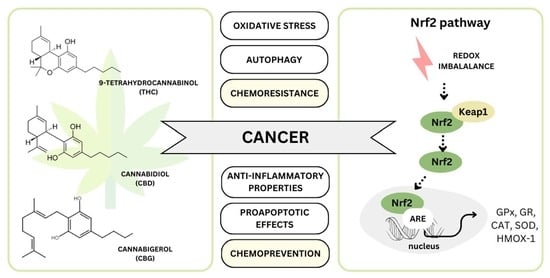
Targeting Nrf2 Signaling Pathway in Cancer Prevention and Treatment: The Role of Cannabis Compounds
Abstract
:1. Introduction
2. Nrf2 Signaling Pathways as a Target
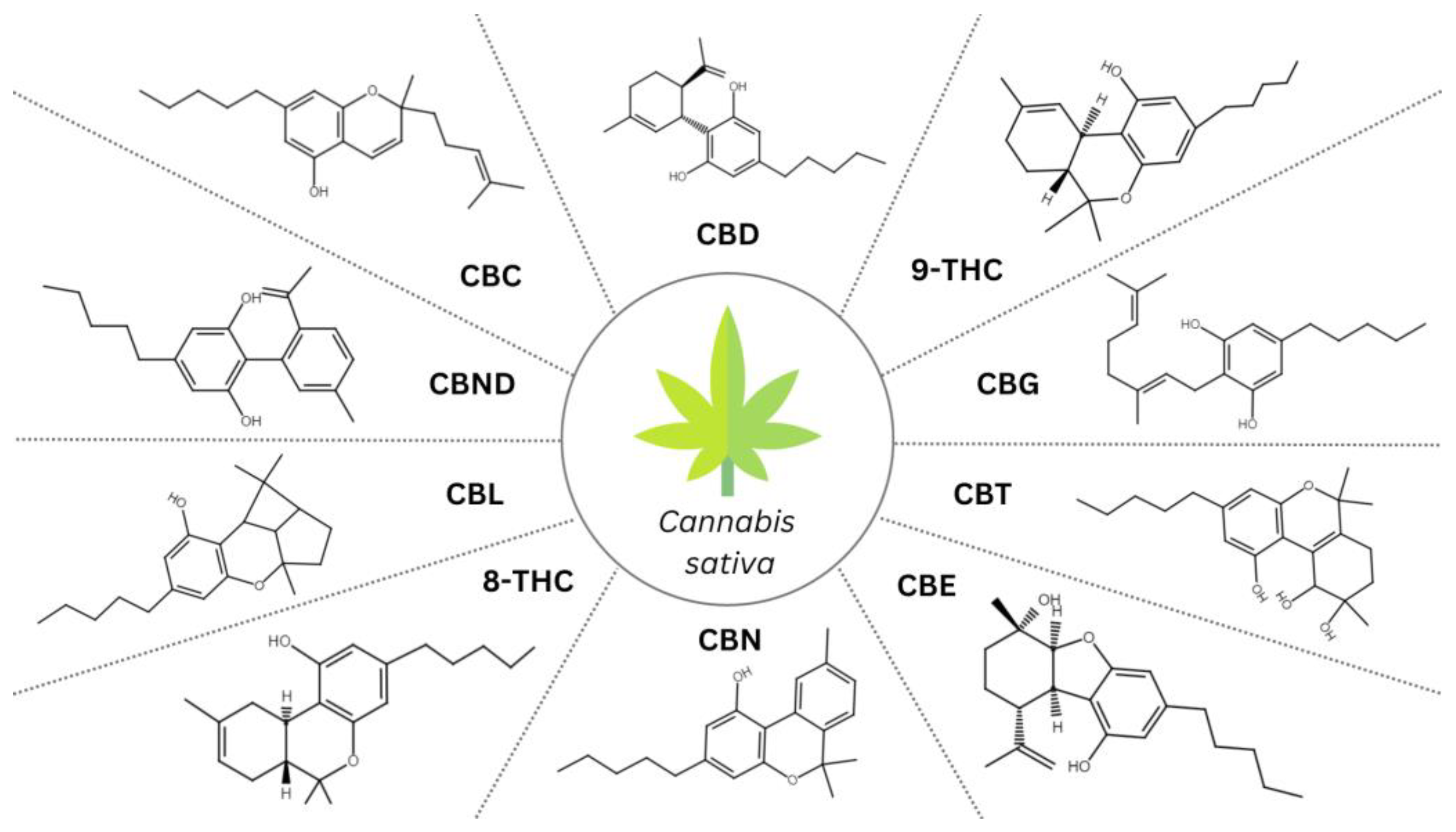
3. Structures and Mechanism of Action of Cannabinoids
4. Cannabinoids and Regulation of the Redox Balance

5. Cannabinoids as Modulators of Nrf2 Pathway—The Role in Chemoprevention and Cancer Therapy
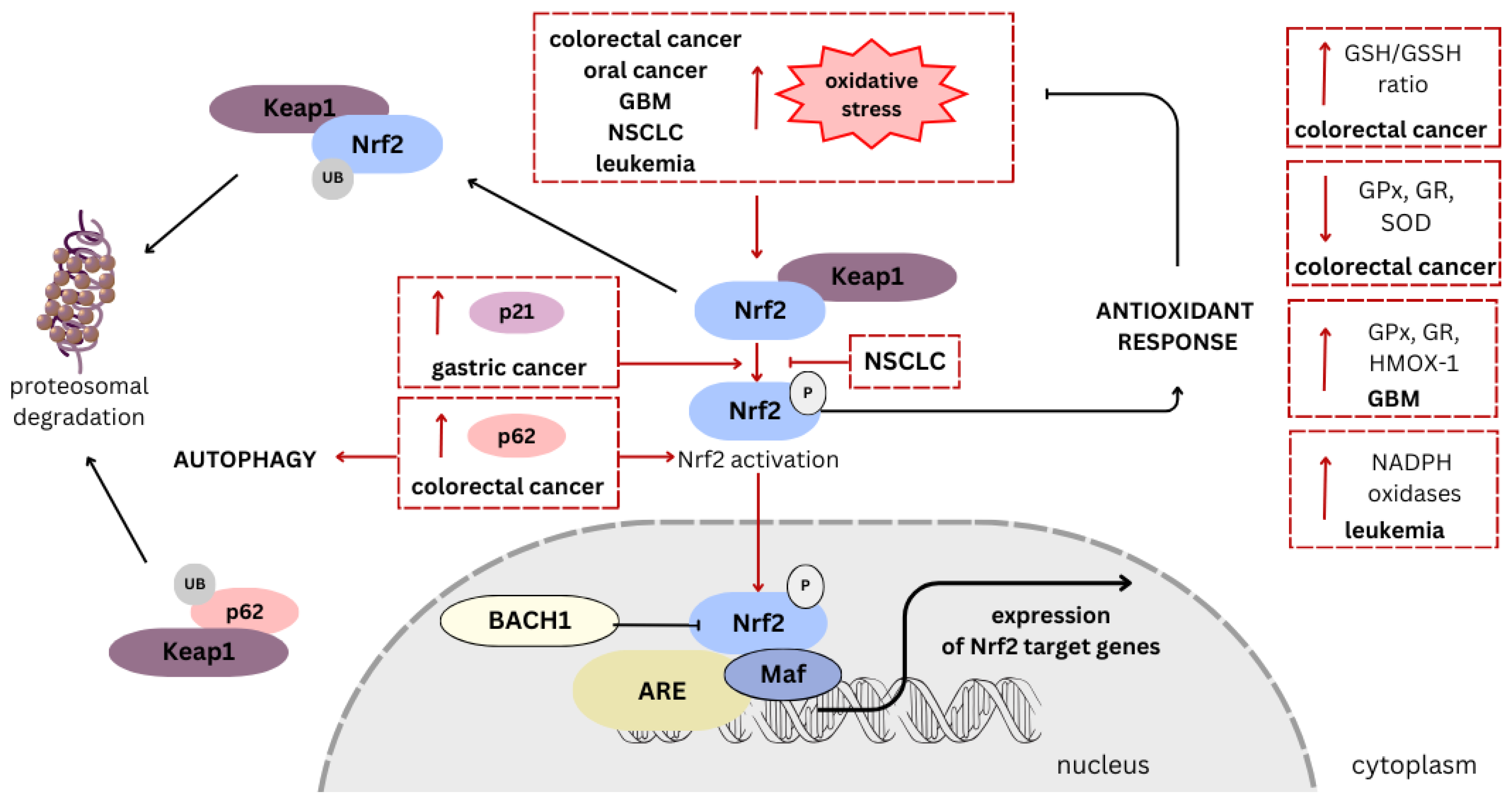
5.1. Colorectal Cancer
5.2. Oral Cancer
5.3. Gastric Cancer
5.4. Non-Small-Cell Lung Cancer (NSCLC)
5.5. Leukemia
5.6. Glioblastoma
6. Targeting the Nrf2 Pathways by Cannabidiol and Its Combination with Other Compounds
| Combination | Condition, Experimental Model | Key Findings of Nrf2 Modulation | References |
|---|---|---|---|
| CBD and tetrahydrocannabivarin (THCV) with doxorubicin (DOX) | Breast cancer Triple-negative breast cancer MDA-MB-231 DOX resistant and wild-type control cells | CBD and THCV downregulated CAT, SP1, NLRP1, SOD2 genes. CBD + DOX and THCV + DOX combo treatment reduced CAT, HMOX-1, SP1, NLRP3 levels. | [108] |
| CBD with moringin | Inflammation Murine macrophage cells RAW 264.7 | The combination of CBD-moringin enhanced Nrf2 level more than CBD alone. | [107] |
| CBD with CBG | Neuroinflammation Motoneuron-like cells NSC-34 treated with medium of LPS-stimulated RAW 264.7 macrophages | Co-administered CBD and CBG increased Nrf2 translocation. | [106] |
| CBD with PES-CI | Colorectal cancer Human adenocarcinoma colon cells HCT116 (p53 wild type) HCT116 (p53 double knockout), SW480, LS174 (p53wild type) SCID mice xenograft model (injected with HCT116 p53 wild-type or p53 double knockout cells) | Hsp70 inhibitor potentiates the anti-tumor effect of CBD with the decrease in ROS and corresponding decrease in the Keap1 expression results in the nuclear translocation of Nrf2 | [71] |
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Adinolfi, S.; Patinen, T.; Jawahar Deen, A.; Pitkänen, S.; Härkönen, J.; Kansanen, E.; Küblbeck, J.; Levonen, A.-L. The KEAP1-NRF2 Pathway: Targets for Therapy and Role in Cancer. Redox Biol. 2023, 63, 102726. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.-I.; Nguyen, L.C.; Oumeslakht, L.; Bensussan, A.; Ben Mkaddem, S. Cannabinoids as Immune System Modulators: Cannabidiol Potential Therapeutic Approaches and Limitations. Cannabis Cannabinoid Res. 2023, 8, 254–269. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell Survival Responses to Environmental Stresses via the Keap1-Nrf2-ARE Pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D. Mechanistic Studies of the Nrf2-Keap1 Signaling Pathway. Drug Metab. Rev. 2006, 38, 769–789. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Chianese, G.; Sirignano, C.; Benetti, E.; Marzaroli, V.; Collado, J.A.; de la Vega, L.; Appendino, G.; Muñoz, E.; Taglialatela-Scafati, O. A Nrf-2 Stimulatory Hydroxylated Cannabidiol Derivative from Hemp (Cannabis Sativa). J. Nat. Prod. 2022, 85, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Hennig, P.; Fenini, G.; Di Filippo, M.; Karakaya, T.; Beer, H.-D. The Pathways Underlying the Multiple Roles of P62 in Inflammation and Cancer. Biomedicines 2021, 9, 707. [Google Scholar] [CrossRef]
- Liu, W.J.; Ye, L.; Huang, W.F.; Guo, L.J.; Xu, Z.G.; Wu, H.L.; Yang, C.; Liu, H.F. P62 Links the Autophagy Pathway and the Ubiqutin–Proteasome System upon Ubiquitinated Protein Degradation. Cell. Mol. Biol. Lett. 2016, 21, 29. [Google Scholar] [CrossRef]
- Jiang, T.; Harder, B.; Rojo de la Vega, M.; Wong, P.K.; Chapman, E.; Zhang, D.D. P62 Links Autophagy and Nrf2 Signaling. Free Radic. Biol. Med. 2015, 88, 199–204. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis Sativa L. Prog. Chem. Org. Nat. Prod. 2017, 103, 1–36. [Google Scholar] [CrossRef]
- Hesami, M.; Pepe, M.; Baiton, A.; Jones, A.M.P. Current Status and Future Prospects in Cannabinoid Production through in Vitro Culture and Synthetic Biology. Biotechnol. Adv. 2023, 62, 108074. [Google Scholar] [CrossRef] [PubMed]
- Berman, P.; Futoran, K.; Lewitus, G.M.; Mukha, D.; Benami, M.; Shlomi, T.; Meiri, D. A New ESI-LC/MS Approach for Comprehensive Metabolic Profiling of Phytocannabinoids in Cannabis. Sci. Rep. 2018, 8, 14280. [Google Scholar] [CrossRef] [PubMed]
