Abstract
In recent decades, there has been growing interest in the fortification of dairy products with antioxidants and phenolics derived from plant byproducts and herbs. The present study focused on the analysis of dairy products, including kefir, cream cheese, yogurt, and vegan yogurt, enhanced with aqueous extracts of plant byproducts (Citrus aurantium peel, Citrus limon peel and Rosa canina seed) and herbs (Sideritis spp., Hypericum perforatum, Origanum dictamnus, Mentha pulegium L., Melissa oficinallis, Mentha spicata L. and Lavandula angustifolia) to characterize their antioxidant content, phenolic profile, and organoleptic characteristics. Antioxidant and phenolic content were determined by Folin–Ciocalteu and ferric reducing antioxidant power (FRAP) assays and presented values up to 46.61 ± 7.22 mmol Fe2+/L and 82.97 ± 4.29 mg gallic acid (GAE)/g, respectively for the aqueous extracts, as well as up to 0.68 ± 0.06 mmol Fe2+/L and 2.82 ± 0.36 mg GAE/g for the fortified dairy products. The bioavailability of antioxidants and phenolics in fortified foods was determined after in vitro digestion and ranged between 4 and 68%. The phytochemical profile of the aqueous extracts was determined by mass spectrometry, and 162 phytochemicals were determined, from which 128 belong to the polyphenol family including flavonoids and phenolic acids. Furthermore, most of the identified compounds have been recorded to possess enhanced antioxidant capacity in correlation to the in vitro findings. Finally, organoleptic evaluation showed an overall acceptability around 3.0 ± 1.0 on a 5-point scale. In conclusion, the studied plants and herbal extracts can be used for the fortification of a variety of dairy products with potential positive effects on human health.
Keywords:
phytochemicals; FRAP; LC-MS; bioavailability; organoleptic evaluation; cream cheese; yogurt; kefir; herbs; plant byproducts 1. Introduction
In the last decade, consumer choice has played an important role in shaping food supply chains worldwide. First, there was a shift in consumer preferences from synthetic to natural food ingredients, and at the same time, interest in foods of high nutritional value. This has led the food industry to utilize plant-based products such as fruits, vegetables, herbs, and spices [1,2]. However, the high demand for food products, such as vegetables and fruits, has generated immense amounts of food waste, including peels and seeds, which has created great concern for their management and environmental footprint [3,4]. Research has shown that these plant-derived byproducts constitute an important source of antioxidants, phenolic compounds, dietary fibers, and other bioactive compounds [5,6], and their use as ingredients in food fortification processes and for the development of functional foods has been studied [7,8].
Food can be considered functional if it improves human health [9]. The term was first used in the 1980s by the Ministry of Health and Welfare of Japan and describes food products that maintain beneficial health effects for human functions while also remaining nutritious [10,11]. Bioactive compounds containing antioxidants, polyphenols, carotenoids, fibers, and phytosterols have been used as sources of functional ingredients [12]. Herbs have therefore been characterized as plant based functional foods, as they constitute a great source of antioxidants [13]. As natural antioxidant compounds have been proven to have multifunctional benefits, bioactive compounds derived from herbs and fruits have been used for the enrichment of food products because of their antimicrobial properties, such as flavor, aroma, and color enhancers, and for their therapeutic properties [14,15].
Although many natural byproducts and herbs have been characterized as good sources of natural antioxidants and phenolic compounds, they exhibit diverse nutritional and organoleptic properties. This can vary depending on their origin and cultivation conditions [16,17,18]. Therefore, it is important to evaluate the quality and nutritional value of these sources for their utilization as functional ingredients. In addition, as consumer acceptance depends highly on the sensory characteristics of foods, it is significant to evaluate sensory characteristics and organoleptic properties of the final functional foods.
The growing interest of consumers in healthier food choices has led the food industry to develop various functional foods. Among them, dairy products including yogurt, cheese, and fermented milk have become increasingly popular as functional food matrixes, due to the fact they are being consumed daily and because health-conscious consumers present a preference over fortified dairy products [19,20,21,22]. Aiming to the production of fortified dairy products, plant bioactive extracts have been used for the food industry [19]. Extracts from plant byproducts such as orange peel, lemon peel, and pomegranate peel and herbs, such as basil, sage, and mountain tea, have been used as natural additives in yogurt, cheese, and kefir products to improve their nutritional and health properties [23,24,25]. However, in order for consumers to purchase these functional foods, the nutritional value and sensory characteristics of the products remain crucial aspects [26].
Data on fortified dairy products containing bioactive compounds derived from plant byproducts or herbs are encouraging and provide useful insights for further studies. Herbal extracts have been used to fortify dairy products to increase the antioxidant and total phenolic properties of the products. Although this fortification did not seem to affect the quality of the studied products, no organoleptic studies have evaluated the responses of consumers [22,27,28,29]. In addition, studies have shown that the addition of fruit and vegetable peel extracts to dairy products by microencapsulation improves the nutritional analysis and organoleptic characteristics of the final products [30,31]. However, more research is needed to overcome technological challenges for new fortified dairy products to be produced on a mass scale.
The aim of the present study was to investigate and characterize the antioxidant, phenolic, and organoleptic characteristics of fortified dairy products, including kefir, cream cheese, yogurt, and vegan yogurt enhanced with plant byproducts and herbal extracts. Specifically, in a series of aqueous extracts of bitter orange peel, lemon peel, rosehip seeds, and herbs, such as mountain tea, St. John’s wort, dittany, pennyroyal, lemon balm, spearmint, and lavender, the total antioxidant and phenolic content as well as their phenolic profile were determined, and these extracts were used for the fortification of dairy products. The phytochemical profile of the aqueous extracts was determined by mass spectrometry. Finally, the bioavailability of antioxidant and phenolic content of the newly developed fortified dairy products was determined, and their sensory characteristics were evaluated.
2. Materials and Methods
In the context of the present study, an in vitro study was conducted to evaluate the antioxidant capacity, total phenolic content, and phenolic profile of plant byproducts and herbs, which have been associated with high antioxidant activity. In addition, an in vitro study simulating gastrointestinal digestion was carried out to evaluate the antioxidant and phenolic content of the extracts and newly developed dairy functional products. Finally, pilot organoleptic characterizations were performed to investigate consumer acceptance of the newly developed products.
2.1. Sample Extract Preparation
Plant byproduct and herb samples were collected from Lemnos Island, North Aegean, Greece, between June and October 2021. The samples consisted of bitter byproducts from orange (Citrus aurantium) peel (n = 5), lemon (Citrus limon) peel (n = 5), rosehip (Rosa canina) seed (n = 5), as well as mountain tea (Sideritis spp.) (n = 10), St. John’s wort (Hypericum perforatum) (n = 10), dittany (Origanum dictamnus) (n = 5), pennyroyal (Mentha pulegium L.) (n = 10), lemon balm (Melissa oficinallis) (n = 5), spearmint (Mentha spicata L.) (n = 10), and lavender (Lavandula angustifolia) (n = 5). At least 5 samples were used from each food product and pooled together. All samples were dried in a drying heating oven (Binder GmbH, Tuttlingen, Germany) at 60 °C for 12 h and kept in sealed bags until extract preparation.
Extracts were prepared by adding in a flask 2 g of each herb or 10 g of each plant byproduct to 100 mL of dH2O. Each flask was then placed in an Elmasonic P 70 H ultrasound water bath (Elma-Hans Schmidbauer GmbH & Co., Singen, Germany) at 70 °C and 80 Hz for 60 min. Filtration of the extracts was performed by filter paper.
2.2. Determination of Antioxidant Capacity and Phenolic Content of Sample Extracts
2.2.1. Total Antioxidant Activity by Ferric Reducing Antioxidant Power Assay
Total antioxidant capacity of the food extracts was determined by the ferric reducing antioxidant power (FRAP) assay [32,33,34]. This method was based on the conversion of the TPTZ-Fe3+ complex to TPTZ-Fe2+, and the absorbance was measured at 595 nm using a SPARK spectrophotometer (TECAN, Zürich, Switzerland). Higher absorbance values were correlated with higher antioxidant capacity by converting the TPTZ-Fe3+ complex to TPTZ-Fe2+. Quantification of the antioxidant activity was performed using a standard FeSO4 curve, and the results of the FRAP assay were quantified in mmol of Fe2+ per L of sample extract. The analysis was performed in triplicate. All chemicals were purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA).
2.2.2. Total Phenolic Content by Folin–Ciocalteu Assay
The total phenolic content of the sample extracts was determined using the Folin–Ciocalteu method. This method is based on the measurement of the reductive capacity of the Folin–Ciocalteu reagent in an alkaline environment, and absorbance was measured at 765 nm using a SPARK spectrophotometer (TECAN, Zürich, Switzerland) [35]. The total phenolic content was determined using a standard gallic acid (GAE) curve, and the results were expressed in milligrams GAE per gram of dried food sample. The analysis was performed in triplicate. All chemicals were purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA).
2.3. Phenolic Profile Determination
2.3.1. Sample Preparation for Phenolic Profile Determination
The sample extracts were prepared according to the methodology described above (Section 2.1). The resulting solution was filtered using a sintered glass filter funnel to remove the solid plant material. The solvent was removed by lyophilization. Phytochemical standards were purchased from Sigma-Aldrich (St. Louis, MO, USA); ethanol and other solvents were purchased from J. T. Baker (J.T.Baker, Randor, PA, USA).
One milligram of each extract was weighed into an Eppendorf tube and was dissolved in 1 mL LC-MS grade water. Samples were further centrifuged at 13,416× g for 10 min. Extracts were diluted to 0.2 mg/mL in water and then filtered with a 0.2 μm syringe filter for LC-MS analysis.
