Abstract
Kidney extraction time has a detrimental effect on post-transplantation outcome. This study aims to improve the flush-out and potentially decrease ischemic injury by the addition of hydrogen sulphide (H2S) to the flush medium. Porcine kidneys (n = 22) were extracted during organ recovery surgery. Pigs underwent brain death induction or a Sham operation, resulting in four groups: donation after brain death (DBD) control, DBD H2S, non-DBD control, and non-DBD H2S. Directly after the abdominal flush, kidneys were extracted and flushed with or without H2S and stored for 13 h via static cold storage (SCS) +/− H2S before reperfusion on normothermic machine perfusion. Pro-inflammatory cytokines IL-1b and IL-8 were significantly lower in H2S treated DBD kidneys during NMP (p = 0.03). The non-DBD kidneys show superiority in renal function (creatinine clearance and FENa) compared to the DBD control group (p = 0.03 and p = 0.004). No differences were seen in perfusion parameters, injury markers and histological appearance. We found an overall trend of better renal function in the non-DBD kidneys compared to the DBD kidneys. The addition of H2S during the flush out and SCS resulted in a reduction in pro-inflammatory cytokines without affecting renal function or injury markers.
1. Introduction
Worldwide, the majority of donor kidneys used for transplantation are derived from deceased brain dead (DBD) donors. Brain death is the potential end result of increased intracranial pressure often caused by cerebral haemorrhage or trauma. When the intracranial pressure exceeds the systemic blood pressure, the subsequent ischemia of the brain results in a release of vast amounts of catecholamines, triggering a cascade of cytokine release to the circulation [1]. The inflammatory response, evoked by brain death, is known to induce organ injury in the donor [2]. In addition to the injurious events in brain dead donors, several unavoidable ischemic episodes occur during kidney transplantation. Extraction time, and cold and warm ischemic time all have a detrimental effect on post-transplantation outcome in kidney transplantation [3,4,5,6,7]. Kidney allografts from brain dead donors are therefore at a higher risk for graft deterioration compared to kidneys from living donors [8,9,10]. To improve the quality of kidneys derived from DBD donors, one could intervene during the ICU phase, but this brings ethical and logistical issues [11]. A more isolated approach, i.e., treatment of the organ during or after retrieval allows target treatment and might be better suitable.
Hydrogen sulphide (H2S) is a gasotransmitter known for its anti-inflammatory capacities [12]. H2S has antioxidant properties [13], and can induce a hypometabolic state in isolated perfused organs [14]. These qualities could result in the protection of kidneys during the first flush and subsequent preservation time. H2S may counteract the inflammatory response induced by brain death and prevent ischemic injury. Kidneys warm up during the procurement surgery, and the capacity of H2S to reduce the metabolic rate could further diminish injury by reducing its metabolism. H2S-enriched flush-out and H2S-enriched cold storage showed beneficial effects in a rat renal transplantation model [15]. However, experimental data on larger mammals, such as pigs and sheep, failed to show hypometabolic action when H2S was administered systemically [16,17]. An isolated administration of H2S might be more effective. The potential therapeutic properties of H2S have not been tested in large brain-dead mammals so far.
The present study aims to improve the flush-out and preservation procedure by decreasing the inflammatory response and ischemic injury through addition of H2S to the preservation solution in kidneys from brain dead and non-brain-dead pigs. We hypothesized that H2S decreases the cytokine release and minimizes the amount of reactive oxygen species (ROS) formed during ischemia, thereby reducing inflammation and ischemia-reperfusion injury. Mitochondrial ROS are important early drivers of ischemia-reperfusion injury, formed by the primary driver succinate, which accumulates during ischemia [18]. In this study, normothermic machine perfusion (NMP) was used as a reperfusion model to test short-term post-preservation renal function [19]. Analysis included metabolic parameters, renal function, injury and oxidative stress markers, cytokines release, and complement activation.
2. Methods
2.1. Study Design
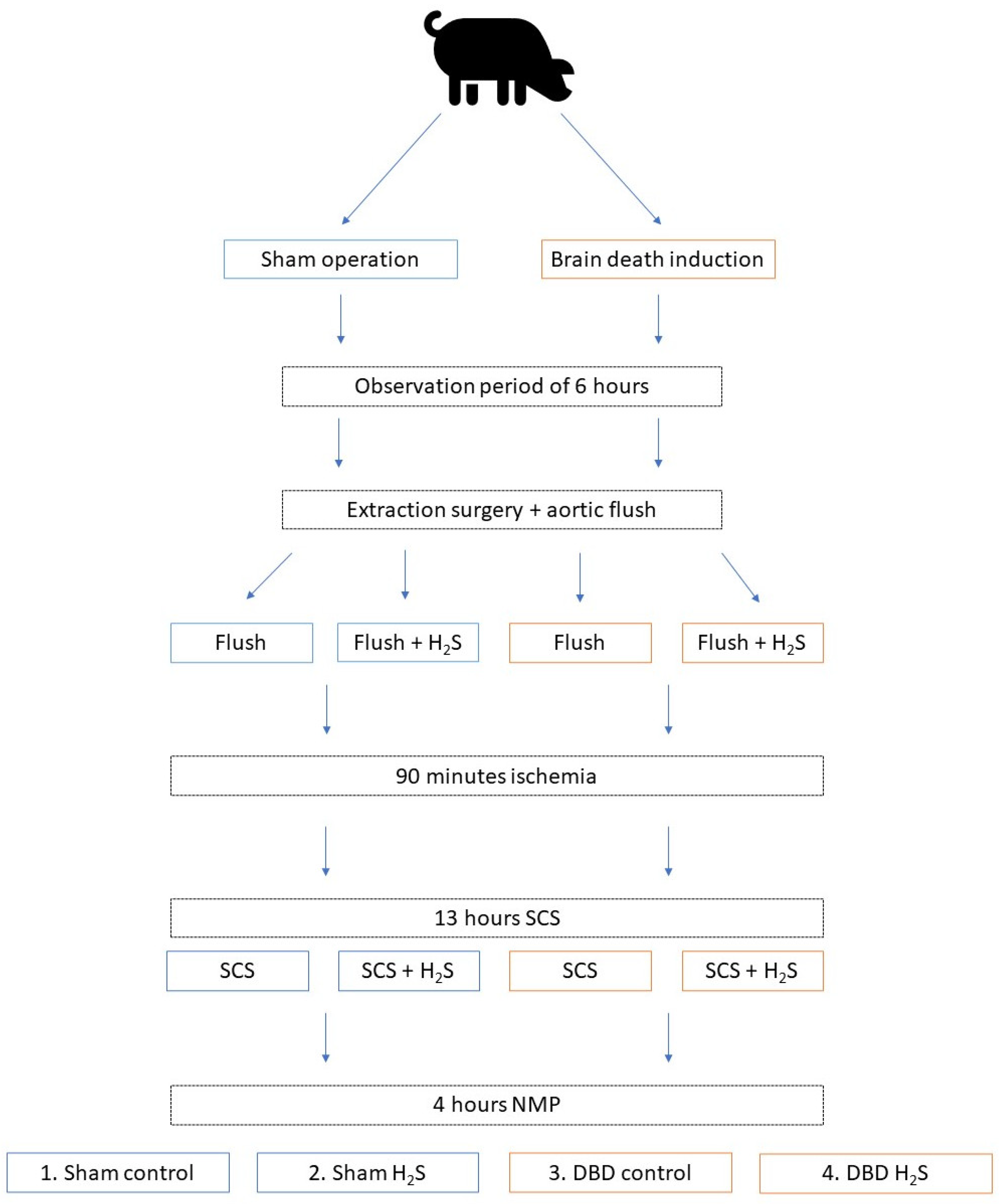
In order to minimize the number of laboratory animals needed for this study, kidneys were included as a sub-study from another larger study from our group. We retrieved kidneys from 6 brain dead and 5 Sham control pigs. The overview of the experimental setup is displayed in Figure 1. After brain death induction or the Sham control procedure, pigs were observed for six hours before extraction surgery. During procurement, the abdominal organs were flushed with 4 L of cold University of Wisconsin-static cold storage solution (UW-SCS) before extraction. For the present study, kidneys were flushed with 500 mL cold UW-SCS with or without H2S (1 mmol/L) and stored at room temperature for 90 min to simulate the prolonged ischemic injury following a human multi-organ donor procedure. All kidneys were cold stored for 13 h in either UW-SCS supplemented with H2S (1 mmol/L) in the H2S groups or UW-SCS without H2S in the control groups. After the storage time, the kidneys were flushed with saline (0.9%, 60 mL) before NMP was performed for 4 h. The experimental setup resulted in four groups: Sham control, Sham H2S, DBD control, and DBD H2S.
