Melatonin in Neurodevelopmental Disorders: A Critical Literature Review
Abstract
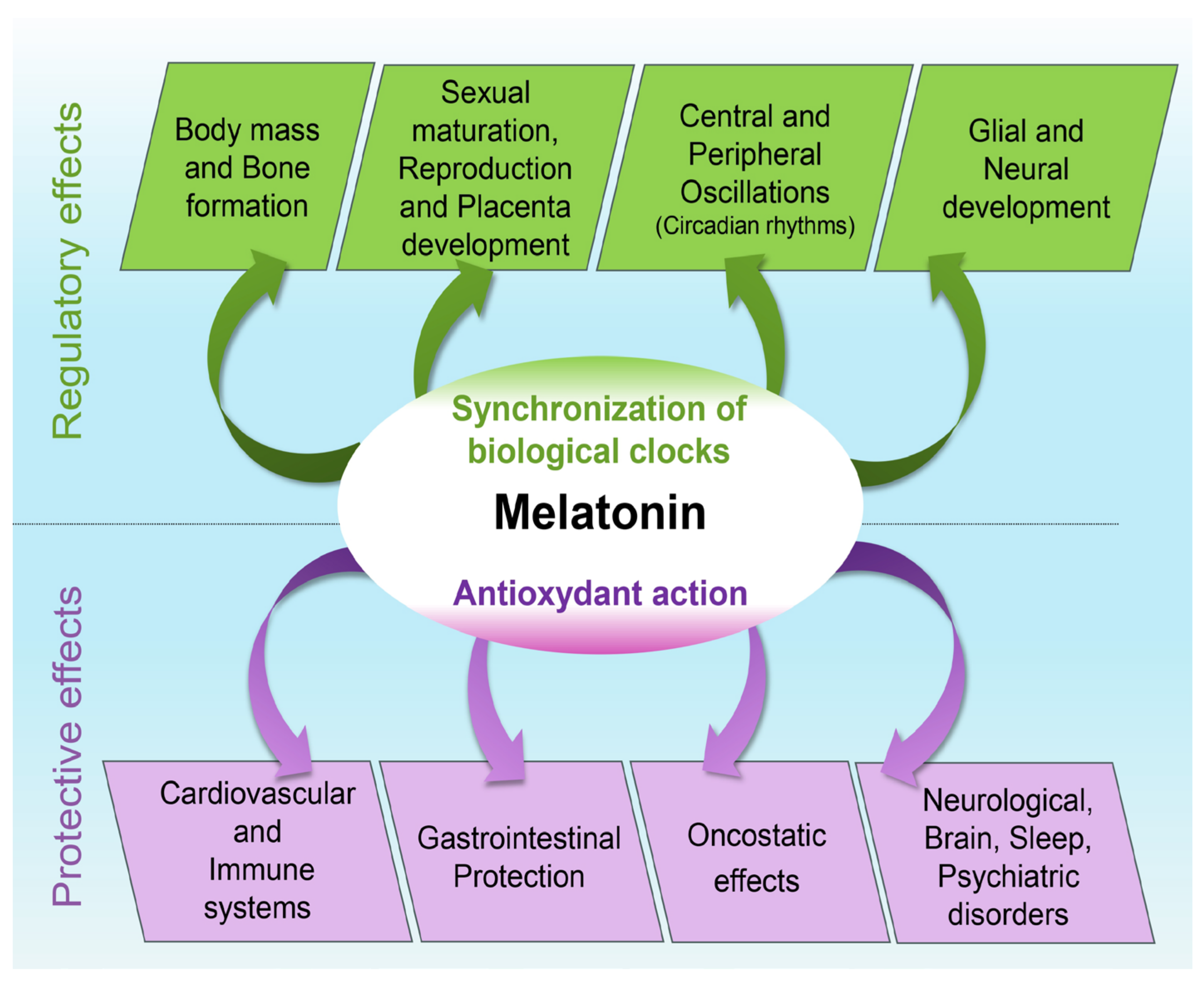
1. Introduction
2. Melatonin and Typical Neurodevelopment
3. Antioxidant Properties and Physiological Effects
4. Melatonin and Atypical Neurodevelopment
5. Melatonin and Neurodevelopmental Disorders
5.1. Relationships between Melatonin and Neurodevelopmental Disorders in Infancy
5.2. Association of Melatonin with Mental Disorders Emerging in Early Adulthood
6. Transnosographic Approach on the Role of Melatonin in Neurodevelopmental Disorders
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abreu, T.; Braganca, M. The bipolarity of light and dark: A review on bipolar disorder and circadian cycles. J. Affect. Disord. 2015, 185, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Abdelgadir, I.S.; Gordon, M.A.; Akobeng, A.K. Melatonin for the management of sleep problems in children with neurodevelopmental disorders: A systematic review and meta-analysis. Arch. Dis. Child. 2018, 103, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.W.; Liu, X.; Pradoldej, S.; Ye, K. N-acetylserotonin activates TrkB receptor in a circadian rhythm. Proc. Natl. Acad. Sci. USA 2010, 107, 3876–3881. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Srinivasan, V.; Maestroni, G.J.M.; Cardinali, D.P.; Poeggeler, B.; Hardeland, R. Melatonin: Nature’s most versatile biological signal? FEBS J. 2006, 273, 2813–2838. [Google Scholar] [CrossRef]
- Whitaker-Azmitia, P.M. Serotonin and brain development: Role in human developmental diseases. Brain Res. Bull. 2001, 56, 479–485. [Google Scholar] [CrossRef]
- Ferlazzo, N.; Andolina, G.; Cannata, A.; Costanzo, M.G.; Rizzo, V.; Currò, M.; Ientile, R.; Caccamo, D. Is Melatonin the Cornucopia of the 21st Century? Antioxidants 2020, 9, 1088. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Kandalepas, P.C.; Mitchell, J.W.; Gillette, M.U. Melatonin signal transduction pathways require E-Box-mediated transcription of Per1 and Per2 to reset the SCN clock at dusk. PLoS ONE 2016, 11, e0157824. [Google Scholar] [CrossRef]
- Dibner, C.; Schibler, U.; Albrecht, U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, functions, and therapeutic benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef]
- Feng, N.Y.; Bass, A.H. ‘Singing’ fish rely on circadian rhythm and melatonin for the timing of nocturnal courtship vocalization. Curr. Biol. 2016, 26, 2681–2689. [Google Scholar] [CrossRef] [PubMed]
- Wilczynski, W.; Lutterschmidt, D.I. Biological rhythms: Melatonin shapes the space–time continuum of social communication. Curr. Biol. 2016, 26, R892–R895. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Voiculescu, S.; Zygouropoulos, N.; Zahiu, C.D.; Zagrean, A.M. Role of melatonin in embryo fetal development. J. Med. Life 2014, 7, 488–492. [Google Scholar] [PubMed]
- Kivela, A.; Kauppila, A.; Lappaluoto, J.; Vakkuri, O. Serum and amniotic fluid melatonin during human labor. J. Clin. Endocrinol. Metab. 1989, 69, 1065–1068. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tamura, H.; Kashida, S.; Takayama, H.; Yamagata, Y.; Karube, A.; Sugino, N.; Kato, H. Changes of serum melatonin level and its relationship to feto-placental unit during pregnancy. J. Pineal Res. 2001, 30, 29–33. [Google Scholar] [CrossRef]
- Supramanian, V.G.; Jenjin, G.; Loose, J. Chronic fetal hypoxia activin: A concentration in the late-pregnant sheep. BJOG Int. J. Obstet. Gyneacol. 2006, 113, 102–109. [Google Scholar] [CrossRef]
- Tricoire, H.; Moller, M.; Chemineau, P.; Malpaux, B. Origin of cerebrospinal fluid melatonin and possible function in the integration of photoperiod. Reprod. Suppl. 2003, 61, 311–321. [Google Scholar] [CrossRef]
- Hardeland, R.; Pandi-Perumal, S.R.; Cardinali, D.P. Melatonin. Int. J. Biochem. Cell Biol. 2006, 38, 313–316. [Google Scholar] [CrossRef]
- Reiter, R.J. Melatonin: The Chemical Expression of Darkness. Mol. Cell. Endocrinol. 1991, 79, C153–C158. [Google Scholar] [CrossRef]
- Prakash, Y.; Thompson, M.A.; Meuchel, L.; Pabelick, C.M.; Mantilla, C.B.; Zaidi, S.; Martin, R.J. Neurotrophins in Lung Health and Disease. Expert Rev. Respir. Med. 2010, 4, 395–411. [Google Scholar] [CrossRef]
