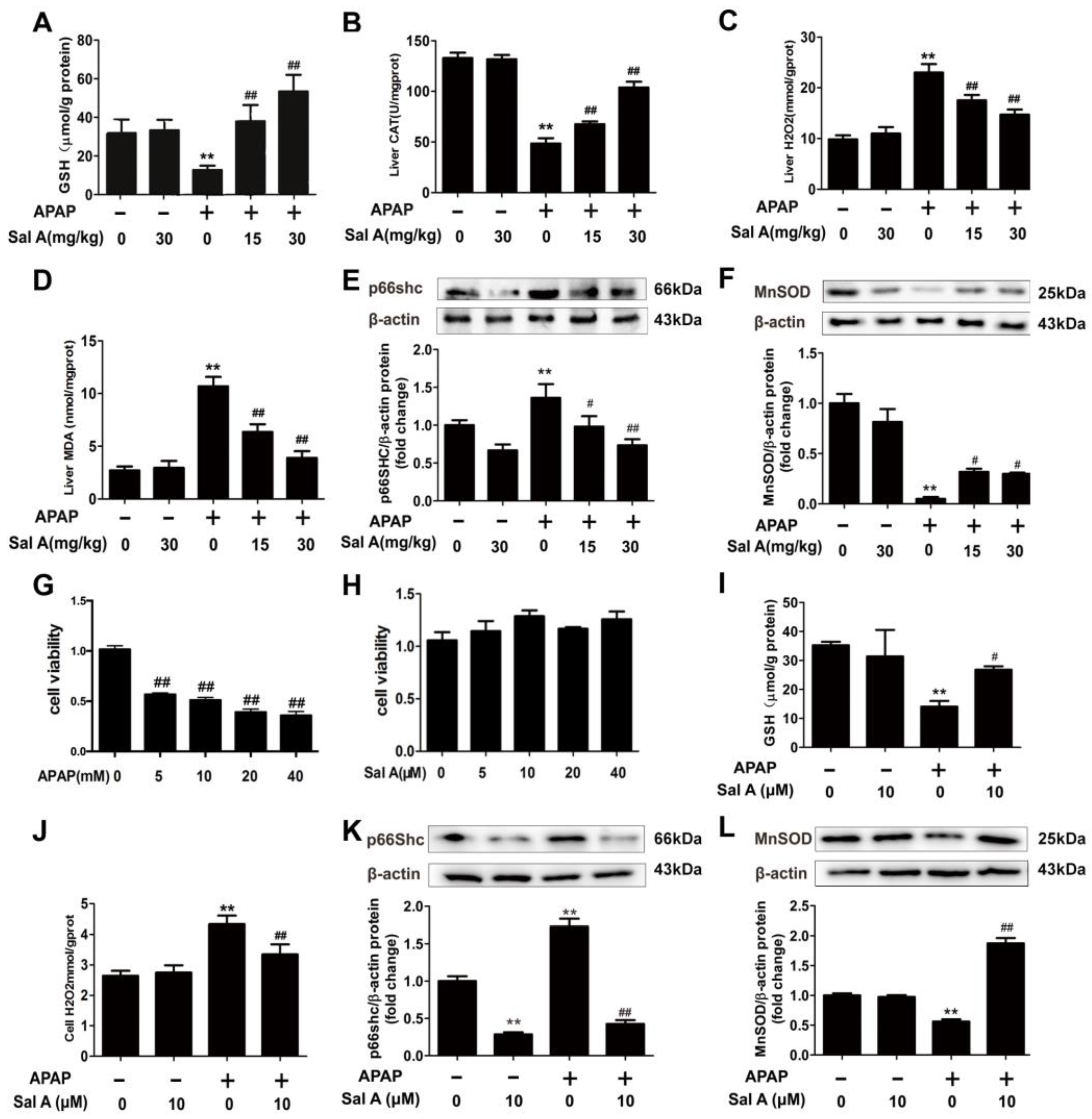
2.4. Analysis of Liver GSH/CAT/H2O2/MDA Levels
2.5. Liver Histological Observation
2.6. Cell Culture
2.7. Cell Viability Assay
2.8. Immunofluorescence Analysis of SIRT1
2.9. Measurement of Cellular ROS
2.10. Cell Transfection
2.11. Western Blot
2.12. Quantitative RT–PCR
2.13. Luciferase Reporter Assay
2.14. Statistical Analysis
3. Results
3.1. Sal A Ameliorates APAP-Induced Liver Injury
3.2. Sal A Ameliorates Hepatic Oxidative Stress In Vivo and In Vitro
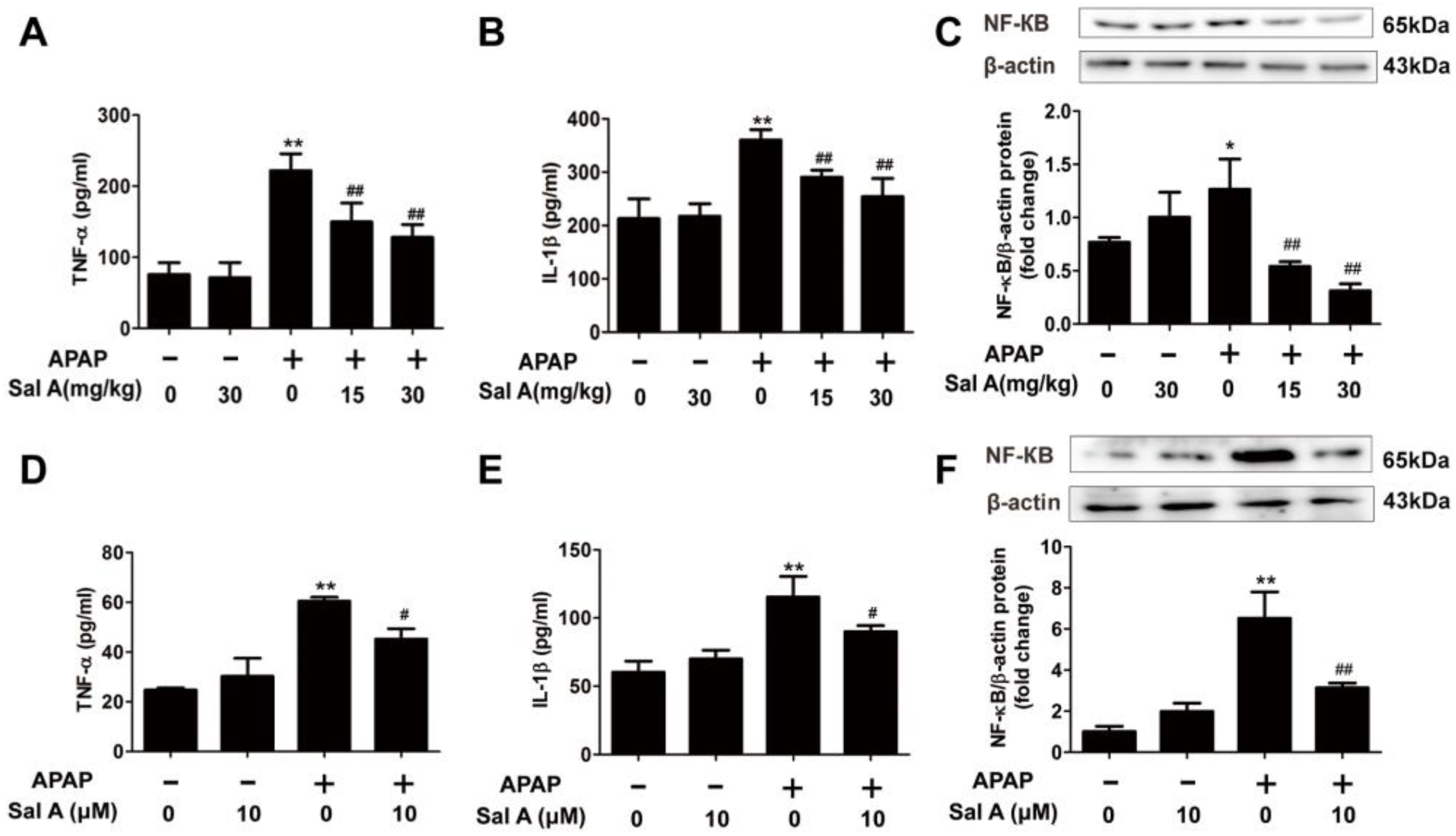
3.3. Sal A Ameliorates Inflammation In Vivo and In Vitro
3.4. Sal A-Mediated Protection against APAP Involves SIRT1 Activation
3.5. Selection of SIRT1-Targeting miRNAs in APAP-Induced Liver Injury
3.6. miR-485-3p Regulates SIRT1 Expression In Vitro
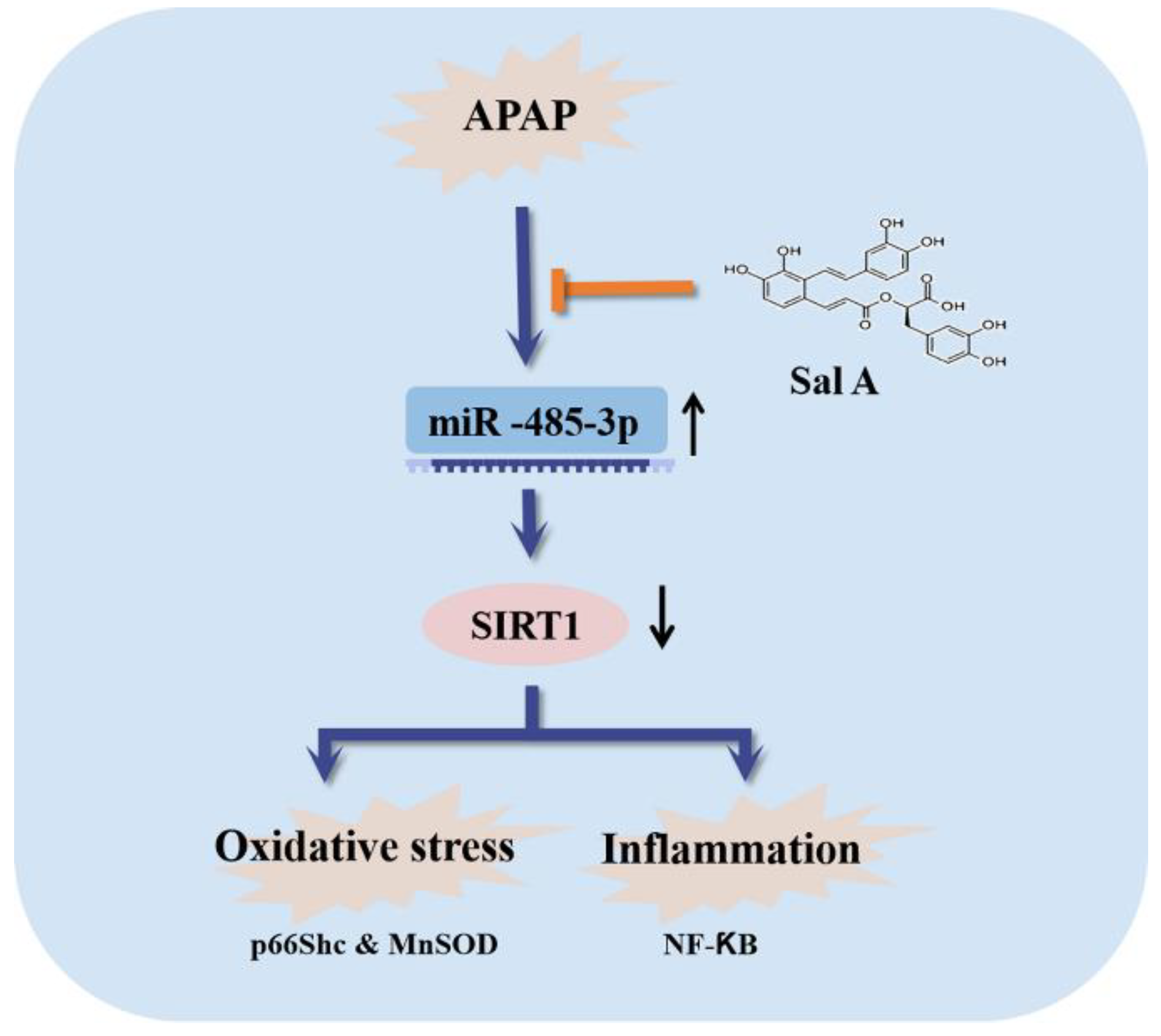
3.7. Sal A Alleviates APAP-Induced Liver Injury though the miR-485-3p/SIRT1 Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaplowitz, N.; Win, S.; Than, T.A.; Liu, Z.X.; Dara, L. Targeting signal transduction pathways which regulate necrosis in acetaminophen hepatotoxicity. J. Hepatol. 2015, 63, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Bohler, S.; Liu, X.; Krauskopf, J.; Caiment, F.; Aubrecht, J.; Nicolaes, G.A.F.; Kleinjans, J.C.S.; Briede, J.J. Acetaminophen Overdose as a Potential Risk Factor for Parkinson’s Disease. Clin. Transl. Sci. 2019, 12, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.M. Acetaminophen (APAP) hepatotoxicity-Isn’t it time for APAP to go away? J. Hepatol. 2017, 67, 1324–1331. [Google Scholar] [CrossRef]
- Rada, P.; Pardo, V.; Mobasher, M.A.; Garcia-Martinez, I.; Ruiz, L.; Gonzalez-Rodriguez, A.; Sanchez-Ramos, C.; Muntane, J.; Alemany, S.; James, L.P.; et al. SIRT1 Controls Acetaminophen Hepatotoxicity by Modulating Inflammation and Oxidative Stress. Antioxid. Redox Signal. 2018, 28, 1187–1208. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Ramachandran, A.; Jaeschke, H. Oxidative stress during acetaminophen hepatotoxicity: Sources, pathophysiological role and therapeutic potential. Redox Biol. 2016, 10, 148–156. [Google Scholar] [CrossRef]