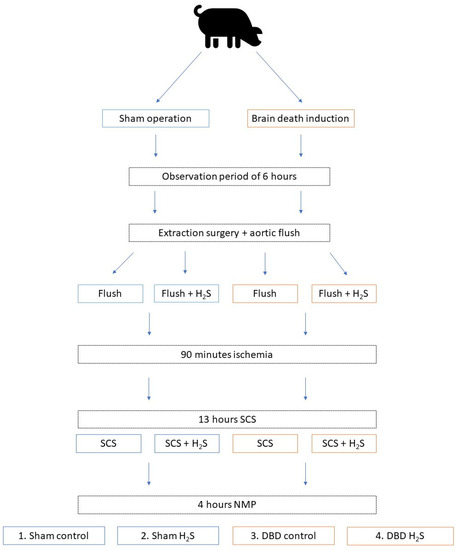
Figure 1.
Experimental overview. Group 1 Sham control, group 2 Sham + H2S treatment, group 3 DBD control, group 4 DBD + H2S treatment. Abbreviations: DBD = donation after brain death. H2S = hydrogen sulphide. NMP = normothermic machine perfusion. SCS = static cold storage.
2.2. Ethics and Animals
A total of 11 female Danish landrace pigs purchased from the same breeder, weighing on average 63.5 kg (range 59.3–68 kg), were used for organ extraction. As previously stated, the pigs were simultaneously used for several research projects to minimize the number of animals used for experiments. The small intestine was used for a luminal preservation experiment. Biopsies from the liver, pancreas, m. psoas, lungs, and heart were used to analyse the effect of brain death on different organ systems. For this project, the kidneys were used and assessed on NMP. All animal care and procedures were conducted in accordance with guidelines by the European Union (directive 2010/63/EU) and local regulations. The study was approved by the Danish Animal Experimentation Council (reference-number 2019-15-0201-00157). All personnel involved had Federation of European Laboratory Animal Science Associations licenses or equivalent.
2.3. Anaesthesia and Induction of Brain Death
Pigs were sedated prior to anaesthesia with an intramuscular injection of Zoletil-mix (Zoletil 50 vet. (125 mg Tiletamin, 125 mg Zolazepam), 125 mg Xylazin, 125 mg Ketamin, 25 mg Butorphanol). An 18G intravenous catheter was placed in each ear and 50–100 mg Propofol was administered i.v. prior to intubation if the sedation with the Zolet-mix was not sufficient for the intubation procedure. Anaesthesia was maintained using Propofol (8 mg/kg/h) (Fresenius Kabi, Bad Homburg von der Höhe, Germany) and Fentanyl (10 µg/kg/h) (B Braun, Melsungen, Germany). Animals were placed in supine position and were intubated. After intubation, a pressure sensor was placed in the coronary artery and a urine catheter was placed in the bladder. Ventilation was achieved with a tidal volume between 8–10 mL/kg and regulated to a PaCO2 between 5.5–6.5 kPa. An arterial sheath was placed in the left common carotid artery and two sheaths were placed in the jugular veins, one on each side. A 14Fr urinary catheter (Teleflex, Wayne, PA, USA) was placed and connected to a two-chamber collection bag (Unomedical, Lejre, Denmark), to monitor urine production. After an infusion of 1000 mL of ringer’s acetate, 400 mL of blood was drawn from the pigs via one of the jugular sheaths, used for perfusion during NMP.
Induction of brain death was performed according to a previously established model [20]. The animal was turned to a prone position and a 10 cm incision was made along the sagittal suture. The skin and periosteum were retracted using a self-retaining retractor. Intracranial pressure monitoring was established in all animals using a RALK hand drill with a 5 mm drill bit, a CH5 bolt, and a NEUROVENT-PTO catheter (Raumedic, Helmbrechts, Germany). A 20 mm Hudson hand drill and back-biting forceps were used for the brain death groups (groups 3 and 4) to make a burr hole where a CH22 urine catheter (Unomedical, Lejre, Denmark) was placed. The balloon of the urinary catheter was filled with saline, at a rate of 1 mL/min for 10 min, followed by 0.5 mL/min for an additional 10 min, and 0.25 mL/min, until a persistent negative cranial perfusion pressure confirmed brain death. Cranial perfusion pressure was defined as the mean arterial pressure minus the intracranial pressure. Following a negative cranial perfusion pressure, an additional 10 mL was injected into the balloon as a bolus to ensure brain death.
2.4. Organ Retrieval and Organ Flush
Before the organ retrieval surgery, pigs were turned to a supine position. Via a midline laparotomy, the abdomen was opened together with the peritoneum. Kidneys were dissected free of the surrounding tissue, together with the ureter, aorta, and vena cava. Kidney biopsies and urine samples were taken. Kidneys were then left in place for further preparation of the remaining organs and vasculature. An amount of 500 IE/kg of heparin (LEO Pharma, Ballerup, Denmark) was administered, and after 3 min, a St. Thomas cannula (Medtronic, Dublin, Ireland) was placed in the ascending aorta. The distal abdominal aorta and vena cava inferior were ligated, and the abdominal aorta was cannulated using a 14F cannula (Bridge to life, London, UK). While the abdominal cannulation was performed, an additional 600 mL of blood was removed via the St. Thomas cannula prior to the cold St. Thomas I solution infusion.
The ascending aorta was cross clamped, 1 L of cold St. Thomas I solution was infused under pressure through the St. Thomas cannula (Medtronic, Dublin, Ireland), and the inferior vena cava was transected above the diaphragm. The abdominal aorta was cross clamped above the liver, 4 L of cold Belzer UW-SCS (Bridge to life, London, England) was infused under pressure via the cannulation of the abdominal aorta. The inferior vena cava was transected proximally to the ligature, preventing the venous return from the legs. Crushed ice (50 mg/mL glucose saline) (Fresenius Kabi, Bad Homburg von der Höhe, Germany) was placed around all transplantable organs. Next, the kidneys were extracted after the other organs were removed. Left and right kidneys were structurally assigned to one group, eliminating any effect of anatomical difference and extraction time on outcomes. One kidney was flushed at the back table with 500 mL of cold UW-SCS, and the other kidney was flushed with UW-SCS supplemented with 1 mmol of H2S. To induce additional ischemic injury, explanted kidneys were placed at room temperature for 90 min in the flush fluid (reaching 20 degrees Celsius at the end). After 90 min, the surrounding solution was replaced with cold fresh UW-SCS (+/− H2S), and the kidneys were stored for 13 h.
2.5. Normothermic Machine Perfusion
NMP was simultaneously performed on each kidney pair to ensure similar ischemic times. The NMP procedure was performed as described earlier [21]. Briefly, a sinusoidal pulse was applied with a mean pressure of 75 mmHg for 4 h using a centrifugal pump (Deltastream DP3®, MEDOS Medizintechnik AG, Heilbronn, Germany) controlled by in-house developed software (Sophistikate®, Labview, National Instruments, Austin, TX, USA). The temperature was regulated using a Jubalo water heating system and was set at 37 degrees Celsius. An integrated heat exchanger (HILITE 1000®, MEDOS Medizintechnik AG, Heilbronn, Germany) was built into the oxygenator. A clamp-on flow sensor was used to measure flow (ME7PXL clamp®, Transonic Systems, Inc., Ithaca, NY, USA). The pressure sensor used was a Truewave® disposable pressure transducer (Edwards Lifesciences, Irvine, CA, USA). As perfusion medium, 170 mL red blood cells (RBCs), 250 mL 5% human albumin, 5 mL 8.4% Sodium bicarbonate, 6 mL 5% glucose, 3 mL 10% calcium gluconate, 5 mL 1000/200 mg Co-Amoxiclav, 31 mg creatinine, 5 IU insulin, and 1 mL (5 mg/2 mL) verapamil was used. Carbogen (95% O2 and 5% CO2) was supplied via the oxygenator at a flow rate of 500 mL/min. Continuous infusion of verapamil (0.25 mg/h) was supplied via the arterial tube. A 60 mg bolus of Co-Amoxiclav (1000/200 mg) was administered every hour. Perfusate and urine samples were taken serially, together with blood gas samples.
2.6. Flush Samples and Biopsies
Flush samples were collected after extraction and flush of the kidney, after cold storage, and the saline flush, and just before NMP. Tissue was collected at the same time points via a punch-biopsy (4 mm). The punch-biopsy was divided into three pieces, one stored in formalin, one in liquid nitrogen, and one in a buffer for ATP analysis (0.372 g EDTA (0.744 g/L) in 130 mL H2O and NaOH (pH 10.9) + 370 mL 96% ethanol). After NMP, two 1 cm3 biopsies were additionally taken and snap-frozen in liquid nitrogen. Periodic acid-Schiff (PAS) staining was performed on the paraffin-embedded biopsies, which were analysed by an experienced pathologist.
2.7. Cytokines and Complement Activation Products
Cytokine and complement activation products were determined in the NMP perfusate, urine produced during NMP, SCS fluid, and flush-out fluids. Fluids were stored in EDTA tubes for the complement analysis and in standard Eppendorf tubes for the cytokine levels. Terminal complement complex (TCC) and C3a were measured using in-house ELISA, as described in detail previously [22,23]. Tumor necrosis factor (TNF), IL-1b, IL-6, IL-8, and IL-10 were measured using commercially available ELISA and multiplex assays as described previously described [24].
