Silver Nanoparticles (AgNPs) as Enhancers of Everolimus and Radiotherapy Sensitivity on Clear Cell Renal Cell Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of AgNPs
2.2. Cell Culture and Treatment Conditions
2.3. AgNPs IC25 and IC50 Determination by Resazurin Assay
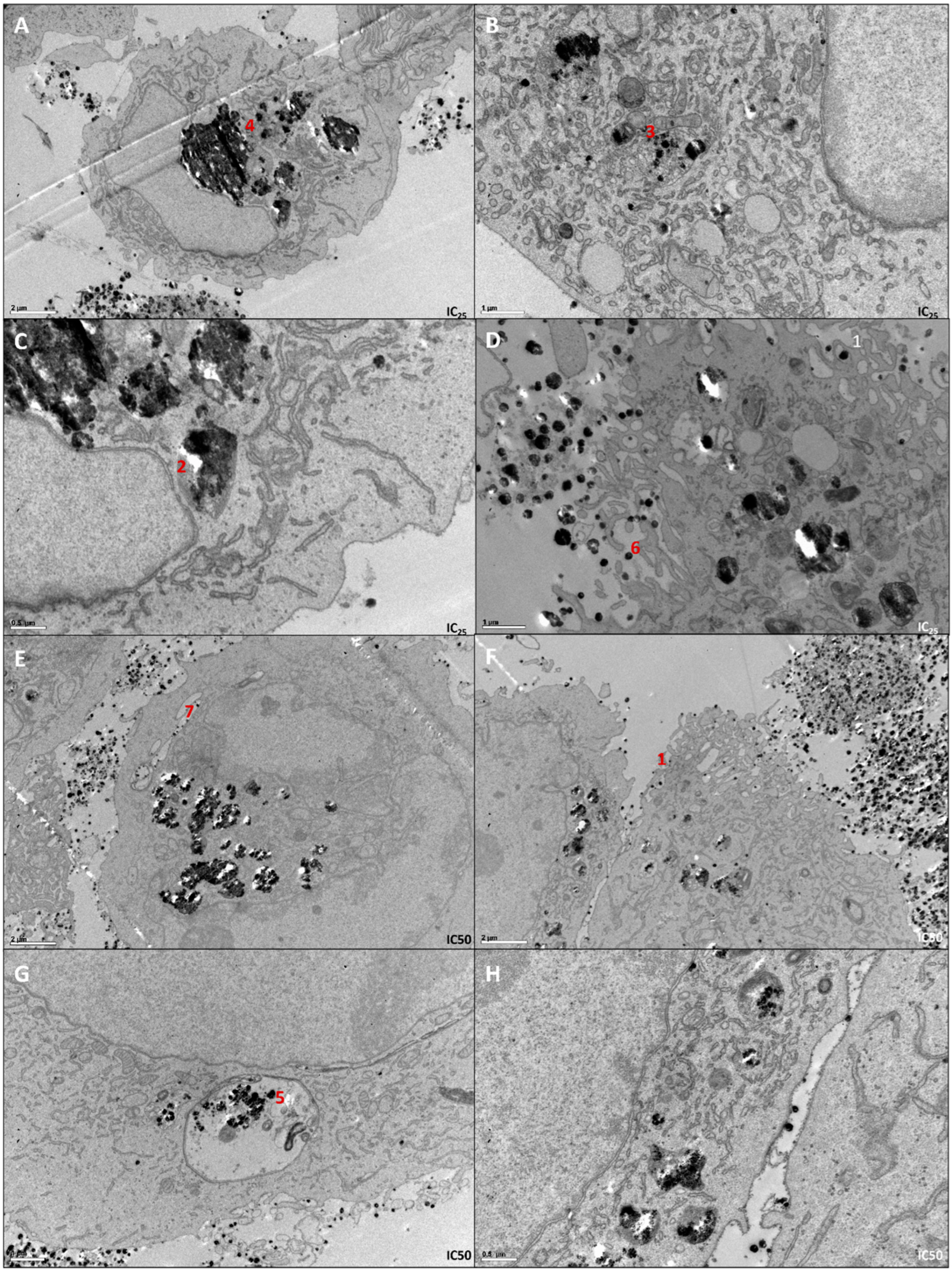
2.4. Determination of Cellular Localization of AgNPs by Transmission Electron Microscopy (TEM)
2.5. Cell Proliferation Assay—BrdU
2.6. Cell Cycle Analysis
2.7. Cell Apoptosis Assay
2.8. Detection of Intracellular Reactive Oxygen Species
2.9. Mitochondrial Membrane Potential Assay (TMRE)
2.10. HepG-2 Toxicity Evaluation—Resazurin Assay
2.11. Everolimus and AgNPs Combined Effect Evaluation
2.12. Irradiation
2.13. Statistical Analysis
3. Results
3.1. AgNPs’ Cytotoxic Potential Screening in RCC Cell Lines
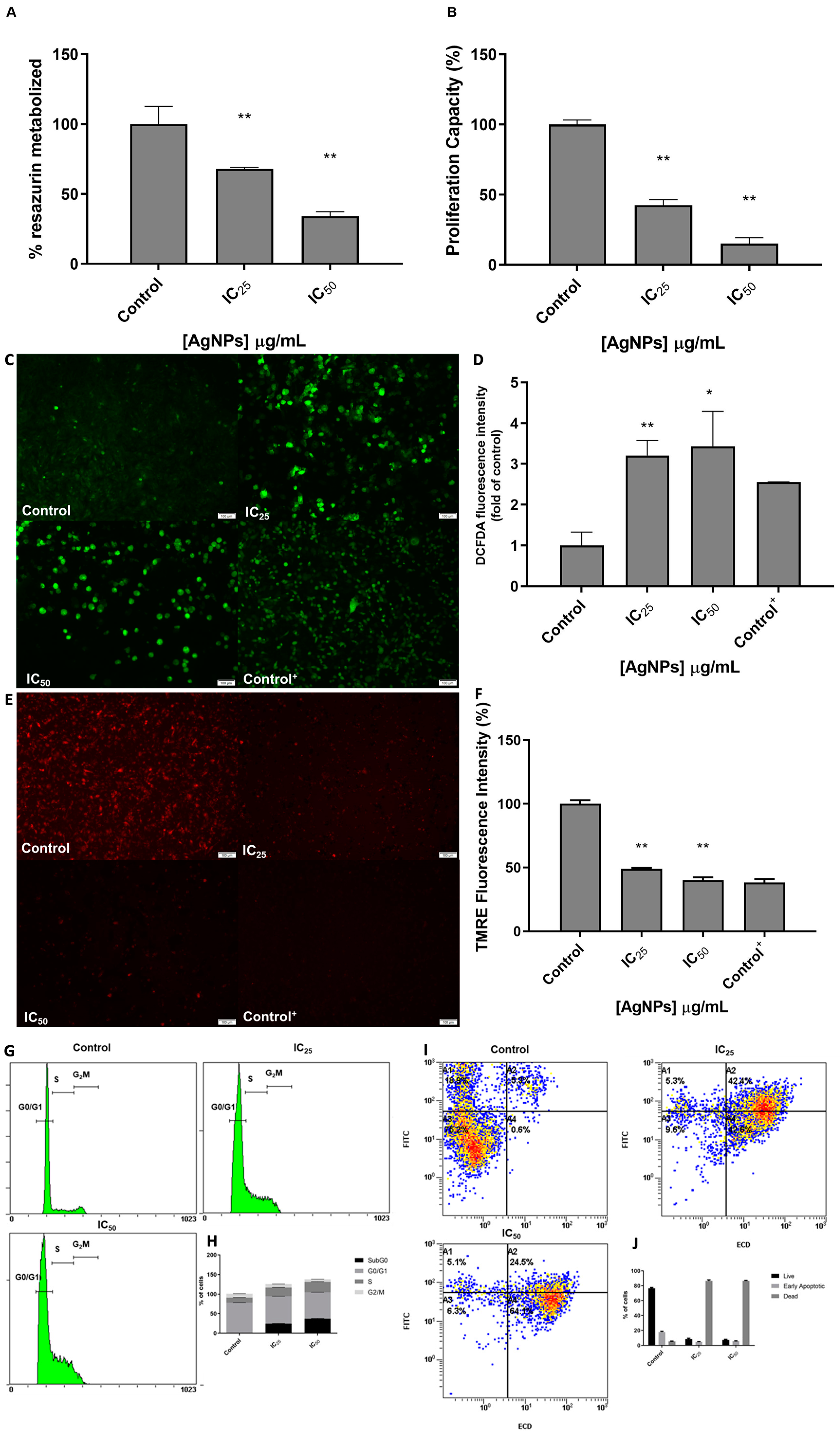
3.2. The Phenotypic Effect of AgNPs in 786-O Cell Line
3.3. AgNPs Biocompatibility in HepG-2 Cell Line
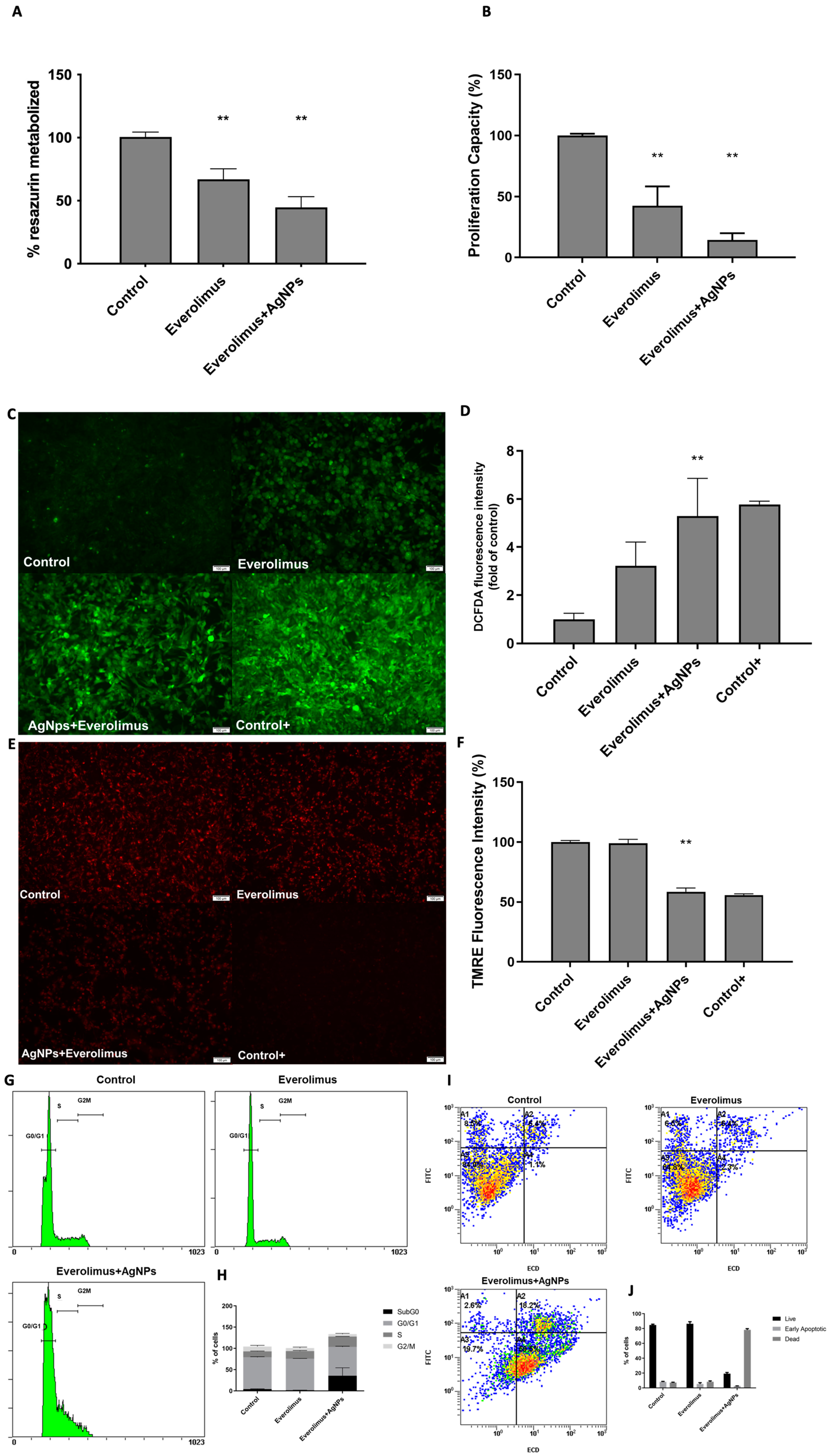
3.4. AgNPs and Everolimus Combined Effect in 786-O Cell Line
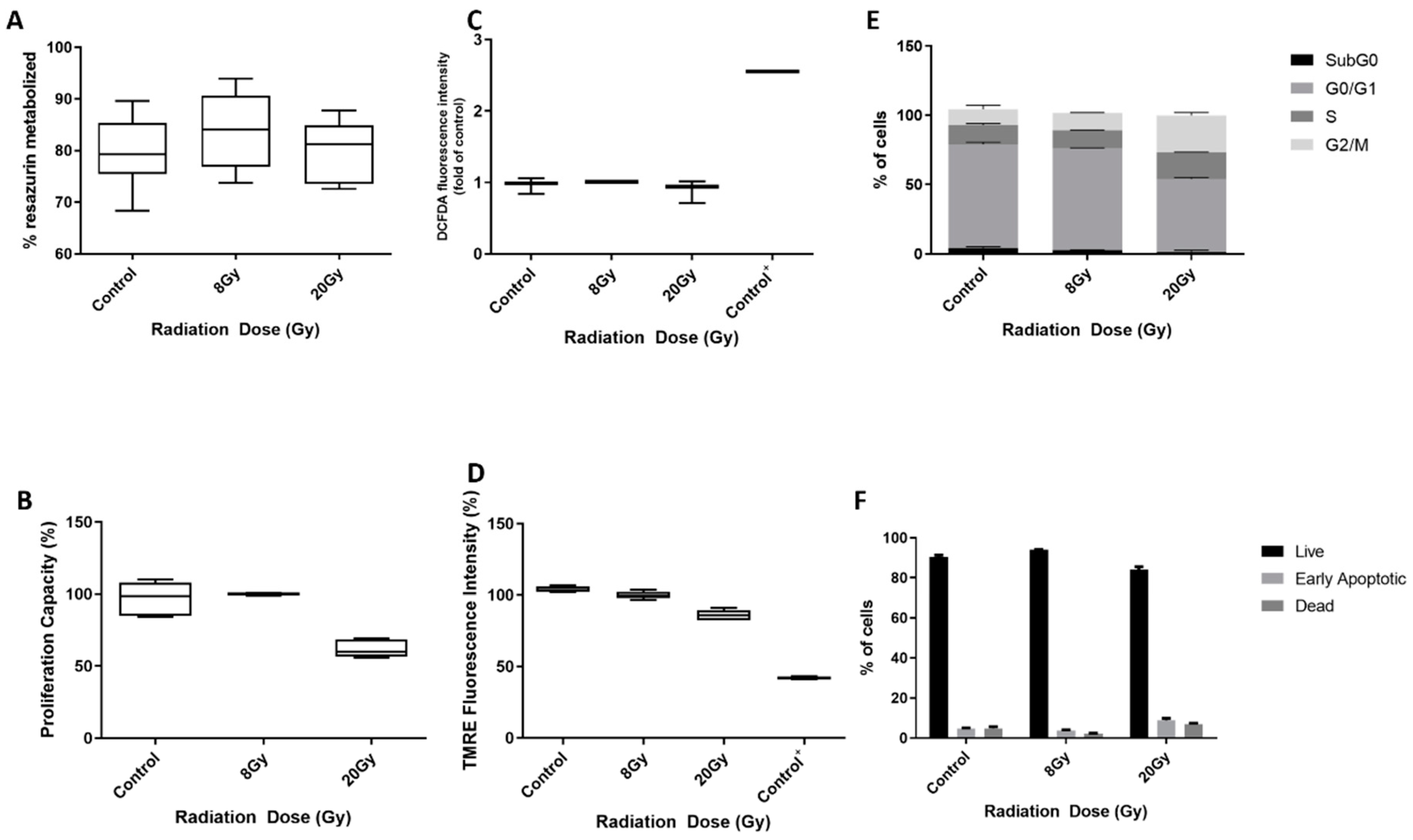
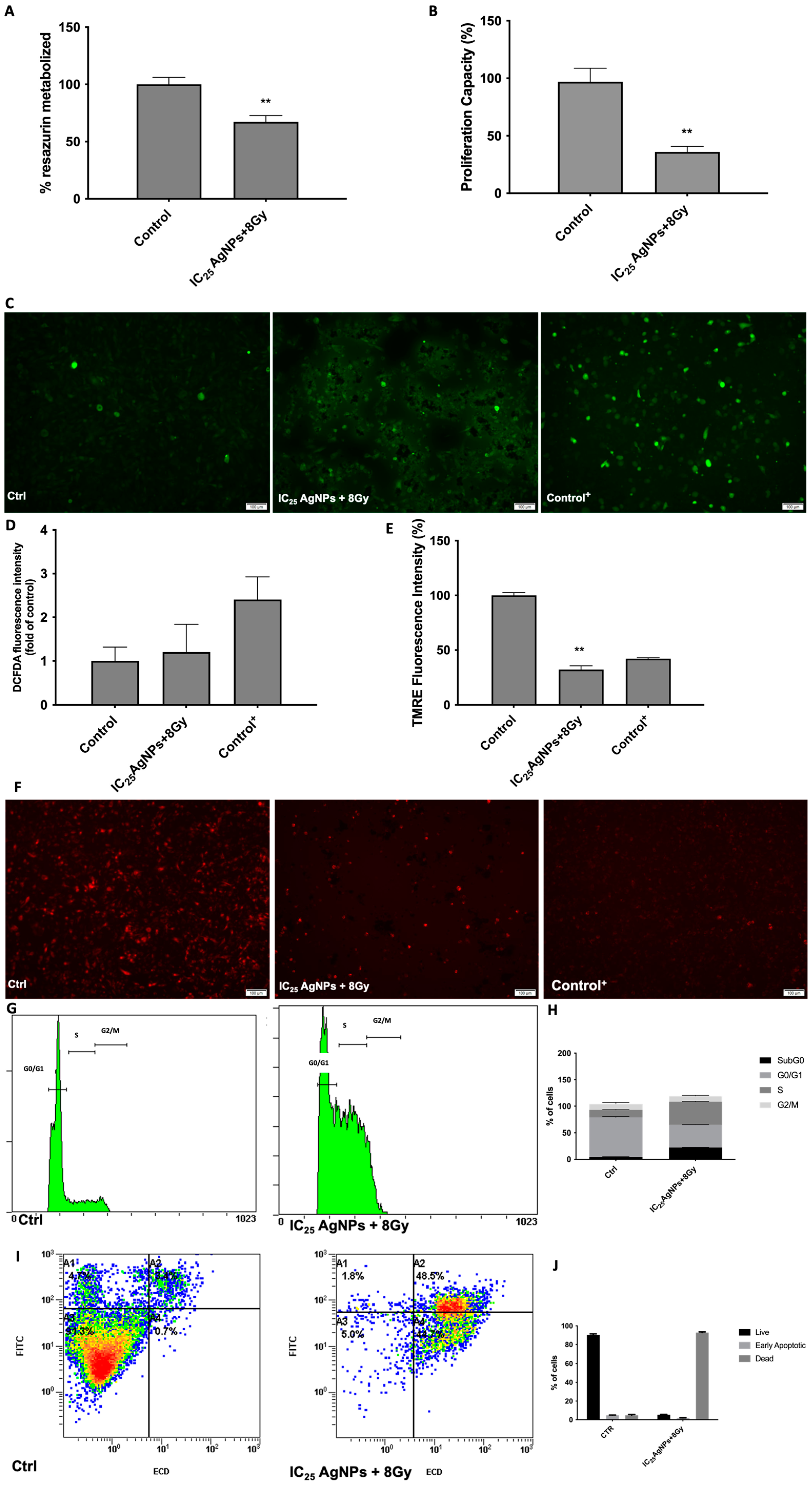
3.5. AgNPs Effect as Sensitizers to Radiation in 786-O Cell Line