- Liu, Z.X.; Govindarajan, S.; Kaplowitz, N. Innate immune system plays a critical role in determining the progression and severity of acetaminophen hepatotoxicity. Gastroenterology 2004, 127, 1760–1774. [Google Scholar] [CrossRef]
- Jaeschke, H.; Williams, C.D.; Ramachandran, A.; Bajt, M.L. Acetaminophen hepatotoxicity and repair: The role of sterile inflammation and innate immunity. Liver Int. 2012, 32, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Mendes, K.L.; Lelis, D.F.; Santos, S.H.S. Nuclear sirtuins and inflammatory signaling pathways. Cytokine Growth Factor. Rev. 2017, 38, 98–105. [Google Scholar] [CrossRef]
- Sosnowska, B.; Mazidi, M.; Penson, P.; Gluba-Brzozka, A.; Rysz, J.; Banach, M. The sirtuin family members SIRT1, SIRT3 and SIRT6: Their role in vascular biology and atherogenesis. Atherosclerosis 2017, 265, 275–282. [Google Scholar] [CrossRef]
- Long, J.K.; Dai, W.; Zheng, Y.W.; Zhao, S.P. miR-122 promotes hepatic lipogenesis via inhibiting the LKB1/AMPK pathway by targeting Sirt1 in non-alcoholic fatty liver disease. Mol. Med. 2019, 25, 26. [Google Scholar] [CrossRef]
- Carthew, R.W.; Sontheimer, E.J. Origins and Mechanisms of miRNAs and siRNAs. Cell 2009, 136, 642–655. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Xiang, Y.; Zhu, N.; Zhao, X.; Ye, S.; Zhong, P.; Zeng, C. Salvianolic acid A protects against myocardial ischemia/reperfusion injury by reducing platelet activation and inflammation. Exp. Ther. Med. 2017, 14, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.Y.; Chuang, C.H.; Chern, C.M.; Liou, K.T.; Liu, D.Z.; Hou, Y.C.; Shen, Y.C. Salvianolic acid A alleviates ischemic brain injury through the inhibition of inflammation and apoptosis and the promotion of neurogenesis in mice. Free Radic. Biol. Med. 2016, 99, 508–519. [Google Scholar] [CrossRef]