- Thoenen, H. Neurotrophins and Neuronal Plasticity. Science 1995, 270, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Motta-Teixeira, L.C.; Machado-Nils, A.V.; Battagello, D.S.; Diniz, G.B.; Andrade-Silva, J.; Silva, S.; Matos, R.A.; do Amaral, F.G.; Xavier, G.F.; Bittencourt, J.C.; et al. The absence of maternal pineal melatonin rhythm during pregnancy and lactation impairs offspring physical growth, neurodevelopment, and behavior. Horm. Behav. 2018, 105, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Kennaway, D.J.; Goble, F.C.; Stamp, G.E. Factors influencing the development of melatonin rhythmicity in humans. J. Clin. Endocrinol. Metab. 1996, 81, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Torres-Farfan, C.; Mendez, N.; Abarzua-Catalan, L.; Vilches, N.; Valenzuela, G.J.; Seron-Ferre, M. A circadian clock entrained by melatonin is ticking in the rat fetal adrenal. Endocrinology 2011, 152, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, F.; Abrial, C.; Gachon, F.; Chevrier, R.; Curé, H.; Chollet, P. Stress, cancer et rythme circadien de la mélatonine. Pathol. Biol. 2004, 53, 269–272. [Google Scholar] [CrossRef]
- Hollway, J.A.; Aman, M.G. Pharmacological treatment of sleep disturbance in developmental disabilities. A review of the literature. Res. Dev. Disabil. 2011, 32, 939–962. [Google Scholar] [CrossRef]
- Joseph, D.; Chong, N.W.; Shanks, M.E.; Rosato, E.; Taub, N.A.; Petersen, S.A. Getting rhythm: How do babies do it? Arch. Dis. Child Fetal Neonatal 2014, 100, F50–F54. [Google Scholar] [CrossRef]
- Price, D.A.; Close, G.C.; Fielding, B.A. Age of appearance of circadian rhythm in salivary cortisol values in infancy. Arch. Dis. Child. 1983, 58, 454–456. [Google Scholar] [CrossRef]
- Paprocka, J.; Kijonka, M.; Rzepka, B.; Sokol, M. Melatonin in hypoxic-ischemic brain injury in term and preterm babies. Int. J. Endocrinol. 2019, 2019, 9626715. [Google Scholar] [CrossRef]
- Touitou, Y. Human aging and melatonin. Clinical relevance. Exp. Gerontol. 2001, 36, 1083–1100. [Google Scholar] [CrossRef]
- Ramirez-Rodriguez, G.; Ortiz-Lopez, L.; Dominguez-Alonso, A.; Benitez-King, G.A.; Kempermann, G. Chronic treatment with melatonin stimulates dendrite maturation and complexity in adult hippocampal neurogenesis of mice. J. Pineal Res. 2011, 50, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Man, H.Y.; Liu, F.; Braunton, J.; Niznik, H.B.; Pang, S.F.; Brown, G.M.; Wang, Y.T. Differential modulation of GABAA receptor function by Mel1a and Mel1b receptors. Nat. Neurosci. 1999, 2, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Yenen, A.S.; Çak, H.T. Melatonin and circadian rhythm in autism spectrum disorders. Turk Psikiyatr. Derg. 2020, 31, 201–211. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Janjetovic, Z.; Kim, T.K.; Böhm, M.; Steinbrink, K.; Reiter, R.J.; Kleszczynski, K.; Slominski, A.T. Protective role of melatonin and its metabolites in skin aging. Int. J. Mol. Sci. 2022, 23, 1238. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Maldonado, M.D. Melatonin as an antioxidant: Physiology versus pharmacology. J. Pineal Res. 2005, 39, 215–216. [Google Scholar] [CrossRef]
- Jockers, R.; Maurice, P.; Boutin, J.A.; Delagrange, P. Melatonin receptors, heterodimerization, signal transduction and binding sites: What’s new? Br. J. Pharmacol. 2008, 154, 1182–1195. [Google Scholar] [CrossRef] [PubMed]
- Arendt, J. Melatonin and human rhythms. Chronobiol. Int. 2006, 29, 21–37. [Google Scholar] [CrossRef]
- Arangino, S.; Cagnacci, A.; Angiolucci, M.; Vacca, A.M.B.; Longu, G.; Volpe, A.; Melis, G.B. Effects of melatonin an vascular reactivity, catecholamine levels, and blood pressure in healthy men. Am. J. Cardiol. 1999, 83, 1417. [Google Scholar] [CrossRef]
- Cagnacci, A.; Arangino, S.; Angiolucci, M.; Maschio, E.; Melis, G.B. Influences of melatonin adminstration on the circulation of women. Am. J. Physiol. 1998, 274, R335–R338. [Google Scholar]
- Doolen, S.; Krause, D.N.; Dubocovich, M.L.; Duckles, S.P. Melatonin mediates two distinct responses in vascular smooth muscle. Eur. J. Pharmacol. 1998, 345, 67–76. [Google Scholar] [CrossRef]
- Iuvone, P.M.; Tosini, G.; Pozdeyev, N.; Haque, R.; Klein, D.C.; Chaurasia, S.S. Circadian clocks, clock networks, arylalkylamine N-acetyltransferase, and melatonin in the retina. Prog. Retin. Eye Res. 2005, 24, 433–456. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, K.; Yasue, H.; Moriyama, Y.; Tsunoda, R.; Ogawa, H.; Yoshimura, M.; Kugiyama, K. Acute effects of melatonin administration on cardiovascular autonomic regulation in healthy men. Am. Heart J. 2001, 141, E9. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.; Van Montfrans, G.A.; Van Someren, E.J.; Mairuhu, G.; Buijs, R.M. Daily night-time melatonin reduces blood pressure in male patients with essential hypertension. Hypertension 2004, 43, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Bubenik, G.A. Gastrointestinal melatonin: Localization, function, and clinical relevance. Dig. Dis. Sci. 2002, 47, 2336–2348. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R.; Esquifino, A.I.; Cardinali, D.P. The role of melatonin in immunoenhancement: Potential application in cancer. Int. J. Exp. Pathol. 2006, 87, 81–87. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Guerrero, J.M.; Lardone, P.J.; Reiter, R.J. A review of the multiple actions of melatonin on the immune system. Endocrine 2005, 27, 189–200. [Google Scholar] [CrossRef]