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F.; Bsc, M.F.B.; Me, J.F.; Soerjomataram, M.I.; et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Capitanio, U.; Bensalah, K.; Bex, A.; Boorjian, S.A.; Bray, F.; Coleman, J.; Gore, J.L.; Sun, M.; Wood, C.; Russo, P. Epidemiology of Renal Cell Carcinoma. Eur. Urol. 2019, 75, 74–84. [Google Scholar] [CrossRef]
- Makino, T.; Kadomoto, S.; Izumi, K.; Mizokami, A. Epidemiology and Prevention of Renal Cell Carcinoma. Cancers 2022, 14, 4059. [Google Scholar] [CrossRef]
- Escudier, B.; Porta, C.; Schmidinger, M.; Rioux-Leclercq, N.; Bex, A.; Khoo, V.; Grünwald, V.; Gillessen, S.; Horwich, A.; ESMO Guidelines Committee. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of Renal Cell Carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Larroquette, M.; Lefort, F.; Heraudet, L.; Bernhard, J.-C.; Ravaud, A.; Domblides, C.; Gross-Goupil, M. Therapeutic Management of Metastatic Clear Cell Renal Cell Carcinoma: A Revolution in Every Decade. Cancers 2022, 14, 6230. [Google Scholar] [CrossRef] [PubMed]
- Ravaud, A.; Gross-Goupil, M. Overcoming resistance to tyrosine kinase inhibitors in renal cell carcinoma. Cancer Treat. Rev. 2012, 38, 996–1003. [Google Scholar] [CrossRef]
- Gherasim, O.; Puiu, R.A.; Bîrcă, A.C.; Burdușel, A.-C.; Grumezescu, A.M. An Updated Review on Silver Nanoparticles in Biomedicine. Nanomaterials 2020, 10, 2318. [Google Scholar] [CrossRef]
- Morais, M.; Teixeira, A.L.; Dias, F.; Machado, V.; Medeiros, R.; Prior, J.A.V. Cytotoxic Effect of Silver Nanoparticles Synthesized by Green Methods in Cancer. J. Med. Chem. 2020, 63, 14308–14335. [Google Scholar] [CrossRef]