2.8. Renal Function and Injury Markers
During NMP, the flow was registered every minute. The sampling of perfusate and urine, and blood gas analysis of the perfusate, were performed immediately after the start of perfusion, after 15 min, 30 min, 60 min, and every following hour. Perfusate and urine samples were stored on ice until centrifuging at 1000× g for 12 min at 4 degrees Celsius. Next, supernatants were aliquoted and stored at −80 degrees Celsius until further analysis.
Renal metabolism and function were calculated with oxygen consumption, metabolic coupling of sodium transport by ATPase in tubular epithelial cells, fractional sodium excretion (FENa), and creatinine clearance using the previously described formulas [25].
Creatinine, sodium, potassium, and protein concentration in both the perfusate and urine samples were analysed via routine clinical procedures at the University Medical Center Groningen (UMCG) clinical chemistry lab. ATP levels were measured as previously described [26] using the ATP Bioluminescence Assay Kit CLS II (Roche Diagnostics, Mannheim, Germany) according to a standardized protocol and expressed relative to the protein concentration (PierceTM BCA Protein Assay Kit, Rockford, IL, USA). Neutrophil gelatinase-associated lipocalin (NGAL) levels were measured in urine using ELISA (pig NGAL ELISA kit 004, BioPorto, Needham, MA, USA). Lipid peroxidation product malondialdehyde (MDA) was measured in tissue homogenates as described previously [27]. BAX/BCL-2 ratios were measured using Real-Time PCR as described previously [28], fragments of these genes were amplified with the primer sets outlined in Table 1.

Table 1.
Primer sequences used for Real-Time PCR.
2.9. Statistics
All data are expressed as median with interquartile range unless stated otherwise. The area under the curve was calculated in data with multiple time points during NMP. Statistical analysis on values during NMP was performed on the calculated AUC during the whole course of NMP. The effect of H2S treatment was evaluated in both the DBD and Sham kidneys. In addition, the effect of donation type was evaluated by comparing the DBD control group with the Sham control group. Differences between the DBD control and DBD H2S group and differences between the Sham control and Sham H2S group were calculated using Wilcoxon matched-pairs signed-rank tests. Differences between the DBD control and Sham control group were calculated using Mann–Whitney U tests. A p value <0.05 was considered statistically significant. Data were analysed with Graphpad PRISM 8.4.2 (GraphPad, San Diego, CA, USA).
3. Results
3.1. Baseline Characteristics
Baseline characteristics of the animals and kidneys are shown in Table 2. No significant differences were seen between the Sham (n = 10) and the DBD (n = 12) kidneys, except for urine production. As expected, DBD pigs produced significantly more urine compared to the Sham pigs during the observation period (4184 mL vs. 963 mL, p < 0.001).

Table 2.
Overview of the characteristics of the pigs.
3.2. Cytokine Release during Preservation and NMP
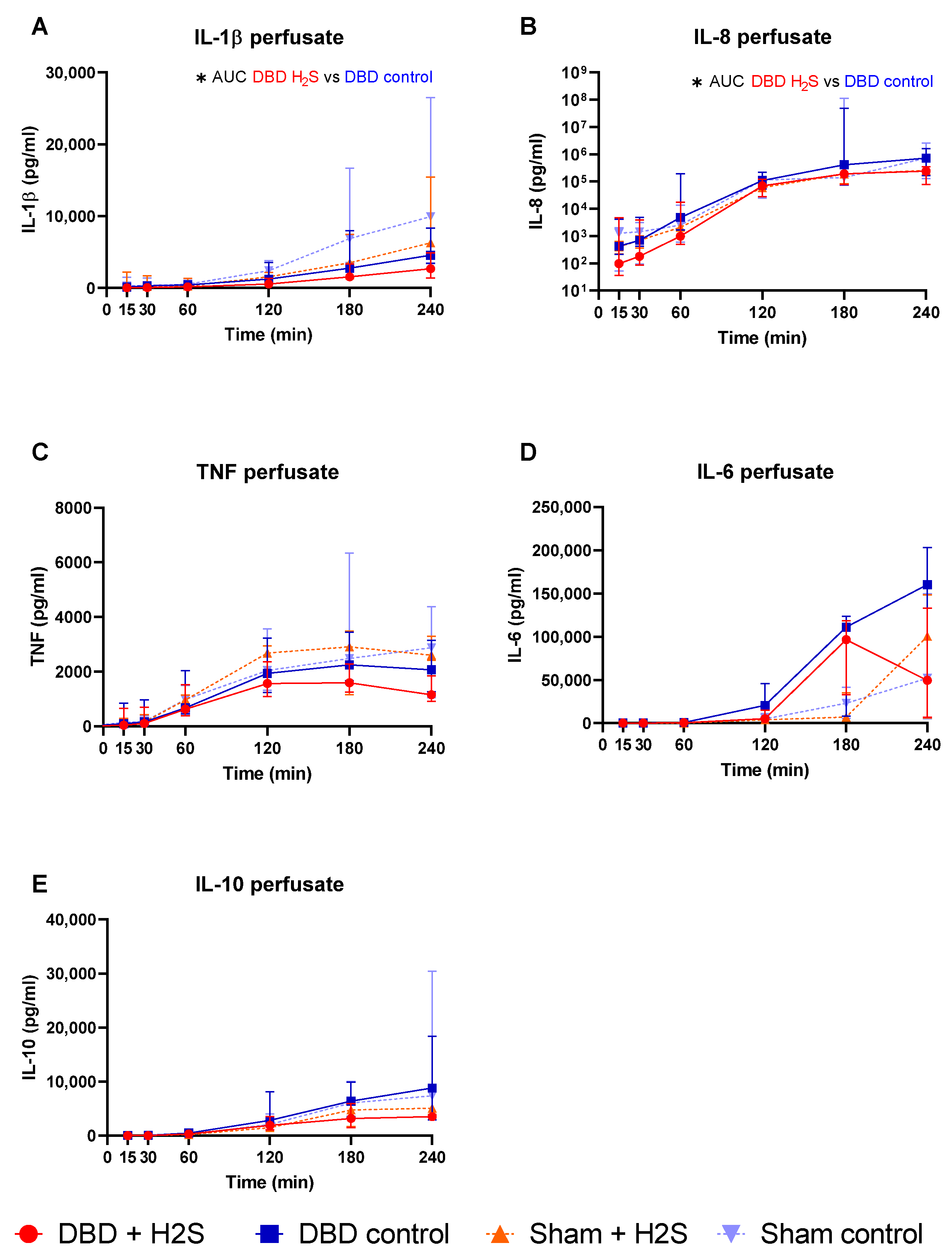
The addition of H2S in the flush-out solution and cold storage solution resulted in lower IL-1β in the H2S-treated DBD kidneys compared to the DBD control kidneys (p = 0.03) (Figure 2A). H2S treatment also decreased IL-8 levels in the perfusate during NMP in the DBD kidneys (p = 0.03) (Figure 2B). H2S treatment in the Sham group did not alter IL-1β or IL-8 levels. No significant effects of H2S or donor type were seen for TNF, IL-6, and IL-10 during NMP (Figure 2C–E).
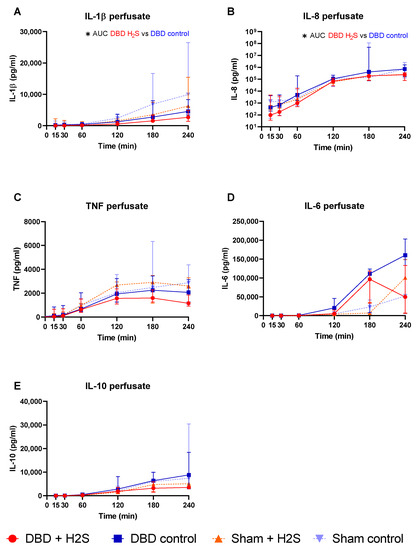
Figure 2.
Cytokine release. (A–E): respectively IL-1b, IL-8, TNF IL-6, and IL-10 levels (pg/mL) in the perfusate during NMP. Data presented as median + interquartile range. * p < 0.05. Abbreviations: AUC = area under the curve. TNF = tumor necrosis factor. NMP = normothermic machine perfusion.