2.3.2. Determination of Phenolic Profile with LC-MS/QToF Analysis
LC-MS/QToF analysis of extract was performed on an Xevo-G2-XS-QToF mass spectrometer coupled to a Waters UPLC I-Class Binary Solvent Manager (Waters Corp., Milford, MA, USA). The MS conditions were as follows: the scan range was set at 50–1200 m/z, the source voltage was 1 kV for negative mode and 0.8 kV for positive mode. The source temperature was 120 °C, and the desolvation gas temperature was 550 °C; the flow of desolvation gas (N2) was set to 1000 L per hour, and cone gas flow was set to 20 L per hour. For MS/MS, collision energy ramp was set from 20 to 40 eV, and the declustering potential was 40 V. The injection volume was 1 μL. The eluent flow was set to 0.4 mL/min, and the column used was the Acquity UPLC® HSS T3 1.8 μM. More specifically, the elution system comprised 2 phases, A and B. Phase A was water (H2O) with 0.1% formic acid (CH3COOH), and phase B was acetonitrile (CH3CN) with 0.1% formic acid (CH3COOH). The gradient was as follows: The run was performed with a constant flow of 400 μL per minute. At the start of the injection, the eluent composition was 1% A that was linearly raised to 100% A by the 10 min mark. This composition (100% A) was held until minute 12.67. The system changed back to initial conditions at minute 12.73 and was held at initial conditions until minute 15, at which point the next injection was performed. The complete chromatographic conditions are summed up in Table S31 from the Supplementary Materials.
For the post processing and analysis of the acquisition data, UNIFY software was used.
2.4. In Vitro Digestion Analysis
2.4.1. In Vitro Digestion Reagents and Chemicals
All chemicals were acquired from Sigma-Aldrich (St. Louis, MO, USA) and from Merck Chemicals (Darmstadt, Germany).
2.4.2. In Vitro Gastrointestinal Digestion (GI)
The in vitro gastrointestinal (GI) digestion assay was conducted according to the method proposed by Kapsokefalou et al. with some modifications [34]. More specifically, the extracts were adjusted to pH 2.8 with HCL 1 M. In 6 well plates, 2 mL of each extract was added to each well and mixed with 0.1 mL of human pepsin. The plates were then placed in a shaking incubator (SKI-4, P.R.C.) for 2 h in 37 °C. After incubation, the dialysis membrane was used in well rings, and piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) buffer reagent was added to each well to acidify the mixture at pH 6.3. Another incubation was performed (1 h, 37 °C), and then, a mixture of pancreatin-bile acids (0.5 mL) was added to each well and incubated for 2 h followed at 37 °C. The digestion samples were centrifuged for 15 min at 4000 rpm at 4 °C.
2.5. Development and Analysis of Fortified Dairy Products with Herbal and Plant Byproduct Extracts
Household utensils and drinking water were used to fortify dairy products. All measures of hygiene and food safety were followed [36]. Dairy products were bought from a local supermarket and included plain kefir milk (Mevgal Kefir, Mevgal, Greece), a spread cream cheese (Philadelphia, Mondelez, Greece), plain Greek yogurt (Total, Fage, Greece), and vegan yogurt (So Soja, Sojade, France). Then, 1 mL of each herbal or plant byproduct extract was added to 20 g of each dairy product, namely the kefir, cream cheese, yogurt and vegan yogurt and mixed well with a spoon. A description scale was used to evaluate the product color, aroma, texture, and flavor. More specifically, the description scale for the intensity of the aroma was defined as ‘not any observed’, ‘low intensity’, ‘intense’ and ‘high intensity’. The liking scale for flavor was coded as ‘not likable’, ‘likable’ and ‘really likable’. To describe the texture of the products, the scale was defined as ‘pleasant’ and ‘not pleasant’. The addition of 1 mL of extracts was then repeated until all organoleptic characteristics of each fortified dairy product were evaluated positively. The final choice and the respective concentration of each extract used for the fortification of the studied dairy products can be found in Section 3.3. The final concentration of the selected extracts was determined in mL/100 g of fortified product.
The new fortified products were then analyzed using an in vitro digestion process as described above, and their total antioxidant capacity, total phenolic compounds, and the respective compound bioavailabilities were determined.
2.6. Sensory Evaluation and Organoleptic Characteristics
The present study was approved by the Research Ethics Committee of the University of Aegean, Greece (no. 13, 18 February 2022). All participants provided written informed consent in accordance with the Declaration of Helsinki [37]. Any information that might reveal the identity of the study participants was omitted, and the participants were number coded.
The study was conducted from March to December 2022. Participants were recruited randomly by the research team via social media and online announcements at the Agricultural University of Athens and the University of Aegean, Lemnos, Island, Greece. All participants were randomly selected, and no further educational/informative leaflets were given about the fortified dairy products.
Two organoleptic evaluations were performed as presented in Figure 1. The first organoleptic study was performed in Athens, Greece (Agricultural University of Athens) with a total number of 22 participants being women (n = 19) and men (n = 3) and the second organoleptic study was implemented in Lemnos Island, Greece (University of Aegean) with a total number of 25 participants, women (n = 18) and men (n = 7). In both studies, unlabeled non-colored disposable plastic containers with 20 g of fortified dairy product were provided to the participants. The studies were conducted in a room with 20–22 °C temperature and 50–65% humidity. Enhanced dairy products were provided to each of the participants, and they did not have any information on which type of fortified product they were censoring. Furthermore, a questionnaire was administered to each participant to evaluate the appearance, taste, flavor, and smell of the product on a scale from ‘1 = I do not like it’ to ‘5 = I highly like it’. Between each sample, the participants were instructed to drink water to clean their mouth.
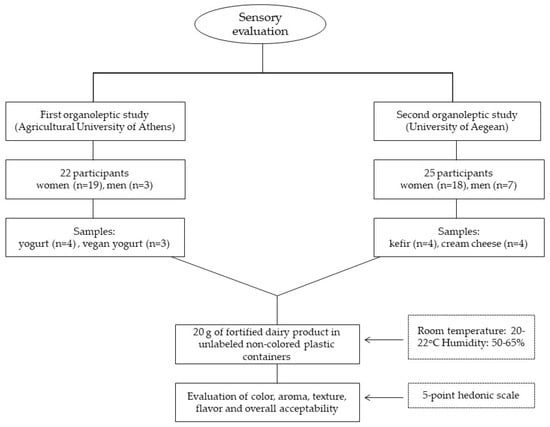
Figure 1.
Flow diagram of organoleptic study.
During the first organoleptic study, the participants tested and evaluated 4 different yogurt samples and 3 vegan yogurt samples. In more detail, plain yogurt without the addition of any extract, yogurt with St. John’s Wort extract, yogurt with dittany extract, yogurt with pennyroyal, and yogurt with lemon balm extract were provided to each participant. After finishing the evaluation of the above samples, participants then tested and evaluated the vegan yogurt samples: a control without any extract added, a vegan yogurt with spearmint, and a vegan yogurt with lavender. The samples were provided in different orders for each participant.
During the second organoleptic study, the participants tested 4 kefir samples and 4 cream cheese samples. Specifically, a control kefir with no added extract, kefir with bitter orange peel extract, kefir with both bitter orange peel and lemon peel extracts, and kefir with bitter orange peel and rosehip seed extracts were evaluated. After finishing with the evaluation of the above samples, participants proceeded with the evaluation of cream cheese samples: plain cream cheese with no added extract, cream cheese with mountain tea extract, cream cheese with St. John’s Wort extract, and cream cheese with both mountain tea and St. John’s wort extracts. The samples were provided in different orders for each participant.
2.7. Statistical Analysis
Statistical analysis was performed using SPSS package, version 16.1 (SPSS Inc., Chicago, IL, USA). Normal distribution of all continuous variables was tested with the parametric Shapiro–Wilk test, and statistical significance was considered at p = 0.05. The total antioxidant and phenolic content of the selected food products after all in vitro analyses are expressed as the mean ± standard deviation (SD). ANOVA was used to investigate the differences between the total antioxidant and phenolic content of the herbal and byproduct extracts. Correlations between the total antioxidant and phenolic content of extracts and their respective content after in vitro digestion were evaluated using Pearson’s correlation test. Organoleptic characteristics as assumed by study participants were presented using descriptive statistics, and ANOVA test was implemented to determine significant statistical differences among participants’ preferences for different food products.
3. Results
3.1. Estimation of Antioxidant and Phenolic Content before and after In Vitro Digestion
Total antioxidant and phenolic content of the selected herbal and plant byproduct extracts before and after in vitro digestion are presented in Table 1. Mean values for total antioxidant capacity of extracts ranged from 2.15 ± 0.17 to 46.61 ± 7.22 mmol Fe2+/L. Lemon balm presented the highest total antioxidant activity, followed by spearmint (22.81 ± 1.40 mmol Fe2+/L) and a combination extract of bitter orange peel and rosehip seed (21.87 ± 1.41 mmol Fe2+/L). Lemon peel presented the lowest antioxidant activity, followed by rosehip seeds (2.85 ± 0.21 mmol Fe2+/L) and bitter orange peel (3.89 ± 0.35 mmol Fe2+/L).

Table 1.
Spectrophotometric determination of total antioxidant and phenolic content of selected plant byproduct and herbal extracts and their bioavailability indices.
Mean values of total phenolic content before digestion varied significantly (p < 0.05) from 4.31 ± 0.54 to 82.97 ± 4.29 mg GAE/g of dried sample. Lemon balm had the highest phenolic content, followed by the combination extracts of bitter orange peel and rosehip seed (67.07 ± 1.67 mg GAE/g) and bitter orange and lemon peels (47.92 ± 2.10 mg GAE/g), while the lowest phenolic content was measured in plant byproduct extracts.