- Judžentienė, A.; Garjonytė, R.; Būdienė, J. Phytochemical Composition and Antioxidant Activity of Various Extracts of Fibre Hemp (Cannabis Sativa L.) Cultivated in Lithuania. Molecules 2023, 28, 4928. [Google Scholar] [CrossRef]
- Ingvardsen, C.R.; Brinch-Pedersen, H. Challenges and Potentials of New Breeding Techniques in Cannabis Sativa. Front. Plant Sci. 2023, 14, 1154332. [Google Scholar] [CrossRef] [PubMed]
- Castaneto, M.S.; Gorelick, D.A.; Desrosiers, N.A.; Hartman, R.L.; Pirard, S.; Huestis, M.A. Synthetic Cannabinoids: Epidemiology, Pharmacodynamics, and Clinical Implications. Drug Alcohol. Depend. 2014, 144, 12–41. [Google Scholar] [CrossRef] [PubMed]
- Le Boisselier, R.; Alexandre, J.; Lelong-Boulouard, V.; Debruyne, D. Focus on Cannabinoids and Synthetic Cannabinoids. Clin. Pharmacol. Ther. 2017, 101, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.P.; Duarte, P.; Calado, M.; Almeida, A.J.; Reis, C.P.; Gaspar, M.M. The Current Role of Cannabis and Cannabinoids in Health: A Comprehensive Review of Their Therapeutic Potential. Life Sci. 2023, 329, 121838. [Google Scholar] [CrossRef]
- Kovalchuk, O.; Kovalchuk, I. Cannabinoids as Anticancer Therapeutic Agents. Cell Cycle 2020, 19, 961–989. [Google Scholar] [CrossRef]
- Saleemi, M.A.; Yahaya, N.; Zain, N.N.M.; Raoov, M.; Yong, Y.K.; Noor, N.S.; Lim, V. Antimicrobial and Cytotoxic Effects of Cannabinoids: An Updated Review with Future Perspectives and Current Challenges. Pharmaceuticals 2022, 15, 1228. [Google Scholar] [CrossRef]
- Filipiuc, S.-I.; Neagu, A.-N.; Uritu, C.M.; Tamba, B.-I.; Filipiuc, L.-E.; Tudorancea, I.M.; Boca, A.N.; Hâncu, M.F.; Porumb, V.; Bild, W. The Skin and Natural Cannabinoids-Topical and Transdermal Applications. Pharmaceuticals 2023, 16, 1049. [Google Scholar] [CrossRef]
- Volmar, M.N.M.; Cheng, J.; Alenezi, H.; Richter, S.; Haug, A.; Hassan, Z.; Goldberg, M.; Li, Y.; Hou, M.; Herold-Mende, C.; et al. Cannabidiol Converts NF-κB into a Tumor Suppressor in Glioblastoma with Defined Antioxidative Properties. Neuro Oncol. 2021, 23, 1898–1910. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.E.; Williams, C.M.; Iversen, L.; Whalley, B.J. Molecular Pharmacology of Phytocannabinoids. Prog. Chem. Org. Nat. Prod. 2017, 103, 61–101. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R. Receptors and Channels Targeted by Synthetic Cannabinoid Receptor Agonists and Antagonists. Curr. Med. Chem. 2010, 17, 1360–1381. [Google Scholar] [CrossRef]
- Martínez, V.; Iriondo De-Hond, A.; Borrelli, F.; Capasso, R.; del Castillo, M.D.; Abalo, R. Cannabidiol and Other Non-Psychoactive Cannabinoids for Prevention and Treatment of Gastrointestinal Disorders: Useful Nutraceuticals? Int. J. Mol. Sci. 2020, 21, 3067. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Morrison, P.D.; Fusar-Poli, P.; Martin-Santos, R.; Borgwardt, S.; Winton-Brown, T.; Nosarti, C.; O’ Carroll, C.M.; Seal, M.; Allen, P.; et al. Opposite Effects of Delta-9-Tetrahydrocannabinol and Cannabidiol on Human Brain Function and Psychopathology. Neuropsychopharmacology 2010, 35, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Leweke, F.M.; Piomelli, D.; Pahlisch, F.; Muhl, D.; Gerth, C.W.; Hoyer, C.; Klosterkötter, J.; Hellmich, M.; Koethe, D. Cannabidiol Enhances Anandamide Signaling and Alleviates Psychotic Symptoms of Schizophrenia. Transl. Psychiatry 2012, 2, e94. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.; Bhattacharyya, S. Cannabidiol as a Potential Treatment for Psychosis. Ther. Adv. Psychopharmacol. 2019, 9, 2045125319881916. [Google Scholar] [CrossRef]
- Esposito, G.; Scuderi, C.; Valenza, M.; Togna, G.I.; Latina, V.; De Filippis, D.; Cipriano, M.; Carratù, M.R.; Iuvone, T.; Steardo, L. Cannabidiol Reduces Aβ-Induced Neuroinflammation and Promotes Hippocampal Neurogenesis through PPARγ Involvement. PLoS ONE 2011, 6, e28668. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y.; Guillevin, R.; Vallée, J.-N. Effects of Cannabidiol Interactions with Wnt/β-Catenin Pathway and PPARγ on Oxidative Stress and Neuroinflammation in Alzheimer’s Disease. Acta Biochim. Biophys. Sin. 2017, 49, 853–866. [Google Scholar] [CrossRef]
- Mecha, M.; Feliú, A.; Iñigo, P.M.; Mestre, L.; Carrillo-Salinas, F.J.; Guaza, C. Cannabidiol Provides Long-Lasting Protection against the Deleterious Effects of Inflammation in a Viral Model of Multiple Sclerosis: A Role for A2A Receptors. Neurobiol. Dis. 2013, 59, 141–150. [Google Scholar] [CrossRef]
- Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Vitoretti, L.B.; Mariano-Souza, D.P.; Quinteiro-Filho, W.M.; Akamine, A.T.; Almeida, V.I.; Quevedo, J.; Dal-Pizzol, F.; et al. Cannabidiol, a Non-Psychotropic Plant-Derived Cannabinoid, Decreases Inflammation in a Murine Model of Acute Lung Injury: Role for the Adenosine A2A Receptor. Eur. J. Pharmacol. 2012, 678, 78–85. [Google Scholar] [CrossRef]
- Fogaça, M.V.; Reis, F.M.C.V.; Campos, A.C.; Guimarães, F.S. Effects of Intra-Prelimbic Prefrontal Cortex Injection of Cannabidiol on Anxiety-like Behavior: Involvement of 5HT1A Receptors and Previous Stressful Experience. Eur. Neuropsychopharmacol. 2014, 24, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Soares, V.d.P.; Campos, A.C.; de Bortoli, V.C.; Zangrossi, H.; Guimarães, F.S.; Zuardi, A.W. Intra-Dorsal Periaqueductal Gray Administration of Cannabidiol Blocks Panic-like Response by Activating 5-HT1A Receptors. Behav. Brain Res. 2010, 213, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Rock, E.; Bolognini, D.; Limebeer, C.; Cascio, M.; Anavi-Goffer, S.; Fletcher, P.; Mechoulam, R.; Pertwee, R.; Parker, L. Cannabidiol, a Non-Psychotropic Component of Cannabis, Attenuates Vomiting and Nausea-like Behaviour via Indirect Agonism of 5-HT1A Somatodendritic Autoreceptors in the Dorsal Raphe Nucleus. Br. J. Pharmacol. 2012, 165, 2620–2634. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, O.; Morelli, M.B.; Annibali, D.; Aguzzi, C.; Zeppa, L.; Tuyaerts, S.; Amantini, C.; Amant, F.; Ferretti, B.; Maggi, F.; et al. The Effects of Cannabidiol and Prognostic Role of TRPV2 in Human Endometrial Cancer. Int. J. Mol. Sci. 2020, 21, 5409. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, M.; Ahirwar, D.; Xiaoli, Z.; Zhou, X.; Lustberg, M.; Nasser, M.W.; Shilo, K.; Ganju, R.K. TRPV2 Is a Novel Biomarker and Therapeutic Target in Triple Negative Breast Cancer. Oncotarget 2018, 9, 33459–33470. [Google Scholar] [CrossRef] [PubMed]
- Maggi, F.; Morelli, M.B.; Tomassoni, D.; Marinelli, O.; Aguzzi, C.; Zeppa, L.; Nabissi, M.; Santoni, G.; Amantini, C. The Effects of Cannabidiol via TRPV2 Channel in Chronic Myeloid Leukemia Cells and Its Combination with Imatinib. Cancer Sci. 2022, 113, 1235–1249. [Google Scholar] [CrossRef]
- Nabissi, M.; Morelli, M.B.; Amantini, C.; Liberati, S.; Santoni, M.; Ricci-Vitiani, L.; Pallini, R.; Santoni, G. Cannabidiol Stimulates Aml-1a-Dependent Glial Differentiation and Inhibits Glioma Stem-like Cells Proliferation by Inducing Autophagy in a TRPV2-Dependent Manner. Int. J. Cancer 2015, 137, 1855–1869. [Google Scholar] [CrossRef]
- Moloney, J.N.; Cotter, T.G. ROS Signalling in the Biology of Cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- de la Harpe, A.; Beukes, N.; Frost, C.L. CBD Activation of TRPV1 Induces Oxidative Signaling and Subsequent ER Stress in Breast Cancer Cell Lines. Biotechnol. Appl. Biochem. 2022, 69, 420–430. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, B.G.; Kim, D.Y.; Kim, B.R.; Kim, J.L.; Park, S.H.; Na, Y.J.; Jo, M.J.; Yun, H.K.; Jeong, Y.A.; et al. Cannabidiol Overcomes Oxaliplatin Resistance by Enhancing NOS3- and SOD2-Induced Autophagy in Human Colorectal Cancer Cells. Cancers 2019, 11, 781. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Xu, T.; Wang, Y.; Zhou, Y.; Yu, D.; Wang, Z.; He, L.; Chen, Z.; Zhang, Y.; Davidson, D.; et al. Cannabidiol Inhibits Human Glioma by Induction of Lethal Mitophagy through Activating TRPV4. Autophagy 2021, 17, 3592–3606. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, A.; Trofimov, Y.A.; Yelshanskaya, M.V.; Khau, J.; Nadezhdin, K.D.; Khosrof, L.S.; Krylov, N.A.; Efremov, R.G.; Sobolevsky, A.I. Molecular Pathway and Structural Mechanism of Human Oncochannel TRPV6 Inhibition by the Phytocannabinoid Tetrahydrocannabivarin. Nat. Commun. 2023, 14, 4630. [Google Scholar] [CrossRef] [PubMed]
