Cytoprotective and Antioxidant Effects of Hydrolysates from Black Soldier Fly (Hermetia illucens)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Insect Breeding
2.3. Protein Hydrolysate Production from BSF Larvae
2.3.1. BSFL Water-Soluble Extract Production (Laboratory Scale)
2.3.2. Process Scale-Up
2.4. Yield of BPHs
2.5. Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.6. NMR Analysis
2.7. Cell Culture
2.8. Cell Viability Assay
2.9. Determination of Intracellular ROS
2.10. Nrf2 Subcellular Localization Assay
2.11. mRNA Levels of nrf2
2.12. Statistical Analysis
3. Results
3.1. Production and Yield of Protein Hydrolysates from BSF Larvae
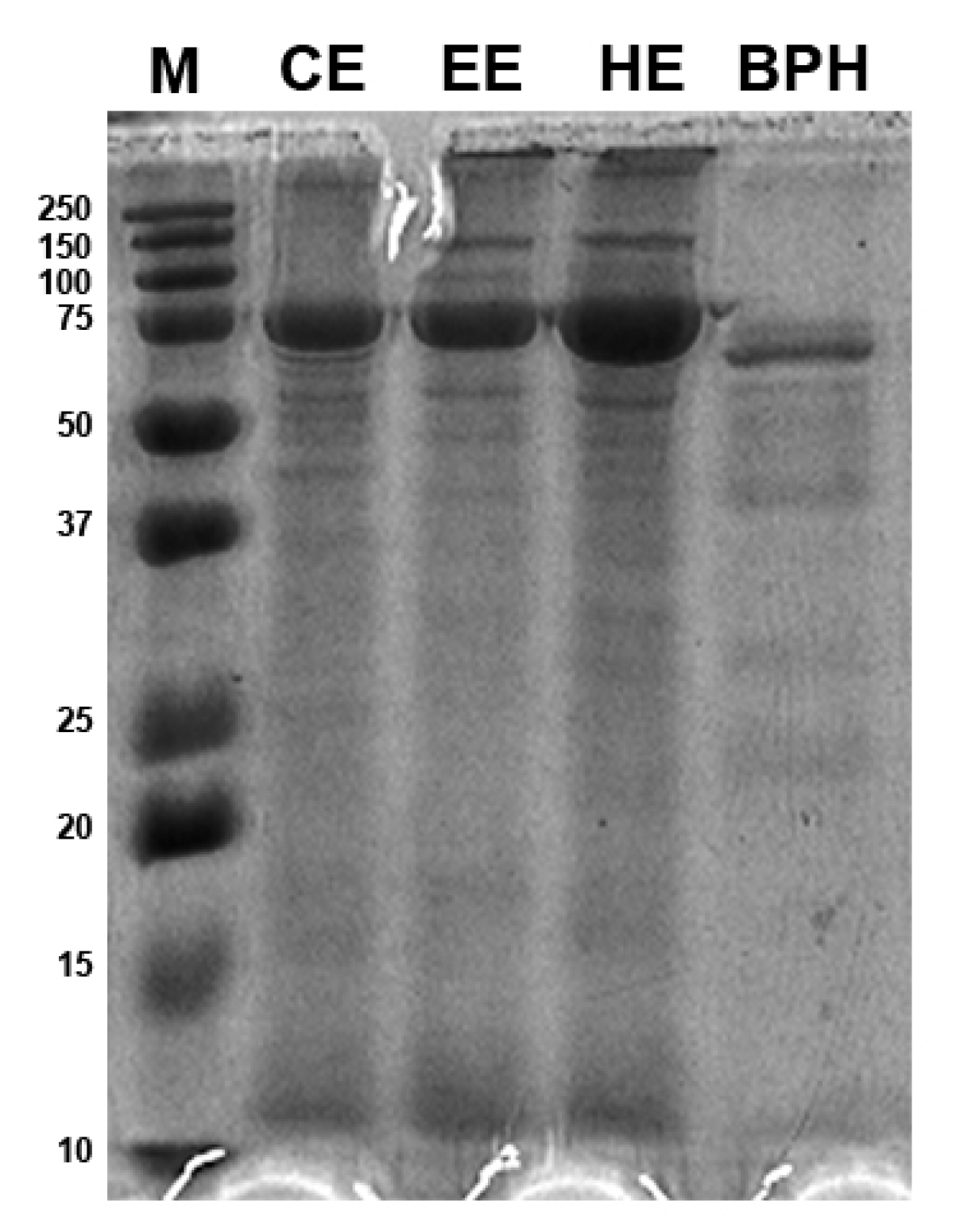
3.2. BPH Molecular Weight Distribution
3.3. NMR Data
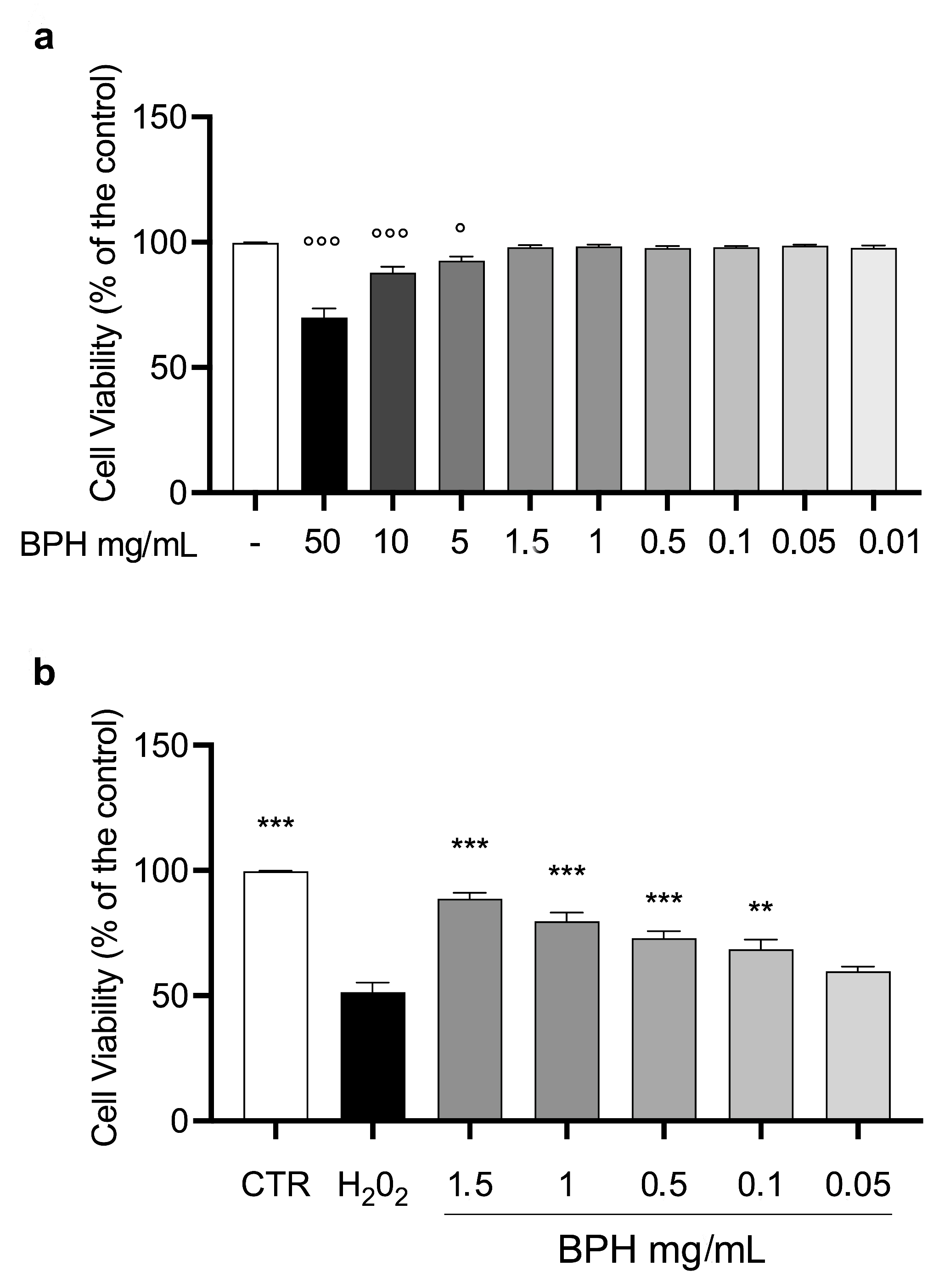
3.4. Effects of BSF Protein Hydrolysates on L-929 Cell Viability In Vitro
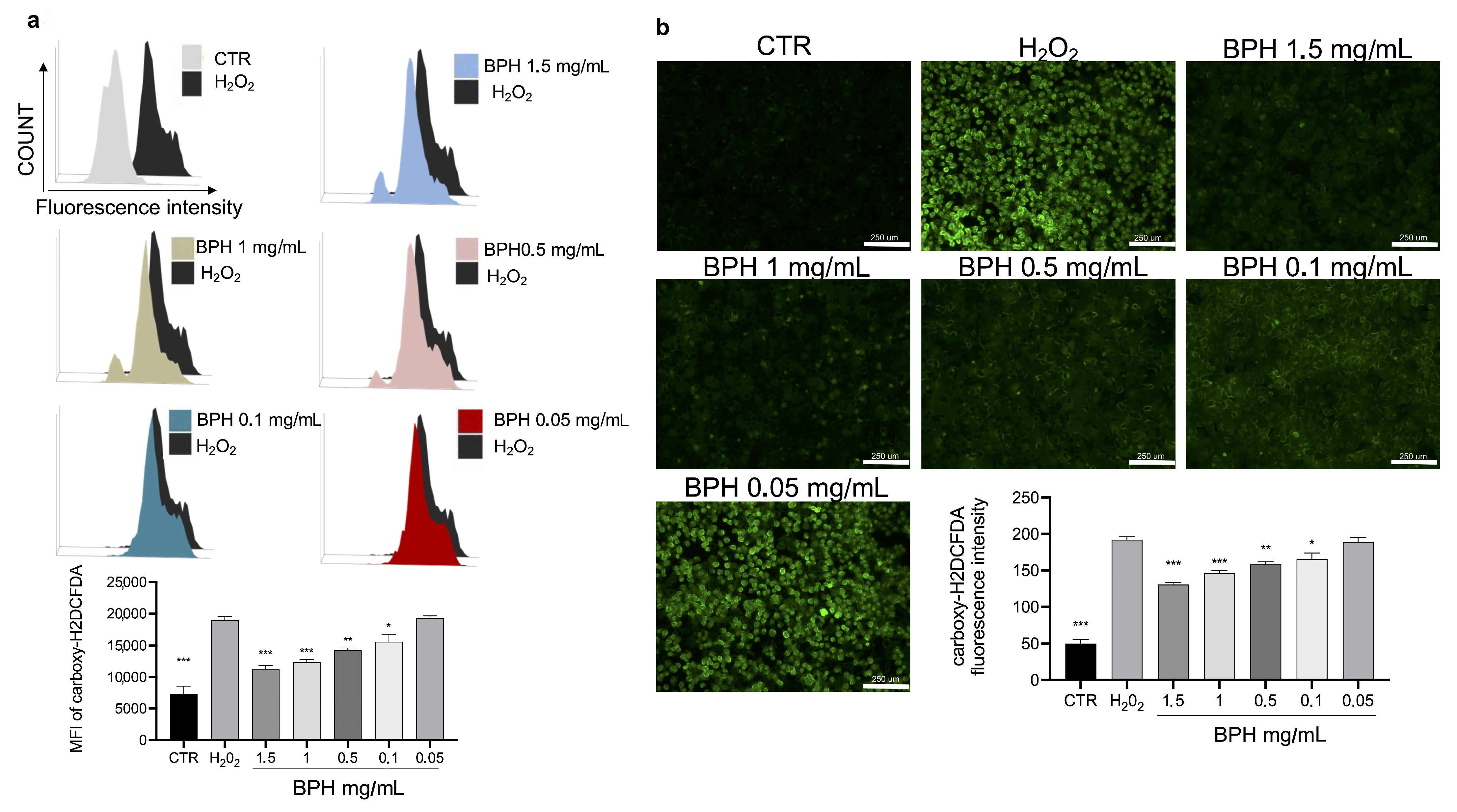
3.5. In Vitro Effects of BPHs against ROS Production in L-929 Cells
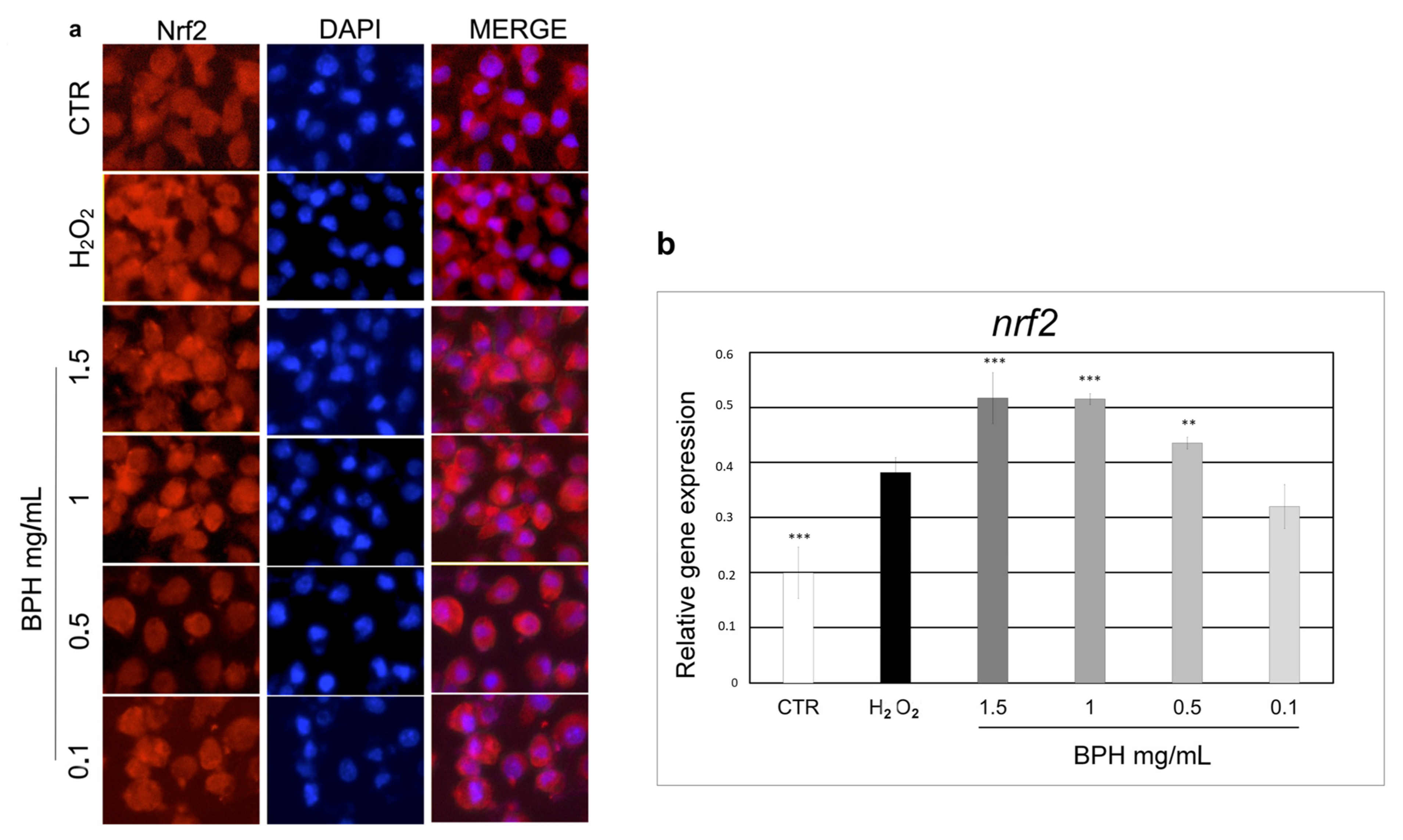
3.6. Effect of BPHs on Nrf2 Activation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Huis, A.; Oonincx, D.G.A.B. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef]
- Vargas, A.; Randazzo, B.; Riolo, P.; Truzzi, C.; Gioacchini, G.; Giorgini, E.; Gasco, L.; Olivotto, I. Rearing zebrafish on black soldier fly (Hermetia illucens): Biometric, histological, spectroscopic, biochemical, and molecular implications. Zebrafish 2018, 15, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Lanes, C.F.; Pedron, F.A.; Bergamin, G.T.; Bitencourt, A.L.; Dorneles, B.E.; Villanova, J.C.; Dias, K.C.; Riolo, K.; Oliva, S.; Savastano, D.; et al. Black Soldier Fly (Hermetia illucens) Larvae and Prepupae Defatted Meals in Diets for Zebrafish (Danio rerio). Animals 2021, 11, 720. [Google Scholar] [CrossRef] [PubMed]