After the implementation of a simulated in vitro digestion model, the values of total antioxidant and phenolic content were generally lower, as presented in Table 1. Total phenolic content ranged from 0.71 ± 0.15 to 10.71 ± 1.20 mmol Fe2+/L after digestion. Regarding the respective estimated bioavailability of total antioxidants of the selected extracts, the extract with the greatest bioavailability was bitter orange peel (42%), followed by lemon peel (36%), whereas those with lower bioavailability were mountain tea (15%) and the combination of bitter orange peel and rosehip seeds (18%).
Total phenolic content after in vitro digestion ranged from 1.33 ± 0.55 to 9.68 ± 4.31 mg GAE/g. Lemon peel had the highest bioavailability (68%) followed by rosehip seeds (62%), whereas spearmint and pennyroyal had the lowest bioavailabilities (4% and 5%, respectively).
Regarding the correlations between the total antioxidants of the samples before and after the in vitro digestion procedure, only lemon peel, mountain tea, and lavender extracts presented statistically significant correlations (p < 0.05). Respectively, in terms of correlations in the phenolic content, prior and after digestion, only dittany extract showed significant correlation (p < 0.05).
Table 2 summarizes the study results published in the literature regarding the total antioxidant and phenolic content of the selected plant byproduct and herbal extracts from the Mediterranean region, as measured with FRAP and Folin–Ciocalteu assays. As presented in Table 2, there is a wide range of values between the different studies.

Table 2.
Mean concentrations of total phenolic content and total antioxidant activity of selected herbs and byproducts reported in literature.
3.2. Determination of Phytochemical Profile of Aqueous Plant Byproduct and Herbal Extracts
To unveil the phytochemical profile of the aqueous extracts of the studied plant materials, LC-MS QTOF spectrometry was performed. The phytochemical profile of the 9 studied plants was identified and a total of 162 different phytochemicals were determined, out of which 128 belong to the polyphenol family (as can be seen in Table S1 of the Supplementary Materials). Each plant was evaluated in two modes (positive and negative), and thus, 18 recordings were performed, and identification data are presented in the Supplementary Materials (Tables S2–S19). The most abundant phytochemicals in bitter orange extracts, namely, were: echinacoside cirsimaritin, leucosceptoside a, luteolin 7-o-rutinoside, 1,2-disinapoylgentiobiose, rutin, orientin, sucrose, kaempferol, 3-o-sophoroside, 5,7-dihydroxychromone, eupatilin, didymin, limonin, nobiletin, rhoifolin, eriocitrin, apigenin-7-o-glucoside, isorhamnetin-3-o-rutinoside, isorhamnetin, 3-o-galactoside, citric acid, luteolin, 7-o-diglucuronide, quercetin-3-o-glucoside, myricetin, 3-alpha-l-arabinopyranosideand manghaslin.
For the dittany extracts, the main compounds were: rosmarinic acid, salvianolic acid c, lithospermic acid b, vanillylmandelic acid, ferulic acid-4′-o-glucoside, diosmin, salvianolic acid b, isoacteoside, 6″-o-malonylgenistin, naringenin 7-o-glucoside, cirsilineol, glucogallin, cafestol, (2-hydroxy-) rutin, 3,4-dicaffeoylquinic acid, 5-feruloylquinic acid, plumieride, orientin, scutellarin, eupatorine and luteolin 7-o-diglucuronide.
As for the lavender aqueous extracts we detected, among others, the following phytochemicals were: rosmarinic acid, luteolin-3-o-glucuronide, vanillylmandelic acid, quercitrin, apigenin, ferulic acid-4′-o-glucoside, salvianolic acid b, luteolin 7-o-diglucuronide, luteolin, melittoside, theaflavin 3-o-gallate, quercetin 3′-o-glucuronide, chicoric acid, scutellarin, salvianolic acid c, 5-feruloylquinic acid, astragalin, 5-o-caffeoylquinic acid, luteolin 7-o-diglucuronide, cynarin, apigenin-7-o-glucoside, pinoresinol-4-o-beta-monoglycoside, acteoside, caffeoyl tartaric acid and hispidulin glucuronide.
Meanwhile, lemon balm produced extracts rich in: rosmarinic acid, lithospermic acid b, vanillylmandelic acid, quercitrin, diosmetin, salvianolic acid b, nicotiflorin, quercetin 3-o-beta-d-glucopyranosyl-7-o-alpha-l-rhamnopyranoside, 1,3-dicaffeoylquinic acid, leucosceptoside a, salvianolic acid a, luteolin, genkwanin, theaflavin 3-o-gallate, chicoric acid, luteolin 7-o-diglucuronide, echinacoside, silydianin, achillolide a, manghaslin, luteolin 7-o-glucoside and silybin.
Lemon peel extracts contained mainly: quercetin-3-o-glucoside, allobetonicoside, geniposidic-acid, barbatoside, luteolin 7-o-glucoside, rutin, limonin, rosmarinic acid, isorhamnetin 3-o-glucoside, azadirachtin, isorhamnetin 3-o-galactoside, chrysoeriol, hesperidin, quercetin 3-o-(6-malonyl-glucoside), quercetin 3-o-beta-d-glucopyranosyl-7-o-alpha-l-rhamnopyranoside, nobiletin, 1,3-dicaffeoylquinic acid, 4-o-caffeoylquinic acid, byakangelicin, myricetin-3-o-α-l-rhamnopyranoside, cirsimaritin, eriocitrin, astilbin, isorhamnetin-3-o-rutinoside and peonidin 3-o-sophoroside.
Roseship admittedly gave a poorer profile: ascorbic acid, apigenin 7-o-diglucuronide, melittoside, valoneic acid, dilactone 5-o-galloylquinic acid, astilbin, taxifolin, protocatechuic acid 4-o-glucoside, nicotiflorin, diosmin, quercetin 3-arabinoside, dihydroferulic acid 4-o-glucuronide, dihydroferulic acid-4′-o-glucuronide, teupolioside alpha-methyl-d-mannopyranoside and genistein 4′,7-o-diglucuronide 6″-o-malonylgenistin.
As expected, sisderitis produced rich extracts: geniposidic-acid, bergenin, teupolioside, leucosceptoside A, lithospermic acid B, 5-feruloylquinic acid, apigenin, kaempferol 3-O-sophoroside, acteoside, hesperidin, luteolin 7-O-diglucuronide, luteolin 7-O-diglucuronide, citric acid, oleuropein, isoacteoside, 1,3-dicaffeoylquinic acid, apigenin-7-O-glucoside, isoscutellarein 4′-methyl ether 7-(6‴-acetylallosyl)(1->2)-glucoside, isoscutellarein 7-O-[6‴-O-acetyl-β-d-allopyranosyl-(1→2)]-β-d-glucopyranoside and cirsilineol.
Spearmint also gave a rich phytochemical profile including, mainly: oleuropein, luteolin, allobetonicoside, 1,3-dicaffeoylquinic acid, luteolin 7-o-glucoside, vanillylmandelic acid, rutin, rosmarinic acid, lithospermic acid b, isorhamnetin 3-o-glucoside, apigenin-7-o-glucuronide, apigenin 7-o-diglucuronide, salvianolic acid a, melittoside, eupatilin, acteoside, manghaslin (quercetin 3-2 g-rhamnosylrutinoside), hesperidin, luteolin 7-o-diglucuronide, kaempferol 3-o-rutinoside, scutellarin, rhoifolin, 3,4-dicaffeoylquinic acid, diosmin, luteolin-3-o-glucuronide and luteolin 7-o-rutinoside.
Lastly, St. John’s wort gave mostly the following compounds: oleuropein, quercetin-3-o-glucoside, allobetonicoside, astragalin, rutin, amentoflavone, apigenin-7-o-glucuronide, kaempferol, cinnamtannin a2, quercetin, chicoric acid, apigenin-7-o-glucoside, luteolin 4′-glucoside, quercetin 3,4′-o-diglucoside, myricetin-3-o-galactopyranoside, kaempferol-3-o-glucuronide, hyperoside, phloridzin, caffeic acid, myricetin-3-o-α-l-rhamnopyranoside, betonicine, pinoresinol-4-o-beta-monoglycoside and quercetin 3-glucuronate.
Some phytochemicals were identified in both modes and/or in more than one studied plant material. These compounds, which were identified more than once, as well as their identification frequency can be seen in Table 3. Some of the most frequently identified compounds within the samples include luteolin 7-o-diglucuronide, salvianolic acid b, rutin, acteoside, nicotiflorin, chrysoeriol 7-o-apiosyl-glucoside, pinoresinol-4-o-beta-monoglycoside, naringenin-4′,5-diglucuronide, cirsilineol and vanillylmandelic acid.

Table 3.
Common phytochemicals identified among the studied extracts and their respective identification frequency.
Out of the 162 different phytochemicals identified, 128 belong to the polyphenol family (Table S1). Based on the number of polyphenolic components, the nine plants were ranked in the following order: 1. bitter orange (52 polyphenols), 2. dittany (41 polyphenols), 3. lemon peel (40 polyphenols), 4. spearmint (39 polyphenols), 5. St. John’s wort (32 polyphenols), 6. lemon balm (31 polyphenols), 7. lavender (29 polyphenols), 8. sideritis (27 polyphenols) and 9. rosehip (13 polyphenols) (Table S30). This order was consistent with the total number of phytochemicals identified in each plant (Table S1). In addition, the number of polyphenolic components in spearmint, St. John’s wort, lavender, sideritis and rosehip is in accordance with the Folin–Ciocalteu assay values.