3.3. Complement Activation during Preservation and NMP
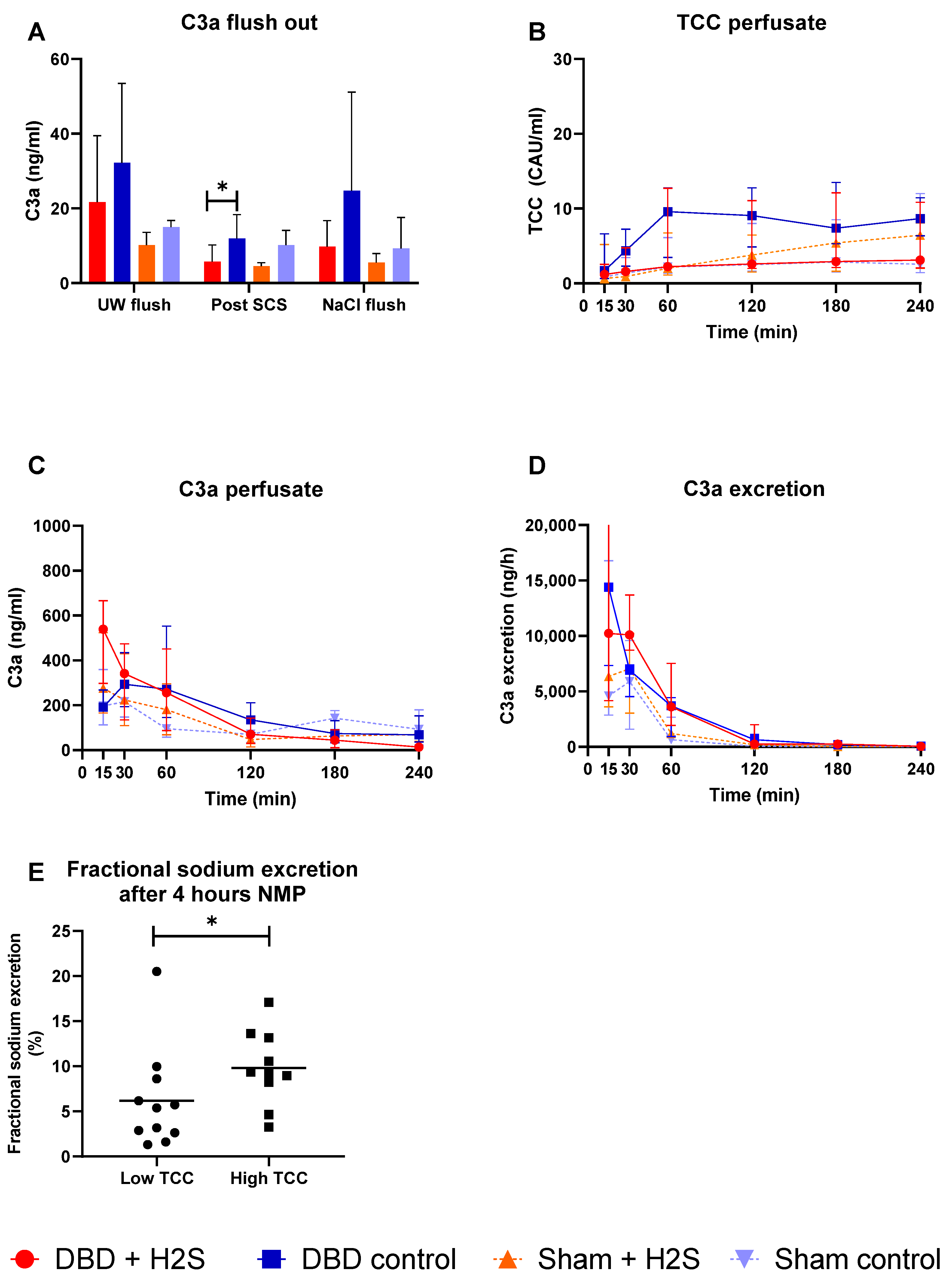
Complement activation was measured in the UW-flush after nephrectomy and in the NaCl-flush before NMP, after SCS, and during different time points of NMP. H2S treatment in the DBD kidneys decreased the C3a levels in the SCS fluid (p = 0.03) (Figure 3A). No effect of H2S treatment was found in the Sham kidneys. TCC levels in the perfusate increased in all groups during NMP (Figure 3B). C3a levels in both the perfusate (Figure 3C) and urine (Figure 3D) started higher and decreased during NMP independent of donation type. To investigate the impact of higher complement activation on renal function, we dichotomized all kidneys in a group with the lowest and the highest TCC values. FENa was significantly lower (better tubular function) in the low TCC group compared to the high TCC group (p = 0.036) (Figure 3E).
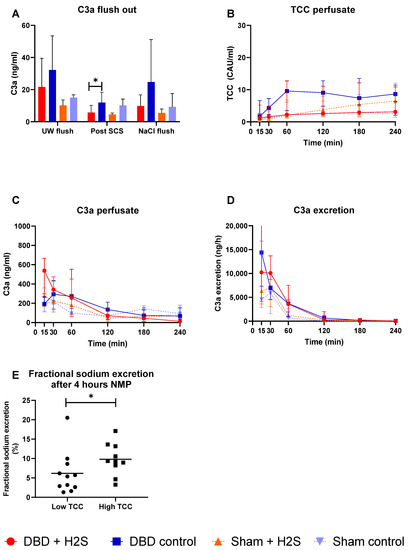
Figure 3.
Complement activation. (A): C3a levels after the flushes and after SCS (ng/mL). (B): TCC in the perfusate during NMP (CAU/mL). (C): C3a in the perfusate during NMP (ng/mL). (D): C3a excretion in the urine (ng/h). (E): Fractional sodium excretion after 4 h NMP in the lowest TCC kidneys and the highest TCC kidneys. Data presented as median + interquartile range. * p < 0.05. Abbreviations: TCC = terminal complement complex. NMP = normothermic machine perfusion. SCS = Static cold storage.
3.4. Metabolic Activity and ROS
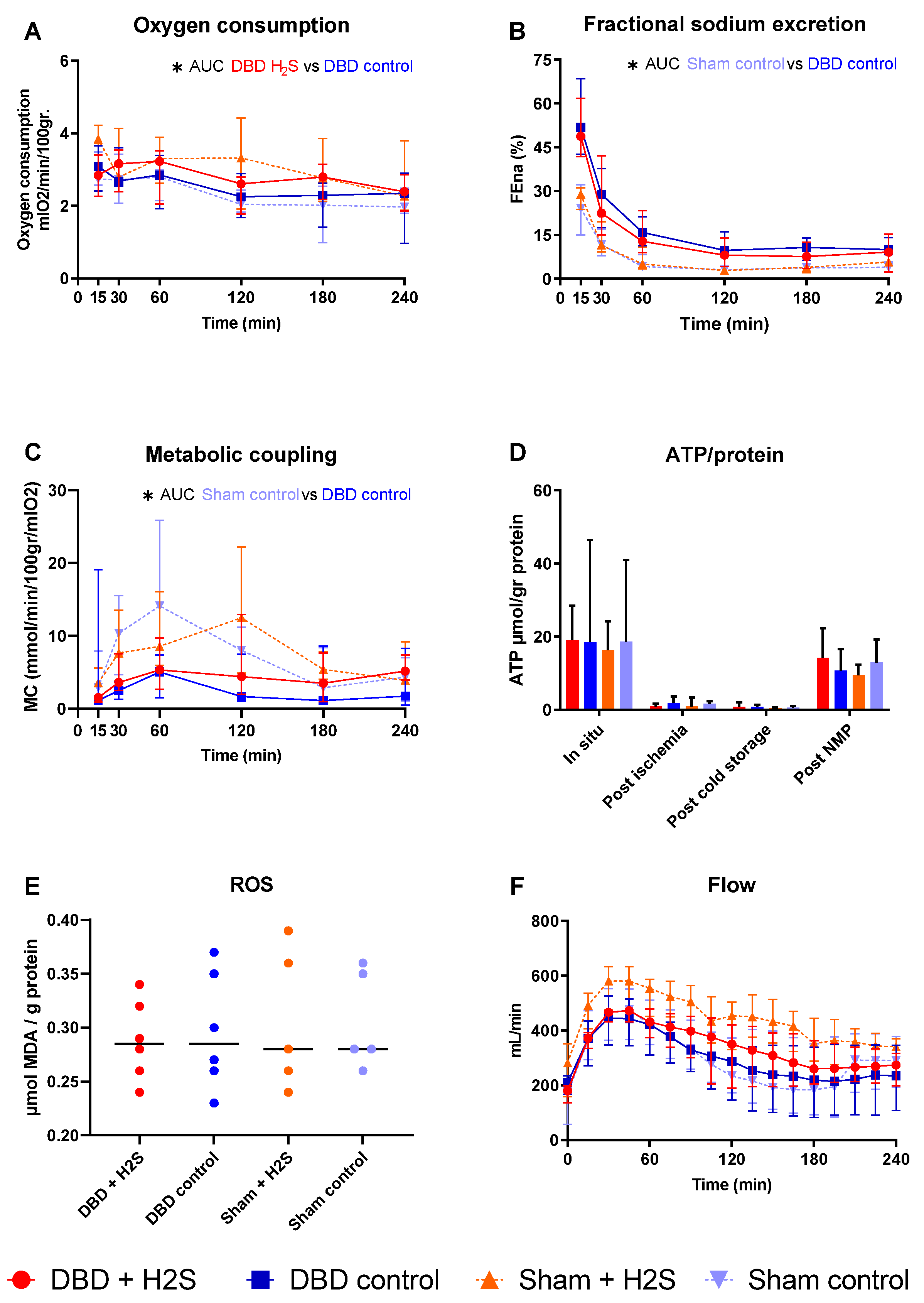
After SCS, kidneys were perfused by NMP for 4 h using an RBC-based perfusate to assess the metabolic activity of the kidneys (Figure 4). There was a significantly higher oxygen consumption in the H2S-treated DBD kidneys compared to the control DBD kidneys (p = 0.03) (Figure 4A). H2S treatment did not impact other metabolic or ROS parameters in the DBD groups. In the Sham groups, H2S did not alter oxygen consumption. FENa was calculated to estimate tubular function (Figure 4B). Metabolic coupling, providing information about how much oxygen is used for sodium transport, was also not affected by H2S treatment (Figure 4C). ATP levels were measured at four different time points, and the kidneys had the highest ATP values while they were still in situ, just before extraction of the kidneys (Figure 4D). After ischemia and cold storage, ATP values were low and comparable between all groups. NMP increased ATP values in all groups. MDA, indicative of the oxidative injury, showed similar values in all groups after 4 h of NMP (Figure 4E). The arterial blood flow showed an increase in the first hour of perfusion, after which it stabilized, independent of the group (Figure 4F). The type of donor affected the tubular function and metabolic coupling. There was significantly lower FENa in the Sham control kidneys than the DBD control kidneys (p = 0.004), and significantly higher metabolic coupling (p = 0.03).