- Giannetto, A.; Oliva, S.; Riolo, K.; Savastano, D.; Parrino, V.; Cappello, T.; Maisano, M.; Fasulo, S.; Mauceri, A. Waste valorization via Hermetia illucens to produce protein-rich Biomass for feed: Insight into the critical nutrient taurine. Animals 2020, 10, 1710. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Huang, X.; Tu, F.; Wang, C.; Yang, F. Preparation, antioxidant activity evaluation, and identification of antioxidant peptide from black soldier fly (Hermetia illucens L.) larvae. J. Food Biochem. 2020, 44, e13186. [Google Scholar] [CrossRef]
- Firmansyah, M.; Abduh, M.Y. Production of protein hydrolysate containing antioxidant activity from Hermetia illucens. Heliyon 2019, 5, e02005. [Google Scholar] [CrossRef] [PubMed]
- Leni, G.; Tedeschi, T.; Faccini, A.; Pratesi, F.; Folli, C.; Puxeddu, I.; Migliorini, P.; Gianotten, N.; Jacobs, J.; Depraetere, S.; et al. Shotgun proteomics, in-silico evaluation and immunoblotting assays for allergenicity assessment of lesser mealworm, black soldier fly and their protein hydrolysates. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Leni, G.; Soetemans, L.; Jacobs, J.; Depraetere, S.; Gianotten, N.; Bastiaens, L.; Caligiani, A.; Sforza, S. Protein hydrolysates from Alphitobius diaperinus and Hermetia illucens larvae treated with commercial proteases. J. Insects Food Feed. 2020, 6, 393–404. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Nam, K.; Huang, X.; Ahn, D.U. Plant-and Animal-Based Antioxidants’ Structure, Efficacy, Mechanisms, and Applications: A Review. Antioxidants 2022, 11, 1025. [Google Scholar] [CrossRef] [PubMed]
- Giannetto, A.; Esposito, E.; Lanza, M.; Oliva, S.; Riolo, K.; Di Pietro, S.; Abbate, J.M.; Briguglio, G.; Cassata, G.; Cicero, L.; et al. Protein hydrolysates from anchovy (Engraulis encrasicolus) waste: In vitro and in vivo biological activities. Mar. Drugs 2020, 18, 86. [Google Scholar] [CrossRef]
- Dai, C.; Ma, H.; Luo, L.; Yin, X. Angiotensin I-converting enzyme (ACE) inhibitory peptide derived from Tenebrio molitor (L.) larva protein hydrolysate. Eur. Food Res. Technol. 2013, 236, 681–689. [Google Scholar] [CrossRef]