- Srinivasan, V.; Pandi-Perumal, S.R.; Maestroni, G.J.M.; Esquifino, A.I.; Hardeland, R.; Cardinali, D.P. Role of melatonin in neurodegenerative diseases. Neurotox. Res. 2005, 7, 293–318. [Google Scholar] [CrossRef]
- Regodon, S.; Martin-Palomino, P.; Fernadez-Montesinos, R.; Herrera, J.L.; Carrascosa-Salmoral, M.P.; Piriz, S.; Vadillo, S.; Guerrero, J.M.; Pozo, D. The use of melatonin as a vaccine agent. Vaccine 2005, 23, 5321–5327. [Google Scholar] [CrossRef]
- Hardeland, R.; Pandi-Perumal, S.R. Melatonin, a potent agent in antioxidative defense: Actions as a natural food constituent, gastrointestinal factor, drug and prodrug. Nutr. Metab. 2005, 2, 22. [Google Scholar] [CrossRef]
- Karbownik, M.; Lewinski, A.; Reiter, R.J. Anticarcinogenic actions of melatonin which involve antioxidative processes: Comparison with other antioxidants. Int. J. Biochem. Cell. Biol. 2001, 33, 735–753. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Xu, Y.; Isenberg, J.S.; Bachowski, S.; Kolaja, K.L.; Jiang, J.; Stevenson, D.E.; Walborg, E.F., Jr. The role of oxidative stress in chemical carcinogenesis. Environ. Health Perspect. 1998, 106, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Polimeni, G.; Esposito, E.; Bevelacqua, V.; Guarneri, C.; Cuzzocrea, S. Role of melatonin supplementation in neurodegenerative disorders. Front. Biosci. 2014, 19, 429–446. [Google Scholar] [CrossRef] [PubMed]
- Tauman, R.; Zisapel, N.; Laudon, M. Melatonin production in infants. Pediatr. Neurol. 2002, 26, 379–382. [Google Scholar] [CrossRef]
- Boeve, B.F.; Silber, M.H.; Ferman, T.J. Melatonin for treatment of REM sleep behavior disorder in neurologic disorders: Results in 14 patients. Sleep Med. 2003, 4, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, M. The Future of Psychiatry and Neurodevelopmental Disorders: A Paradigm Shift; Intech Open: London, UK, 2019; ISBN 978-1-78923-826-6. [Google Scholar]
- Morris-Rosendahl, D.; Crocq, M. Neurodevelopmental disorders—The history and future of a diagnostic concept. Dialogues Clin. Neurosci. 2020, 22, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Charrier, A.; Olliac, B.; Roubertoux, P.; Tordjman, S. Clock genes and altered sleep-wake rhythms: Their role in the development of psychiatric disorders. Int. J. Mol. Sci. 2017, 18, 938. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, S.; Cohen, D.; Anderson, G.M.; Botbol, M.; Coulon, N.; Roubertoux, P. Reframing autism as a behavioral syndrome and not a specific mental disorder: Implications of genetic and phenotypic heterogeneity. Neurosci. Biobehav. Rev. 2018, 89, 132–150. [Google Scholar] [CrossRef]
- Lalanne, S.; Fougerou-Leurent, C.; Anderson, G.M.; Schroder, C.; Nir, T.; Chokron, S.; Delorme, R.; Claustrat, B.; Bellissant, E.; Kermarrec, S.; et al. Melatonin: From pharmacokinetics to clinical use in autism spectrum disorder. Int. J. Mol. Sci. 2021, 22, 1490. [Google Scholar] [CrossRef]
- Kloeckner, A.; Justard, C.; Bullinger, A.; Nicoulaud, L.; Tordjman, S.; Cohen, D. Intérêt d l’abord sensorimoteur dans les pathologies autistiques sévères: Introduction aux travaux d’André Bullinger. Neuropsychol. Enf. Adolesc. 2009, 57, 154–159. [Google Scholar] [CrossRef]
- Tordjman, S.; Celume, M.P.; Denis, L.; Motillon, T.; Keromnes, G. Reframing schizophrenia and autism as bodily self-consciousness disorders leading to a deficit of theory of mind and empathy with social communication impairments. Neurosci. Biobehav. Rev. 2019, 103, 401–413. [Google Scholar] [CrossRef]
- Grandgeorge, M.; Hausberger, M.; Tordjman, S.; Deleau, M.; Lazartigues, A.; Lemonnier, E. Environmental factors influence language development in children with autism spectrum disorders. PLoS ONE 2009, 4, e4683. [Google Scholar] [CrossRef] [PubMed]
- Keromnes, G.; Chokron, S.; Celume, M.P.; Berthoz, A.; Botbol, M.; Canitano, L.; Du Boisgueheneuc, F.; Jaafari, N.; Lavenne-Collot, N.; Martin, B.; et al. Exploring self-consciousness from self- and other-image recognition in the mirror: Concepts and evaluation. Front. Psychol. 2019, 10, 719. [Google Scholar] [CrossRef] [PubMed]
- Benabou, M.; Rolland, T.; Leblond, C.S.; Millot, G.A.; Huguet, G.; Delorme, R.; Leboyer, M.; Pagan, C.; Callebert, J.; Maronde, E.; et al. Heritability of the melatonin synthesis variability in autism spectrum disorders. Sci. Rep. 2017, 7, 17746. [Google Scholar] [CrossRef] [PubMed]