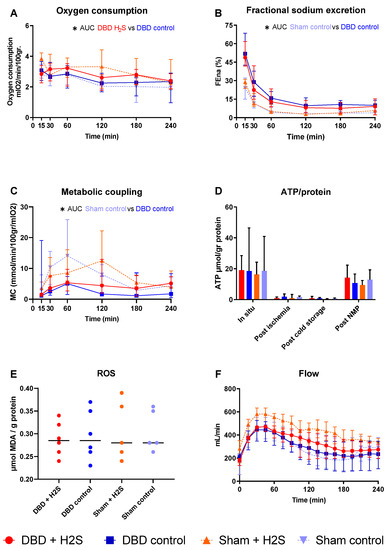
Figure 4.
Metabolic activity and ROS. (A): Oxygen consumption (mlO2/min/100 gr). (B): Fractional sodium excretion (%). (C): Metabolic coupling (mmol/min/100 gr/mlO2). (D): ATP values during preservation and after NMP (µmol/gr protein). (E): MDA after NMP, measured in tissue (µmol MDA/gram protein). (F): Arterial blood flow during NMP (mL/min). Data presented as median + interquartile range. * p < 0.05. Abbreviations: AUC = area under the curve. MDA = malondialdehyde.
3.5. Renal Function and Injury Markers
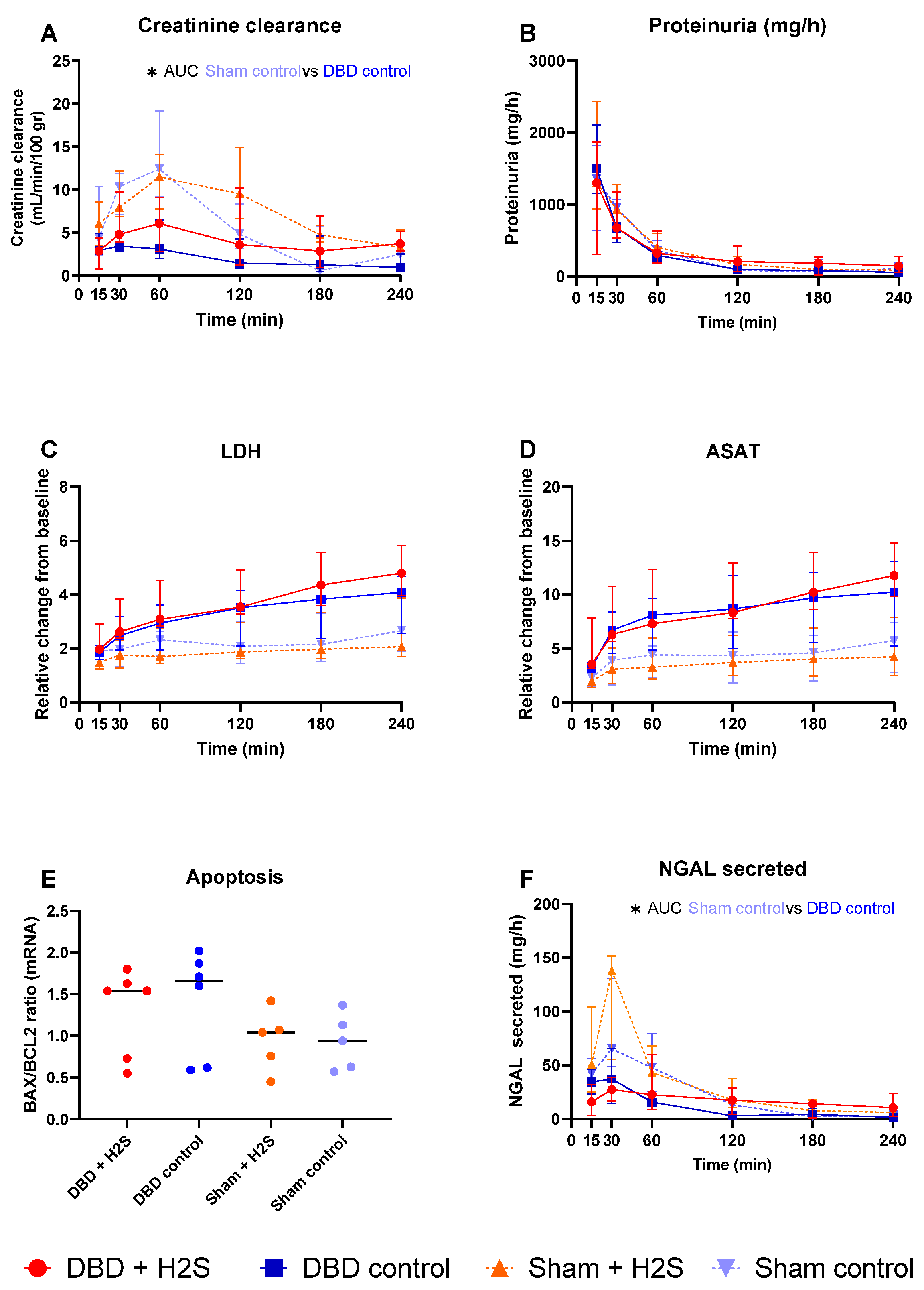
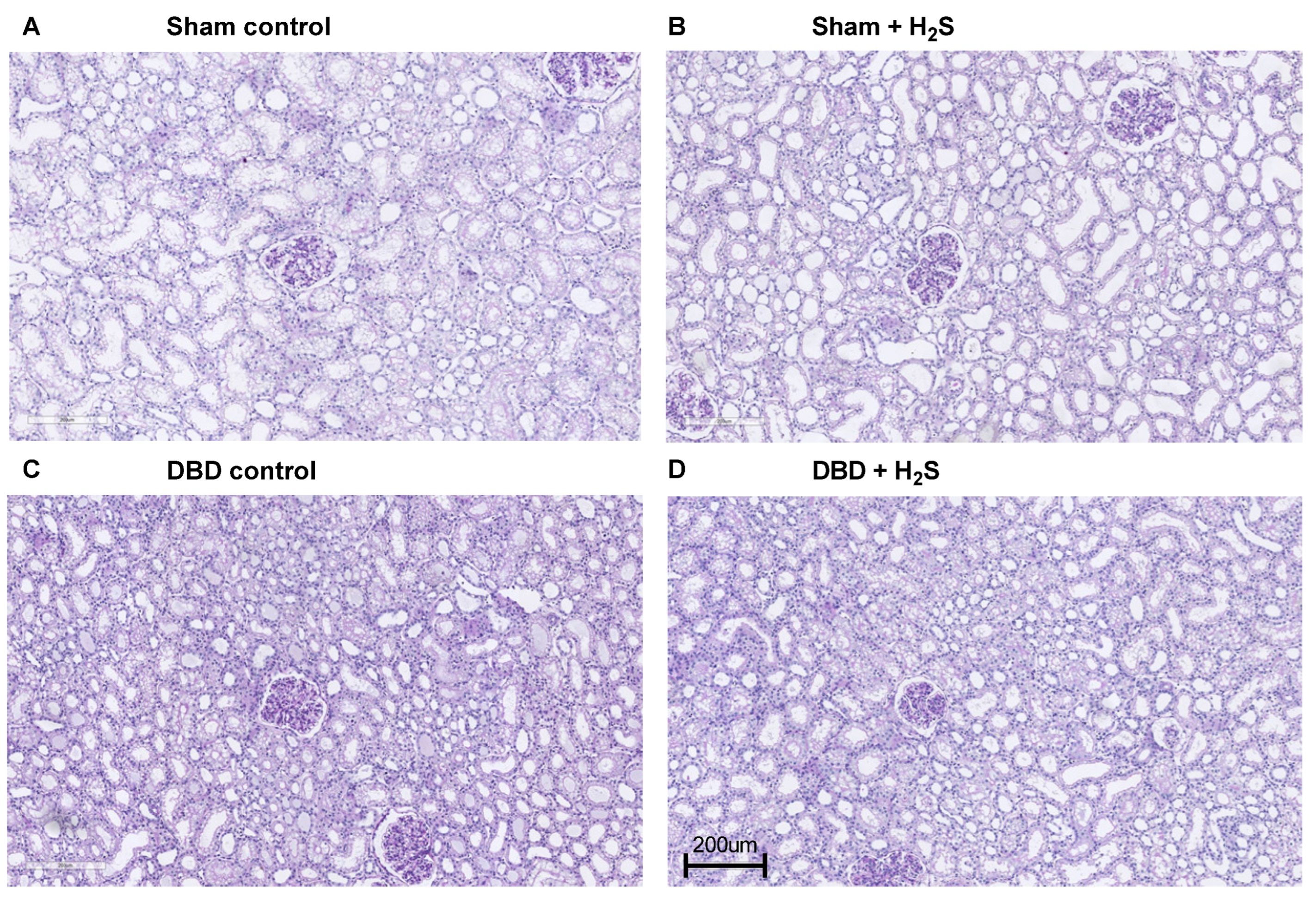
Creatinine clearance was calculated as an estimation of glomerular filtration (Figure 5A). The H2S enriched flush and subsequent cold storage did not alter creatinine clearance in the DBD groups. H2S treatment also did not affect creatinine clearance in the Sham kidneys. All kidneys had similar proteinuria during NMP (Figure 5B). Lactate dehydrogenase (LDH) (Figure 5C) and aspartate aminotransferase (ASAT) (Figure 5D) increased over time during NMP. BAX/BCL-2 ratios, indicative of the amount of apoptosis present in the biopsy, showed similar values after NMP, independent of H2S treatment or donor type (Figure 5E). The Sham control kidneys showed a higher creatinine clearance compared to the DBD control kidney, indicating an adverse effect of brain death on kidney function (p = 0.03) (Figure 5A). NGAL secretion levels were measured in the urine as a marker for renal injury and were significantly higher in the Sham control group vs. the DBD control group (p = 0.03) (Figure 5F). Kidneys were histologically examined, we did not find any structural changes in the kidneys. Kidneys were evaluated for glomerular, vascular, and interstitial damage (Figure 6).