- Hoffmann, L.; Rawski, M.; Nogales-Merida, S.; Mazurkiewicz, J. Dietary inclusion of Tenebrio molitor meal in sea trout larvae rearing: Effects on fish growth performance, survival, condition, and GIT and liver enzymatic activity. Ann. Anim. Sci. 2020, 20, 579–598. [Google Scholar] [CrossRef]
- Mikołajczak, Z.; Rawski, M.; Mazurkiewicz, J.; Kierończyk, B.; Józefiak, D. The effect of hydrolyzed insect meals in sea trout fingerling (Salmo trutta m. trutta) diets on growth performance, microbiota and biochemical blood parameters. Animals 2020, 10, 1031. [Google Scholar] [CrossRef] [PubMed]
- Roques, S.; Deborde, C.; Guimas, L.; Marchand, Y.; Richard, N.; Jacob, D.; Skiba-Cassy, S.; Moing, A.; Fauconneau, B. Integrative Metabolomics for Assessing the Effect of Insect (Hermetia illucens) Protein Extract on Rainbow Trout Metabolism. Metabolites 2020, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Terrey, D.; James, J.; Tankovski, I.; Dalim, M.; van Spankeren, M.; Chakraborty, A.; Schmitt, E.; Paul, A. Palatability Enhancement Potential of Hermetia illucens Larvae Protein Hydrolysate in Litopenaeus vannamei Diets. Molecules 2021, 26, 1582. [Google Scholar] [CrossRef] [PubMed]
- Batish, I.; Brits, D.; Valencia, P.; Miyai, C.; Rafeeq, S.; Xu, Y.; Galanopoulos, M.; Sismour, E.; Ovissipour, R. Effects of Enzymatic Hydrolysis on the Functional Properties, Antioxidant Activity and Protein Structure of Black Soldier Fly (Hermetia illucens) Protein. Insects 2020, 11, 876. [Google Scholar] [CrossRef]
- Ravi, H.K.; Degrou, A.; Costil, J.; Trespeuch, C.; Chemat, F.; Vian, M.A. Effect of devitalization techniques on the lipid, protein, antioxidant, and chitin fractions of black soldier fly (Hermetia illucens) larvae. Eur. Food Res. Technol. 2020, 246, 2549–2568. [Google Scholar] [CrossRef]
- Mintah, B.K.; He, R.; Dabbour, M.; Agyekum, A.A.; Xing, Z.; Golly, M.K.; Ma, H. Sonochemical action and reaction of edible insect protein: Influence on enzymolysis reaction-kinetics, free-Gibbs, structure, and antioxidant capacity. J. Food Biochem. 2019, 43, e12982. [Google Scholar] [CrossRef]
