Impact of Chlorogenic Acid on Peripheral Blood Mononuclear Cell Proliferation, Oxidative Stress, and Inflammatory Responses in Racehorses during Exercise
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Blood Sampling
2.2. Chlorogenic Acid
2.3. Cell Isolation and Culture
2.4. Cell Staining
2.5. Flow Cytometry Analysis
2.6. ELISA
2.7. Statistical Analysis
3. Results
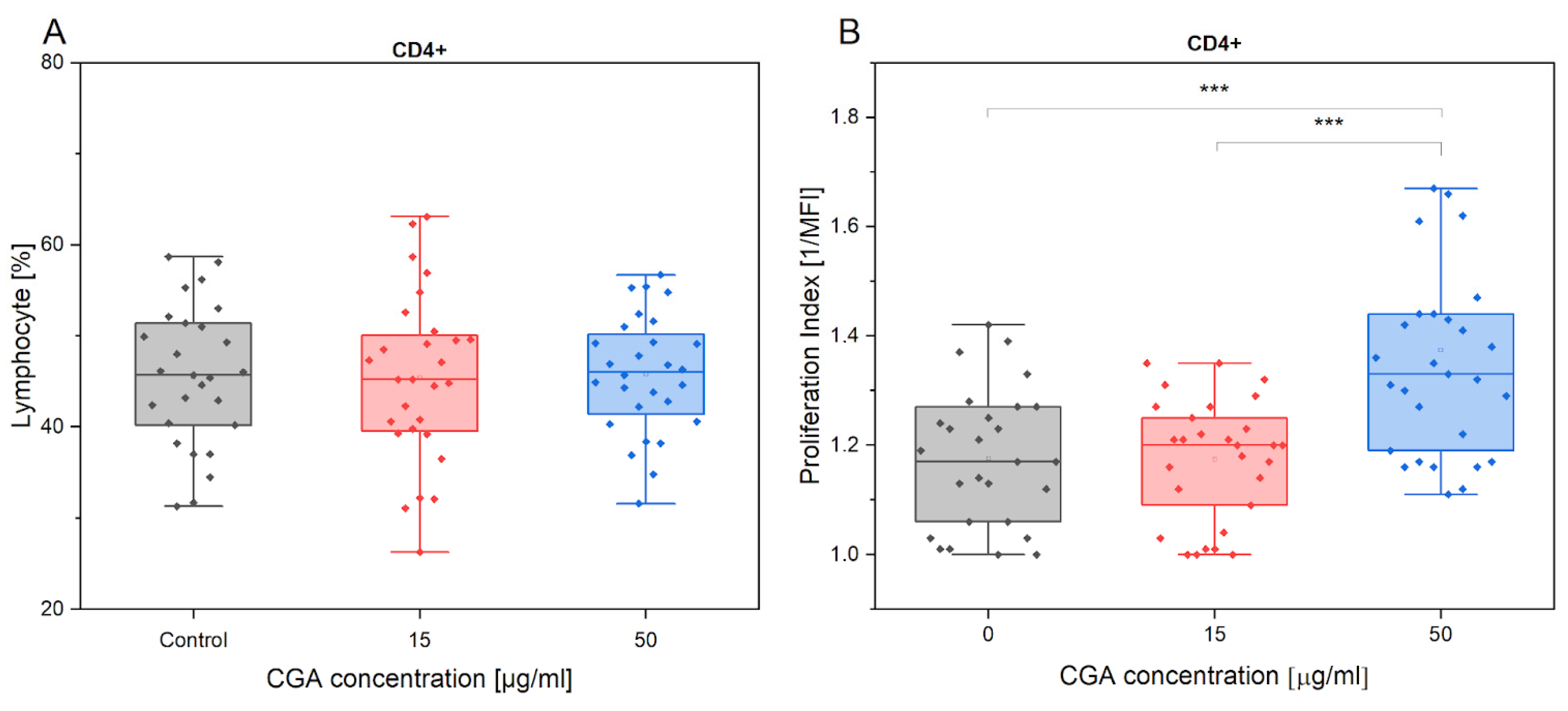
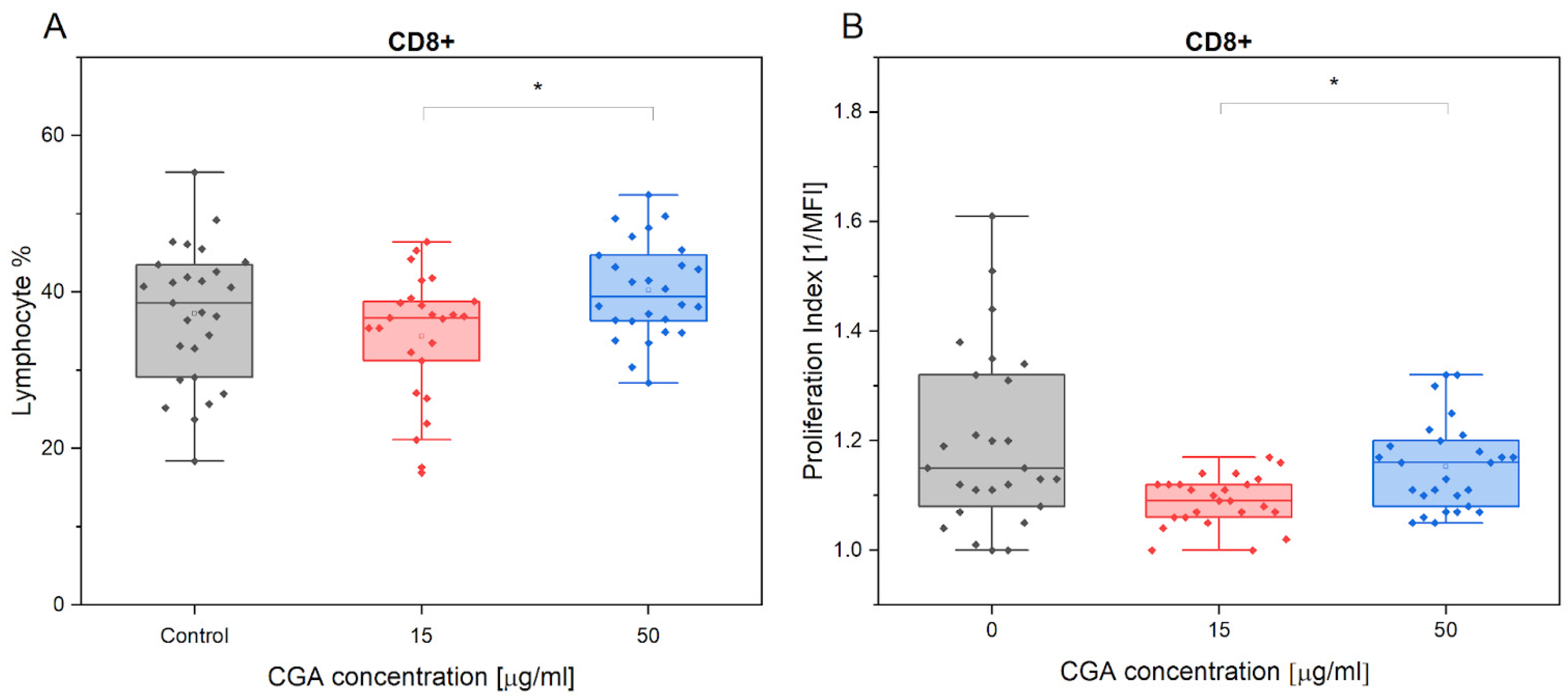
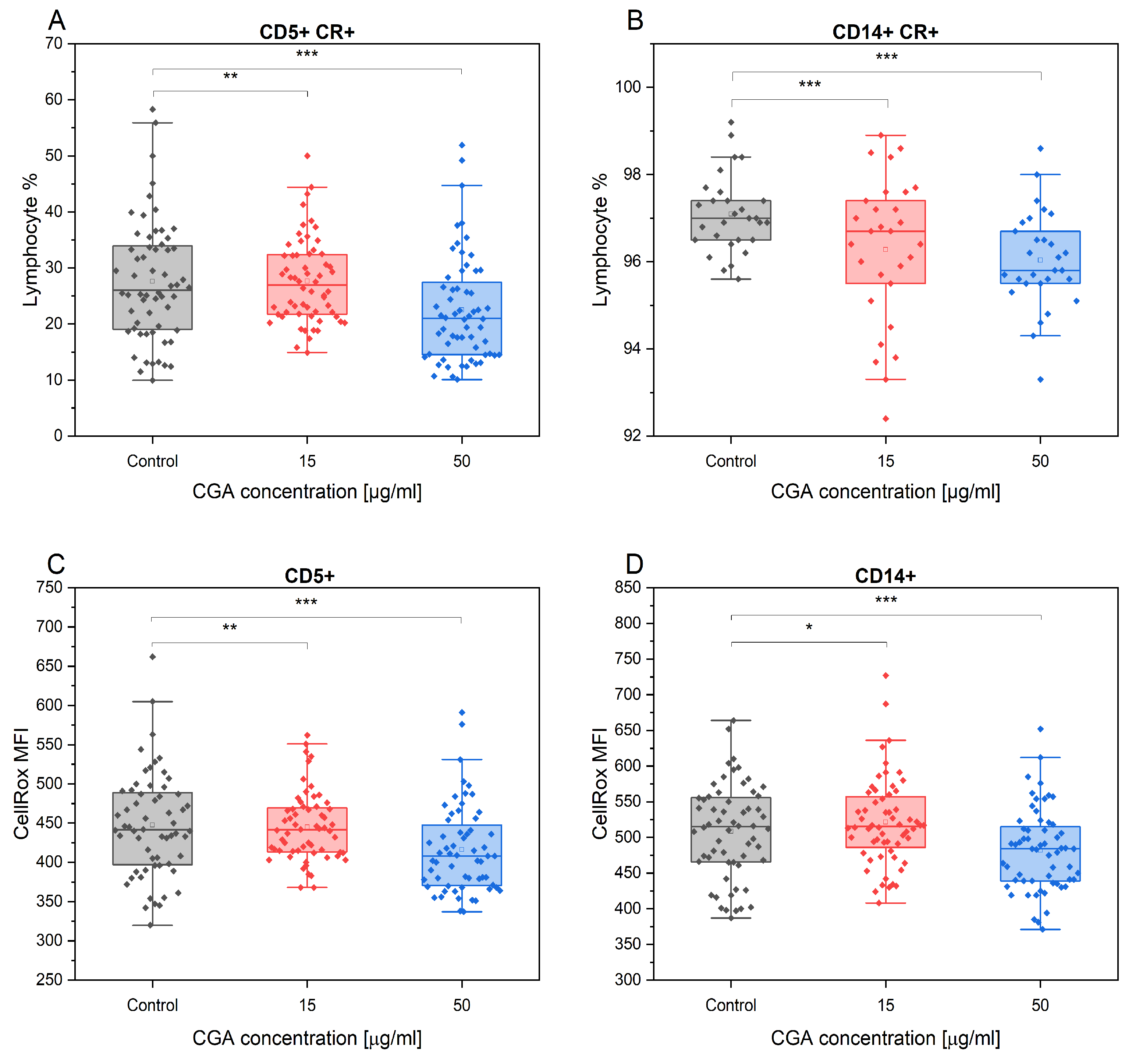
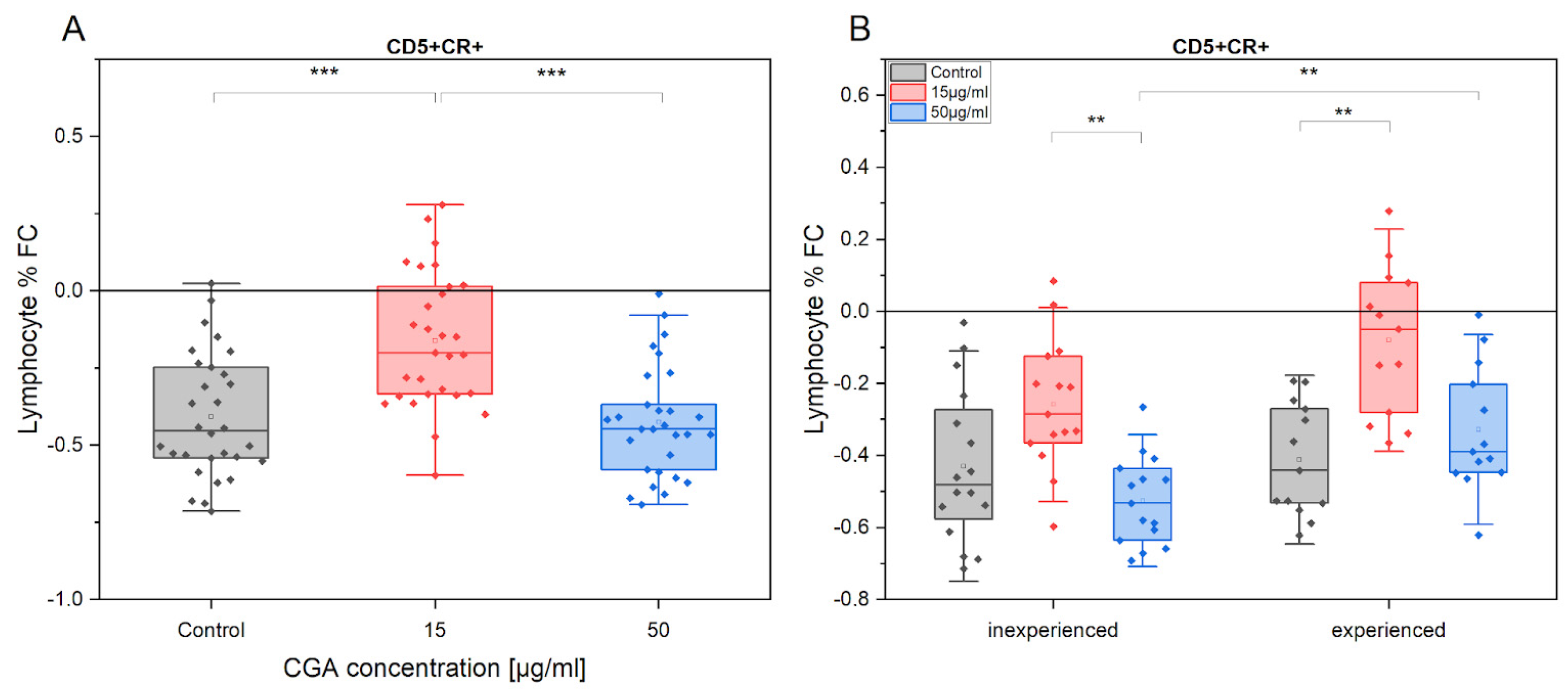