- Maestro, S.; Casella, C.; Milone, A.; Muratori, F.; Palacio-Espasa, F. Study of the onset of autism through home movies. Psychopathology 1999, 32, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Saint-Georges, C.; Cassel, R.S.; Cohen, D.; Chetouani, M.; Laznik, M.C.; Maestro, S.; Muratori, F. What studies of family home movies can teach us about autistic infants: A literature review. Res. Autism Spectr. Disord. 2010, 4, 355–366. [Google Scholar] [CrossRef]
- Stern, D. Le Monde Interpersonnel du Nourrisson; PUF: Paris, France, 1989. [Google Scholar]
- Tordjman, S.; Najjar, I.; Bellissant, E.; Anderson, G.M.; Barburoth, M.; Cohen, D.; Jaafari, N.; Schischmanoff, O.; Fagard, R.; Lagdas, I.; et al. Advances in the research of melatonin in autism spectrum disorders: Literature review and new perspectives. Int. J. Mol. Sci. 2013, 14, 20508–20542. [Google Scholar] [CrossRef]
- Moon, E.; Kim, K.; Partonen, T.; Linnaranta, O. Role of melatonin in the management of sleep and circadian disorders in the context of psychiatric illness. Curr. Psychiatry Rep. 2022, 24, 623–634. [Google Scholar] [CrossRef]
- Fang, Y.; Zhao, C.; Xiang, H.; Zhao, X.; Zhong, R. Melatonin inhibits formation of mitochondrial permeability transition pores and improves oxidative phosphorylation of frozen-thawed ram sperm. Front. Endocrinol. 2020, 10, 896. [Google Scholar] [CrossRef]
- Nir, I.; Meir, D.; Zilber, N.; Knobler, H.; Hadjez, J.; Lerner, Y. Brief report: Circadian melatonin, thyroid-stimulating hormone, prolactin, and cortisol levels in serum of young adults with autism. J. Autism Dev. Disord. 1995, 25, 641–654. [Google Scholar] [CrossRef]
- Hu, V.W.; Sarachana, T.; Kim, K.S.; Nguyen, A.; Kulkarni, S.; Steinberg, M.E.; Luu, T.; Lai, Y.; Lee, N.H. Gene expression profiling differentiates autism case–controls and phenotypic variants of autism spectrum disorders: Evidence for circadian rhythm dysfunction in severe autism. Autism Res. 2009, 2, 78–97. [Google Scholar] [CrossRef]
- Tordjman, S.; Anderson, G.M.; Bellissant, E.; Botbol, M.; Charbuy, H.; Camus, F.; Graignic, R.; Kermarrec, S.; Fougerou, C.; Cohen, D.; et al. Day and nighttime excretion of 6-sulphatoxymelatonin in adolescents and young adults with autistic disorder. Psychoneuroendocrinology 2012, 37, 1990–1997. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, S.; Anderson, G.M.; Pichard, N.; Charbuy, H.; Touitou, Y. Nocturnal excretion of 6-sulphatoxymelatonin in children and adolescents with autistic disorder. Biol. Psychiatry 2005, 57, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Abdul, F.; Sreenivas, N.; Kommu, J.V.S.; Banerjee, M.; Berk, M.; Maes, M.; Leboyer, M.; Debnath, M. Disruption of circadian rhythm and risk of autism spectrum disorder: Role of immune-inflammatory, oxidative stress, metabolic and neurotransmitter pathways. Rev. Neurosci. 2021, 33, 93–109. [Google Scholar] [CrossRef]
- Bonnot, O.; Herrera, P.M.; Tordjman, S.; Walterfang, M. Secondary psychosis induced by metabolic disorders. Front. Neurosci. 2015, 9, 177. [Google Scholar] [CrossRef]
- Rose Meyer, R. A review of the serotonin transporter and prenatal cortisol in the development of autism spectrum disorders. Mol. Autism 2013, 4, 37. [Google Scholar] [CrossRef] [PubMed]
- Eslinger, P.J.; Anders, S.; Ballarini, T.; Boutros, S.; Krach, S.; Mayer, A.V.; Zahn, R. The neuroscience of social feelings: Mechanisms of adaptive social functioning. Neurosci. Biobehav. Rev. 2021, 128, 592–620. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.C.; McGavran, L.; Robinson, J.; Waldstein, G.; Macfarlane, J.; Zonona, J.; Reiss, J.; Lahr, M.; Allen, L.; Magenis, E. Interstitial deletion of (17) (P11.2p11.2) in nine patients. Am. J. Hum. Genet. 1986, 24, 393–414. [Google Scholar] [CrossRef]
- Vostanis, P.; Harrington, R.; Prendergast, M.; Farndon, P. Case reports of autism with interstitial deletion of chromosome 17 (p11.2 p11.2) and monosomy of chromosome 5 (5pter > 5p15.3). Psychiatr. Genet. 1994, 4, 109–111. [Google Scholar] [CrossRef]
- Rinaldi, B.; Villa, R.; Sironi, A.; Garavelli, L.; Finelli, P.; Bedeschi, M.F. Smith-Magenis Syndrome: Clinical Review, Biological Background and Related Disorders. Genes 2022, 13, 335. [Google Scholar] [CrossRef]
- Elsea, S.H.; Girirajan, S. Smith-Magenis syndrome. Eur. J. Hum. Genet. 2008, 16, 412–421. [Google Scholar] [CrossRef]
- GHR. Smith Magenis Syndrome. 2018. Available online: https://rarediseases.info.nih.gov/diseases/8197/smith-magenis-syndrome (accessed on 2 November 2023).