Sideritis, spearmint, dittany, lavender and lemon balm belong to the Lamiacea family and were screened for compounds that were constitutively shared among them. The analysis of those plants showed that the most common antioxidants in the Lamiaceae family samples were luteolin 7-O-diglucuronide, acteoside, vanillylmandelic acid, scutellarin, salvianolic acid B, lithospermic acid B, rosmarinic acid, 3,4-dicaffeoylquinic acid, luteolin, chicoric acid, 5-feruloylquinic acid, apigenin-7-O-glucoside, nicotiflorin, pinoresinol-4-O-beta-monoglycoside, leucosceptoside A, pectolinarigenin, silybin, rutin, hesperidin, oleuropein, 1,3-dicaffeoylquinic acid, diosmin, kaempferol 3-O-rutinoside, salvianolic acid C, cirsilineol, theaflavin 3-O-gallate, luteolin 7-O-glucoside, cynarin. The most frequently identified antioxidants in the Lamiacea family are shown in Table 4.

Table 4.
Most common antioxidants identified in Lamicaea family of studied plant materials.
Bitter orange and lemon peel belong to the Rutaceae family and were screened for compounds that were constitutively shared among them. The most abundant antioxidants in the Rutaceae family were rutin, limonin, eriocitrin_1, isorhamnetin-3-O-rutinoside, nicotiflorin, orientin, kaempferol 3-O-sophoroside, isorhamnetin 3-O-galactoside, citric acid, didymin, cirsimaritin, pinoresinol-4-O-beta-monoglycoside, astilbin, nobiletin, D-(+)-mannose, azadirachtin, quercetin-3-O-glucoside, allobetonicoside, rhoifolin, eupatilin, 1,2-disinapoylgentiobiose and hesperidin. The most frequently identified antioxidants in the Rutaceae family are shown in Table 5.

Table 5.
Most common, by count, antioxidants identified in the Rutaceae family of studied plant materials.
The total number of different identified phytochemicals for each plant studied was as follows: 65 different compounds were identified in bitter orange, 51 in dittany and lemon peel, 47 in spearmint, 42 in lavender, 39 in lemon balm, sideritis and St. John’s wort and only 20 in rosehip (Table S20). Bitter orange showed the richest phytochemical profile followed by dittany and lemon peel, while rosehip presented the poorest profile. Based on the number of their antioxidant components recorded (Tables S21–S29), the nine plants were ranked in the following order: 1. bitter orange (44 antioxidants), 2. dittany (37 antioxidants), 3. lemon peel (31 antioxidants), 4. spearmint (30 anti-oxidants), 5. lavender (26 antioxidants), 6. lemon balm (25 antioxidants), 7. sideritis (24 antioxidants), 8. St. John’s wort (20 antioxidants) and 9. rosehip (12 antioxidants). This order is consistent with the total number of phytochemicals identified in each plant material.
Some of the most commonly found antioxidants among the studied plant byproduct and herbal extracts include kaempferol 3-o-sophoroside, isorhamnetin 3-o-galactoside, luteolin 7-o-diglucuronide, salvianolic acid b, rutin, luteolin-3-o-glucuronide, quercetin 3-arabinoside and isoscutellarein 7-o-[6‴-o-acetyl-β-d-allopyranosyl-(1→2)]-β-d-glucopyranoside.
3.3. Estimation of Total Antioxidant and Phenolic Content in Fortified Foods after In Vitro Digestion
Total antioxidant capacity and total phenolic content of the above-selected extracts were determined in specific food samples, namely kefir, cream cheese, yogurt, and a vegan yogurt, after simulation of in vitro digestion. The values of dairy products fortified with the respective extracts are presented in Table 6.

Table 6.
Total antioxidant and phenolic content in fortified food products after in vitro digestion.
The antioxidant content was higher in kefir fortified with bitter orange and rosehip extract, compared to plain kefir (control sample). No statistically significant difference (p > 0.05) was observed between the different kefir samples in terms of phenolic content.
Cream cheese showed higher antioxidant and phenolic content when fortified with St. John’s wort extract, with a value of 0.53 ± 0.16 mmol Fe2+/L and 56.36 ± 7.13 mg GAE/g, respectively. A statistically significant difference (p < 0.05) was observed in both antioxidant capacity and phenolic content.
In yogurt, antioxidant capacity was higher when fortified with lemon balm (1.21 ± 0.12 mmol Fe2+/L) yogurt, while no statistical important differences were observed in the phenolic content of samples (p > 0.05).
Vegan yogurt fortified with spearmint showed the highest antioxidant activity (0.32 ± 0.04 mmol Fe2+/L), while vegan yogurt fortified with lavender and spearmint presented higher phenolic content (2.01 ± 0.40, 2.00 ± 0.41 mg GAE/g, respectively) compared to control sample.
3.4. Evaluation of the Organoleptic Characteristics of the Fortified Food Products
The evaluation of the organoleptic characteristics (color, aroma, texture, and flavor) of the fortified food products are presented in Table 7. The ratings were on a five-point hedonic scale: 5 (liked a lot) to 1 (did not like at all).

Table 7.
Evaluation of color, aroma, texture and flavor of the fortified new dairy products.
As presented in Table 4, values for the overall acceptability of kefir ranged from 3.2 ± 1.1 in control sample (plain kefir) to 2.6 ± 1.2 in fortified bitter orange and lemon peels kefir. However, no statistically significant difference (p > 0.05) was observed between the samples.
Regarding cream cheese, total acceptability was 4.0 ± 1.1 for control and 2.6 ± 1.0, the lowest value, for cream cheese fortified with mountain tea extract. In addition, a statistically significant difference (p < 0.05) was observed between the samples for texture, flavor, and overall acceptability, but not for color and aroma (p > 0.05).
Yogurt’s overall acceptability ranged from 3.9 ± 0.8 for the blank yogurt to 3.2 ± 0.9 for yogurt with dittany extract. Statistically significant difference (p < 0.05) were presented for color, texture, flavor, and overall acceptability but not for aroma (p > 0.05)
As for the vegan yogurt, the highest value (2.8 ± 1.2) for total acceptability was that without any extract, and the lowest value (2.4 ± 1.1) was that with the lavender extract. A statistically significant difference (p < 0.05) was presented between samples only for texture.
In general, in all cases, products without any extract had the highest value for overall acceptability.
4. Discussion
Several studies have been conducted on herbs and their extracts with the aim of determining their antioxidant capacity and total phenolic content and characterizing the respective phenolic components [58]. However, variations exist, even among the same plant byproducts and/or species. This is attributed to differences in the extraction method during sample preparation [59], differences in harvest time [60], differences in the variety of the analyzed sample [61], as well as differences in the climatic and soil conditions and their origins [62]. Therefore, it remains important to determine the antioxidant and phenolic content of plants cultivated in different places. In the present study, we focused on the North Aegean region and evaluated the total antioxidant and phenolic content of selected aqueous extracts of herbs, byproducts, and several combinations of them.
Among them, lemon balm presented the highest value for antioxidants and total phenolics before and after in vitro digestion; however, it had lower bioavailability compared to other herbal extracts. In general, a decrease in the concentrations of both antioxidants and phenolics was observed in all extracts after simulation of gastrointestinal digestion. This could be due to the degradation of some polyphenols and flavonoids during the transition from acidic gastric conditions to mildly alkaline intestinal conditions, especially under the impact of bile acids and pancreatin [63,64]. Specifically, it is suggested that approximately 15% of polyphenols are lost during the transition from the acidic gastric environment to the mild alkaline intestinal environment [65].
The bioavailability of polyphenols has been a major problem limiting their use as a dietary intervention for different disease factors that polyphenols have been found to prevent or manage [66]. It has been estimated that only 5–10% of the total polyphenol intake is absorbed in the small intestine, whereas the remaining polyphenols (90–95% of total polyphenol intake) may accumulate in the lumen of the large intestine [67,68,69,70]. However, the poor bioavailability of polyphenols favors interactions with gut microbes, whereas microbes may modulate the activity of polyphenols by converting naturally occurring polyphenols into metabolites that can exert different effects [71]. In the large intestine, colonic bacteria act enzymatically on the polyphenolic backbone of the remaining unabsorbed polyphenols (90–95% of the total polyphenol intake), sequentially producing metabolites with different physiological significance [72]. This suggests that biοaccessibility of phenolics may be an important factor in bioavailability, and in the case of food fortification, the concentration of polyphenols in the fortification extracts must be examined alongside the bioavailability rates.
Of the total phytochemicals identified in the studied samples (162), 119 were found to have some level of antioxidant activity according to the published literature [73,74,75,76,77,78,79,80,81,82,83]. Furthermore, 86 of these 119 compounds were identified in more than one plant, and the extracts from the studied plants showed satisfactory results for the bioavailability of total antioxidants (>20%) and total phenolics (>10%). Therefore, we can assume that the studied extracts can be used as potential sources of antioxidants and phenolic compounds for the enrichment of dairy products, which are beneficial to human health [84]. Furthermore, the data show that the greatest benefits of antioxidant and phenol bioavailability can be obtained when they are consumed in food products rather than in supplement forms [85].
According to the results obtained from the FRAP assay for total antioxidant content, lemon balm had the highest antioxidant activity, although it was ranked low in terms of the number of its identified antioxidants. A plausible explanation for this could be that in the specific LC-MS set-up, the recorded phytochemical profile is qualitative, while the results of the FRAP assay are quantity-dependent. The total antioxidant activity depends not only on the total number of different antioxidants (qualitative information) but also on their concentration (quantitative information) and individual antioxidant capacity [86].