- Xu, X.; Hu, Y.; Zhai, X.; Lin, M.; Chen, Z.; Tian, X.; Zhang, F.; Gao, D.; Ma, X.; Lv, L.; et al. Salvianolic acid A preconditioning confers protection against concanavalin A-induced liver injury through SIRT1-mediated repression of p66shc in mice. Toxicol. Appl. Pharmacol. 2013, 273, 68–76. [Google Scholar] [CrossRef]
- Lin, M.; Zhai, X.; Wang, G.; Tian, X.; Gao, D.; Shi, L.; Wu, H.; Fan, Q.; Peng, J.; Liu, K.; et al. Salvianolic acid B protects against acetaminophen hepatotoxicity by inducing Nrf2 and phase II detoxification gene expression via activation of the PI3K and PKC signaling pathways. J. Pharmacol. Sci. 2015, 127, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fan, X.; Jiao, T.; Li, W.; Chen, P.; Jiang, Y.; Sun, J.; Chen, Y.; Chen, P.; Guan, L.; et al. SIRT6 as a key event linking P53 and NRF2 counteracts APAP-induced hepatotoxicity through inhibiting oxidative stress and promoting hepatocyte proliferation. Acta. Pharm. Sin. B 2021, 11, 89–99. [Google Scholar] [CrossRef]
- Hu, S.; Yao, Y.; Wei, Z.Y.; Wang, S.X.; Wu, Y.C.; Hu, Y.; Yang, C.C.; Min, J.L.; Li, L.Y.; Zhou, H.; et al. Deletion of p38γ attenuates ethanol consumption- and acetaminophen-induced liver injury in mice through promoting Dlg1. Acta. Pharmacol. Sin. 2022, 43, 1733–1748. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Y.; Sun, R.; Sun, Y.; Liu, D.; Lin, M.; Chen, Z.; Zhou, J.; Lv, L.; Tian, X.; et al. circ-CBFB upregulates p66Shc to perturb mitochondrial dynamics in APAP-induced liver injury. Cell Death. Dis. 2020, 11, 953. [Google Scholar] [CrossRef]
- Pang, C.; Zheng, Z.; Shi, L.; Sheng, Y.; Wei, H.; Wang, Z.; Ji, L. Caffeic acid prevents acetaminophen-induced liver injury by activating the Keap1-Nrf2 antioxidative defense system. Free Radic. Biol. Med. 2016, 91, 236–246. [Google Scholar] [CrossRef]
- Di Lisa, F.; Giorgio, M.; Ferdinandy, P.; Schulz, R. New aspects of p66Shc in ischaemia reperfusion injury and other cardiovascular diseases. Br. J. Pharmacol. 2017, 174, 1690–1703. [Google Scholar] [CrossRef]
- Trinei, M.; Migliaccio, E.; Bernardi, P.; Paolucci, F.; Pelicci, P.; Giorgio, M. p66Shc, mitochondria, and the generation of reactive oxygen species. Methods Enzym. 2013, 528, 99–110. [Google Scholar] [CrossRef]