- Robert, C.; Pasquier, L.; Cohen, D.; Fradin, M.; Canitano, R.; Damaj, L.; Odent, S.; Tordjman, S. Role of genetics in the etiology of Autistic Spectrum Disorder: Towards a hierarchical diagnostic strategy. Int. J. Mol. Sci. 2017, 18, 618. [Google Scholar] [CrossRef] [PubMed]
- Gropman, A.L.; Duncan, W.C.; Smith, A.C.M. Neurologic and developmental features of the Smith-Magenis Syndrome (del 17p11.2). Pediatr. Neurol. 2006, 34, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Laje, G.; Morse, R.; Richter, W.; Ball, J.; Pao, M.; Smith, A.C.M. Autism spectrum features in smith-magenis syndrome. Am. J. Med. Genet. 2010, 154, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.C.M.; Dykens, E.; Greenberg, F. Behavioral Phenotype of Smith-Magenis Syndrome (del 17 p11.2). J. Med. Genet. Neuropsychiatry 1998, 81, 179–185. [Google Scholar] [CrossRef]
- De Leersnyder, H.; de Blois, M.C.; Claustrat, B.; Romana, S.; Albrecht, U.; von Kleist-Retzow, J.-C.; Delobel, B.; Viot, G.; Lyonnet, S.; Vekemans, M.; et al. Inversion of the circadian rhythm of melatonin in the Smith-Magenis syndrome. J. Pediatr. 2001, 139, 111–116. [Google Scholar] [CrossRef]
- De Leersnyder, H. Inverted rhythm of melatonin secretion in Smith-Magenis syndrome: From symptoms to treatment. Trends Endocrinol. Metab. 2006, 17, 291–298. [Google Scholar] [CrossRef]
- Chamberlain, S.J.; Chen, P.F.; Ng, K.Y.; Bourgois-Rocha, F.; Lemtiri-Chlieh, F.; Levine, E.S.; Lalande, M. Induced pluripotent stem cell models of the genomic imprinting disorders Angelman and Prader-Willi syndromes. Proc. Natl. Acad. Sci. USA 2010, 107, 17668–17673. [Google Scholar] [CrossRef]
- Mabb, A.M.; Judson, M.C.; Zylka, M.J.; Philpot, B.D. Angelman syndrome: Insights into genomic imprinting and neurodevelopmental phenotypes. Trends Neurosci. 2011, 34, 293–303. [Google Scholar] [CrossRef]
- Erickson, C.A.; Wink, L.K.; Baindu, B.; Ray, B.; Schaefer, T.L.; Pedapati, E.V.; Lahiri, D.K. Analysis of peripheral amyloid precursor protein in Angelman syndrome. Am. J. Med. Genet. 2016, 170, 2334–2337. [Google Scholar] [CrossRef]
- Li, W.; Yao, A.; Zhi, H.; Kaur, K.; Zhu, Y.; Jia, M.; Zhao, H.; Wang, Q.; Jin, S.; Zhao, G.; et al. Angelman syndrome protein Ube3a regulates synaptic growth and endocytosis by inhibiting BMP signaling in Drosophila. PLoS Genet. 2016, 12, e1006062. [Google Scholar] [CrossRef]
- Williams, C.A.; Driscoll, D.J.; Dagli, A.I. Clinical and genetic aspects of Angelman syndrome. Genet. Med. 2010, 12, 385–395. [Google Scholar] [CrossRef] [PubMed]
- NORD. Angelman Syndrome. 2018. Available online: https://rarediseases.org/rare-diseases/angelman-syndrome/ (accessed on 8 November 2023).
- Williams, C.A.; Beaudet, A.L.; Clayton-Smith, J.; Knoll, J.H.; Kyllerman, M.; Laan, L.A.; Magenis, E.; Moncla, A.; Schinzel, A.A.; Summers, J.A.; et al. Conference Report. Angelman Syndrome 2005: Updated consensus for diagnostic criteria. Am. J. Med. Genet. 2006, 140A, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Clayton-Smith, J. Clinical research on Angelman syndrome in the United Kingdom: Observations on 82 affected individuals. Am. J. Med. Genet. 1993, 46, 12–15. [Google Scholar] [CrossRef]
- Summers, J.A.; Allison, D.B.; Lynch, P.S.; Sandler, L. Behaviour problems in Angelman syndrome. J. Intellect. Disabil. Res. 1995, 39, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Walz, N.C. Parent report of stereotyped behaviors, social interaction, and developmental disturbances in individuals with Angelman syndrome. J. Autism Dev. Disord. 2007, 37, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Paprocka, J.; Kijonka, M.; Wojcieszek, P.; Pęcka, M.; Emich-Widera, E.; Sokół, M. Melatonin and Angelman syndrome: Implications and mathematical model of diurnal secretion. Int. J. Endocrinol. 2017, 2017, 5853167. [Google Scholar] [CrossRef]
- Braam, W.; Didden, R.; Smits, M.G.; Curfs, L.M.G. Melatonin for chronic insomnia in Angelman syndrome: A randomized placebo-controlled trial. J. Child Neurol. 2008, 23, 649–654. [Google Scholar] [CrossRef]
- Curatolo, P.; Bombardieri, R.; Jozwiak, S. Tuberous sclerosis. Lancet 2008, 372, 657–668. [Google Scholar] [CrossRef]
- GHR. Tuberous Sclerosis Complex. 2018. Available online: https://rarediseases.info.nih.gov/diseases/7830/tuberous-sclerosis (accessed on 17 November 2023).
- Staley, B.A.; Vail, E.A.; Thiele, E.A. Tuberous sclerosis complex: Diagnostic challenges, presenting symptoms, and commonly missed signs. Pediatrics 2011, 127, e117–e125. [Google Scholar] [CrossRef]
- Bolton, P.F.; Park, R.J.; Higgins, J.N.; Griffiths, P.D.; Pickles, A. Neuro-epileptic determinants of autism spectrum disorders in tuberous sclerosis complex. Brain 2002, 125, 1247–1255. [Google Scholar] [CrossRef]
- Muto, Y.; Sasaki, H.; Sumitomo, M.; Inagaki, H.; Kato, M.; Kato, T.; Miyai, S.; Kurahashi, H.; Shiroki, R. Genotype-phenotype correlation of renal lesions in the tuberous sclerosis complex. Hum. Genome Var. 2022, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Jeste, S.S.; Varcin, K.J.; Hellemann, G.S.; Gulsrud, A.C.; Bhatt, R.; Kasari, C.; Wu, J.Y.; Sahin, M.; Nelson, C.A. Symptom profiles of autism spectrum disorder in tuberous sclerosis complex. Neurology 2016, 87, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Bruni, O.; Cortesi, F.; Giannotti, F.; Curatolo, P. Sleep disorders in tuberous sclerosis: A polysomnographic study. Brain Dev. 1995, 17, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Hancock, E.; O’Callaghan, F.; English, J.; Osborne, J.P. Melatonin excretion in normal children and in tuberous sclerosis complex with sleep disorder responsive to melatonin. J. Child Neurol. 2005, 20, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Hancock, E.; O’Callaghan, F.; Osborne, J.P. Effect of melatonin dosage on sleep disorder in tuberous sclerosis complex. J. Child Neurol. 2005, 20, 78–80. [Google Scholar] [CrossRef]
- Amir, R.E.; Van Den Veyver, I.B.; Wan, M.; Tran, C.Q.; Francke, U.; Zoghbi, H.Y. Rett syndrome is cause by mutations in X-linked MECP2, encoding methil-CpG-binding protein 2. Nat. Genet. 1999, 23, 185–188. [Google Scholar] [CrossRef]
- Gadalla, K.; Bailey, M.E.; Cobb, S.R. MeCP2 and Rett syndrome: Reversibility and potential avenues for therapy. Biochem. J. 2011, 439, 1–14. [Google Scholar] [CrossRef]
- NORD. Rett Syndrome. 2015. Available online: https://rarediseases.org/rare-diseases/rett-syndrome/ (accessed on 8 November 2023).