- Ferro, R.; Adamska, A.; Lattanzio, R.; Mavrommati, I.; Edling, C.E.; Arifin, S.A.; Fyffe, C.A.; Sala, G.; Sacchetto, L.; Chiorino, G.; et al. GPR55 Signalling Promotes Proliferation of Pancreatic Cancer Cells and Tumour Growth in Mice, and Its Inhibition Increases Effects of Gemcitabine. Oncogene 2018, 37, 6368–6382. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Jo, M.J.; Yun, H.K.; Kim, D.Y.; Kim, B.R.; Kim, J.L.; Park, S.H.; Na, Y.J.; Jeong, Y.A.; Kim, B.G.; et al. Cannabidiol Promotes Apoptosis via Regulation of XIAP/Smac in Gastric Cancer. Cell Death Dis. 2019, 10, 846. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Yun, H.K.; Jeong, Y.A.; Jo, M.J.; Kang, S.H.; Kim, J.L.; Kim, D.Y.; Park, S.H.; Kim, B.R.; Na, Y.J.; et al. Cannabidiol-Induced Apoptosis Is Mediated by Activation of Noxa in Human Colorectal Cancer Cells. Cancer Lett. 2019, 447, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Lukhele, S.T.; Motadi, L.R. Cannabidiol Rather than Cannabis Sativa Extracts Inhibit Cell Growth and Induce Apoptosis in Cervical Cancer Cells. BMC Complement. Altern. Med. 2016, 16, 335. [Google Scholar] [CrossRef]
- Kim, M.J.; Huang, Y.; Park, J.-I. Targeting Wnt Signaling for Gastrointestinal Cancer Therapy: Present and Evolving Views. Cancers 2020, 12, 3638. [Google Scholar] [CrossRef]
- Atalay Ekiner, S.; Gęgotek, A.; Skrzydlewska, E. The Molecular Activity of Cannabidiol in the Regulation of Nrf2 System Interacting with NF-κB Pathway under Oxidative Stress. Redox Biol. 2022, 57, 102489. [Google Scholar] [CrossRef]
- Pereira, S.R.; Hackett, B.; O’Driscoll, D.N.; Sun, M.C.; Downer, E.J. Cannabidiol Modulation of Oxidative Stress and Signalling. Neuronal. Signal. 2021, 5, NS20200080. [Google Scholar] [CrossRef]
- Musetti, B.; González-Ramos, H.; González, M.; Bahnson, E.M.; Varela, J.; Thomson, L. Cannabis Sativa Extracts Protect LDL from Cu2+-Mediated Oxidation. J. Cannabis Res. 2020, 2, 37. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.S.; Batista, J.; Viana, R.B.; Baetas, A.C.; Orestes, E.; Andrade, M.A.; Honório, K.M.; da Silva, A.B.F. Understanding the Molecular Aspects of Tetrahydrocannabinol and Cannabidiol as Antioxidants. Molecules 2013, 18, 12663–12674. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.M.; Masteikova, R.; Lazauskas, R.; Bernatoniene, J. Cannabis Sativa L. Bioactive Compounds and Their Protective Role in Oxidative Stress and Inflammation. Antioxidants 2022, 11, 660. [Google Scholar] [CrossRef] [PubMed]
- Tura, M.; Mandrioli, M.; Gallina Toschi, T. Preliminary Study: Comparison of Antioxidant Activity of Cannabidiol (CBD) and α-Tocopherol Added to Refined Olive and Sunflower Oils. Molecules 2019, 24, 3485. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, J.; Liu, H.; Ma, W.; Yu, L.; Tan, X.; Wang, S.; Ren, F.; Li, X.; Li, X. Cannabidiol Attenuates Pulmonary Arterial Hypertension by Improving Vascular Smooth Muscle Cells Mitochondrial Function. Theranostics 2021, 11, 5267–5278. [Google Scholar] [CrossRef] [PubMed]
- Hamelink, C.; Hampson, A.; Wink, D.A.; Eiden, L.E.; Eskay, R.L. Comparison of Cannabidiol, Antioxidants, and Diuretics in Reversing Binge Ethanol-Induced Neurotoxicity. J. Pharmacol. Exp. Ther. 2005, 314, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Mohanraj, R.; Mukhopadhyay, P.; Bátkai, S.; Patel, V.; Horváth, B.; Wink, D.A.; Pacher, P.; Mechoulam, R. Cannabidiol Attenuates Cardiac Dysfunction, Oxidative Stress, Fibrosis, Inflammatory and Cell Death Signaling Pathways in Diabetic Cardiomyopathy. FASEB J. 2010, 25, 2115–2125. [Google Scholar] [CrossRef]
- Ni, B.; Liu, Y.; Dai, M.; Zhao, J.; Liang, Y.; Yang, X.; Han, B.; Jiang, M. The Role of Cannabidiol in Aging. Biomed. Pharmacother. 2023, 165, 115074. [Google Scholar] [CrossRef]
- Liu, C.; Li, H.; Xu, F.; Jiang, X.; Ma, H.; Seeram, N.P. Cannabidiol Protects Human Skin Keratinocytes from Hydrogen-Peroxide-Induced Oxidative Stress via Modulation of the Caspase-1-IL-1β Axis. J. Nat. Prod. 2021, 84, 1563–1572. [Google Scholar] [CrossRef]
- Dos-Santos-Pereira, M.; Guimarães, F.S.; Del-Bel, E.; Raisman-Vozari, R.; Michel, P.P. Cannabidiol Prevents LPS-Induced Microglial Inflammation by Inhibiting ROS/NF-κB-Dependent Signaling and Glucose Consumption. Glia 2020, 68, 561–573. [Google Scholar] [CrossRef]
- Mabou Tagne, A.; Marino, F.; Legnaro, M.; Luini, A.; Pacchetti, B.; Cosentino, M. A Novel Standardized Cannabis Sativa L. Extract and Its Constituent Cannabidiol Inhibit Human Polymorphonuclear Leukocyte Functions. Int. J. Mol. Sci. 2019, 20, 1833. [Google Scholar] [CrossRef]
- Chen, J.; Hou, C.; Chen, X.; Wang, D.; Yang, P.; He, X.; Zhou, J.; Li, H. Protective Effect of Cannabidiol on Hydrogen Peroxide-induced Apoptosis, Inflammation and Oxidative Stress in Nucleus Pulposus Cells. Mol. Med. Rep. 2016, 14, 2321–2327. [Google Scholar] [CrossRef]