- Morais, M.; Machado, V.; Dias, F.; Figueiredo, P.; Palmeira, C.; Martins, G.; Fernandes, R.; Malheiro, A.R.; Mikkonen, K.S.; Teixeira, A.L.; et al. Glucose-Functionalized Silver Nanoparticles as a Potential New Therapy Agent Targeting Hormone-Resistant Prostate Cancer cells. Int. J. Nanomed. 2022, 17, 4321–4337. [Google Scholar] [CrossRef]
- Nogueira, I.; Dias, F.; Morais, M.; Teixeira, A.L.; Medeiros, R. Everolimus resistance in clear cell renal cell carcinoma: miRNA-101 and HIF-2α as molecular triggers? Future Oncol. 2019, 15, 2361–2370. [Google Scholar] [CrossRef]
- Bahadoram, S.; Davoodi, M.; Hassanzadeh, S.; Bahadoram, M.; Barahman, M.; Mafakher, L. Renal cell carcinoma: An overview of the epidemiology, diagnosis, and treatment. G. Ital. Nefrol. 2022, 39, 1. [Google Scholar]
- Grange, C.; Brossa, A.; Bussolati, B. Extracellular Vesicles and Carried miRNAs in the Progression of Renal Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 1832. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.; Bauleth-Ramos, T.; Hirvonen, J.; Sarmento, B.; Santos, H.A. Chapter 1—The Emerging Role of Multifunctional Theranostic Materials in Cancer Nanomedicine. In Handbook of Nanomaterials for Cancer Theranostics; Conde, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–31. [Google Scholar]