3.1. Chlorogenic Acid Effect on Lymphocyte Phenotype and Proliferation
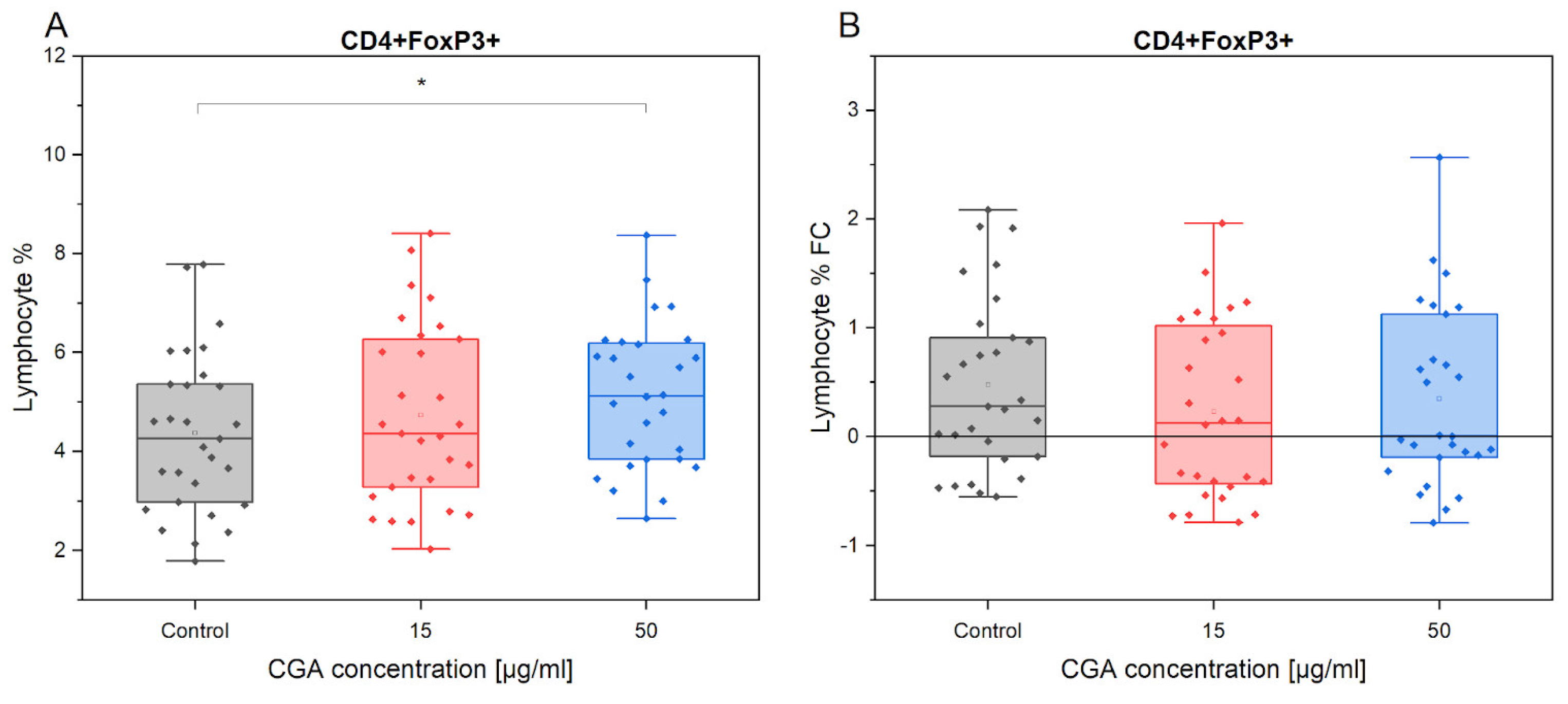
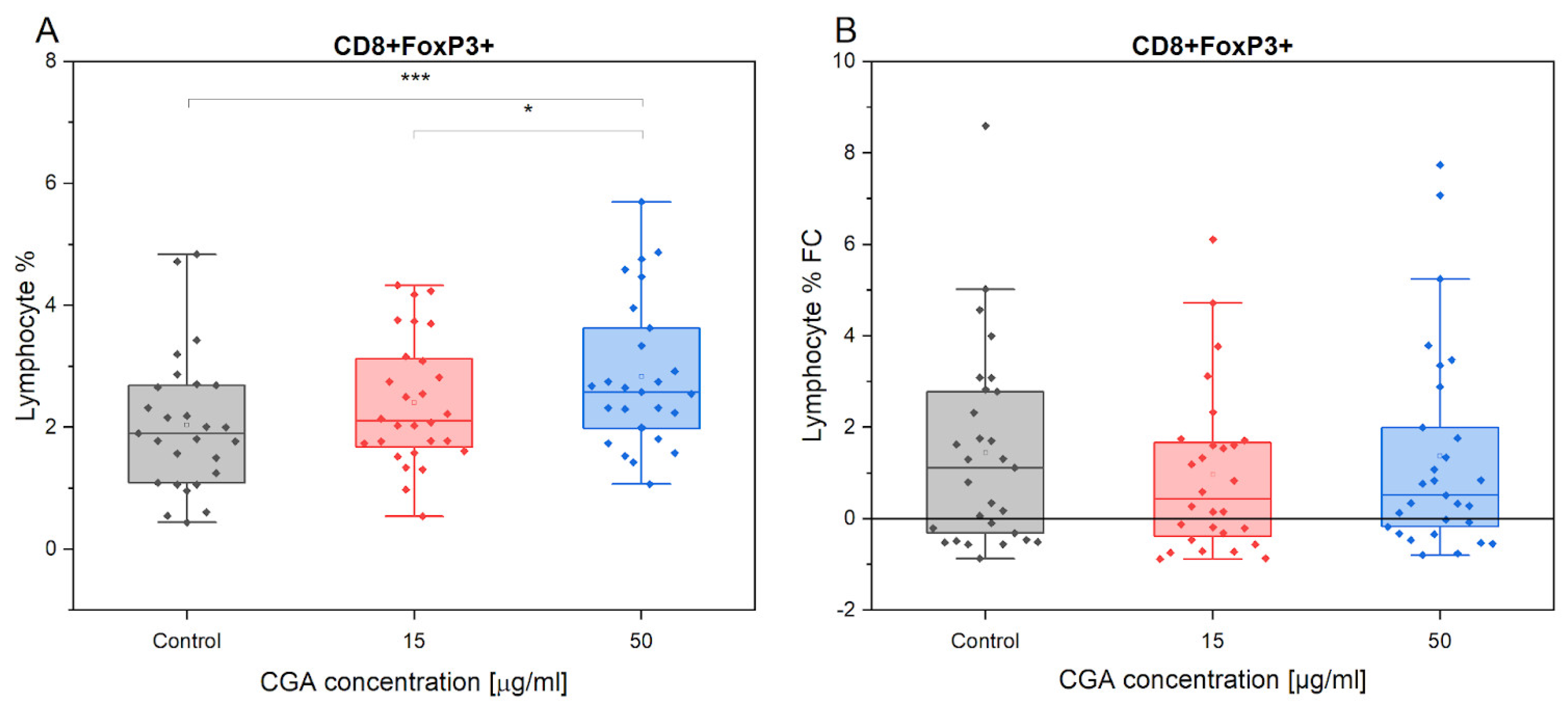
3.2. Chlorogenic Acid Effect on T Regulatory Cells
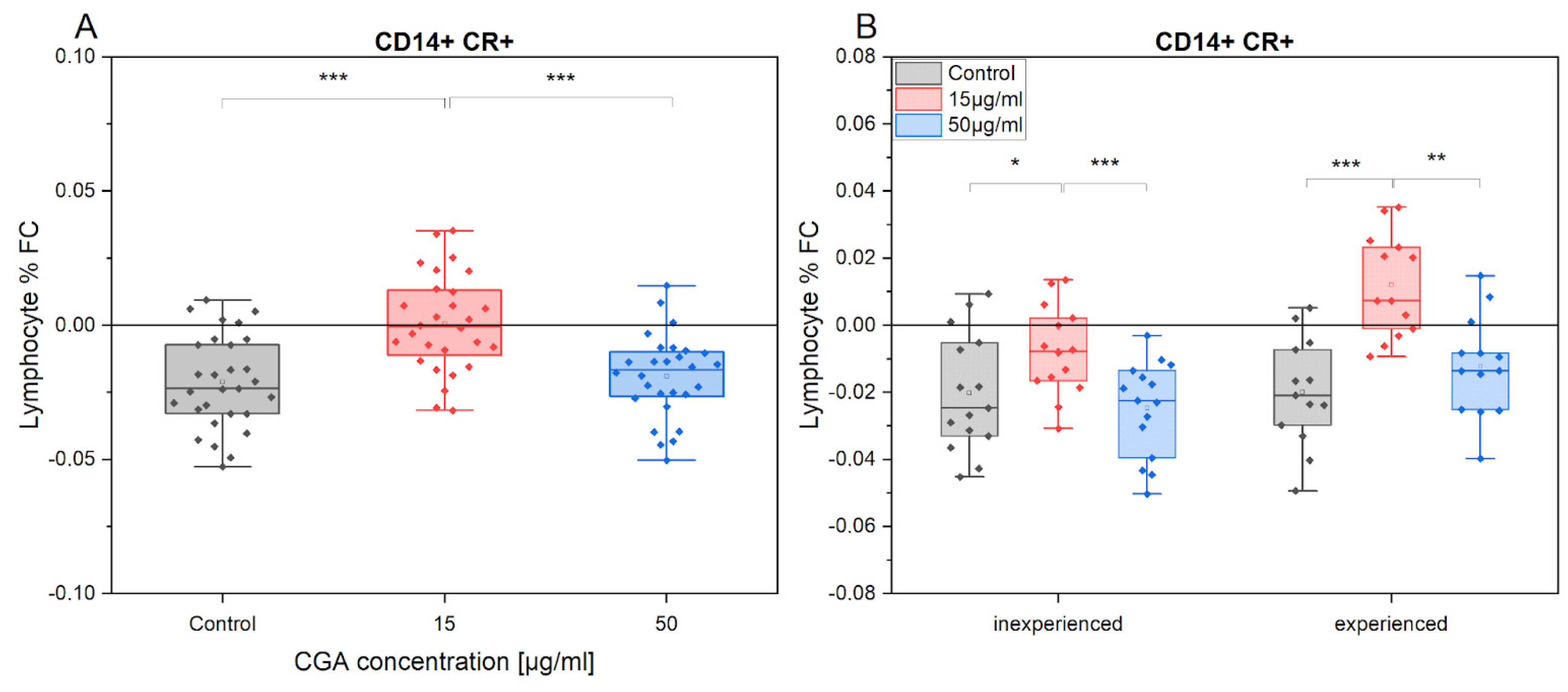
3.3. Chlorogenic Acid Effect on Monocyte Phenotype