- Baeeri, M.; Rahimifard, M.; Daghighi, S.; Khan, F.; Salami, S.A.; Moini-Nodeh, S.; Haghi-Aminjan, H.; Bayrami, Z.; Rezaee, F.; Abdollahi, M. Cannabinoids as Anti-ROS in Aged Pancreatic Islet Cells. Life Sci. 2020, 256, 117969. [Google Scholar] [CrossRef] [PubMed]
- Hampson, A.J.; Grimaldi, M.; Axelrod, J.; Wink, D. Cannabidiol and (-)Delta9-Tetrahydrocannabinol Are Neuroprotective Antioxidants. Proc. Natl. Acad. Sci. USA 1998, 95, 8268–8273. [Google Scholar] [CrossRef] [PubMed]
- Branca, J.J.V.; Morucci, G.; Becatti, M.; Carrino, D.; Ghelardini, C.; Gulisano, M.; Di Cesare Mannelli, L.; Pacini, A. Cannabidiol Protects Dopaminergic Neuronal Cells from Cadmium. Int. J. Environ. Res. Public Health 2019, 16, 4420. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, A.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. In Vitro Model of Neuroinflammation: Efficacy of Cannabigerol, a Non-Psychoactive Cannabinoid. Int. J. Mol. Sci. 2018, 19, 1992. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Typek, R.; Olszowy-Tomczyk, M. Natural vs. Artificial Cannabinoid Oils: The Comparison of Their Antioxidant Activities. Eur. Food Res. Technol. 2023, 249, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Stasiłowicz-Krzemień, A.; Sip, S.; Szulc, P.; Cielecka-Piontek, J. Determining Antioxidant Activity of Cannabis Leaves Extracts from Different Varieties—Unveiling Nature’s Treasure Trove. Antioxidants 2023, 12, 1390. [Google Scholar] [CrossRef]
- Juknat, A.; Pietr, M.; Kozela, E.; Rimmerman, N.; Levy, R.; Gao, F.; Coppola, G.; Geschwind, D.; Vogel, Z. Microarray and Pathway Analysis Reveal Distinct Mechanisms Underlying Cannabinoid-Mediated Modulation of LPS-Induced Activation of BV-2 Microglial Cells. PLoS ONE 2013, 8, e61462. [Google Scholar] [CrossRef]
- Cerretani, D.; Collodel, G.; Brizzi, A.; Fiaschi, A.I.; Menchiari, A.; Moretti, E.; Moltoni, L.; Micheli, L. Cytotoxic Effects of Cannabinoids on Human HT-29 Colorectal Adenocarcinoma Cells: Different Mechanisms of THC, CBD, and CB83. Int. J. Mol. Sci. 2020, 21, 5533. [Google Scholar] [CrossRef]
- Wang, F.; Dezfouli, A.B.; Khosravi, M.; Sievert, W.; Stangl, S.; Schwab, M.; Wu, Z.; Steiger, K.; Ma, H.; Multhoff, G. Cannabidiol-Induced Crosstalk of Apoptosis and Macroautophagy in Colorectal Cancer Cells Involves P53 and Hsp70. Cell Death Discov. 2023, 9, 286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qin, Y.; Pan, Z.; Li, M.; Liu, X.; Chen, X.; Qu, G.; Zhou, L.; Xu, M.; Zheng, Q.; et al. Cannabidiol Induces Cell Cycle Arrest and Cell Apoptosis in Human Gastric Cancer SGC-7901 Cells. Biomolecules 2019, 9, 302. [Google Scholar] [CrossRef] [PubMed]
- Misri, S.; Kaul, K.; Mishra, S.; Charan, M.; Verma, A.K.; Barr, M.P.; Ahirwar, D.K.; Ganju, R.K. Cannabidiol Inhibits Tumorigenesis in Cisplatin-Resistant Non-Small Cell Lung Cancer via TRPV2. Cancers 2022, 14, 1181. [Google Scholar] [CrossRef] [PubMed]
- McKallip, R.J.; Jia, W.; Schlomer, J.; Warren, J.W.; Nagarkatti, P.S.; Nagarkatti, M. Cannabidiol-Induced Apoptosis in Human Leukemia Cells: A Novel Role of Cannabidiol in the Regulation of P22phox and Nox4 Expression. Mol. Pharmacol. 2006, 70, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Massi, P.; Vaccani, A.; Bianchessi, S.; Costa, B.; Macchi, P.; Parolaro, D. The Non-Psychoactive Cannabidiol Triggers Caspase Activation and Oxidative Stress in Human Glioma Cells. Cell. Mol. Life Sci. 2006, 63, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
- Singer, E.; Judkins, J.; Salomonis, N.; Matlaf, L.; Soteropoulos, P.; McAllister, S.; Soroceanu, L. Reactive Oxygen Species-Mediated Therapeutic Response and Resistance in Glioblastoma. Cell Death Dis. 2015, 6, e1601. [Google Scholar] [CrossRef] [PubMed]
- Juknat, A.; Kozela, E.; Kaushansky, N.; Mechoulam, R.; Vogel, Z. Anti-Inflammatory Effects of the Cannabidiol Derivative Dimethylheptyl-Cannabidiol—Studies in BV-2 Microglia and Encephalitogenic T Cells. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 289–296. [Google Scholar] [CrossRef]
- Kozela, E.; Pietr, M.; Juknat, A.; Rimmerman, N.; Levy, R.; Vogel, Z. Cannabinoids Delta(9)-Tetrahydrocannabinol and Cannabidiol Differentially Inhibit the Lipopolysaccharide-Activated NF-kappaB and Interferon-Beta/STAT Proinflammatory Pathways in BV-2 Microglial Cells. J. Biol. Chem. 2010, 285, 1616–1626. [Google Scholar] [CrossRef]
- Juknat, A.; Gao, F.; Coppola, G.; Vogel, Z.; Kozela, E. miRNA Expression Profiles and Molecular Networks in Resting and LPS-Activated BV-2 Microglia—Effect of Cannabinoids. PLoS ONE 2019, 14, e0212039. [Google Scholar] [CrossRef]