- Kowalczyk, P.; Szymczak, M.; Maciejewska, M.; Laskowski, Ł.; Laskowska, M.; Ostaszewski, R.; Skiba, G.; Franiak-Pietryga, I. All That Glitters Is Not Silver—A New Look at Microbiological and Medical Applications of Silver Nanoparticles. Int. J. Mol. Sci. 2021, 22, 854. [Google Scholar] [CrossRef] [PubMed]
- Rocha Amorim, M.O.; Gomes, D.L.; Dantas, L.A.; Viana, R.L.S.; Chiquetti, S.C.; Almeida-Lima, J.; Costa, L.S.; Rocha, H.A.O. Fucan-coated silver nanoparticles synthesized by a green method induce human renal adenocarcinoma cell death. Int. J. Biol. Macromol. 2016, 93 Pt A, 57–65. [Google Scholar] [CrossRef]
- Singh, D.; Yadav, E.; Kumar, V.; Verma, A. Madhuca longifolia Embedded Silver Nanoparticles Attenuate Diethylnitrosamine (DEN)-Induced Renal Cancer via Regulating Oxidative Stress. Curr. Drug Deliv. 2021, 18, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Brodaczewska, K.K.; Szczylik, C.; Fiedorowicz, M.; Porta, C.; Czarnecka, A.M. Choosing the right cell line for renal cell cancer research. Mol. Cancer 2016, 15, 83. [Google Scholar] [CrossRef]
- Hsiao, I.L.; Hsieh, Y.-K.; Wang, C.-F.; Chen, I.-C.; Huang, Y.-J. Trojan-horse mechanism in the cellular uptake of silver nanoparticles verified by direct intra- and extracellular silver speciation analysis. Environ. Sci. Technol. 2015, 49, 3813–3821. [Google Scholar] [CrossRef]