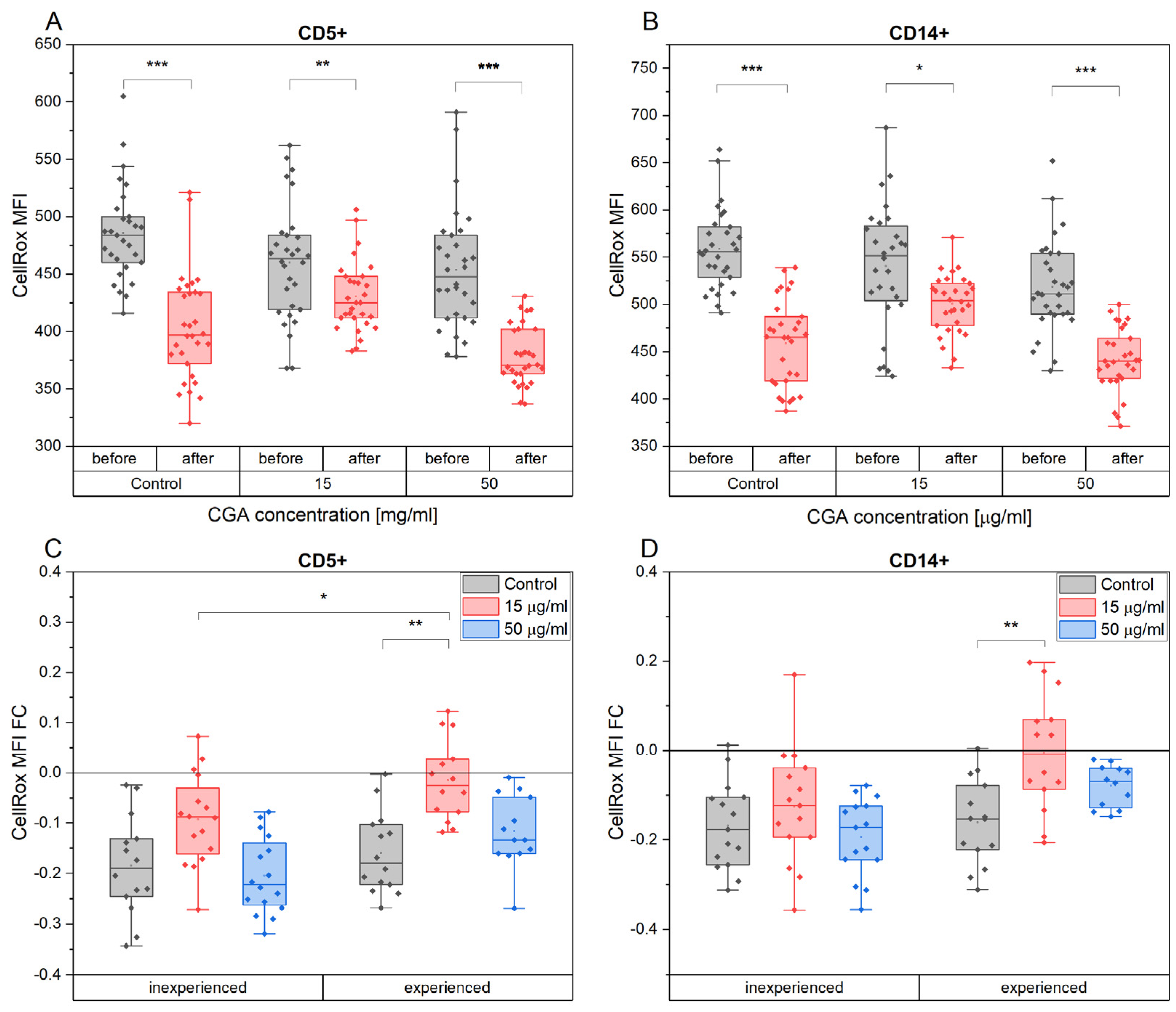
3.4. Chlorogenic Acid Effect on ROS Production in Monocytes and Lymphocytes
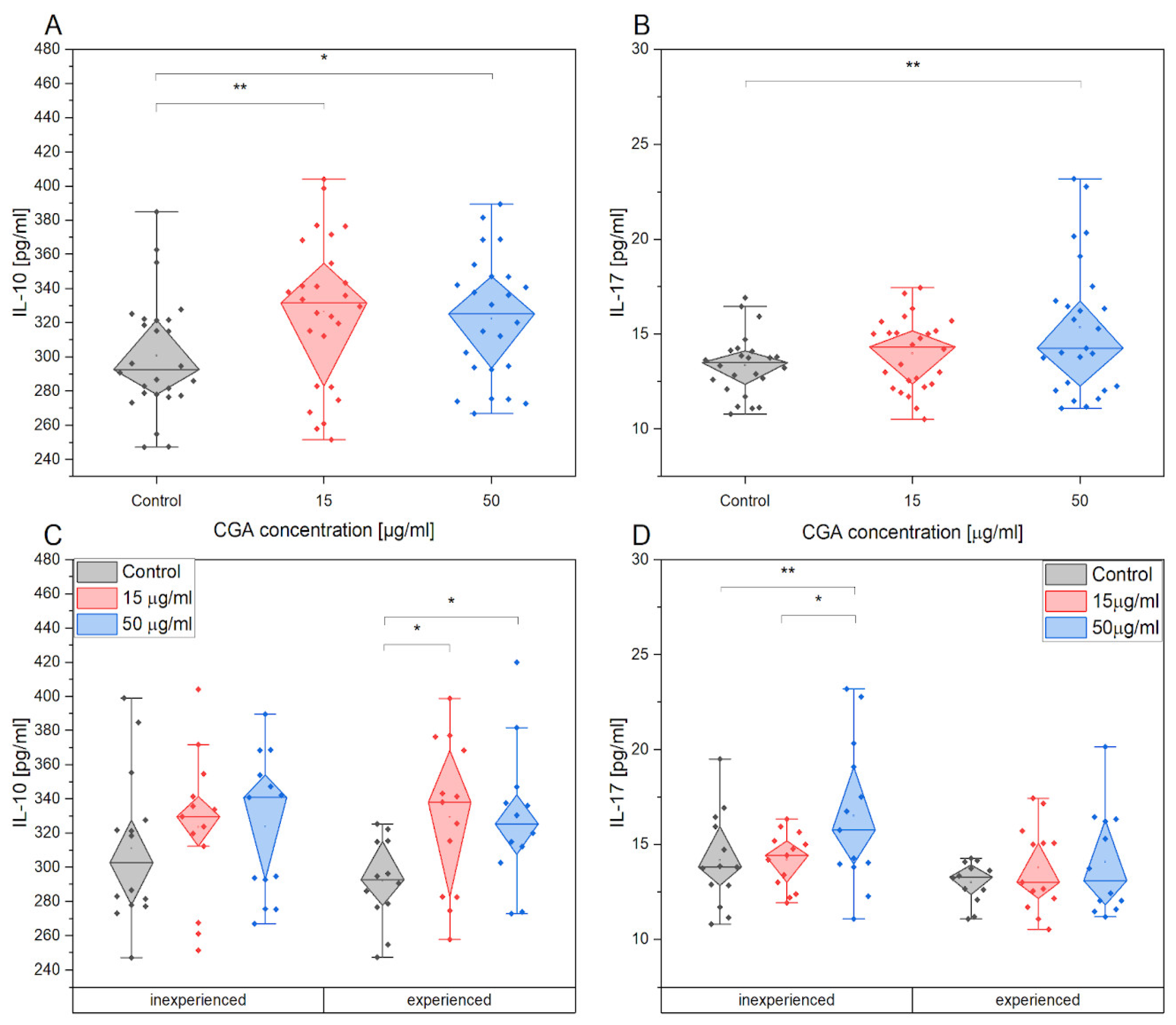
3.5. Chlorogenic Acid Effect on PBMCs Cytokine Production after Training
4. Discussion
4.1. Lymphocyte Phenotype and Proliferation
4.2. ROS Production by T Lymphocytes and Monocytes
4.3. Cytokines
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Coffee Organization—Monthly Coffee Market Report (2022/23). 2022. Available online: https://www.ico.org/Market-Report-22-23-e.asp (accessed on 13 July 2023).