- Juknat, A.; Pietr, M.; Kozela, E.; Rimmerman, N.; Levy, R.; Coppola, G.; Geschwind, D.; Vogel, Z. Differential Transcriptional Profiles Mediated by Exposure to the Cannabinoids Cannabidiol and Δ9-Tetrahydrocannabinol in BV-2 Microglial Cells. Br. J. Pharmacol. 2012, 165, 2512–2528. [Google Scholar] [CrossRef]
- Li, L.; Xuan, Y.; Zhu, B.; Wang, X.; Tian, X.; Zhao, L.; Wang, Y.; Jiang, X.; Wen, N. Protective Effects of Cannabidiol on Chemotherapy-Induced Oral Mucositis via the Nrf2/Keap1/ARE Signaling Pathways. Oxidative Med. Cell. Longev. 2022, 2022, e4619760. [Google Scholar] [CrossRef] [PubMed]
- Böckmann, S.; Hinz, B. Cannabidiol Promotes Endothelial Cell Survival by Heme Oxygenase-1-Mediated Autophagy. Cells 2020, 9, 1703. [Google Scholar] [CrossRef] [PubMed]
- Casares, L.; García, V.; Garrido-Rodríguez, M.; Millán, E.; Collado, J.A.; García-Martín, A.; Peñarando, J.; Calzado, M.A.; de la Vega, L.; Muñoz, E. Cannabidiol Induces Antioxidant Pathways in Keratinocytes by Targeting BACH1. Redox Biol. 2020, 28, 101321. [Google Scholar] [CrossRef] [PubMed]
- Atalay, S.; Gęgotek, A.; Wroński, A.; Domigues, P.; Skrzydlewska, E. Therapeutic Application of Cannabidiol on UVA and UVB Irradiated Rat Skin. A Proteomic Study. J. Pharm. Biomed. Anal. 2021, 192, 113656. [Google Scholar] [CrossRef] [PubMed]
- Jastrząb, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Regulates the Expression of Keratinocyte Proteins Involved in the Inflammation Process through Transcriptional Regulation. Cells 2019, 8, 827. [Google Scholar] [CrossRef] [PubMed]
- Hamad, H.; Olsen, B.B. Cannabidiol Induces Cell Death in Human Lung Cancer Cells and Cancer Stem Cells. Pharmaceuticals 2021, 14, 1169. [Google Scholar] [CrossRef] [PubMed]
- Loubaki, L.; Rouabhia, M.; Zahrani, M.A.; Amri, A.A.; Semlali, A. Oxidative Stress and Autophagy Mediate Anti-Cancer Properties of Cannabis Derivatives in Human Oral Cancer Cells. Cancers 2022, 14, 4924. [Google Scholar] [CrossRef]
- Jana, S.; Patra, K.; Jana, J.; Mandal, D.P.; Bhattacharjee, S. Nrf-2 Transcriptionally Activates P21Cip/WAF1 and Promotes A549 cell Survival against Oxidative Stress Induced by H2O2. Chem. Biol. Interact. 2018, 285, 59–68. [Google Scholar] [CrossRef]
- Nagahashi, M.; Abe, M.; Sakimura, K.; Takabe, K.; Wakai, T. The Role of Sphingosine-1-phosphate in Inflammation and Cancer Progression. Cancer Sci. 2018, 109, 3671–3678. [Google Scholar] [CrossRef]
- Dan, W.-Y.; Zhou, G.-Z.; Peng, L.-H.; Pan, F. Update and Latest Advances in Mechanisms and Management of Colitis-Associated Colorectal Cancer. World J. Gastrointest. Oncol. 2023, 15, 1317–1331. [Google Scholar] [CrossRef]
- Couch, D.G.; Tasker, C.; Theophilidou, E.; Lund, J.N.; O’Sullivan, S.E. Cannabidiol and Palmitoylethanolamide Are Anti-Inflammatory in the Acutely Inflamed Human Colon. Clin. Sci. 2017, 131, 2611–2626. [Google Scholar] [CrossRef] [PubMed]
- Honarmand, M.; Namazi, F.; Mohammadi, A.; Nazifi, S. Can Cannabidiol Inhibit Angiogenesis in Colon Cancer? Comp. Clin. Pathol. 2019, 28, 165–172. [Google Scholar] [CrossRef]
- Greenhough, A.; Patsos, H.A.; Williams, A.C.; Paraskeva, C. The Cannabinoid Delta(9)-Tetrahydrocannabinol Inhibits RAS-MAPK and PI3K-AKT Survival Signalling and Induces BAD-Mediated Apoptosis in Colorectal Cancer Cells. Int. J. Cancer 2007, 121, 2172–2180. [Google Scholar] [CrossRef] [PubMed]
- Aviello, G.; Romano, B.; Borrelli, F.; Capasso, R.; Gallo, L.; Piscitelli, F.; Di Marzo, V.; Izzo, A.A. Chemopreventive Effect of the Non-Psychotropic Phytocannabinoid Cannabidiol on Experimental Colon Cancer. J. Mol. Med. 2012, 90, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Raup-Konsavage, W.M.; Sepulveda, D.E.; Morris, D.P.; Amin, S.; Vrana, K.E.; Graziane, N.M.; Desai, D. Efficient Synthesis for Altering Side Chain Length on Cannabinoid Molecules and Their Effects in Chemotherapy and Chemotherapeutic Induced Neuropathic Pain. Biomolecules 2022, 12, 1869. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.-K.; Chung, D.-J.; Chang, L.-C.; Luo, C.-K.; Jwo, S.-H.; Lee, Y.-H.; Lin, J.-S.; Wang, C.-H.; Wei, T.-T. The Protective Effect of Cannabinoids against Colorectal Cancer Cachexia through Modulation of Inflammation and Immune Responses. Biomed. Pharmacother. 2023, 161, 114467. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Salam, O.M.E.; Salama, R.A.A.; El-Denshary, E.-E.; Sleem, A.A.; El-Shamarka, M.E.-S.; Hassan, N.S. Effect of Cannabis Sativa Extract on Gastric Acid Secretion, Oxidative Stress and Gastric Mucosal Integrity in Rats. Comp. Clin. Pathol. 2015, 24, 1417–1434. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Khodakarami, A.; Adibfar, S.; Karpisheh, V.; Abolhasani, S.; Jalali, P.; Mohammadi, H.; Gholizadeh Navashenaq, J.; Hojjat-Farsangi, M.; Jadidi-Niaragh, F. The Molecular Biology and Therapeutic Potential of Nrf2 in Leukemia. Cancer Cell Int. 2022, 22, 241. [Google Scholar] [CrossRef]