- Mouithys-Mickalad, A.; Schmitt, E.; Dalim, M.; Franck, T.; Tome, N.M.; van Spankeren, M.; Serteyn, D.; Paul, A. Black soldier fly (Hermetia illucens) larvae protein derivatives: Potential to promote animal health. Animals 2020, 10, 941. [Google Scholar] [CrossRef] [PubMed]
- Surendra, K.C.; Tomberlin, J.K.; van Huis, A.; Cammack, J.A.; Heckmann, L.-H.L.; Khanal, S.K. Rethinking organic wastes bioconversion: Evaluating the potential of the black soldier fly (Hermetia illucens (L.)) (Diptera: Stratiomyidae) (BSF). Waste Manag. 2020, 117, 58–80. [Google Scholar] [CrossRef]
- Mangano, V.; Gervasi, T.; Rotondo, A.; De Pasquale, P.; Dugo, G.; Macrì, F.; Salvo, A. Protein hydrolysates from anchovy waste: Purification and chemical characterization. Nat. Prod. Res. 2021, 35, 399–406. [Google Scholar] [CrossRef]
- Rotondo, A.; Rigano, F.; Mondello, L. Comprehensive Chemical Characterization of the Pistacia vera Fruits through Original NMR Quantification Methods. Appl. Sci. 2020, 10, 5523. [Google Scholar] [CrossRef]
- Lanza, M.; Casili, G.; La Torre, G.L.; Giuffrida, D.; Rotondo, A.; Esposito, E.; Ardizzone, A.; Rando, R.; Bartolomeo, G.; Albergamo, A.; et al. Properties of a new food supplement containing Actinia equina extract. Antioxidants 2020, 9, 945. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, A.; Mannina, L.; Salvo, A. Multiple Assignment Recovered Analysis (MARA) NMR for a Direct Food Labeling: The Case Study of Olive Oils. Food Anal. Methods 2019, 12, 1238–1245. [Google Scholar] [CrossRef]
- Vasantharaja, R.; Stanley Abraham, L.; Gopinath, V.; Hariharan, D.; Smita, K.M. Attenuation of oxidative stress induced mitochondrial dysfunction and cytotoxicity in fibroblast cells by sulfated polysaccharide from Padina gymnospora. Int. J Biol. Macromol. 2019, 124, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Chen, Q.; Wang, L.; Gao, X.; Zhu, W.; Mu, P.; Deng, Y. Aflatoxin B1 Induces Neurotoxicity through Reactive Oxygen Species Generation, DNA Damage, Apoptosis, and S-Phase Cell Cycle Arrest. Int. J. Mol. Sci. 2020, 21, 6517. [Google Scholar] [CrossRef]