- Johnson, D.G.; Walker, C.L. Cyclins and Cell Cycle Checkpoints. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 295–312. [Google Scholar] [CrossRef]
- Kammerer, S.; Küpper, J.-H. Human hepatocyte systems for in vitro toxicology analysis. J. Cell. Biotechnol. 2018, 3, 85–93. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Fan, Y.; Feng, Q.; Cui, F.-Z. Biocompatibility and Toxicity of Nanoparticles and Nanotubes. J. Nanomater. 2012, 2012, 548389. [Google Scholar] [CrossRef]
- Ramachandran, R.; Parthasarathy, R.; Dhayalan, S. Silver nanoparticles synthesized by Euphorbia hirta exhibited antibacterial activity and induced apoptosis through downregulation of PI3Kγ mediated PI3K/Akt/mTOR/p70S6K in human lung adenocarcinoma A549 cells. Environ. Toxicol. 2022, 37, 2865–2876. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Atique Ullah, A.K.M.; Banik, S.; Sikder, M.T.; Hosokawa, T.; Saito, T.; Kurasaki, M. Green Synthesized Silver Nanoparticles-Mediated Cytotoxic Effect in Colorectal Cancer Cells: NF-κB Signal Induced Apoptosis Through Autophagy. Biol. Trace Elem. Res. 2021, 199, 3272–3286. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhao, L.; Tang, H.; He, X.; Ye, G.; Shi, F.; Kang, M.; Chen, H.; Li, Y. Silver Nanoparticles Induced Oxidative Stress and Mitochondrial Injuries Mediated Autophagy in HC11 Cells Through Akt/AMPK/mTOR Pathway. Biol. Trace Elem. Res. 2021, 199, 1062–1073. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, M.; Zhang, T.; Du, E.; Liu, Y.; Qi, S.; Xu, Y.; Zhang, Z. Autophagic effects and mechanisms of silver nanoparticles in renal cells under low dose exposure. Ecotoxicol. Environ. Saf. 2018, 166, 71–77. [Google Scholar] [CrossRef]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef]
- Houdaihed, L.; Evans, J.C.; Allen, C. In Vivo Evaluation of Dual-Targeted Nanoparticles Encapsulating Paclitaxel and Everolimus. Cancers 2019, 11, 752. [Google Scholar] [CrossRef]
- Chiesa, E.; Dorati, R.; Conti, B.; Modena, T.; Cova, E.; Meloni, F.; Genta, I. Hyaluronic Acid-Decorated Chitosan Nanoparticles for CD44-Targeted Delivery of Everolimus. Int. J. Mol. Sci. 2018, 19, 2310. [Google Scholar] [CrossRef]
- Houdaihed, L.; Evans, J.C.; Allen, C. Dual-Targeted Delivery of Nanoparticles Encapsulating Paclitaxel and Everolimus: A Novel Strategy to Overcome Breast Cancer Receptor Heterogeneity. Pharm. Res. 2020, 37, 39. [Google Scholar] [CrossRef]
- Ishikawa, K.; Koyama-Saegusa, K.; Otsuka, Y.; Ishikawa, A.; Kawai, S.; Yasuda, K.; Suga, T.; Michikawa, Y.; Suzuki, M.; Iwakawa, M.; et al. Gene expression profile changes correlating with radioresistance in human cell lines. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 234–245. [Google Scholar] [CrossRef]