- Velasco, G.; Hernández-Tiedra, S.; Dávila, D.; Lorente, M. The Use of Cannabinoids as Anticancer Agents. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 259–266. [Google Scholar] [CrossRef]
- Tomko, A.M.; Whynot, E.G.; Ellis, L.D.; Dupré, D.J. Anti-Cancer Potential of Cannabinoids, Terpenes, and Flavonoids Present in Cannabis. Cancers 2020, 12, 1985. [Google Scholar] [CrossRef] [PubMed]
- López-Valero, I.; Saiz-Ladera, C.; Torres, S.; Hernández-Tiedra, S.; García-Taboada, E.; Rodríguez-Fornés, F.; Barba, M.; Dávila, D.; Salvador-Tormo, N.; Guzmán, M.; et al. Targeting Glioma Initiating Cells with A Combined Therapy of Cannabinoids and Temozolomide. Biochem. Pharmacol. 2018, 157, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Marcu, J.P.; Christian, R.T.; Lau, D.; Zielinski, A.J.; Horowitz, M.P.; Lee, J.; Pakdel, A.; Allison, J.; Limbad, C.; Moore, D.H.; et al. Cannabidiol Enhances the Inhibitory Effects of Δ9-Tetrahydrocannabinol on Human Glioblastoma Cell Proliferation and Survival. Mol. Cancer Ther. 2010, 9, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Benito, S.; Seijo-Vila, M.; Caro-Villalobos, M.; Tundidor, I.; Andradas, C.; García-Taboada, E.; Wade, J.; Smith, S.; Guzmán, M.; Pérez-Gómez, E.; et al. Appraising the “Entourage Effect”: Antitumor Action of a Pure Cannabinoid versus a Botanical Drug Preparation in Preclinical Models of Breast Cancer. Biochem. Pharmacol. 2018, 157, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.L.; Hill, D.S.; McKee, C.S.; Hernandez-Tiedra, S.; Lorente, M.; Lopez-Valero, I.; Eleni Anagnostou, M.; Babatunde, F.; Corazzari, M.; Redfern, C.P.F.; et al. Exploiting Cannabinoid-Induced Cytotoxic Autophagy to Drive Melanoma Cell Death. J. Investig. Dermatol. 2015, 135, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Mammana, S.; Cavalli, E.; Gugliandolo, A.; Silvestro, S.; Pollastro, F.; Bramanti, P.; Mazzon, E. Could the Combination of Two Non-Psychotropic Cannabinoids Counteract Neuroinflammation? Effectiveness of Cannabidiol Associated with Cannabigerol. Medicina 2019, 55, 747. [Google Scholar] [CrossRef] [PubMed]
- Chiricosta, L.; Silvestro, S.; Pizzicannella, J.; Diomede, F.; Bramanti, P.; Trubiani, O.; Mazzon, E. Transcriptomic Analysis of Stem Cells Treated with Moringin or Cannabidiol: Analogies and Differences in Inflammation Pathways. Int. J. Mol. Sci. 2019, 20, 6039. [Google Scholar] [CrossRef]
- Kalvala, A.K.; Nimma, R.; Bagde, A.; Surapaneni, S.K.; Patel, N.; Arthur, P.; Sun, L.; Singh, R.; Kommineni, N.; Nathani, A.; et al. The Role of Cannabidiol and Tetrahydrocannabivarin to Overcome Doxorubicin Resistance in MDA-MB-231 Xenografts in Athymic Nude Mice. Biochimie 2023, 208, 19–30. [Google Scholar] [CrossRef]
| Condition | Compound | Experimental Model | Key Findings of Nrf2 Modulation | References |
|---|---|---|---|---|
| Colorectal cancer | ∆9-THC (natural) CBD (natural) CB83 (synthetic) | Human colorectal carcinoma cells HT-29 | Significantly reduced glutathione/ oxidized glutathione ratio in CBD-treated cells and significantly increased in CB83-treated cells. CBD, ∆9-THC, and CB83 reduced catalase activity. The activities of glutathione reductase and glutathione peroxidase were significantly increased in cells exposed to ∆9-THC and significantly decreased in those treated with CBD. | [70] |
| CBD (natural) | Human adenocarcinoma colon cells: HCT116 (p53 wild type) HCT116 (p53 double knockout), SW480, LS174 (p53wild-type) SCID mice xenograft model (injected with HCT116 p53 wild-type or p53 double knockout cells) | CBD treatment induces ROS production and stimulation of the Keap1-Nrf2 antioxidant pathway in p53 wild-type cells. | [71] | |
| Gastric cancer | CBD (natural) | Human gastric cancer cells SGC-7901 | CBD markedly enhanced ROS intracellular levels and increased p21 level. | [72] |