- Agarwal, R.; MacMillan-Crow, L.A.; Rafferty, T.M.; Saba, H.; Roberts, D.W.; Fifer, E.K.; James, L.P.; Hinson, J.A. Acetaminophen-induced hepatotoxicity in mice occurs with inhibition of activity and nitration of mitochondrial manganese superoxide dismutase. J. Pharmacol. Exp. Ther. 2011, 337, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Woolbright, B.L.; Jaeschke, H. Role of the inflammasome in acetaminophen-induced liver injury and acute liver failure. J. Hepatol. 2017, 66, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G.; Petrasek, J. Inflammasome activation and function in liver disease. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Sen, R.; Smale, S.T. Selectivity of the NF-{kappa}B response. Cold Spring Harb. Perspect. Biol. 2010, 2, a000257. [Google Scholar] [CrossRef]
- Parsamanesh, N.; Asghari, A.; Sardari, S.; Tasbandi, A.; Jamialahmadi, T.; Xu, S.; Sahebkar, A. Resveratrol and endothelial function: A literature review. Pharmacol. Res. 2021, 170, 105725. [Google Scholar] [CrossRef]
- Wang, G.; Yao, J.; Li, Z.; Zu, G.; Feng, D.; Shan, W.; Li, Y.; Hu, Y.; Zhao, Y.; Tian, X. miR-34a-5p Inhibition Alleviates Intestinal Ischemia/Reperfusion-Induced Reactive Oxygen Species Accumulation and Apoptosis via Activation of SIRT1 Signaling. Antioxid. Redox Signal. 2016, 24, 961–973. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, S.; Marzolf, B.; Troisch, P.; Brightman, A.; Hu, Z.; Hood, L.E.; Galas, D.J. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc. Natl. Acad. Sci. USA 2009, 106, 4402–4407. [Google Scholar] [CrossRef]
- Brillant, N.; Elmasry, M.; Burton, N.C.; Rodriguez, J.M.; Sharkey, J.W.; Fenwick, S.; Poptani, H.; Kitteringham, N.R.; Goldring, C.E.; Kipar, A.; et al. Dynamic and accurate assessment of acetaminophen-induced hepatotoxicity by integrated photoacoustic imaging and mechanistic biomarkers in vivo. Toxicol. Appl. Pharmacol. 