- Hosokawa, Y.; Okumura, K.; Terashima, S.; Sakakura, Y. Radiation protective effect of hypoxia-inducible factor-1α (HIF-1α) on human oral squamous cell carcinoma cell lines. Radiat. Prot. Dosim. 2012, 152, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lin, J.; Liu, P.; Huang, Z.; Zhao, P.; Jin, H.; Ma, J.; Wen, L.; Gu, N. Reactive oxygen species acts as executor in radiation enhancement and autophagy inducing by AgNPs. Biomaterials 2016, 101, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tan, H.; Zhang, X.; Chen, F.; Zhou, Z.; Hu, X.; Chang, S.; Liu, P.; Zhang, H. Enhancement of radiotherapy efficacy by silver nanoparticles in hypoxic glioma cells. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S3), S922–S930. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-X.; Zhou, P.-K. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct. Target. Ther. 2020, 5, 60. [Google Scholar] [CrossRef]
- Wang, B. Analyzing cell cycle checkpoints in response to ionizing radiation in mammalian cells. Methods Mol. Biol. 2014, 1170, 313–320. [Google Scholar]
- Dabestani, S.; Thorstenson, A.; Lindblad, P.; Harmenberg, U.; Ljungberg, B.; Lundstam, S. Renal cell carcinoma recurrences and metastases in primary non-metastatic patients: A population-based study. World J. Urol. 2016, 34, 1081–1086. [Google Scholar] [CrossRef]
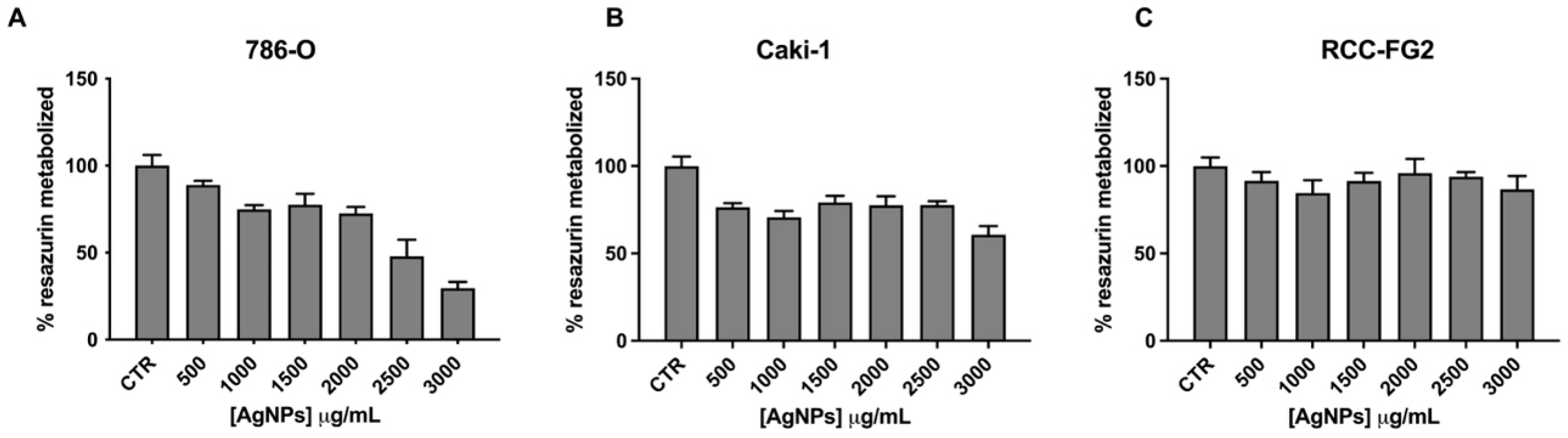

| IC25 | IC50 |
|---|---|