| Non-small-cell lung cancer (NSCLC) | CBD (natural) | Large cell carcinoma cells H460 (cisplatin-resistant) Adenocarcinoma cells A549 (cisplatin-resistant) NSC mice xenograft model (injected with H460 cells) | CBD treatment decreased Nrf2 expression in cisplatin-resistant NSCLC cells. Reduction in tumor progression and metastasis through inhibition of cell growth by reducing Nrf2 expression, increasing ROS generation, and targeting TRPV2. | [73] |
| Leukemia | CBD (natural) | Murine lymphoma cells EL-4 Human leukemia cells Jurkat and MOLT-4 C57BL/6 mice model (injected with EL-4 cells) | CBD increased production of ROS as well as upregulated the NAD(P)H oxidases -Nox4 and p22phox. | [74] |
| Glioblastoma | CBD (natural) | Human glioma cells U87 | CBD induced production of ROS, depletion of intracellular glutathione and increased activity of glutathione reductase and glutathione peroxidase enzymes. | [75] |
Human glioma cells U251 Tissue-derived glioma stem cells (GSC lines 387 and 3832) Athymic nu/nu mice model (injected with GSC lines 3832 or 387) | CBD induced nuclear translocation and activation of Nrf2. Inhibited expression of Sox2 but upregulated expression levels of SLC7A11 (xCT) and HMOX-1. | [76] | ||
| Neuroinflammation (microglia) | CBD (natural) ∆9-THC (natural) Dimethylheptyl-cannabidiol (DMH-CBD) (synthetic) | Immortalized murine microglial cells BV-2 stimulated with lipopolysaccharide (LPS) | CBD induced HMOX-1, Slc7a11 (xCT) and Bach1 upregulation. CBD and less THC treatment caused Herpud, Gclm, Gstm6, HMOX-1, NQO1 and Gstm1 upregulation. In cells treated with DMH-CBD the expression of Trb3, Slc7a11 (xCT), HMOX-1, Atf4, Chop, and p8 were upregulated. | [77,78,79,80] |
| Neuroinflammation (motor neurons) | CBG (natural) | Motor neurons NSC-34 treated with medium of LPS-stimulated RAW 264.7 macrophages | CBG pre-treatment reduced SOD1 levels and restored Nrf2 levels in cells treated with medium of LPS-stimulated macrophages. | [66] |
| Chemoprevention | CBD (natural) Hexocannabitriol (synthetic) | Human epidermal keratinocyte-ARE-luciferase cells (HaCaT-ARE-Luc) | Hexocannabitriol showed a very potent Nrf2 activation, greater than CBD-treated keratinocytes. | [6] |
| Oral mucositis | CBD (natural) | Human oral keratinocytes from 5-fluorouracil-induced oral mucositis; C57BL/6N mice model (treated with 5-fluorouracil) | CBD caused increasing expression and nuclear translocation of Nrf2 and decreasing Keap1. Upregulated the expression levels of HMOX-1and NAD(P)H quinine oxidoreductase 1 (NQO1). | [81] |
| Atherosclerosis | CBD (natural) | Human Umbilical Vein Endothelial Cells (HUVEC) | CBD showed a concentration-dependent increase of Nrf2 as well as HMOX-1 mRNA and protein level. | [82] |
| Skin inflammation | CBD (natural) | Normal human epidermal keratinocytes (NHEK); HaCaT-ARE-Luc cells | CBD dramatically reduced BACH1 total and nuclear levels and enhanced HMOX-1 and p62 gene expression. | [83] |
| CBD (natural) | RH-FOXN1RNU rats irradiated with UVA/B | CBD reduced the dramatic Nrf2 increase and NADPH-dependent diflavin oxido reductase 1 (D4ABT4) and SOD after UVA/UVB exposure | [84] | |
| Diabetic cardiomyopathy | CBD (natural) | C57/BL6J mice model (treated with streptozotocin) | CBD reduced the increased activity of NADPH oxidases, SOD and reversed GSH/GSSG ratio. | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rybarczyk, A.; Majchrzak-Celińska, A.; Krajka-Kuźniak, V. Targeting Nrf2 Signaling Pathway in Cancer Prevention and Treatment: The Role of Cannabis Compounds. Antioxidants 2023, 12, 2052. https://doi.org/10.3390/antiox12122052
Rybarczyk A, Majchrzak-Celińska A, Krajka-Kuźniak V. Targeting Nrf2 Signaling Pathway in Cancer Prevention and Treatment: The Role of Cannabis Compounds. Antioxidants. 2023; 12(12):2052. https://doi.org/10.3390/antiox12122052
Chicago/Turabian StyleRybarczyk, Anna, Aleksandra Majchrzak-Celińska, and Violetta Krajka-Kuźniak. 2023. "Targeting Nrf2 Signaling Pathway in Cancer Prevention and Treatment: The Role of Cannabis Compounds" Antioxidants 12, no. 12: 2052. https://doi.org/10.3390/antiox12122052
APA StyleRybarczyk, A., Majchrzak-Celińska, A., & Krajka-Kuźniak, V. (2023). Targeting Nrf2 Signaling Pathway in Cancer Prevention and Treatment: The Role of Cannabis Compounds. Antioxidants, 12(12), 2052. https://doi.org/10.3390/antiox12122052