Concerning the phenolic profile of the studied plant materials, some results of the Folin–Ciocalteu assay were not in complete accordance with the total number of polyphenolic components. Bitter orange, dittany, and lemon peel were found to contain the most polyphenols out of the nine plant species but gave only poor results when tested using the Folin–Ciocalteu assay. On the other hand, lemon balm showed great results with the Folin–Ciocalteu assay although it was ranked only sixth according to the identified polyphenols (Table S30). These differences may be attributed to the fact that the Folin–Ciocalteu assay is quantity-dependent [87], while as mentioned above, the results of the specific LC-MS set-up are qualitative. Furthermore, the Folin–Ciocalteu assay is based on a redox reaction in which a chromophore complex is formed [88]. Except for polyphenols, numerous reducing agents can produce the complex and thus lead to ambiguous results regarding the total phenolic capacity [89,90].
As suggested by the above, the use of plant byproduct and herbal extracts for fortifying of dairy products has been of great interest in recent years [27,91]. At the same time, dairy products remain high in consumer preferences [21,92], whereas dairy products derived from fermentation are an ideal basis for the incorporation of bioactive ingredients [29]. The use of plant extracts for the fortification of dairy products can be used to increase their antioxidant content [93] and therefore for the development of functional dairy products. Meanwhile, it seems that the combined use of extracts derived from different herbs can lead to higher bioavailability of bioactive ingredients [94]. This is in accordance with the present study, in which the combination of herbal or byproduct extracts showed higher concentrations of antioxidants and total phenolics before and after in vitro digestion.
To increase the antioxidant capacity and bioavailability of food products such as meat and bakery products, antioxidant compounds have been added to them using encapsulation methods [95,96,97,98]. Nevertheless, it should be considered that the design and development of such products are expensive and time consuming [92]. The present study determined the higher antioxidant content of fortified dairy products compared to that of control samples, while exploiting a simple, cost-effective method for fortification. Specifically, plant byproducts and herbal extracts were utilized to prepare aqueous extracts that were easily incorporated into the final food products. However, an important factor that remains is the final organoleptic characteristics of fortified dairy products, as consumers usually choose taste over the health benefits of functional foods [12].
The pilot organoleptic evaluation implemented in the context of the present study indicated that the overall acceptability of all the newly developed dairy products was average, as most participants stated that they slightly liked the fortified products (scored as an average of three in the five-point scale). Although the dairy products that did not contain any extract presented the highest values for overall acceptability, the evaluation score given by the study participants for organoleptic characteristics between the control and fortified products did not present statistically significant differences, except in the case of the fortified cream cheese products and the yogurt fortified with St. John’s Wort. This may be attributed to the relatively low concentration of the added extract. Specifically, participants positively evaluated the organoleptic characteristics of plain kefir as well as kefir fortified with extracts. In contrast, the participants did not seem to like the flavor of the fortified cream cheese samples compared to plain cream cheese. Participants also identified differences in the color, texture, and flavor of yogurt, but not vegan yogurt, compared with the control samples. Further research is needed on dairy products to obtain favorable organoleptic characteristics. Studies have shown that desirable changes can be achieved through the addition of goat milk and/or different starting bacterial cultures [99,100,101]. This can lead to desirable changes and low-cost solutions for sensory improvement.
Finally, some limitations of the present study should be considered. The in vitro digestion methodology aims to simulate gastrointestinal conditions for the estimation of the bioavailability of different compounds. In the present study, we used an in vitro digestion model to estimate antioxidant and phenolic bioavailability; however, the human digestive system is quite complex and therefore remains challenging to recreate outside the human body [102]. To obtain safer conclusions, further study including clinical interventions is needed. It should also be noted that well-designed organoleptic studies, with training panelists, for the sensory evaluation of novel foods remain crucial. In our study, a pilot organoleptic characterization was implemented with the scope of evaluating the sensory characteristics of the fortified dairy products to obtain information on the potential of developing the studied dairy products and their respective consumer acceptance.
5. Conclusions
In conclusion, the fortification of dairy products with aqueous extracts from plant byproducts and herbs can lead to innovative food products with high antioxidant and phenolic content. In the present study, vegan yogurt fortified with lavender or spearmint as well as kefir fortified with a mixture of bitter orange peel and rosehip seed presented high antioxidant and phenolic content compared to plain products, while also being evaluated closely in terms of overall organoleptic characteristics and flavor compared to plain products that can be found in the market. Although these products are promising, organoleptic characteristics can be assumed to be critical for consumer acceptability and therefore for final adaptation from the food industry. Future research on the development of tasteful dairy products and further organoleptic evaluation of consumer preferences for these products is needed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox12020500/s1. Table S1: The 162 different identified phytochemicals in the 9 studied plant materials. The compounds in blue color belong to the family of polyphenols; Table S2: Identified compounds in bitter orange aqueous extract (ESI negative mode); Table S3: Identified compounds in bitter orange aqueous extract (ESI positive mode); Table S4: Identified compounds in dittany aqueous extract (ESI negative mode); Table S5: Identified compounds in dittany aqueous extract (ESI positive mode); Table S6: Identified compounds in lavender aqueous extract (ESI negative mode); Table S7: Identified compounds in lavender aqueous extract (ESI positive mode); Table S8: Identified compounds in lemon balm aqueous extract (ESI negative mode); Table S9: Identified compounds in lemon balm aqueous extract (ESI positive mode); Table S10: Identified compounds in lemon peel aqueous extract (ESI negative mode); Table S11: Identified compounds in lemon peel aqueous extract (ESI positive mode); Table S12: Identified compounds in rosehip aqueous extract (ESI negative mode); Table S13: Identified compounds in rosehip aqueous extract (ESI positive mode); Table S14: Identified compounds in sideritis aqueous extract (ESI negative mode); Table S15: Identified compounds in sideritis aqueous extract (ESI positive mode); Table S16: Identified compounds in spearmint aqueous extract (ESI negative mode); Table S17: Identified compounds in spearmint aqueous extract (ESI positive mode); Table S18: Identified compounds in St. John’s wort aqueous extract (ESI negative mode); Table S19: Identified compounds in St. John’s wort aqueous extract (ESI positive mode); Table S20: Total phytochemical profile of the 9 studied plants; Table S21: Phytochemicals of bitter orange with antioxidant activity according to international literature; Table S22: Phytochemicals of dittany with antioxidant activity according to international literature; Table S23: Phytochemicals of lemon peel with antioxidant activity according to international literature.; Table S24: Phytochemicals of spearmint with antioxidant activity according to international literature; Table S25: Phytochemicals of lavender with antioxidant activity according to international literature; Table S26: Phytochemicals of lemon balm with antioxidant activity according to international literature; Table S27: Phytochemicals of sideritis with antioxidant activity according to international literature; Table S28: Phytochemicals of St. John’s wort with antioxidant activity according to international literature; Table S29: Phytochemicals of rosehip with antioxidant activity according to international literature; Table S30: Polyphenolic components of each studied plant; Table S31: Chromatographic Conditions including elution program and mobile phases. Phase A was water (H2O) with 0.1% formic acid (CH3COOH) and phase B was acetonitrile (CH3CN) with 0.1% formic acid (CH3COOH); Figure S1: St. John’s wort’s total ion chromatogram (negative ionization mode); Figure S2: St. John’s wort’s total ion chromatogram (positive ionization mode); Figure S3: Lemon balm’s total ion chromatogram (negative ionization mode); Figure S4: Lemon balm’s total ion chromatogram (positive ionization mode); Figure S5: Dittany’s total ion chromatogram (negative ionization mode); Figure S5: Dittany’s total ion chromatogram (negative ionization mode); Figure S7: Rosehip’s total ion chromatogram (negative ionization mode); Figure S8: Rosehip’s total ion chromatogram (positive ionization mode; Figure S9: Bitter orange’s total ion chromatogram (negative ionization mode); Figure S10: Bitter orange’s total ion chromatogram (positive ionization mode); Figure S11: Spearmint’s total ion chromatogram (negative ionization mode); Figure S12: Spearmint’s total ion chromatogram (positive ionization mode); Figure S13: Sideritis’s total ion chromatogram (negative ionization mode); Figure S14: Sideritis’s total ion chromatogram (positive ionization mode); Figure S15: Lemon peel’s total ion chromatogram (negative ionization mode); Figure S16: Lemon peel’s total ion chromatogram (positive ionization mode); Figure S17: Lavender’s total ion chromatogram (negative ionization mode); Figure S18: Lemon peel’s total ion chromatogram (positive ionization mode).
Author Contributions
Conceptualization, A.E.K., A.G.T. and A.K., methodology, A.E.K., A.G.T. and A.K.; software, A.E.K.; validation, A.K., A.E.K. and A.G.T.; formal analysis, P.P., P.B., C.K., K.A., M.C., A.M., C.K.F., L.V.P. and V.K.G.; investigation, A.K., P.P., P.B., C.K., K.A., M.C., A.M., C.K.F., L.V.P. and V.K.G.; resources, A.E.K. and A.G.T.; data curation, P.P., P.B., C.K., K.A., M.C., A.M., C.K.F., L.V.P. and V.K.G.; writing—original draft preparation, A.K., P.B., P.P., C.K. and V.K.G.; writing—review and editing, A.E.K., A.G.T. and A.K.; visualization, A.K., P.B., P.P., C.K. and V.K.G.; supervision, A.E.K. and A.G.T.; project administration, A.E.K., A.G.T. and A.K.; funding acquisition, A.E.K. and A.G.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research is part of the project, “Infrastructure of Microbiome Applications in Food Systems-FoodBiomes”, which is co-funded by the European Regional Development Fund (ERDF), under the Operational Program “Competitiveness, Entrepreneurship and Innovation-EPANEK 2014–2020”, Call 111 “Support for Regional Excellence”.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of University of Aegean, Greece (no. 13, 18 February 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in the present article and the Supplementary Material.