2017, 332, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zhao, Y.; Shi, X.; Zhang, N.; Zu, G.; Li, Z.; Zhou, J.; Gao, D.; Lv, L.; Tian, X.; et al. New insights into salvianolic acid A action: Regulation of the TXNIP/NLRP3 and TXNIP/ChREBP pathways ameliorates HFD-induced NAFLD in rats. Sci. Rep. 2016, 6, 28734. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Z.; Tang, F.; Zhao, Y.; Feng, D.; Li, Y.; Hu, Y.; Wang, C.; Zhou, J.; Tian, X.; et al. Carnosol-mediated Sirtuin 1 activation inhibits Enhancer of Zeste Homolog 2 to attenuate liver fibrosis. Pharmacol. Res. 2018, 128, 327–337. [Google Scholar] [CrossRef]
- Shan, W.; Gao, L.; Zeng, W.; Hu, Y.; Wang, G.; Li, M.; Zhou, J.; Ma, X.; Tian, X.; Yao, J. Activation of the SIRT1/p66shc antiapoptosis pathway via carnosic acid-induced inhibition of miR-34a protects rats against nonalcoholic fatty liver disease. Cell Death Dis. 2015, 6, e1833. [Google Scholar] [CrossRef] [PubMed]
- Starkey Lewis, P.J.; Dear, J.; Platt, V.; Simpson, K.J.; Craig, D.G.; Antoine, D.J.; French, N.S.; Dhaun, N.; Webb, D.J.; Costello, E.M.; et al. Circulating microRNAs as potential markers of human drug-induced liver injury. Hepatology 2011, 54, 1767–1776. [Google Scholar] [CrossRef]
- Antoine, D.J.; Dear, J.W.; Lewis, P.S.; Platt, V.; Coyle, J.; Masson, M.; Thanacoody, R.H.; Gray, A.J.; Webb, D.J.; Moggs, J.G.; et al. Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced acute liver injury at first presentation to hospital. Hepatology 2013, 58, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Sangokoya, C.; Doss, J.F.; Chi, J.T. Iron-responsive miR-485-3p regulates cellular iron homeostasis by targeting ferroportin. PLoS Genet. 2013, 9, e1003408. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.; Baulies, A.; Insausti-Urkia, N.; Alarcon-Vila, C.; Fucho, R.; Solsona-Vilarrasa, E.; Nunez, S.; Robles, D.; Ribas, V.; Wakefield, L.; et al. Endoplasmic Reticulum Stress-Induced Upregulation of STARD1 Promotes Acetaminophen-Induced Acute Liver Failure. Gastroenterology 2019, 157, 552–568. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Li, C.; Yu, S.; Liu, G.; Zou, J.; Zhang, D.; Jiang, C.; Wang, X.; He, L.; Huang, P.; et al. Protective effect of platinum nano-antioxidant and nitric oxide against hepatic ischemia-reperfusion injury. Nat. Commun. 2022, 13, 2513. [Google Scholar] [CrossRef]