| 1712.15 µg/mL | 2629.63 µg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morais, M.; Machado, V.; Figueiredo, P.; Dias, F.; Craveiro, R.; Lencart, J.; Palmeira, C.; Mikkonen, K.S.; Teixeira, A.L.; Medeiros, R. Silver Nanoparticles (AgNPs) as Enhancers of Everolimus and Radiotherapy Sensitivity on Clear Cell Renal Cell Carcinoma. Antioxidants 2023, 12, 2051. https://doi.org/10.3390/antiox12122051
Morais M, Machado V, Figueiredo P, Dias F, Craveiro R, Lencart J, Palmeira C, Mikkonen KS, Teixeira AL, Medeiros R. Silver Nanoparticles (AgNPs) as Enhancers of Everolimus and Radiotherapy Sensitivity on Clear Cell Renal Cell Carcinoma. Antioxidants. 2023; 12(12):2051. https://doi.org/10.3390/antiox12122051
Chicago/Turabian StyleMorais, Mariana, Vera Machado, Patrícia Figueiredo, Francisca Dias, Rogéria Craveiro, Joana Lencart, Carlos Palmeira, Kirsi S. Mikkonen, Ana Luísa Teixeira, and Rui Medeiros. 2023. "Silver Nanoparticles (AgNPs) as Enhancers of Everolimus and Radiotherapy Sensitivity on Clear Cell Renal Cell Carcinoma" Antioxidants 12, no. 12: 2051. https://doi.org/10.3390/antiox12122051
APA StyleMorais, M., Machado, V., Figueiredo, P., Dias, F., Craveiro, R., Lencart, J., Palmeira, C., Mikkonen, K. S., Teixeira, A. L., & Medeiros, R. (2023). Silver Nanoparticles (AgNPs) as Enhancers of Everolimus and Radiotherapy Sensitivity on Clear Cell Renal Cell Carcinoma. Antioxidants, 12(12), 2051. https://doi.org/10.3390/antiox12122051