- Giannetto, A.; Nagasawa, K.; Fasulo, S.; Fernandes, J.M. Influence of photoperiod on expression of DNA (cytosine-5) methyltransferases in Atlantic cod. Gene 2013, 519, 222–230. [Google Scholar] [CrossRef]
- Nagasawa, K.; Giannetto, A.; Fernandes, J.M. Photoperiod influences growth and mll (mixed-lineage leukaemia) expression in Atlantic cod. PLoS ONE 2012, 7, e36908. [Google Scholar] [CrossRef]
- Pan, J.; Xu, H.; Cheng, Y.; Mintah, B.K.; Dabbour, M.; Yang, F.; Chen, W.; Zhang, Z.; Dai, C.; He, R.; et al. Recent Insight on Edible Insect Protein: Extraction, Functional Properties, Allergenicity, Bioactivity, and Applications. Foods 2022, 11, 2931. [Google Scholar] [CrossRef]
- Hartmann, R.; Meisel, H. Food-derived peptides with biological activity: From research to food applications. Curr. Opin. Biotechnol. 2007, 18, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Mintah, B.K.; He, R.; Dabbour, M.; Golly, M.K.; Agyekum, A.A.; Ma, H. Effect of sonication pretreatment parameters and their optimization on the antioxidant activity of Hermetia illucens larvae meal protein hydrolysates. J. Food Process Preserv. 2019, 43, e14093. [Google Scholar] [CrossRef]
- Khayrova, A.; Lopatin, S.; Varlamov, V. Black Soldier Fly Hermetia illucens as a Novel Source of Chitin and Chitosan. Int. J. Sci. 2019, 8, 81–86. [Google Scholar] [CrossRef]
- Almeida, C.; Rijo, P.; Rosado, C. Bioactive Compounds from Hermetia Illucens Larvae as Natural Ingredients for Cosmetic Application. Biomolecules 2020, 10, 976. [Google Scholar] [CrossRef]
- Leni, G.; Caligiani, A.; Sforza, S. Killing method affects the browning and the quality of the protein fraction of Black Soldier Fly (Hermetia illucens) prepupae: A metabolomics and proteomic insight. Food Res. Int. 2019, 115, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.N.; Klanrit, P.; Hanboonsong, Y.; Yordpratum, U.; Suksawat, M.; Kulthawatsiri, T.; Jirahiranpat, A.; Deewai, S.; Mackawan, P.; Sermswan, R.W.; et al. Bacterial challenge-associated metabolic phenotypes in Hermetia illucens defining nutritional and functional benefits. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Giannetto, A.; Oliva, S.; Lanes, C.F.C.; de Araújo Pedron, F.; Savastano, D.; Baviera, C.; Parrino, V.; Lo Paro, G.; Spanò, N.C.; Cappello, T.; et al. Hermetia illucens (Diptera: Stratiomydae) larvae and prepupae: Biomass production, fatty acid profile and expression of key genes involved in lipid metabolism. J. Biotechnol. 2020, 307, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Wu, L.; Li, B.; Zhang, D. Reproductive potential and nutritional composition of Hermetia illucens (Diptera: Stratiomyidae) prepupae reared on different organic wastes. J. Econ. Entomol. 2020, 113, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Lalander, C.; Diener, S.; Zurbrügg, C.; Vinnerås, B. Effects of feedstock on larval development and process efficiency in waste treatment with black soldier fly (Hermetia illucens). J. Clean Prod. 2019, 208, 211–219. [Google Scholar] [CrossRef]
- Zeisel, S.H.; DaCosta, K.A.; Fox, J.G. Endogenous formation of dimethylamine. Biochem J. 1985, 232, 403–408. [Google Scholar] [CrossRef]
- Oliver, N.J.; Rabinovitch-Deere, C.A.; Carroll, A.L.; Nozzi, N.E.; Case, A.E.; Atsumi, S. Cyanobacterial metabolic engineering for biofuel and chemical production. Curr. Opin. Chem. Biol. 2016, 35, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, E.; Baraniak, B.; Karaś, M. Antioxidant and Anti-Inflammatory Activities of Hydrolysates and Peptide Fractions Obtained by Enzymatic Hydrolysis of Selected Heat-Treated Edible Insects. Nutrients 2017, 9, 970. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Guo, Y.; Muhmood, A.; Zeng, B.; Qiu, Y.; Wang, P.; Ren, L. Probing the antioxidant activity of functional proteins and bioactive peptides in Hermetia illucens larvae fed with food wastes. Sci. Rep. 2022, 12, 2799. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.B.; He, T.P.; Li, H.B.; Tang, H.W.; Xia, E.Q. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