- Richards, C.; Jones, C.; Groves, L.; Moss, J.; Oliver, C. Prevalence of autism spectrum disorder phenomenology in genetic disorders: A systematic review and meta-analysis. Lancet Psychiatry 2015, 2, 909–916. [Google Scholar] [CrossRef]
- Ramocki, M.B.; Peters, S.U.; Tavyev, Y.J.; Zhang, F.; Carvalho, C.M.; Schaaf, C.P.; Richman, R.; Fang, P.; Glaze, D.G.; Lupski, J.R.; et al. Autism and other neuropsychiatric symptoms are prevalent in individuals with MeCP2 duplication syndrome. Ann. Neurol. 2009, 66, 771–782. [Google Scholar] [CrossRef]
- Young, D.; Nagarajan, L.; De Klerk, N.; Jacoby, P.; Ellaway, C.; Leonard, H. Sleep problems in Rett syndrome. Brain Dev. 2007, 29, 609–616. [Google Scholar] [CrossRef]
- Miyamoto, A.; Oki, J.; Takahashi, S.; Okuno, A. Serum melatonin kinetics and long-term melatonin treatment for sleep disorders in Rett syndrome. Brain Dev. 1999, 21, 59–62. [Google Scholar] [CrossRef]
- McArthur, A.; Budden, S. Sleep dysfunction in Rett syndrome: A trial of exogenous melatonin treatment. Dev. Med. Child Neurol. 1998, 40, 186–192. [Google Scholar] [CrossRef] [PubMed]
- GHR. Williams Beuren Syndrome. 2018. Available online: https://rarediseases.info.nih.gov/espanol/13102/sindrome-de-williams (accessed on 12 November 2023).
- Edelmann, L.; Prosnitz, A.; Pardo, S.; Bhatt, J.; Cohen, N.; Lauriat, T.; Ouchanov, L.; González, P.J.; Manghi, E.R.; Bondy, P.; et al. An atypical deletion of the Williams-Beuren syndrome interval implicates genes associated with defective visuospatial processing and autism. J. Med. Genet. 2007, 44, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Sadler, L.; O’Riordan, M.A.; Robin, N.H. Delay in diagnosis of Williams syndrome. Clin. Pediatr. 2002, 41, 257–261. [Google Scholar] [CrossRef]
- Morris, C.; Janssens, A.; Shilling, V.; Allard, A.; Fellowes, A.; Tomlinson, R.; Williams, J.; Coon, J.T.; Rogers, M.; Beresford, B.; et al. Meaningful health outcomes for paediatric neurodisability: Stakeholder prioritisation and appropriateness of patient reported outcome measures. Health Qual. Life Outcomes 2015, 13, 87. [Google Scholar] [CrossRef]
- Gagliardi, C.; Bonaglia, M.C.; Selicorni, A.; Borgatti, R.; Giorda, R. Unusual cognitive and behavioural profile in a Williams syndrome patient with atypical 7q11.23 deletion. J. Med. Genet. 2003, 40, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, A.J.; Searcy, Y.M.; Jones, W.; Lord, C. Social interaction behaviors discriminate young children with autism and Williams syndrome. J. Am. Acad. Child Adolesc. Psychiatry 2007, 46, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, S.; Anderson, G.M.; Botbol, M.; Toutain, A.; Sarda, P.; Carlier, M.; Saugier-Veber, P.; Baumann, C.; Cohen, D.; Lagneaux, C.; et al. Autistic disorder in patients with Williams-Beuren syndrome: A reconsideration of the Williams-Beuren syndrome phenotype. PLoS ONE 2012, 7, e30778. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, S.; Anderson, G.M.; Cohen, D.; Kermarrec, S.; Carlier, M.; Touitou, Y.; Saugier-Veber, P.; Lagneaux, C.; Chevreuil, C.; Verloes, A. Presence of autism, hyperserotonemia, and severe expressive language impairment in Williams-Beuren syndrome. Mol. Autism 2013, 4, 29. [Google Scholar] [CrossRef]
- Martens, M.A.; Wilson, S.J.; Reutens, D.C. Research Review: Williams syndrome: A critical review of the cognitive, behavioral, and neuroanatomical phenotype. J. Child Psychol. Psychiatry 2008, 49, 576–608. [Google Scholar] [CrossRef]
- Santoro, S.D.; Giacheti, C.M.; Rossi, N.F.; Guissoni Campos, L.M.; Pinato, L. Correlations between behavior, memory, sleep-wake and melatonin in Williams-Beuren syndrome. Physiol. Behav. 2016, 159, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Sniecinska-Cooper, A.M.; Iles, R.K.; Butler, S.A.; Jones, H.; Bayford, R.; Dimitriou, D. Abnormal secretion of melatonin and cortisol in relation to sleep disturbances in children with Williams syndrome. Sleep Med. 2015, 16, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Sniecinska-Cooper, A.M.; Iles, R.K.; Butler, S.A.; Jones, H.; Bayford, R.; Dimitriou, D. Response to the letter “Sleep characteristics of children with Williams syndrome in relation to saliva melatonin and cortisol”. Sleep Med. 2015, 16, 1177. [Google Scholar] [CrossRef] [PubMed]
- Martens, M.A.; Seyfer, D.L.; Andridge, R.R.; Coury, D.L. Use and effectiveness of sleep medications by parent report in individuals with Williams syndrome. J. Dev. Behav. Pediatr. 2017, 38, 765–771. [Google Scholar] [CrossRef]
- Reilly, C.; Senior, J.; Murtagh, L. ASD, ADHD, mental health conditions and psychopharmacology in neurogenetic syndromes: Parent survey. J. Intellect. Disabil. Res. 2015, 59, 307–318. [Google Scholar] [CrossRef]
- Farrow, T.F.D.; Whitford, T.J.; Williams, L.M.; Gomes, L.; Harris, A.W.F. Diagnosis-related regional gray matter loss over two years in first episode of schizophrenia and bipolar disorder. Biol. Psychiatry 2005, 58, 713–723. [Google Scholar] [CrossRef]
- Nath, M.; Wong, T.P.; Srivastava, L.K. Neurodevelopmental insights into circuit dysconnectivity in schizophrenia. Biol. Psychiatry 2021, 104, 110047. [Google Scholar] [CrossRef]
- Schaefer, J.; Giangrande, E.; Weinberger, D.R.; Dickinson, D. The global cognitive impairment in schizophrenia: Consistent over decades and around the world. Schizo. Res. 2013, 150, 42–50. [Google Scholar] [CrossRef]
- Morera-Fumero, A.L.; Abreu-Gonzalez, P. Role of melatonin in schizophrenia. Int. J. Mol. Sci. 2013, 14, 9037–9050. [Google Scholar] [CrossRef]
- Monteleone, P.; Natale, M.; La Rocca, A.; Maj, M. Decreased nocturnal secretion of melatonin in drug-free schizophrenics: No change after subchronic treatment with antipsychotics. Neuropsychobiology 1997, 36, 159–163. [Google Scholar] [CrossRef]
- Rao, M.L.; Gross, G.; Strebel, B.; Halaris, A.; Huber, G.; Bräunig, P.; Marler, M. Circadian rhythm of tryptophan, serotonin, melatonin, and pituitary hormones in schizophrenia. Biol. Psychiatry 1994, 35, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Galván-Arrieta, T.; Trueta, C.; Cercós, M.G.; Valdés-Tovar, M.; Alarcón, S.; Oikawa, J.; Zamudio-Meza, H.; Benítez-King, G. The role of melatonin in the neurodevelopmental etiology of schizophrenia: A study in human olfactory neuronal precursors. J. Pineal Res. 2017, 63, e12421. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Mee, T.J.; Padwick, D.J.; Spokes, E.G. Human post-mortem pineal enzyme activity. Clin. Endocrinol. 1981, 14, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Petursson, H.; Lader, M.H. Withdrawal from long-term benzodiazepine treatment. Br. Med. J. 1981, 283, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, H.; Wetterberg, L.; Gattaz, W.F. Melatonin immunoreactivity in cerebrospinal fluid of schizophrenic patients and healthy controls. Psychiatry Res. 1984, 11, 107–110. [Google Scholar] [CrossRef]