- Shimizu, D.; Ishitsuka, Y.; Miyata, K.; Tomishima, Y.; Kondo, Y.; Irikura, M.; Iwawaki, T.; Oike, Y.; Irie, T. Protection afforded by pre- or post-treatment with 4-phenylbutyrate against liver injury induced by acetaminophen overdose in mice. Pharmacol. Res. 2014, 87, 26–41. [Google Scholar] [CrossRef]
- Xu, Q.; Fan, Y.; Loor, J.J.; Liang, Y.; Sun, X.; Jia, H.; Zhao, C.; Xu, C. Cardamonin Reduces Acetaminophen-Induced Acute Liver Injury in Mice via Activating Autophagy and NFE2L2 Signaling. Front Pharmacol. 2020, 11, 601716. [Google Scholar] [CrossRef]
- Ghanem, C.I.; Pérez, M.J.; Manautou, J.E.; Mottino, A.D. Acetaminophen from liver to brain: New insights into drug pharmacological action and toxicity. Pharmacol. Res. 2016, 109, 119–131. [Google Scholar] [CrossRef]
- Tripathy, D.; Grammas, P. Acetaminophen inhibits neuronal inflammation and protects neurons from oxidative stress. J. Neuroinflamm. 2009, 6, 10. [Google Scholar] [CrossRef] [PubMed]



| Name | Sequences (5′-3′) | Accession Number |
|---|---|---|
| agomir targeting miR-485-3p | AGUCAUACACGGCUCUCCUCUC GAGGAGAGCCGUGUAUGACUUU | MIMAT0003129 |
| agomir negative control | UUCUCCGAACGUGUCACGUTT ACGUGACACGUUCGGAGAATT | MIMAT0000295 |
| antagomir targeting miR-485-3p | GAGAGGAGAGCCGUGUAUGACU | MIMAT0003129 |
| antagomir negative control | CAGUACUUUUGUGUAGUACAA | MIMAT0000295 |
| siRNA targeting SIRT1 | CCCUGUAAAGCUUUCAGAA (TT) UUCUGAAAGCUUUACAGGG (TT) | NM_019812.3 |
| siRNA negative control | ACGUGACACGUUCGGAGAA (TT) UUCUCCGAACGUGUCACGU (TT) | M403861200 |
| SIRT1 mRNA--F | CCCAGCTCCAGTCAGAACTAT | NM_019812.3 |
| SIRT1 mRNA--R | TTGGCACCGATCCTCGAAC | |
| β-actin mRNA--F | TTCGTTGCCGGTCCACACCC | NM_001101.5 |
| β-actin mRNA--R | GCTTTGCACATGCCGGAGCC |
| miRNA Name | ||||
|---|---|---|---|---|
| Increased miRNA | mmu-miR-297a | mmu-miR-483 | mmu-let-7d * | mghv-miR-M1-2 |