- Davis, A.P.; Govaerts, R.; Bridson, D.M.; Stoffelen, P. An annotated taxonomic conspectus of the genus Coffea (Rubiaceae). Bot. J. Linn. Soc. 2006, 152, 465–512. [Google Scholar] [CrossRef]
- Ginz, M.; Balzer, H.H.; Bradbury, A.G.W.; Maier, H.G. Formation of aliphatic acids by carbohydrate degradation during roasting of coffee. Eur. Food Res. Technol. 2000, 211, 404–410. [Google Scholar] [CrossRef]
- Vio, M.B.E.M.F.A.; Luisa, P.F.; Fabiana, C.R.; Eacute, H.S.T.J.; Gerson, S.G.; Terezinha, J.G.S. The relationship between organic acids, sucrose and the quality of specialty coffees. Afr. J. Agric. Res. 2016, 11, 709–717. [Google Scholar] [CrossRef]
- Rune, C.J.; Giacalone, D.; Steen, I.; Duelund, L.; Münchow, M.; Clausen, M.P. Acids in brewed coffees: Chemical composition and sensory threshold. Curr. Res. Food Sci. 2023, 6, 100485. [Google Scholar] [CrossRef]
- Almoosawi, S.; Dickinson, A.; Fyfe, L.; Kenyon, C.J.; Al-Dujaili, E.A.S. Effect of green coffee bean extract and chlorogenic acid consumption on 11[beta]HSD activity in humans and mice. Endocr. Abstr. 2023, 19, 122. [Google Scholar]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2019, 97, 67–74. [Google Scholar] [CrossRef]
- Watanabe, T.; Arai, Y.; Mitsui, Y.; Kusaura, T.; Okawa, W.; Kajihara, Y.; Saito, I. The blood pressure-lowering effect and safety of chlorogenic acid from green coffee bean extract in essential hypertension. Clin. Exp. Hypertens. 2006, 28, 439–449. [Google Scholar] [CrossRef]
- Upadhyay, R.; Mohan Rao, L.J. An outlook on chlorogenic acids-occurrence, chemistry, technology, and biological activities. Crit. Rev. Food Sci. Nutr. 2013, 53, 968–984. [Google Scholar] [CrossRef]
- Shafaghatlonbar, M.; Bagherzade, G. Dual role of chlorogenic acid as an influential precursor in synthesizing nano-sized Cu(II) complexes and investigating its catalytic role in the oxidation of alcohols and its antibacterial activity. J. Organomet. Chem. 2023, 996, 122758. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Cifuentes-Gomez, T.; Tabatabaee, S.; Lecras, C.; Spencer, J.P.E. Procyanidin, Anthocyanin, and Chlorogenic Acid Contents of Highbush and Lowbush Blueberries. J. Agric. Food Chem. 2012, 60, 5772–5778. [Google Scholar] [CrossRef]
- Clifford, M.N. Chlorogenic acids and other cinnamates—Nature, occurrence and dietary burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N. Chlorogenic acids and other cinnamates—Nature, occurrence, dietary burden, absorption and metabolism. J. Sci. Food Agric. 2000, 80, 1033–1043. [Google Scholar] [CrossRef]
- Chiang, H.-M.; Chen, C.-W.; Chen, C.-C.; Wang, H.-W.; Jhang, J.-H.; Huang, Y.-H.; Wen, K.-C. Chapter 58—Role of Coffea arabica Extract and Related Compounds in Preventing Photoaging and Photodamage of the Skin. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 523–530. [Google Scholar] [CrossRef]
- Krakauer, T. The polyphenol chlorogenic acid inhibits staphylococcal exotoxin-induced inflammatory cytokines and chemokines. Immunopharmacol. Immunotoxicol. 2002, 24, 113–119. [Google Scholar] [CrossRef] [PubMed]
- de Magalhães, P.M.; Dupont, I.; Hendrickx, A.; Joly, A.; Raas, T.; Dessy, S.; Sergent, T.; Schneider, Y.-J. Anti-inflammatory effect and modulation of cytochrome P450 activities by Artemisia annua tea infusions in human intestinal Caco-2 cells. Food Chem. 2012, 134, 864–871. [Google Scholar] [CrossRef]
- Yun, N.; Kang, J.-W.; Lee, S.-M. Protective effects of chlorogenic acid against ischemia/reperfusion injury in rat liver: Molecular evidence of its antioxidant and anti-inflammatory properties. J. Nutr. Biochem. 2012, 23, 1249–1255. [Google Scholar] [CrossRef]
- Hwang, S.J.; Kim, Y.-W.; Park, Y.; Lee, H.-J.; Kim, K.-W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef]
- Shin, H.S.; Satsu, H.; Bae, M.-J.; Zhao, Z.; Ogiwara, H.; Totsuka, M.; Shimizu, M. Anti-inflammatory effect of chlorogenic acid on the IL-8 production in Caco-2 cells and the dextran sulphate sodium-induced colitis symptoms in C57BL/6 mice. Food Chem. 2015, 168, 167–175. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial activity and mechanism of action of chlorogenic acid. J Food Sci 2011, 76, M398–M403. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, X.; Wu, H.; Wang, H.; Bian, H.; Zhu, Y.; Xu, W.; Liu, F.; Wang, D.; Fu, L. Antibacterial activity and action mode of chlorogenic acid against Salmonella Enteritidis, a foodborne pathogen in chilled fresh chicken. World J. Microbiol. Biotechnol. 2020, 36, 24. [Google Scholar] [CrossRef]
- Wu, Y.; Liang, S.; Zhang, M.; Wang, Z.; Wang, Z.; Ren, X. The Effect of Chlorogenic Acid on Bacillus subtilis Based on Metabolomics. Molecules 2020, 25, 4038. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Peng, C.; Chi, F.; Yu, C.; Yang, Q.; Li, Z. Antibacterial and Antibiofilm Activities of Chlorogenic Acid Against Yersinia enterocolitica. Front. Microbiol. 2022, 13, 885092. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lan, W.; Xie, J. Antimicrobial and anti-biofilm activities of chlorogenic acid grafted chitosan against Staphylococcus aureus. Microb. Pathog. 2022, 173, 105748. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.Y.; Yang, H.R.; Zhang, J.; Li, D.; Lin, J.; Wang, L.; Xu, X. The Studies of Chlorogenic Acid Antitumor Mechanism by Gene Chip Detection: The Immune Pathway Gene Expression. J. Anal. Methods Chem. 2013, 2013, 617243. [Google Scholar] [CrossRef]