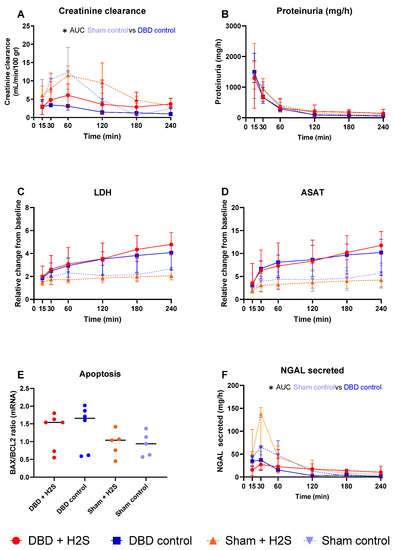
Figure 5.
Renal function and injury markers. (A): Creatinine clearance (mL/min/100 gr). (B): Proteinuria (mg/h). (C): LDH (relative change from baseline). (D): ASAT (relative change from baseline). (E). BAX/BCL2 ratios, measured in tissue after NMP. (F): NGAL secretion in urine (mg/h). Data presented as median + interquartile range. * p < 0.05. Abbreviations: AUC = area under the curve. LDH = lactate dehydrogenase. ASAT = aspartate aminotransferase. NGAL = urinary neutrophil gelatinase-associated lipocalin. BAX = bcl-2-like protein 4. BCL2 = B-cell lymphoma 2.
Figure 6.
Representative histopathological images.
4. Discussion
The objective of our study was to examine the potential of the addition of H2S to the flush-out and cold storage solution, to reduce inflammation and ischemic injury in kidneys retrieved from brain dead donors and non-brain-dead donors. In our approach, the addition of H2S reduced levels of the pro-inflammatory cytokines IL-1β and IL-8 during NMP without affecting renal function or renal injury markers.
Anti-inflammatory characteristics of H2S have been studied extensively in vitro, with a suggested role for the attenuation of the nuclear-factor-kappa B (NF-kB) pathway [29] and activation of the protective Keap1/NRF2 signalling pathway. A decrease in cytokine levels due to H2S has previously been described in IRI and transplantation models. For example, Sun et al. showed lower levels of TNF and IL-1β after reperfusion in NaHS-enriched UW in a rat heart transplantation model [30]. Additionally, NaHS treatment in a rabbit model of urinary-derived sepsis showed downregulation of pro-inflammatory markers TNF and NF-kB, and upregulation of anti-inflammatory IL-10 [31]. Our data support these findings with a lower level of pro-inflammatory cytokines IL-1β and IL-8 in the perfusate of the H2S treated DBD kidneys during NMP. No response to the H2S treatment was seen in the Sham kidneys. The lack of impact of H2S in the Sham kidneys could not result from higher cytokine levels in the DBD groups, since the Sham group had comparable levels of both IL-1β and IL-8. IL-1β is strongly proinflammatory and is released at the early stages of the immune response to infections or stress [32]. IL-8 is a potent chemoattractant for neutrophils, produced by macrophages [32]. The source of these cytokines might differ between the DBD and Sham pigs, resulting in equal levels of cytokines during NMP, but a stronger effect of H2S on the DBD group compared to the Sham group. Brain death and NMP are known to induce a release of cytokines, possibly indicating the different triggers of cytokine release in both groups [33,34].
Reperfusion induces activation of the innate immune system, with an essential role in complement activation and cytokine release [35]. The complement system, consisting of three different pathways, the classical, lectin, and alternative pathway, is activated after reperfusion. The lectin pathway has been suggested as a primary role of renal complement activation following ischemia reperfusion injury (IRI) [36]. Brain death has also been shown to affect the innate immune system, probably by the release of alarmins triggering both complement and Toll-like receptors, leading to a systemic cytokine storm affecting distant organs, including the kidneys [37]. Treatment of H2S in both DBD and non-DBD donors could identify a possible decrease in injury initiated by the complement system. Complement levels of C3a were lower in the H2S treated DBD group after SCS. A similar impact of H2S treatment was not seen in the Sham control kidneys. Excessive complement activation can lead to cell lysis and necrosis [38], but the decrease in complement activation by H2S was not seen during/after NMP. This might account for the lack of differences in apoptotic markers after NMP. Comparing DBD and Sham kidneys could distinguish between the impact of brain death and ischemia-induced immune system activation. Although our group described an increase in complement activation during different phases of transplantation [39], in this study, no significantly higher activation of complement during NMP in the DBD group compared to the Sham control group were observed. In all groups, TCC showed an increase in the first 2–3 h of reperfusion, whereafter it seemed to stabilize. For C3a, the opposite was the case. C3a showed a higher level at the start of reperfusion with a decrease over time, which can be explained by different activation and turn-over kinetics. There is evidence for complement activation in proximal tubular epithelial cells [40], which might be responsible for the production of TCC over time. The decrease of C3a during NMP might be due to a higher excretion than production during NMP, especially visible in the high secretion values at the start of NMP. When dichotomizing all kidneys in a group with high and low TCC, we showed that the low TCC group had a significantly improved FENa (Figure 4E). The higher FENa in the high TCC group implies that more complement activation might result in worse tubular function in these kidneys, independent of donation type. Treatment of kidneys on NMP with complement inhibitors could lead to improved renal quality prior to transplantation.
It should be noted that kidneys from all groups showed increased cytokines and TCC during NMP over time. With NMP being increasingly tested for several purposes, including preconditioning, and with clinical implementation around the corner [19], one should contemplate which kidney should be put on NMP and for what reason. Cytokines and complement activation products during NMP might harm rather than improve the kidney before it is transplanted. Especially in living donors, where injury is minimal, the added value of NMP is very limited compared to the risk. On the other hand, NMP could provide a platform and opportunity to counteract or alter the activation of complement and release of cytokines, decreasing possible future injury. Pre-clinical research on this topic shows promising results in decreasing renal injury in bran-dead rats [41].