Acknowledgments
We acknowledge the support of this work by the project ‘Infrastructure of Microbiome Applications in FoodSystems-FOODBIOMES’ (MIS 5047291), which is implemented under the Action ‘Regional Excellence in R&D Infrastructures’, funded by the Operational Program ‘Competitiveness, Entrepreneurship and Innovation’ (NSRF 2014-2020) and co-financed by Greece and the European Union (European Regional Development Fund). We would like to also acknowledge the Project ‘BEST—Addressing joint Agro-and Aqua-Biodiversity pressures Enhancing SuSTainable Rural Development’; of the European Territorial Cooperation Program ‘Interreg Greece-Italy 2014-2020′ which co-financed by the European Union for acquiring an MS unit.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Jokar, N.K.; Noorhosseini, S.A.; Allahyari, M.S.; Damalas, C.A. Consumers’ Acceptance of Medicinal Herbs: An Application of the Technology Acceptance Model (TAM). J. Ethnopharmacol. 2017, 207, 203–210. [Google Scholar] [CrossRef]
- Chaboud, G.; Daviron, B. Food Losses and Waste: Navigating the Inconsistencies. Glob. Food Secur. 2017, 12, 1–7. [Google Scholar] [CrossRef]
- Kummu, M.; de Moel, H.; Porkka, M.; Siebert, S.; Varis, O.; Ward, P.J. Lost Food, Wasted Resources: Global Food Supply Chain Losses and Their Impacts on Freshwater, Cropland, and Fertiliser Use. Sci. Total Environ. 2012, 438, 477–489. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of High Added-Value Components from Food Wastes: Conventional, Emerging Technologies and Commercialized Applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization: Fruit and Vegetable Waste. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, V.; Spinelli, S.; Angiolillo, L.; Del Nobile, M.A.; Conte, A. Emerging Techniques Applied to By-Products for Food Fortification. J. Food Sci. Technol. 2020, 57, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Picciotti, U.; Massaro, A.; Galiano, A.; Garganese, F. Cheese Fortification: Review and Possible Improvements. Food Rev. Int. 2021, 38, 474–500. [Google Scholar] [CrossRef]
- Brown, L.; Poudyal, H.; Panchal, S.K. Functional Foods as Potential Therapeutic Options for Metabolic Syndrome: Foods as the Treatment of Obesity. Obes. Rev. 2015, 16, 914–941. [Google Scholar] [CrossRef] [PubMed]
- Elmaliklis, I.N.; Miserli, E.; Filipatou, M.; Tsikouras, I.; Dimou, C.; Koutelidakis, A. Association of Mediterranean Diet Adherence, Functional Food Consumption and Anthropometric Characteristics with Anxiety and Depression Indexes in a Sample of Healthy Greek Adults: A Cross-Sectional Study. Psychiatry Int. 2020, 1, 135–149. [Google Scholar] [CrossRef]
- Konstantinidi, M.; Koutelidakis, A.E. Functional Foods and Bioactive Compounds: A Review of Its Possible Role on Weight Management and Obesity’s Metabolic Consequences. Medicines 2019, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Kandyliari, A.; Elmaliklis, I.-N.; Kontopoulou, O.; Tsafkopoulou, M.; Komninos, G.; Ntzatha, C.; Petsas, A.; Karantonis, H.C.; Koutelidakis, A.E. An Epidemiological Study Report on the Antioxidant and Phenolic Content of Selected Mediterranean Functional Foods, Their Consumption Association with the Body Mass Index, and Consumers Purchasing Behavior in a Sample of Healthy Greek Adults. Appl. Sci. 2021, 11, 7818. [Google Scholar] [CrossRef]
- Bishnoi, S. Herbs as Functional Foods. In Functional Foods: Sources and Health Benefits; Mudgil, D., Barak, S., Eds.; Scientific Publishers: Jodhpur, India, 2016; pp. 141–172. [Google Scholar]
- Paswan, V.K.; Rose, H.; Singh, C.S.; Yamini, S.; Rathaur, A. Herbs and Spices Fortified Functional Dairy Products; IntechOpen: London, UK, 2021; ISBN 978-1-83969-609-1. [Google Scholar]
- Alenisan, M.A.; Alqattan, H.H.; Tolbah, L.S.; Shori, A.B. Antioxidant Properties of Dairy Products Fortified with Natural Additives: A Review. J. Assoc. Arab. Univ. Basic Appl. Sci. 2017, 24, 101–106. [Google Scholar] [CrossRef]
- Meng, X.; Chen, S.; Wang, X. [Dao-di herbs and its change of cultivated origin place]. Zhongguo Zhong Yao Za Zhi 2011, 36, 1687–1692. [Google Scholar]
- Luca, V.S.; Stan, A.-M.; Trifan, A.; Miron, A.; Aprotosoaie, A.C. Catechins profile, caffeine content and antioxidant activity of camellia sinensis teas commercialized in romania. Rev. Med. Chir. Soc. Med. Nat. Iasi 2016, 120, 457–463. [Google Scholar]
- Komes, D.; Belščak-Cvitanović, A.; Horžić, D.; Rusak, G.; Likić, S.; Berendika, M. Phenolic Composition and Antioxidant Properties of Some Traditionally Used Medicinal Plants Affected by the Extraction Time and Hydrolysis. Phytochem. Anal. 2011, 22, 172–180. [Google Scholar] [CrossRef]
- Martins, N.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Development of Functional Dairy Foods. In Bioactive Molecules in Food; Mérillon, J.M., Ramawat, K.G., Eds.; Reference Series in Phytochemistry; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–19. ISBN 978-3-319-54528-8. [Google Scholar]
- Granato, D.; Branco, G.F.; Cruz, A.G.; de Faria, J.A.F.; Shah, N.P. Probiotic Dairy Products as Functional Foods. Compr. Rev. Food Sci. Food Saf. 2010, 9, 455–470. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Fakhri, O.; Farhoodi, A.; Kaboudari, A.; Pir-Mahalleh, S.; Tahapour, K.; Khayyati, M.; Chegini, R. A Review on Probiotic Dairy Products as Functional Foods Reported from Iran. Int. J. Food Nutr. Saf. 2015, 6, 94–105. [Google Scholar]
- Granato, D.; Santos, J.S.; Salem, R.D.; Mortazavian, A.M.; Rocha, R.S.; Cruz, A.G. Effects of Herbal Extracts on Quality Traits of Yogurts, Cheeses, Fermented Milks, and Ice Creams: A Technological Perspective. Curr. Opin. Food Sci. 2018, 19, 1–7. [Google Scholar] [CrossRef]
- Dabija, A.; Codină, G.G.; Ropciuc, S.; Gâtlan, A.-M.; Rusu, L. Assessment of the Antioxidant Activity and Quality Attributes of Yogurt Enhanced with Wild Herbs Extracts. J. Food Qual. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Marinho, M.T.; Zielinski, A.A.F.; Demiate, I.M.; dos Bersot, L.S.; Granato, D.; Nogueira, A. Ripened Semihard Cheese Covered with Lard and Dehydrated Rosemary (Rosmarinus officinalis L.) Leaves: Processing, Characterization, and Quality Traits: Marinho et al. J. Food Sci. 2015, 80, S2045–S2054. [Google Scholar] [CrossRef] [PubMed]
- Aiello, F.; Restuccia, D.; Spizzirri, U.G.; Carullo, G.; Leporini, M.; Loizzo, M.R. Improving Kefir Bioactive Properties by Functional Enrichment with Plant and Agro-Food Waste Extracts. Fermentation 2020, 6, 83. [Google Scholar] [CrossRef]
- Topolska, K.; Florkiewicz, A.; Filipiak-Florkiewicz, A. Functional Food—Consumer Motivations and Expectations. Int. J. Environ. Res. Public Health 2021, 18, 5327. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, S.M.; Youssef, A.M. Potential Application of Herbs and Spices and Their Effects in Functional Dairy Products. Heliyon 2019, 5, e01989. [Google Scholar] [CrossRef] [PubMed]
- Shori, A.B.; Muniandy, P.; Baba, A.S. Changes in Phenolic Compounds Profiles in Tea Extracts and the Composition of These Phenolic Compounds in Yogurt. Recent Patents Food Nutr. Agric. 2021, 12, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Gruskiene, R.; Bockuviene, A.; Sereikaite, J. Microencapsulation of Bioactive Ingredients for Their Delivery into Fermented Milk Products: A Review. Molecules 2021, 26, 4601. [Google Scholar] [CrossRef]
- El-Messery, T.M.; El-Said, M.M.; Demircan, E.; Ozçelik, B. Microencapsulation of Natural Polyphenolic Compounds Extracted from Apple Peel and Its Application in Yoghurt. Acta Sci. Pol. Technol. Aliment. 2019, 18, 25–34. [Google Scholar] [CrossRef]
- Šeregelj, V.; Tumbas Šaponjac, V.; Lević, S.; Kalušević, A.; Ćetković, G.; Čanadanović-Brunet, J.; Nedović, V.; Stajčić, S.; Vulić, J.; Vidaković, A. Application of Encapsulated Natural Bioactive Compounds from Red Pepper Waste in Yogurt. J. Microencapsul. 2019, 36, 704–714. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Cao, G.; Prior, R.L. Comparison of Different Analytical Methods for Assessing Total Antioxidant Capacity of Human Serum. Clin. Chem. 1998, 44, 1309–1315. [Google Scholar] [CrossRef]