- Yan, Y.; Li, J.; Han, J.; Hou, N.; Song, Y.; Dong, L. Chlorogenic acid enhances the effects of 5-fluorouracil in human hepatocellular carcinoma cells through the inhibition of extracellular signal-regulated kinases. Anticancer Drugs 2015, 26, 540–546. [Google Scholar] [CrossRef]
- Zhang, F.; Yin, G.; Han, X.; Jiang, X.; Bao, Z. Chlorogenic acid inhibits osteosarcoma carcinogenesis via suppressing the STAT3/Snail pathway. J. Cell. Biochem. 2019, 120, 10342–10350. [Google Scholar] [CrossRef]
- Sapio, L.; Salzillo, A.; Illiano, M.; Ragone, A.; Spina, A.; Chiosi, E.; Pacifico, S.; Catauro, M.; Naviglio, S. Chlorogenic acid activates ERK1/2 and inhibits proliferation of osteosarcoma cells. J. Cell. Physiol. 2020, 235, 3741–3752. [Google Scholar] [CrossRef]
- Gupta, A.; Atanasov, A.G.; Li, Y.; Kumar, N.; Bishayee, A. Chlorogenic acid for cancer prevention and therapy: Current status on efficacy and mechanisms of action. Pharmacol. Res. 2022, 186, 106505. [Google Scholar] [CrossRef]
- Thom, E. The effect of chlorogenic acid enriched coffee on glucose absorption in healthy volunteers and its effect on body mass when used long-term in overweight and obese people. J. Int. Med. Res. 2007, 35, 900–908. [Google Scholar] [CrossRef]
- Jin, S.; Chang, C.; Zhang, L.; Liu, Y.; Huang, X.; Chen, Z. Huang, and Z. Chen. Chlorogenic Acid Improves Late Diabetes through Adiponectin Receptor Signaling Pathways in db/db Mice. PLoS ONE 2015, 10, e0120842. [Google Scholar] [CrossRef]
- Roshan, H.; Nikpayam, O.; Sedaghat, M.; Sohrab, G. Sedaghat, and G. Sohrab. Effects of green coffee extract supplementation on anthropometric indices, glycaemic control, blood pressure, lipid profile, insulin resistance and appetite in patients with the metabolic syndrome: A andomized clinical trial. Br. J. Nutr. 2018, 119, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Asbaghi, O.; Sadeghian, M.; Nasiri, M.; Khodadost, M.; Shokri, A.; Panahande, B.; Pirouzi, A.; Sadeghi, O. The effects of green coffee extract supplementation on glycemic indices and lipid profile in adults: A systematic review and dose-response meta-analysis of clinical trials. Nutr. J. 2020, 19, 71. [Google Scholar] [CrossRef] [PubMed]
- Pourmasoumi, M.; Hadi, A.; Marx, W.; Najafgholizadeh, A.; Kaur, S.; Sahebkar, A. The Effect of Green Coffee Bean Extract on Cardiovascular Risk Factors: A Systematic Review and Meta-analysis. Adv. Exp. Med. Biol. 2021, 1328, 323–345. [Google Scholar] [CrossRef] [PubMed]
- Urso, M.L.; Sawka, M.N. Inflammation: Sustaining the balance to optimize recovery of skeletal muscle, connective tissue, and exertional injuries. J. Appl. Physiol. 1985, 115, 877–878. [Google Scholar] [CrossRef][Green Version]
- Witkowska-Piłaszewicz, O.; Bąska, P.; Czopowicz, M.; Żmigrodzka, M.; Szarska, E.; Szczepaniak, J.; Nowak, Z.; Winnicka, A.; Cywińska, A. Anti-Inflammatory State in Arabian Horses Introduced to the Endurance Training. Animals 2019, 9, 616. [Google Scholar] [CrossRef]
- Plisak, U.; Szczepaniak, J.; Żmigrodzka, M.; Giercuszkiewicz-Hecold, B.; Witkowska-Piłaszewicz, O. Changes in novel anti-infalmmatory cytokine concetration in the bood of endurance and race horses at different levels of training. Comput. Struct. Biotechnol. J. 2023, 21, 418–424. [Google Scholar] [CrossRef]
- Horohov, D.W.; Sinatra, S.T.; Chopra, R.K.; Jankowitz, S.; Betancourt, A.; Bloomer, R.J. The Effect of Exercise and Nutritional Supplementation on Proinflammatory Cytokine Expression in Young Racehorses During Training. J. Equine Vet. Sci. 2012, 32, 805–815. [Google Scholar] [CrossRef]
- Fallon, K.E. The acute phase response and exercise: The ultramarathon as prototype exercise. Clin. J. Sport. Med. 2001, 11, 38–43. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Toft, A.D. Effects of exercise on lymphocytes and cytokines. Br. J. Sports Med. 2000, 34, 246–251. [Google Scholar] [CrossRef]
- Capomaccio, S.; Cappelli, K.; Spinsanti, G.; Mencarelli, M.; Muscettola, M.; Felicetti, M.; Supplizi, A.V.; Bonifazi, M. Athletic humans and horses: Comparative analysis of interleukin-6 (IL-6) and IL-6 receptor (IL-6R) expression in peripheral blood mononuclear cells in trained and untrained subjects at rest. BMC Physiol. 2011, 11, 3. [Google Scholar] [CrossRef]
- Cappelli, K.; Felicetti, M.; Capomaccio, S.; Nocelli, C.; Silvestrelli, M.; Verini-Supplizi, A. Effect of training status on immune defence related gene expression in Thoroughbred: Are genes ready for the sprint? Vet. J. 2013, 195, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, A.I.; Shephard, R.J.; Shek, P.N. The cytokine response to physical activity and training. Sports Med. 2001, 31, 115–144. [Google Scholar] [CrossRef]
- Walsh, N.P.; Gleeson, M.; Shephard, R.J.; Gleeson, M.; A Woods, J.; Bishop, N.C.; Fleshner, M.; Green, C.; Pedersen, B.K.; Hoffman-Goetz, L.; et al. Position statement. Part one: Immune function and exercise. Exerc. Immunol. Rev. 2011, 17, 6–63. [Google Scholar] [PubMed]