| Measured Weight (g) | Yield Dry Matter (%) | Yield Total Matter (%) | |
|---|---|---|---|
| Water-soluble species | 126.40 | 30.83 | 12.99 |
| Extracted water-soluble amino acids | 85.89 | 20.95 | 8.83 |
| n-Hexane-soluble species (fat fraction) | 83.40 | 20.34 | 8.57 |
| Insoluble solid (polymers–chitins) | 150.30 | 36.66 | 15.45 |
| Compound/Specie | Code Name | Concentration in 1.6 L Solution (mM) | Relative % in Weight for FAAs | Relative % of FAA Particles | W/W of Raw Larvae (mg/g) | W/W of Dry Matter (mg/g) | Total Millimoles in the 1.6 L Solution |
|---|---|---|---|---|---|---|---|
| Leucine | LEU | 32.8 ± 0.5 | 8.0 | 7.2 | 7.1 | 16.8 | 52.6 |
| Isoleucine | ILE | 35.6 ± 0.6 | 8.7 | 7.8 | 7.7 | 18.2 | 56.9 |
| Valine | VAL | 43.1 ± 0.8 | 9.4 | 9.4 | 8.3 | 19.7 | 68.9 |
| Threonine | THR | 21.7 ± 0.7 | 4.8 | 4.7 | 4.2 | 10.1 | 34.6 |
| Lactate | LAC | 23.6 ± 0.5 | 3.9 | 5.2 | 3.5 | 8.3 | 37.7 |
| Alanine | ALA | 80.7 ± 0.8 | 13.4 | 17.7 | 11.8 | 28.0 | 129.2 |
| Proline | PRO | 36.4 ± 0.3 | 7.8 | 8.0 | 6.9 | 16.3 | 58.2 |
| Glutamate | GLU | 47.1 ± 1.0 | 12.9 | 10.3 | 11.4 | 27.0 | 75.4 |
| Methionine | MET | 12.4 ± 0.3 | 3.4 | 2.7 | 3.0 | 7.2 | 19.9 |
| Aspartate | ASP | 0.7 ± 0.1 | 0.2 | 0.2 | 0.2 | 0.4 | 1.1 |
| Serine | SER | 12.0 ± 0.4 | 2.3 | 2.6 | 2.1 | 4.9 | 19.1 |
| Phenylalanine | PHE | 18.0 ± 0.6 | 5.5 | 3.9 | 4.9 | 11.6 | 28.8 |
| Tryptophan | TRP | 8.1 ± 0.2 | 3.1 | 1.8 | 2.7 | 6.5 | 13.0 |
| Tyrosine | TYR | 15.9 ± 0.8 | 5.4 | 3.5 | 4.7 | 11.2 | 25.4 |
| Glycine | GLY | 40.6 ± 0.5 | 5.7 | 8.9 | 5.0 | 11.9 | 65.0 |
| 4-aminobutytrate | GABA | 28.6 ± 0.7 | 5.5 | 6.2 | 4.8 | 11.5 | 45.7 |
| Ethanol | EtOH | 28.3 ± 0.5 | - | - | 2.1 | 5.1 | 45.2 |
| Propanediol | PRDO | 10.7 ± 0.4 | - | - | 1.3 | 3.2 | 17.1 |
| Acetate | AcO | 92.3 ± 1.5 | - | - | 9.1 | 21.6 | 147.7 |
| Succinate | SUC | 55.8 ± 1.5 | - | - | 10.8 | 25.7 | 89.3 |
| 3-Hydroxypropanoate | 3-HPRO | 11.8 ± 0.3 | - | - | 1.8 | 4.2 | 18.9 |
| Dimethylamine | DMA | 0.5 ± 0.2 | - | - | 0.0 | 0.1 | 0.8 |