- Kapsokefalou, M.; Zhu, L.; Miller, D.D. Adding Iron to Green Tea May Decrease Its Antioxidant Capacity in Rats after an Oral Dose of the Mixture. Nutr. Res. 2006, 26, 480–485. [Google Scholar] [CrossRef]
- Spanos, G.A.; Wrolstad, R.E. Influence of Variety, Maturity, Processing and Storage on the Phenolic Composition of Pear Juice. J. Agric. Food Chem. 1990, 38, 817–824. [Google Scholar] [CrossRef]
- Ropkins, K.; Beck, A.J. Application of hazard analysis critical control points (HACCP) to organic chemical contaminants in food. Crit Rev Food Sci Nutr. 2002, 2, 123–149. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [CrossRef]
- Gayoso, L.; Roxo, M.; Cavero, R.Y.; Calvo, M.I.; Ansorena, D.; Astiasarán, I.; Wink, M. Bioaccessibility and Biological Activity of Melissa Officinalis, Lavandula Latifolia and Origanum Vulgare Extracts: Influence of an in Vitro Gastrointestinal Digestion. J. Funct. Foods 2018, 44, 146–154. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Sousa, M.J.; Ferreira, I.C.F.R. Systematic Comparison of Nutraceuticals and Antioxidant Potential of Cultivated, in Vitro Cultured and Commercial Melissa Officinalis Samples. Food Chem. Toxicol. 2012, 50, 1866–1873. [Google Scholar] [CrossRef] [PubMed]
- Spiridon, I.; Colceru, S.; Anghel, N.; Teaca, C.A.; Bodirlau, R.; Armatu, A. Antioxidant Capacity and Total Phenolic Contents of Oregano (Origanum vulgare), Lavender (Lavandula angustifolia) and Lemon Balm (Melissa officinalis) from Romania. Nat. Prod. Res. 2011, 25, 1657–1661. [Google Scholar] [CrossRef] [PubMed]
- Møller, J.K.S.; Lindberg Madsen, H.; Aaltonen, T.; Skibsted, L.H. Dittany (Origanum Dictamnus) as a Source of Water-Extractable Antioxidants. Food Chem. 1999, 64, 215–219. [Google Scholar] [CrossRef]
- Oztürk, N.; Tunçel, M.; Potoğlu-Erkara, İ. Phenolic Compounds and Antioxidant Activities of Some Hypericum Species: A Comparative Study with H. Perforatum. Pharm. Biol. 2009, 47, 120–127. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Batista, I.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. European Pennyroyal (Mentha pulegium) from Portugal: Chemical Composition of Essential Oil and Antioxidant and Antimicrobial Properties of Extracts and Essential Oil. Ind. Crops Prod. 2012, 36, 81–87. [Google Scholar] [CrossRef]
- Kaloteraki, C.; Almpounioti, K.; Potsaki, P.; Bousdouni, P.; Kandyliari, A.; Koutelidakis, A.E. Total Antioxidant Capacity and Phenolic Content of 17 Mediterranean Functional Herbs and Wild Green Extracts from North Aegean, Greece. Biol. Life Sci. Forum 2021, 6, 43. [Google Scholar] [CrossRef]
- Chen, G.L.; Chen, S.G.; Xie, Y.Q.; Chen, F.; Zhao, Y.Y.; Luo, C.X.; Gao, Y.Q. Total Phenolic, Flavonoid and Antioxidant Activity of 23 Edible Flowers Subjected to in Vitro Digestion. J. Funct. Foods 2015, 17, 243–259. [Google Scholar] [CrossRef]
- Kara, M.; Sahin, H.; Turumtay, H.; Dinc, S.; Gumuscu, A. The Phenolic Composition and Antioxidant Activity of Tea with Different Parts of Sideritis Condensate at Different Steeping Conditions. J. Food Nutr. Res. 2014, 2, 258–262. [Google Scholar] [CrossRef]
- Linardaki, Z.I.; Vasilopoulou, C.G.; Constantinou, C.; Iatrou, G.; Lamari, F.N.; Margarity, M. Differential Antioxidant Effects of Consuming Tea from Sideritis Clandestina Subsp. Peloponnesiaca on Cerebral Regions of Adult Mice. J. Med. Food 2011, 14, 1060–1064. [Google Scholar] [CrossRef]
- de la Puerta, R.; Fernández-Arche, M.A.; Lopez-Lazaro, M.; Garcia, M.D. Antioxidant and Cytotoxic Activities of Sideritis Perezlarae (Borja) Roselló, Stübing and Peris. Nat. Prod. Res. 2013, 27, 1602–1606. [Google Scholar] [CrossRef] [PubMed]
- Jabri Karoui, I.; Marzouk, B. Characterization of Bioactive Compounds in Tunisian Bitter Orange (Citrus aurantium L.) Peel and Juice and Determination of Their Antioxidant Activities. BioMed Res. Int. 2013, 2013, e345415. [Google Scholar] [CrossRef] [PubMed]
- Farahmandfar, R.; Tirgarian, B.; Dehghan, B.; Nemati, A. Comparison of Different Drying Methods on Bitter Orange (Citrus aurantium L.) Peel Waste: Changes in Physical (Density and Color) and Essential Oil (Yield, Composition, Antioxidant and Antibacterial) Properties of Powders. J. Food Meas. Charact. 2020, 14, 862–875. [Google Scholar] [CrossRef]
- Rahman, N.F.A.; Shamsudin, R.; Ismail, A.; Shah, N.N.A.K.; Varith, J. Effects of Drying Methods on Total Phenolic Contents and Antioxidant Capacity of the Pomelo (Citrus grandis (L.) Osbeck) Peels. Innov. Food Sci. Emerg. Technol. 2018, 50, 217–225. [Google Scholar] [CrossRef]
- Dong, X.; Hu, Y.; Li, Y.; Zhou, Z. The Maturity Degree, Phenolic Compounds and Antioxidant Activity of Eureka Lemon [Citrus limon (L.) Burm. f.]: A Negative Correlation between Total Phenolic Content, Antioxidant Capacity and Soluble Solid Content. Sci. Hortic. 2019, 243, 281–289. [Google Scholar] [CrossRef]
- Casquete, R.; Castro, S.M.; Martín, A.; Ruiz-Moyano, S.; Saraiva, J.A.; Córdoba, M.G.; Teixeira, P. Evaluation of the Effect of High Pressure on Total Phenolic Content, Antioxidant and Antimicrobial Activity of Citrus Peels. Innov. Food Sci. Emerg. Technol. 2015, 31, 37–44. [Google Scholar] [CrossRef]
- Aladedunye, F.; Kersting, H.J.; Matthäus, B. Phenolic Extract from Wild Rose Hip with Seed: Composition, Antioxidant Activity, and Performance in Canola Oil. Eur. J. Lipid Sci. Technol. 2014, 116, 1025–1034. [Google Scholar] [CrossRef]
- Tabaszewska, M.; Najgebauer-Lejko, D. The Content of Selected Phytochemicals and in Vitro Antioxidant Properties of Rose Hip (Rosa canina L.) Tinctures. NFS J. 2020, 21, 50–56. [Google Scholar] [CrossRef]
- Agourram, A.; Ghirardello, D.; Rantsiou, K.; Zeppa, G.; Belviso, S.; Romane, A.; Oufdou, K.; Giordano, M. Phenolic Content, Antioxidant Potential, and Antimicrobial Activities of Fruit and Vegetable By-Product Extracts. Int. J. Food Prop. 2013, 16, 1092–1104. [Google Scholar] [CrossRef]
- Koczka, N.; Stefanovits-Bányai, É.; Ombódi, A. Total Polyphenol Content and Antioxidant Capacity of Rosehips of Some Rosa Species. Medicines 2018, 5, 84. [Google Scholar] [CrossRef]
- Gonçalves, S.; Moreira, E.; Andrade, P.B.; Valentão, P.; Romano, A. Effect of in Vitro Gastrointestinal Digestion on the Total Phenolic Contents and Antioxidant Activity of Wild Mediterranean Edible Plant Extracts. Eur. Food Res. Technol. 2019, 245, 753–762. [Google Scholar] [CrossRef]
- Silva, B.N.; Cadavez, V.; Ferreira-Santos, P.; Alves, M.J.; Ferreira, I.C.F.R.; Barros, L.; Teixeira, J.A.; Gonzales-Barron, U. Chemical Profile and Bioactivities of Extracts from Edible Plants Readily Available in Portugal. Foods 2021, 10, 673. [Google Scholar] [CrossRef]
- Farhat, M.B.; Chaouch-Hamada, R.; Sotomayor, J.A.; Landoulsi, A.; Jordán, M.J. Antioxidant Potential of Salvia Officinalis L. Residues as Affected by the Harvesting Time. Ind. Crops Prod. 2014, 54, 78–85. [Google Scholar] [CrossRef]
- Erdogan-Orhan, I.; Baki, E.; Şenol, S.; Yilmaz, G. Sage-Called Plant Species Sold in Turkey and Their Antioxidant Activities. J. Serb. Chem. Soc. 2010, 75, 1491–1501. [Google Scholar] [CrossRef]
- Yesiloglu, Y.; Sit, L.; Kilic, I. In Vitro Antioxidant Activity and Total Phenolic Content of Various Extracts of Satureja Hortensis L. Collected from Turkey. Asian J. Chem. 2013, 25, 8311–8316. [Google Scholar] [CrossRef]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Rupasinghe, H.P.V. Change of Phenolics, Carotenoids, and Antioxidant Capacity Following Simulated Gastrointestinal Digestion and Dialysis of Selected Edible Green Leaves. Food Chem. 2018, 245, 371–379. [Google Scholar] [CrossRef]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total Phenolics, Flavonoids, Anthocyanins and Antioxidant Activity Following Simulated Gastro-Intestinal Digestion and Dialysis of Apple Varieties: Bioaccessibility and Potential Uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In Vitro Bio-Accessibility and Antioxidant Activity of Grape Polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Adebooye, O.C.; Alashi, A.M.; Aluko, R.E. A Brief Review on Emerging Trends in Global Polyphenol Research. J. Food Biochem. 2018, 42, e12519. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, Dietary Sources and Bioavailability. Ann. Ist. Super. Sanita. 2007, 43, 348–361. [Google Scholar]