After the initial paper by Blackstone et al. [42], we and others investigated the hypometabolic capacity of H2S. H2S can induce a hypometabolic state when administered to small mammals, such as mice. H2S competes with O2 for binding to cytochrome c oxidase (complex IV) of the electron transport chain in mitochondria. As a result, O2 cannot react with H+ to form H2O, blocking ATP synthase [43], thereby inducing a hypometabolic state. We previously showed that this form of hypometabolism is also conductible in isolated perfused kidneys from large animals otherwise not responsive to H2S-indcued hypometabolism [14]. Impairing the metabolism of the kidney ex vivo during the flush could diminish metabolic activity and decrease organ injury. During NMP, the H2S treated kidneys were more metabolically active, displayed by oxygen consumption. These results are in line with our previous experiment where H2S treatment resulted in higher oxygen consumption after the hypometabolic effect of H2S had passed [14]. In our experimental setup, the addition of H2S did not result in a decrease in MDA levels, apoptosis or NGAL levels or better preservation of the renal and tubular function within the time frame studied. Although the induction of brain death highly affected renal function, alterations by H2S treatment were not visible. H2S therapy has shown beneficial results in counteracting ischemic conditions in previous experiments in small mammals [15,44]. Although promising results are also shown in porcine kidneys regarding ameliorating ischemic injury [45], testing in an extensive transplantation model is still lacking. Although we did not see clear evidence of the efficacy of H2S to improve renal function, it cannot be excluded that this is due to the model used. In our model we applied the H2S flush at the back table after the initial systemic flush. The application of H2S in the first flush could be beneficial, but it would also reach other transplantable organs with unknown effects. The second disadvantage of our setup is the short observation time of 4 h of NMP. NMP is a valuable option to test the early function of organs after retrieval from the donor, as can be observed in the impaired renal function in the DBD group. Longer follow-up in the NMP model is challenging, and therefore, transplantation experiments are needed to examine the long-term effects. The potential direct ischemia reducing characteristics of H2S might be limited, and a longer follow-up should elucidate if a decrease in cytokines reduces, for example, fibrosis on the long term. Lastly, the sample size that was used is small, and therefore, the power of the study is limited.
5. Conclusions
The addition of H2S in flush-out and storage conditions showed a reduction in inflammatory markers, without affecting renal function or injury markers. At this stage, H2S cannot be recommended as an ischemia reducing treatment strategy during a second flush-out after extraction of the kidney. This study may provide a base for future research that focuses on anti-inflammatory and anti-ischemic therapies in more compromised conditions, such as those observed in DBD donor kidneys.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox12030749/s1.
Author Contributions
Conceptualization, H.M., L.H.V., M.G.W., H.S.H., A.K.K., B.J., H.v.G. and H.G.D.L.; methodology, H.M., T.E.M., M.E., S.E.P., B.J., H.v.G. and H.G.D.L.; formal analysis, H.M., M.G.W., T.M.H., T.E.M., M.E. and S.E.P.; investigation, H.M., L.H.V., M.G.W., T.M.H., A.K.K. and M.E.; resources, H.S.H., A.K.K., T.E.M., M.E., S.E.P., B.J., H.v.G. and H.G.D.L.; data curation, H.M., M.G.W., H.v.G. and H.G.D.L.; writing—original draft preparation, H.M., L.H.V., M.G.W. and T.M.H.; writing—review and editing, H.S.H., A.K.K., T.E.M., M.E., S.E.P., B.J., H.v.G. and H.G.D.L.; supervision, H.S.H., A.K.K., T.E.M., M.E., S.E.P., B.J., H.v.G. and H.G.D.L.; project administration, H.M., B.J., H.v.G. and H.G.D.L.; funding acquisition, H.S.H., A.K.K., T.E.M., M.E., S.E.P., B.J., H.v.G. and H.G.D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by de Cock-Hadders stichting (grand number: COC 2020-37), the South-Eastern Norway Regional Health Authority (grant number: 2020117), The Novo Nordic Foundation (grand number: NNF19OC0056466), Aase and Ejnar Danielsen’s Foundation (grant number: 19-10-0392), A.P. Møller Maersk Foundation (grant number: 19-L-0132), the Research foundation of the Danish Renal Association, and was supported by GUIDE, and the University Medical Centre Groningen.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Animal Experimentation Council of Denmark (reference-number 2019-15-0201-00157).
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw data can be found in Supplementary Material.
Acknowledgments
The authors express their gratitude to Petra Ottens, Anna Lammerts, Maria Arge, Birgitte Kildevæld Sahl, Judith Anita Ludviksen, Åse Emblem, and Kristin Pettersen for their support and help during the experiments and analysis afterwards.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Westendorp, W.H.; Leuvenink, H.G.; Ploeg, R.J. Brain death induced renal injury. Curr. Opin. Organ Transpl. 2011, 16, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Bouma, H.R.; Ploeg, R.J.; Schuurs, T.A. Signal transduction pathways involved in brain death-induced renal injury. Am. J. Transpl. 2009, 9, 989–997. [Google Scholar] [CrossRef]
- Osband, A.J.; James, N.T.; Segev, D.L. Extraction Time of Kidneys From Deceased Donors and Impact on Outcomes. Am. J. Transpl. 2016, 16, 700–703. [Google Scholar] [CrossRef]
- Osband, A.J.; Zaki, R.F. Extraction time of kidneys during organ procurement impacts function. Clin. Transpl. 2011, 25, 235–238. [Google Scholar] [CrossRef]
- Heylen, L.; Pirenne, J.; Samuel, U.; Tieken, I.; Coemans, M.; Naesens, M.; Sprangers, B.; Jochmans, I. Effect of donor nephrectomy time during circulatory-dead donor kidney retrieval on transplant graft failure. Br. J. Surg. 2019, 107, 87–95. [Google Scholar] [CrossRef]
- Debout, A.; Foucher, Y.; Trébern-Launay, K.; Legendre, C.; Kreis, H.; Mourad, G.; Garrigue, V.; Morelon, E.; Buron, F.; Rostaing, L.; et al. Each additional hour of cold ischemia time significantly increases the risk of graft failure and mortality following renal transplantation. Kidney Int. 2015, 87, 343–349. [Google Scholar] [CrossRef]
- Tennankore, K.K.; Kim, S.J.; Alwayn, I.P.; Kiberd, B.A. Prolonged warm ischemia time is associated with graft failure and mortality after kidney transplantation. Kidney Int. 2016, 89, 648–658. [Google Scholar] [CrossRef]
- Pratschke, J.; Wilhelm, M.J.; Kusaka, M.; Beato, F.; Milford, E.L.; Hancock, W.W.; Tilney, N.L. Accelerated Rejection of Renal Allografts From Brain-Dead Donors. Ann. Surg. 2000, 232, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Pratschke, J.; Wilhelm, M.J.; Laskowski, I.; Kusaka, M.; Beato, F.; Tullius, S.G.; Neuhaus, P.; Hancock, W.W.; Tilney, N.L. Influence of Donor Brain Death on Chronic Rejection of Renal Transplants in Rats. J. Am. Soc. Nephrol. 2001, 12, 2474–2481. [Google Scholar] [CrossRef]
- Terasaki, P.I.; Cecka, J.M.; Gjertson, D.W.; Takemoto, S. High survival rates of kidney transplants from spousal and living unrelated donors. N. Engl. J. Med. 1995, 333, 333–336. [Google Scholar] [CrossRef]
- van Erp, A.C.; van Dullemen, L.F.; Ploeg, R.J.; Leuvenink, H.G. Systematic review on the treatment of deceased organ donors. Transpl. Rev. 2018, 32, 194–206. [Google Scholar] [CrossRef]
- Hendriks, K.; Maassen, H.; van Dijk, P.R.; Henning, R.; van Goor, H.; Hillebrands, J.-L. Gasotransmitters in health and disease: A mitochondria-centered view. Curr. Opin. Pharmacol. 2019, 45, 87–93. [Google Scholar] [CrossRef]
- Koning, A.M.; Frenay, A.-R.S.; Leuvenink, H.G.D.; van Goor, H. Hydrogen sulfide in renal physiology, disease and transplantation—The smell of renal protection. Nitric Oxide 2015, 46, 37–49. [Google Scholar] [CrossRef]
- Maassen, H.; Hendriks, K.D.W.; Venema, L.H.; Henning, R.H.; Hofker, S.H.; Van Goor, H.; Leuvenink, H.G.D.; Coester, A.M. Hydrogen sulphide-induced hypometabolism in human-sized porcine kidneys. PLoS ONE 2019, 14, e0225152. [Google Scholar] [CrossRef]