- Kloiber, S.; Rosenblat, J.D.; Husain, M.I.; Ortiz, A.; Berk, M.; Quevedo, J.; Vieta, E.; Maes, M.; Birmaher, B.; Soares, J.C.; et al. Neurodevelopmental pathways in bipolar disorder. Neurosci. Biobehav. Rev. 2020, 112, 213–226. [Google Scholar] [CrossRef]
- Leboyer, M.; Kupfer, D.J. Bipolar disorder: New perspectives in health care and prevention. J. Clin. Psychiatry 2010, 71, 1689–1695. [Google Scholar] [CrossRef]
- Salvatore, P.; Ghidini, S.; Zita, G.; De Panfilis, C.; Lambertino, S.; Maggini, C.; Baldessarini, R.J. Circadian activity rhythm abnormalities in ill and recovered bipolar I disorder patients. Bipolar Disord. 2008, 10, 256–265. [Google Scholar] [CrossRef]
- Lewy, A.J. Circadian misalignment in mood disturbances. Curr. Psychiatry Rep. 2009, 11, 459–465. [Google Scholar] [CrossRef]
- Gonzalez, R.; Tamminga, C.A.; Tohen, M.; Suppes, T. The relationship between affective state and the rhythmicity of activity in bipolar disorder. J. Clin. Psychiatry 2014, 75, 317–322. [Google Scholar] [CrossRef]
- López-Munoz, F.; Molina, J.D.; Rubio, G.; Alamo, C. An historical view of the pineal gland and mental disorders. J. Clin. Neurosci. 2011, 18, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R.; Moscovitch, A.; Srinivasan, V.; Spence, D.W.; Cardinali, D.P.; Brown, G.M. Bidirectional communication between sleep and circadian rhythms and its implications for depression: Lessons from agomelatine. Prog. Neurobiol. 2009, 88, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Savino, R.; Polito, A.N.; Marsala, G.; Ventriglio, A.; Di Salvatore, M.; De Stefano, M.I.; Valenzano, A.; Marinaccio, L.; Bellomo, A.; Cibelli, G.; et al. Agomelatine: A potential multitarget compound for neurodevelopmental disorders. Brain Sci. 2023, 13, 734. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, S.; Davlantis, K.S.; Georgieff, N.; Geoffray, M.M.; Speranza, M.; Anderson, G.M.; Xavier, J.; Botbol, M.; Oriol, C.; Bellissant, E. Autism as a disorder of biological and behavioral rhythms: Toward new therapeutic perspectives. Front. Pediatr. 2015, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Lamont, E.W.; Legault-Coutu, D.; Cermakian, N.; Boivin, D.B. The role of circadian clock genes in mental disorders. Dialogues Clin. Neurosci. 2007, 9, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.W.; McClung, C.A. Rhythms of life: Circadian disruption and brain disorders across the lifespan. Nat. Rev. Neurosci. 2019, 20, 49–65. [Google Scholar] [CrossRef]
- Murray, R.M.; Lewis, S.W. Is schizophrenia a neurodevelopmental disorder? Br. Med. J. 1987, 295, 681–682. [Google Scholar] [CrossRef]
- Owen, M.J.; O’Donovan, M.C.; Thapar, A.; Craddock, N. Neurodevelopmental hypothesis of schizophrenia. Br. J. Psychiatry 2011, 198, 173–175. [Google Scholar] [CrossRef]
- Rutter, M.; Kim-Cohen, J.; Maughan, B. Continuities and discontinuities in psychopathology between childhood and adult life. J. Child Psychol. Psychiatry 2006, 47, 276–295. [Google Scholar] [CrossRef]
- Stahlberg, O.; Soderstrom, H.; Rastam, M.; Gillberg, C. Bipolar disorder, schizophrenia, and other psychotic disorders in adults with childhood onset AD/HD and/or autism spectrum disorders. J. Neural. Transm. 2004, 111, 891–902. [Google Scholar] [CrossRef]
- Ayano, G.; Maravilla, J.C.; Alati, R. Risk of autistic spectrum disorder in offspring with parental mood disorders: A systematic review and meta-analysis. J. Affect. Disord. 2019, 248, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Ripke, S.; Neale, B.M.; Faraone, S.V.; Purcell, S.M.; Perlis, R.H.; Mowry, B.J.; Thapar, A.; Goddard, M.E.; Witte, J.S. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat. Genet. 2013, 45, 984–994. [Google Scholar] [CrossRef]
- Cannon, M.; Caspi, A.; Moffitt, T.E.; Harrington, H.; Taylor, A.; Murray, R.M.; Poulton, R. Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: Results from a longitudinal birth cohort. Arch. Gen. Psychiatry 2002, 59, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Lipska, B.K.; Jaskiw, G.E.; Weinberger, D.R. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: A potential animal model of schizophrenia. Neuropsychopharmacology 1993, 9, 67–75. [Google Scholar] [CrossRef]
- Singh, T.; Walters, J.T.; Johnstone, M.; Curtis, D.; Suvisaari, J.; Torniainen, M.; Rees, E.; Lyzgbe, C.; Blackwood, D.; Mclntosh, A.; et al. The contribution of rare variants to risk of schizophrenia in individuals with and without intellectual disability. Nat. Genet. 2017, 49, 1167–1173. [Google Scholar] [CrossRef]
- Le Roy, I.; Roubertoux, P.L.; Jamot, L.; Maarouf, F.; Tordjman, S.; Mortaud, S.; Blanchard, C.; Martin, B.; Guillot, P.V.; Duquenne, V. Neuronal and behavioral differences between Mus musculus domesticus (C57BL/6JBy) and Mus musculus castaneus (CAST/Ei). Behav. Brain Res. 1998, 95, 135–142. [Google Scholar] [CrossRef]
- Walker, W.H.; Walton, J.C.; DeVries, A.C.; Nelson, R.J. Circadian rhythm disruption and mental health. Transl. Psychiatry 2020, 10, 28. [Google Scholar] [CrossRef]
| Neurogenetic Disorder | Neurogenetic Disorder Frequency, Estimated Rate (%) of Autism in the Disorder, and Estimated Rate (%) of the Disorder in Autism | Age of Diagnosis | Phenotype (Including Autistic Behaviors and Intellectual Functioning) | Sleep Problems | Melatonin Abnormality | Response to Melatonin Therapy |
|---|---|---|---|---|---|---|
| Smith-Magenis syndrome (SMS) Chromosome 17p11.2 microdeletion encompassing retinoic acid-induced 1 (RAI1) or a mutation in the RAI1 gene [79,80,81,82] |