- Urpi-Sarda, M.; Garrido, I.; Monagas, M.; Gómez-Cordovés, C.; Medina-Remón, A.; Andres-Lacueva, C.; Bartolomé, B. Profile of Plasma and Urine Metabolites after the Intake of Almond [Prunus Dulcis (Mill.) D.A. Webb] Polyphenols in Humans. J. Agric. Food Chem. 2009, 57, 10134–10142. [Google Scholar] [CrossRef]
- Jacobs, D.M.; Gaudier, E.; van Duynhoven, J.; Vaughan, E.E. Non-Digestible Food Ingredients, Colonic Microbiota and the Impact on Gut Health and Immunity: A Role for Metabolomics. Curr. Drug. Metab. 2009, 10, 41–54. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. Interactions of Gut Microbiota with Dietary Polyphenols and Consequences to Human Health. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 471–476. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of Polyphenols on Gut Microbiota and Implications in Human Health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- Piao, X.L.; Kim, H.Y.; Yokozawa, T.; Lee, Y.A.; Piao, X.S.; Cho, E.J. Protective Effects of Broccoli (Brassica oleracea) and Its Active Components against Radical-Induced Oxidative Damage. J. Nutr. Sci. Vitaminol. 2005, 51, 142–147. [Google Scholar] [CrossRef]
- Chien, L.H.; Wu, C.T.; Deng, J.S.; Jiang, W.P.; Huang, W.C.; Huang, G.J. Salvianolic Acid C Protects against Cisplatin-Induced Acute Kidney Injury through Attenuation of Inflammation, Oxidative Stress and Apoptotic Effects and Activation of the CaMKK–AMPK–Sirt1-Associated Signaling Pathway in Mouse Models. Antioxidants 2021, 10, 1620. [Google Scholar] [CrossRef] [PubMed]
- Shiraiwa, M.; Kitakaze, T.; Yamashita, Y.; Ukawa, Y.; Mukai, K.; Ashida, H. Pectolinarigenin Induces Antioxidant Enzymes through Nrf2/ARE Pathway in HepG2 Cells. Antioxidants 2022, 11, 675. [Google Scholar] [CrossRef] [PubMed]
- Cherkas, A.; Holota, S.; Mdzinarashvili, T.; Gabbianelli, R.; Zarkovic, N. Glucose as a Major Antioxidant: When, What for and Why It Fails? Antioxidants 2020, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Cádiz-Gurrea, M.d.l.L.; Pinto, D.; Delerue-Matos, C.; Rodrigues, F. Olive Fruit and Leaf Wastes as Bioactive Ingredients for Cosmetics—A Preliminary Study. Antioxidants 2021, 10, 245. [Google Scholar] [CrossRef] [PubMed]
- Lue, B.; Sørensen, A.M.; Jacobsen, C.; Guo, Z.; Xu, X. Antioxidant Efficacies of Rutin and Rutin Esters in Bulk Oil and Oil-in-water Emulsion. Eur. J. Lipid Sci. Technol. 2017, 119, 1600049. [Google Scholar] [CrossRef]
- Plumb, G.W.; Price, K.R.; Modes, M.J.C.; Williamson, G. Antioxidant Properties of the Major Polyphenolic Compounds in Broccoli. Free. Radic. Res. 1997, 27, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.M.; Zahri, S.; Zarrini, G.; Nazemiyeh, H.; Mohammadi, S. Biological Activity of Quercetin-3-O-Glucoside, a Known Plant Flavonoid. Russ. J. Bioorganic Chem. 2009, 35, 376–378. [Google Scholar] [CrossRef]
- Rehecho, S.; Hidalgo, O.; García-Iñiguez de Cirano, M.; Navarro, I.; Astiasarán, I.; Ansorena, D.; Cavero, R.Y.; Calvo, M.I. Chemical Composition, Mineral Content and Antioxidant Activity of Verbena officinalis L. LWT—Food Sci. Technol. 2011, 44, 875–882. [Google Scholar] [CrossRef]
- Cho, Y.-C.; Park, J.; Cho, S. Anti-Inflammatory and Anti-Oxidative Effects of Luteolin-7-O-Glucuronide in LPS-Stimulated Murine Macrophages through TAK1 Inhibition and Nrf2 Activation. Int. J. Mol. Sci. 2020, 21, 2007. [Google Scholar] [CrossRef]
- Sprenger, R.d.F.; Cass, Q.B. Characterization of Four Phyllanthus Species Using Liquid Chromatography Coupled to Tandem Mass Spectrometry. J. Chromatogr. A 2013, 1291, 97–103. [Google Scholar] [CrossRef]
- Ulewicz-Magulska, B.; Wesolowski, M. Total Phenolic Contents and Antioxidant Potential of Herbs Used for Medical and Culinary Purposes. Plant Foods Hum. Nutr. 2019, 74, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Markosyan, A.; McCluskey, J.J.; Wahl, T.I. Consumer Response to Information about a Functional Food Product: Apples Enriched with Antioxidants. Can. J. Agric. Econ. Can. d’agroeconomie 2009, 57, 325–341. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Predoi, G. Antioxidant Capacity Determination in Plants and Plant-Derived Products: A Review. Oxidative Med. Cell. Longev. 2016, 2016, 9130976. [Google Scholar] [CrossRef] [PubMed]
- Schofield, P.; Mbugua, D.M.; Pell, A.N. Analysis of Condensed Tannins: A Review. Anim. Feed. Sci. Technol. 2001, 91, 21–40. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; de Mello, J.C.P. Application and Analysis of the Folin Ciocalteu Method for the Determination of the Total Phenolic Content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough Study of Reactivity of Various Compound Classes toward the Folin-Ciocalteu Reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Oxidants and Antioxidants Part A; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Cosentino, C.; Colonna, M.A.; Musto, M.; Dimotta, A.; Freschi, P.; Tarricone, S.; Ragni, M.; Paolino, R. Effects of Dietary Supplementation with Extruded Linseed and Oregano in Autochthonous Goat Breeds on the Fatty Acid Profile of Milk and Quality of Padraccio Cheese. J. Dairy Sci. 2021, 104, 1445–1453. [Google Scholar] [CrossRef]
- Granato, D.; Barba, F.J.; Bursać Kovačević, D.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional Foods: Product Development, Technological Trends, Efficacy Testing, and Safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef]
- Nguyen, L.; Hwang, E.S. Quality Characteristics and Antioxidant Activity of Yogurt Supplemented with Aronia (Aronia melanocarpa) Juice. Prev. Nutr. Food Sci. 2016, 21, 330–337. [Google Scholar] [CrossRef]
- Malongane, F.; McGaw, L.J.; Mudau, F.N. The Synergistic Potential of Various Teas, Herbs and Therapeutic Drugs in Health Improvement: A Review. J. Sci. Food Agric 2017, 97, 4679–4689. [Google Scholar] [CrossRef]
- Zhang, D.; Ivane, N.M.A.; Haruna, S.A.; Zekrumah, M.; Elysé, F.K.R.; Tahir, H.E.; Wang, G.; Wang, C.; Zou, X. Recent Trends in the Micro-Encapsulation of Plant-Derived Compounds and Their Specific Application in Meat as Antioxidants and Antimicrobials. Meat Sci. 2022, 191, 108842. [Google Scholar] [CrossRef]
- Najafi-Soulari, S.; Shekarchizadeh, H.; Kadivar, M. Encapsulation Optimization of Lemon Balm Antioxidants in Calcium Alginate Hydrogels. J. Biomater. Sci. Polym. Ed. 2016, 27, 1631–1644. [Google Scholar] [CrossRef] [PubMed]
- Nanditha, B.; Prabhasankar, P. Antioxidants in Bakery Products: A Review. Crit. Rev. Food Sci. Nutr. 2009, 49, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A Review of Microencapsulation Methods for Food Antioxidants: Principles, Advantages, Drawbacks and Applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Genevois, C.E.; Castellanos Fuentes, A.P.; Flores, S.K.; de Escalada Pla, M.F. The Functional and Organoleptic Characterization of a Dairy-Free Dessert Containing a Novel Probiotic Food Ingredient. Food Funct. 2018, 9, 5697–5706. [Google Scholar] [CrossRef]
- De Santis, D.; Giacinti, G.; Chemello, G.; Frangipane, M.T. Improvement of the Sensory Characteristics of Goat Milk Yogurt. J. Food Sci. 2019, 84, 2289–2296. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.G.; Beltrão Filho, E.M.; de Sousa, S.; da Cruz, G.R.B.; de Queiroga, R.C.R.; da Cruz, E.N. Physicochemical and Sensory Characteristics of Yoghurts Made from Goat and Cow Milk. Anim. Sci. J. 2016, 87, 703–709. [Google Scholar] [CrossRef]
- Kristensen, K.; David-Rogeat, N.; Alshammari, N.; Liu, Q.; Muleya, M.; Muttakin, S.; Marciani, L.; Bakalis, S.; Foster, T.J.; Gouseti, O. Chapter 10—Food Digestion Engineering. In Sustainable Food Processing and Engineering Challenges; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 343–368. ISBN 978-0-12-822714-5. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).