- Lobb, I.; Jiang, J.; Lian, D.; Liu, W.; Haig, A.; Saha, M.N.; Torregrossa, R.; Wood, M.E.; Whiteman, M.; Sener, A. Hydrogen Sulfide Protects Renal Grafts Against Prolonged Cold Ischemia–Reperfusion Injury via Specific Mitochondrial Actions. Am. J. Transpl. 2017, 17, 341–352. [Google Scholar] [CrossRef]
- Dirkes, M.C.; Milstein, D.M.; Heger, M.; van Gulik, T.M. Absence of Hydrogen Sulfide-Induced Hypometabolism in Pigs: A Mechanistic Explanation in Relation to Small Nonhibernating Mammals. Eur. Surg. Res. 2015, 54, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Haouzi, P.; Notet, V.; Chenuel, B.; Chalon, B.; Sponne, I.; Ogier, V.; Bihain, B. H2S induced hypometabolism in mice is missing in sedated sheep. Respir. Physiol. Neurobiol. 2008, 160, 109–115. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Hamelink, T.L.; Ogurlu, B.; De Beule, J.; Lantinga, V.A.; Pool, M.B.F.; Venema, L.H.; Leuvenink, H.G.D.; Jochmans, I.; Moers, C. Renal Normothermic Machine Perfusion: The Road Toward Clinical Implementation of a Promising Pretransplant Organ Assessment Tool. Transplantation 2021. ahead of print. [Google Scholar] [CrossRef]
- Barklin, A.; Larsson, A.; Vestergaard, C.; Koefoed-Nielsen, J.; Bach, A.; Nyboe, R.; Wogensen, L.; Tønnesen, E. Does brain death induce a pro-inflammatory response at the organ level in a porcine model? Acta Anaesthesiol. Scand. 2008, 52, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, S.; Pool, M.B.; Rozenberg, K.M.; Keller, A.K.; Moers, C.; Møldrup, U.; Møller, B.K.; Lignell, S.J.; Krag, S.; Sierra-Parraga, J.M.; et al. Mesenchymal stromal cell treatment of donor kidneys during ex vivo normothermic machine perfusion: A porcine renal autotransplantation study. Am. J. Transpl. 2020, 21, 2348–2359. [Google Scholar] [CrossRef]
- Høgåsen, K.; Jansen, J.; Mollnes, T.; Harboe, M. Extensive complement activation in hereditary porcine membranoproliferative glomerulonephritis type II (porcine dense deposit disease). Mol. Immunol. 1993, 30, 17. [Google Scholar] [CrossRef]
- Nilsson, P.H.; Pettersen, K.; Oppermann, M.; Skjeflo, E.W.; Fure, H.; Christiansen, D.; Mollnes, T.E. Quantification of Porcine Complement Activation Fragment C3a by a Neoepitope-Based Enzyme-Linked Immunosorbent Assay. Methods Mol. Biol. 2021, 2227, 51–59. [Google Scholar] [CrossRef]
- Ueland, N.L.; Ludvigsen, J.K.; Hellerud, B.C.; Mollnes, T.E.; Skjeflo, E.W. Choice of immunoassay to evaluate porcine cytokine levels. Veter-Immunol. Immunopathol. 2020, 230, 110129. [Google Scholar] [CrossRef]
- Venema, L.H.; Brat, A.; Moers, C.; Hart, N.A.; Ploeg, R.J.; Hannaert, P.; Minor, T.; Leuvenink, H. Effects of Oxygen During Long-term Hypothermic Machine Perfusion in a Porcine Model of Kidney Donation After Circulatory Death. Transplantation 2019, 103, 2057–2064. [Google Scholar] [CrossRef]
- Mahboub, P.; Ottens, P.; Seelen, M.; t Hart, N.; Van Goor, H.; Ploeg, R.; Martins, P.N.; Leuvenink, H. Gradual rewarming with gradual increase in pressure during machine perfusion after cold static preservation reduces kidney ischemia reperfusion injury. PLoS ONE 2015, 10, e0143859. [Google Scholar] [CrossRef]
- van Erp, A.C.; Qi, H.; Jespersen, N.R.; Hjortbak, M.V.; Ottens, P.J.; Wiersema-Buist, J.; Nørregaard, R.; Pedersen, M.; Laustsen, C.; Leuvenink, H.G.D.; et al. Organ-specific metabolic profiles of the liver and kidney during brain death and afterwards during normothermic machine perfusion of the kidney. Am. J. Transpl. 2020, 20, 2425–2436. [Google Scholar] [CrossRef]
- Rebolledo, R.A.; Liu, B.; Akhtar, M.Z.; Ottens, P.J.; Zhang, J.-N.; Ploeg, R.J.; Leuvenink, H.G.D. Steroid Anti-Inflammatory Effects Did Not Improve Organ Quality in Brain-Dead Rats. BioMed Res. Int. 2015, 2015, 207534. [Google Scholar] [CrossRef]
- Sivarajah, A.; Collino, M.; Yasin, M.; Benetti, E.; Gallicchio, M.; Mazzon, E.; Cuzzocrea, S.; Fantozzi, R.; Thiemermann, C. Anti-apoptotic and anti-inflammatory effects of hydrogen sulfide in a rat model of regional myocardial I/R. Shock 2009, 31, 267–274. [Google Scholar] [CrossRef]
- Sun, X.; Wang, W.; Dai, J.; Huang, J.; Shi, M.; Chu, X.; Wang, F.; Guo, C.; Wang, C.; Pang, L.; et al. Donor heart preservation with a novel long-term and slow-releasing hydrogen sulfide system. Nitric Oxide 2018, 81, 1–10. [Google Scholar] [CrossRef]
- Chen, X.; Xu, W.; Wang, Y.; Luo, H.; Quan, S.; Zhou, J.; Yang, N.; Zhang, T.; Wu, L.; Liu, J.; et al. Hydrogen sulfide reduces kidney injury due to urinary-derived sepsis by inhibiting NF-κB expression, decreasing TNF-α levels and increasing IL-10 levels. Exp. Ther. Med. 2014, 8, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Duque, G.A.; Descoteaux, A. Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [PubMed]
- Kusaka, M.; Pratschke, J.; Wilhelm, M.J.; Ziai, F.; Zandi-Nejad, K.; Mackenzie, H.S.; Hancock, W.W.; Tilney, N.L. Activation of inflammatory mediators in rat renal isografts by donor brain death. Transplantation 2000, 69, 405–410. [Google Scholar] [CrossRef]
- Stone, J.P.; Ball, A.L.; Critchley, W.R.; Major, T.; Edge, R.J.; Amin, K.; Clancy, M.J.; Fildes, J.E. Ex Vivo Normothermic Perfusion Induces Donor-Derived Leukocyte Mobilization and Removal Prior to Renal Transplantation. Kidney Int. Rep. 2016, 1, 230–239. [Google Scholar] [CrossRef]
- Nieuwenhuijs-Moeke, G.J.; Pischke, S.E.; Berger, S.P.; Sanders, J.S.F.; Pol, R.A.; Struys, M.M.R.F.; Ploeg, R.J.; Leuvenink, H.G.D. Ischemia and Reperfusion Injury in Kidney Transplantation: Relevant Mechanisms in Injury and Repair. J. Clin. Med. 2020, 9, 253. [Google Scholar] [CrossRef]
- Nauser, C.L.; Farrar, C.A.; Sacks, S.H. Complement Recognition Pathways in Renal Transplantation. J. Am. Soc. Nephrol. 2017, 28, 2571–2578. [Google Scholar] [CrossRef]
- Damman, J.; Seelen, M.A.; Moers, C.; Daha, M.R.; Rahmel, A.; Leuvenink, H.G.; Paul, A.; Pirenne, J.; Ploeg, R.J. Systemic Complement Activation in Deceased Donors Is Associated With Acute Rejection After Renal Transplantation in the Recipient. Transplantation 2011, 92, 163–169. [Google Scholar] [CrossRef]
- Trouw, L.A.; Bengtsson, A.A.; Gelderman, K.A.; Dahlbäck, B.; Sturfelt, G.; Blom, A.M. C4b-binding Protein and Factor H Compensate for the Loss of Membrane-bound Complement Inhibitors to Protect Apoptotic Cells against Excessive Complement Attack. J. Biol. Chem. 2007, 282, 28540–28548. [Google Scholar] [CrossRef]
- Jager, N.M.; Poppelaars, F.; Daha, M.R.; Seelen, M.A. Complement in renal transplantation: The road to translation. Mol. Immunol. 2017, 89, 22–35. [Google Scholar] [CrossRef]
- Gerritsma, J.S.; van Kooten, C.; Gerritsen, A.F.; van Es, L.A.; Daha, M.R. Transforming growth factor-β1 regulates chemokine and complement production by human proximal tubular epithelial cells. Kidney Int. 1998, 53, 609–616. [Google Scholar] [CrossRef]
- Poppelaars, F.; Jager, N.M.; Kotimaa, J.; Leuvenink, H.G.; Daha, M.R.; van Kooten, C.; Seelen, M.A.; Damman, J. C1-Inhibitor Treatment Decreases Renal Injury in an Established Brain-Dead Rat Model. Transplantation 2018, 102, 79–87. [Google Scholar] [CrossRef]
- Blackstone, E.; Morrison, M.; Roth, M.B. H2S Induces a Suspended Animation-Like State in Mice. Science 2005, 308, 518. [Google Scholar] [CrossRef]
- Cooper, C.E.; Brown, G.C. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: Chemical mechanism and physiological significance. J. Bioenerg. Biomembr. 2008, 40, 533–539. [Google Scholar] [CrossRef]
- Snijder, P.M.; De Boer, R.A.; Bos, E.M.; van den Born, J.C.; Ruifrok, W.-P.T.; Vreeswijk-Baudoin, I.; Van Dijk, M.C.R.F.; Hillebrands, J.-L.; Leuvenink, H.G.; Van Goor, H. Gaseous Hydrogen Sulfide Protects against Myocardial Ischemia-Reperfusion Injury in Mice Partially Independent from Hypometabolism. PLoS ONE 2013, 8, e63291. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Nicholson, M.L. Hydrogen sulphide ameliorates ischaemia-reperfusion injury in an experimental model of non-heart-beating donor kidney transplantation. Br. J. Surg. 2010, 97, 202–209. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).