| mmu-miR-574-3p | mmu-miR-709 | mmu-miR-466 g | mmu-miR-466 h | |
| mmu-miR-466f-3p | mmu-miR-1224 | mmu-miR-574-5p | mmu-miR-467a * | |
| mmu-miR-671-5p | mmu-miR-467b * | mmu-miR-207 | mmu-miR-669c | |
| mmu-miR-483 * | mmu-miR-877 * | mmu-miR-467e * | mmu-miR-468 | |
| mmu-miR-297b-3p | mmu-miR-197 | mmu-miR-672 | mmu-miR-328 | |
| mmu-miR-466c-5p | mmu-miR-485 * | mmu-miR-689 | mmu-miR-188-5p | |
| mmu-miR-669a | mmu-miR-721 | mmu-miR-710 | mmu-miR-711 | |
| mmu-miR-466d-3p | ||||
| SIRT1-targeted miRNAs | miR-483 | miR-467b * | miR-467e * | miR-297b-3p |
| miR-485 * | miR-466d-3p | miR-672 | ||
| Conservation | miR-485 * | |||
| Predicted Consequential Pairing of Target Region (Top) and miRNA (Bottom) | Accession Number | |
|---|---|---|
| Position 232-238 of SIRT1 3′ UTR | 5′…CUUUCAAGGUUCAUUUGUAUGAU… | NM_019812.3 |
 | ||
| mmu-miR-485-3p | 3′ UCUCUCCUCUCGGCACAUACUG | MIMAT0003129 |
| Position 280-286 of SIRT1 3′ UTR | 5′…UUUUAAAGGUUCAUUUGUAUGAU… | NM_012238.5 |
 | ||
| hsa-miR-485-3p | 3′ UCUCUCCUCUCGGCACAUACUG | MIMAT0002176 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, F.; Wang, Z.; Zhou, J.; Yao, J. Salvianolic Acid A Protects against Acetaminophen-Induced Hepatotoxicity via Regulation of the miR-485-3p/SIRT1 Pathway. Antioxidants 2023, 12, 870. https://doi.org/10.3390/antiox12040870
Tang F, Wang Z, Zhou J, Yao J. Salvianolic Acid A Protects against Acetaminophen-Induced Hepatotoxicity via Regulation of the miR-485-3p/SIRT1 Pathway. Antioxidants. 2023; 12(4):870. https://doi.org/10.3390/antiox12040870
Chicago/Turabian StyleTang, Fan, Zhecheng Wang, Junjun Zhou, and Jihong Yao. 2023. "Salvianolic Acid A Protects against Acetaminophen-Induced Hepatotoxicity via Regulation of the miR-485-3p/SIRT1 Pathway" Antioxidants 12, no. 4: 870. https://doi.org/10.3390/antiox12040870
APA StyleTang, F., Wang, Z., Zhou, J., & Yao, J. (2023). Salvianolic Acid A Protects against Acetaminophen-Induced Hepatotoxicity via Regulation of the miR-485-3p/SIRT1 Pathway. Antioxidants, 12(4), 870. https://doi.org/10.3390/antiox12040870