| Trimethylamine | TMA | 3.9 ± 0.5 | - | - | 0.4 | 0.9 | 6.3 |
| Ethanolamine | ETN | 12.1 ± 1.8 | - | - | 1.2 | 2.9 | 19.4 |
| Choline | CHO | 5.6 ± 0.6 | - | - | 1.0 | 2.3 | 8.9 |
| Betaine | BETA | 24.9 ± 1.0 | - | - | 4.8 | 11.4 | 39.9 |
| Glycerol | GLYOH | 28.5 ± 1.4 | - | - | 4.3 | 10.2 | 45.5 |
| Uracil | URA | 4.1 ± 0.5 | - | - | 0.7 | 1.8 | 6.5 |
| Tyramine | TYM | 14.9 ± 1.0 | - | - | 3.4 | 8.0 | 23.9 |
| Formate | FOR | 27.8 ± 1.0 | - | - | 2.1 | 4.9 | 44.5 |
| Total | TOT | 778.5 ± 6.2 | - | - | 131.3 | 311.6 | 1245.5 |
| Total aminoacidic composition | TOT FAAs | 457.3 ± 1.8 | 100.0 | 100.0 | 88.3 | 209.5 | 731.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riolo, K.; Rotondo, A.; La Torre, G.L.; Marino, Y.; Franco, G.A.; Crupi, R.; Fusco, R.; Di Paola, R.; Oliva, S.; De Marco, G.; et al. Cytoprotective and Antioxidant Effects of Hydrolysates from Black Soldier Fly (Hermetia illucens). Antioxidants 2023, 12, 519. https://doi.org/10.3390/antiox12020519
Riolo K, Rotondo A, La Torre GL, Marino Y, Franco GA, Crupi R, Fusco R, Di Paola R, Oliva S, De Marco G, et al. Cytoprotective and Antioxidant Effects of Hydrolysates from Black Soldier Fly (Hermetia illucens). Antioxidants. 2023; 12(2):519. https://doi.org/10.3390/antiox12020519
Chicago/Turabian StyleRiolo, Kristian, Archimede Rotondo, Giovanna Loredana La Torre, Ylenia Marino, Gianluca Antonio Franco, Rosalia Crupi, Roberta Fusco, Rosanna Di Paola, Sabrina Oliva, Giuseppe De Marco, and et al. 2023. "Cytoprotective and Antioxidant Effects of Hydrolysates from Black Soldier Fly (Hermetia illucens)" Antioxidants 12, no. 2: 519. https://doi.org/10.3390/antiox12020519
APA StyleRiolo, K., Rotondo, A., La Torre, G. L., Marino, Y., Franco, G. A., Crupi, R., Fusco, R., Di Paola, R., Oliva, S., De Marco, G., Savastano, D., Cuzzocrea, S., Gugliandolo, E., & Giannetto, A. (2023). Cytoprotective and Antioxidant Effects of Hydrolysates from Black Soldier Fly (Hermetia illucens). Antioxidants, 12(2), 519. https://doi.org/10.3390/antiox12020519
