| Many of the features of SMS are subtle in infancy and early childhood and become more recognizable with advancing age. Despite increased clinical awareness of SMS as well as improved cytogenetic technologies, many children are not definitively diagnosed until early childhood or even school age [85] | Facial dysmorphism, peripheral neuropathy, hypotonia, early feeding problems. Tantrums, self-injurious and stereotyped behaviors, sameness, developmental delay in vocalizations but possible social contact. Normal intellectual functioning to moderate intellectual disability [84,86,87] | Disrupted sleep patterns with shortened sleep cycles are characteristic of SMS and begin typically during the months after birth. Reports of excessive daytime sleepiness, increased sleep latency, frequent nocturnal and early morning awakenings due to an inverted circadian rhythm of melatonin [88] | Inverted circadian rhythm of melatonin secretion [88] | Melatonin therapy is used to regulate sleep problems. Combined with exogenous PRM (prolonged release melatonin), blockade of endogenous melatonin production during the day by the adrenergic antagonist acebutolol can improve impaired sleep and behaviors, and increase melatonin concentrations [89] Patients aged 3–18 years were given PRM (4 to 6 mg/day) as a single evening dose over a treatment duration of 6–72 months. Within 3 months, parents report improvement in sleep duration, sleep latency, number of midnight awakenings and sleep quality. No serious adverse events [88] |
| Angelman syndrome (AS) Maternal 15q11-q13 deletion, paternal uniparental disomy, mutations of UBE3A that encodes ubiquitin protein ligase (UBE3A) [90,91,92,93,94] |
| Developmental delays, between about 6 and 12 months of age, are usually the first signs, and seizures begin often between the age of 2 and 3 years old [96] | Facial dysmorphism, microcephaly, seizures (>1 year), ataxia and walking disturbance, Attention Deficit with Hyperactivity Disorder (ADHD), paroxysmal laughter, tantrums No language, stereotypies, sameness. Severe intellectual disability [84,96,97,98,99,100] | Severe sleep disturbances are common in Angelman syndrome, and are included in the diagnostic criteria [96] | The melatonin secretion profile of patients with Angelman syndrome is impaired, leading to a variety of sleep problems, most prominently in the areas of sleep-wake patterns and sleep duration [100] | Melatonin therapy significantly advanced sleep onset by 28 min, decreased sleep latency by 32 min, increased total sleep time by 56 min, and reduced the number of nights with awakenings from 3.1 to 1.6 nights per week [101] |
| Tuberous sclerosis complex (TSC), synonym: Bourneville disease (TSC1, 9q34) (TSC2, 16p13.3) Pathogenic variants in TSC1 and TSC2 genes: 31% and 69%, respectively [102] |
| The average age at diagnosis of TSC is 7.5 years with 81% of patients diagnosed before the age of 10. Diagnosis may be difficult because symptoms are not present in all patients, and none are pathognomonic [104] | Autosomal dominant neurocutaneous disorder with ectodermal anomalies clinically diagnosed, renal lesions, seizures, learning disorder. Severe autistic syndrome. Variable intellectual disability [84,103,105,106,107] | Sleep problems are considered one of the most common behavioral manifestations in children with TSC [108] | Significant differences between TSC melatonin secretion profiles and control ones. Melatonin rhythm but not its amplitude was related to the total number of seizures [109] | Treatment improvement in sleep latency, total sleep time, and sleep fragmentation reported with melatonin at 5 mg dose [110] |
| Rett’s syndrome (RS) Mutation in the MECP2 gene coding for the methyl CpG binding protein 2 and located at Xq28 [111,112] |
| Because of the apparent normal developmental course in early childhood, diagnosis may be delayed [116] | Developmental course: - Stagnation stage in girls (6–18 months); - Regression stage (12–36 months) with head growth deceleration, appearance of progressive motor symptoms (gait and truncal apraxia, ataxia, decreasing mobility) and respiratory symptoms (hyperventilation, breath holding, apnea); - Pseudo-stationary stage (2–10 years); - Late motor deterioration (>10 years). Autistic behaviors: stereotyped hand movements, absence of language, loss of social engagement. Severe intellectual Disability [84] | Sleep problems are common in Rett’s syndrome but there is some variation with age and mutation type [116] | Impaired secretion of melatonin [117] | Exogenous melatonin improved the sleep-wake cycle and sleep onset. The effect was maintained over 2 years without any adverse effects [117,118] |
| Williams Beuren syndrome (WBS) 7q11.23 deletion including 26 to 28 genes (typically CLIP2, ELN, GTF2I, GTF2IRD1, and LIMK1) [119,120] |
| The mean age at initial concerns is 0.98 year (Standard Deviation: 1.24), and the mean age at diagnosis may be delayed to 3.66 years (Standard Deviation: 4.13) [121] | Facial dysmorphism, short stature, heart and endocrine malformations, hypercalcemia, feeding problems, hyperacusis, visual spatial deficit, risk for attention deficit. Autistic syndrome but overfriendliness with social disinhibition and overtalkativeness. Mild to moderate intellectual disability [122,123,124,125,126,127] | Sleep disorders are common in individuals with Williams syndrome [128] | The WBS group had shallower drops in cortisol and less pronounced increase in melatonin at bedtime compared to the control group [129,130] | Melatonin was the most frequently reported medication taken for sleep problems in WBS, with 91% of parents reporting benefits for their child with WBS, and very few, if any, side effects [131,132] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feybesse, C.; Chokron, S.; Tordjman, S. Melatonin in Neurodevelopmental Disorders: A Critical Literature Review. Antioxidants 2023, 12, 2017. https://doi.org/10.3390/antiox12112017
Feybesse C, Chokron S, Tordjman S. Melatonin in Neurodevelopmental Disorders: A Critical Literature Review. Antioxidants. 2023; 12(11):2017. https://doi.org/10.3390/antiox12112017
Chicago/Turabian StyleFeybesse, Cyrille, Sylvie Chokron, and Sylvie Tordjman. 2023. "Melatonin in Neurodevelopmental Disorders: A Critical Literature Review" Antioxidants 12, no. 11: 2017. https://doi.org/10.3390/antiox12112017
APA StyleFeybesse, C., Chokron, S., & Tordjman, S. (2023). Melatonin in Neurodevelopmental Disorders: A Critical Literature Review. Antioxidants, 12(11), 2017. https://doi.org/10.3390/antiox12112017