- Peake, J.M.; Neubauer, O.; Walsh, N.P.; Simpson, R.J. Recovery of the immune system after exercise. J. Appl. Physiol. 2017, 122, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Witkowska-Piłaszewicz, O.; Pingwara, R.; Winnicka, A. The Effect of Physical Training on Peripheral Blood Mononuclear Cell Ex Vivo Proliferation, Differentiation, Activity, and Reactive Oxygen Species Production in Racehorses. Antioxidants 2020, 9, 1155. [Google Scholar] [CrossRef]
- Beiter, T.; Hoene, M.; Prenzler, F.; Mooren, F.C.; Steinacker, J.M.; Weigert, C.; Nieß, A.M.; Munz, B. Exercise, skeletal muscle and inflammation: ARE-binding proteins as key regulators in inflammatory and adaptive networks. Exerc. Immunol. Rev. 2015, 21, 42–57. [Google Scholar]
- Şimşek, A.; Çiçek, B.; Turan, E. The effect of chlorogenic acid from green coffee as a natural antioxidant on the shelf life and composition of hazelnut paste. Eur. Food Res. Technol. 2023, 8, 2077–2086. [Google Scholar] [CrossRef]
- Abaidullah, M.; Peng, S.; Song, X.; Zou, Y.; Li, L.; Jia, R.; Yin, Z. Chlorogenic acid is a positive regulator of MDA5, TLR7 and NF-κB signaling pathways mediated antiviral responses against Gammacoronavirus infection. Int. Immunopharmacol. 2021, 96, 107671. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef]
- Zeng, L.; Xiang, R.; Fu, C.; Qu, Z.; Liu, C. The Regulatory effect of chlorogenic acid on gut-brain function and its mechanism: A systematic review. Biomed. Pharmacother. 2022, 149, 112831. [Google Scholar] [CrossRef]
- Zeng, A.; Liang, X.; Zhu, S.; Liu, C.; Wang, S.; Zhang, Q.; Zhao, J.; Song, L. Chlorogenic acid induces apoptosis, inhibits metastasis and improves antitumor immunity in breast cancer via the NF-κB signaling pathway. Oncol. Rep. 2021, 45, 717–727. [Google Scholar] [CrossRef]
- Yuan, Y.; Gong, X.; Zhang, L.; Jiang, R.; Yang, J.; Wang, B.; Wan, J. Chlorogenic acid ameliorated concanavalin A-induced hepatitis by suppression of Toll-like receptor 4 signaling in mice. Int. Immunopharmacol. 2017, 44, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Folsom, R.W.; Littlefield-Chabaud, M.A.; French, D.D.; Pourciau, S.S.; Mistric, L.; Horohov, D.W. Exercise alters the immune response to equine influenza virus and increases susceptibility to infection. Equine Vet. J. 2012, 33, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S. The origin of FOXP3-expressing CD4+ regulatory T cells: Thymus or periphery. J. Clin. Investig. 2003, 112, 1310–1312. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gong, R.; Zhao, C.; Lei, K.; Sun, X.; Ren, H. Human FOXP3 and tumour microenvironment. Immunology 2023, 168, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.T.; Floess, S.; Baru, A.M.; Lahl, K.; Huehn, J.; Sparwasser, T. CD8+ Foxp3+ T cells share developmental and phenotypic features with classical CD4+ Foxp3+ regulatory T cells but lack potent suppressive activity. Eur. J. Immunol. 2011, 41, 716–725. [Google Scholar] [CrossRef]
- Thannickal, V.J.; Fanburg, B.L.; Yasuoka, H.; Garrett, S.M.; Nguyen, X.-X.; Artlett, C.M.; Feghali-Bostwick, C.A.; Zimmer, A.; Bagchi, A.K.; Vinayak, K.; et al. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [CrossRef]
- Yarosz, E.L.; Chang, C.-H. The Role of Reactive Oxygen Species in Regulating T Cell-mediated Immunity and Disease. Immune Netw. 2018, 18, e14. [Google Scholar] [CrossRef]
- Jacinto, T.A.; Meireles, G.S.; Dias, A.T.; Aires, R.; Porto, M.L.; Gava, A.L.; Vasquez, E.C.; Pereira, T.M.C.; Campagnaro, B.P.; Meyrelles, S.S. Increased ROS production and DNA damage in monocytes are biomarkers of aging and atherosclerosis. Biol. Res. 2018, 51, 33. [Google Scholar] [CrossRef]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J. Sport. Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Zada, S.; Pham, T.M.; Hwang, J.S.; Ahmed, M.; Lai, T.H.; Elashkar, O.; Kim, J.-H.; Kim, D.H.; Kim, D.R. Chlorogenic acid protects human chondrocyte C28/I2 cells from oxidative stress-induced cell death through activation of autophagy. Life Sci. 2021, 285, 119968. [Google Scholar] [CrossRef] [PubMed]
- Le, Y.-J.; He, L.-Y.; Li, S.; Xiong, C.-J.; Lu, C.-H.; Yang, X.-Y. Chlorogenic acid exerts antibacterial effects by affecting lipid metabolism and scavenging ROS in Streptococcus pyogenes. FEMS Microbiol. Lett. 2022, 369, fnac061. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Lee, D.G. Depletion of reactive oxygen species induced by chlorogenic acid triggers apoptosis-like death in Escherichia coli. Free Radic. Res. 2018, 52, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Shi, A.; Dong, L.; Lu, X.; Wang, Y.; Zhao, J.; Dai, F.; Guo, X. Chlorogenic acid protects against liver fibrosis in vivo and in vitro through inhibition of oxidative stress. Clin. Nutr. 2016, 35, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; He, X.; Wu, L.; Yan, D.; Yan, S. Chlorogenic Acid, the Main Antioxidant in Coffee, Reduces Radiation-Induced Apoptosis and DNA Damage via NF-E2-Related Factor 2 (Nrf2) Activation in Hepatocellular Carcinoma. Oxid. Med. Cell Longev. 2022, 2022, 4566949. [Google Scholar] [CrossRef]
- Lawler, J.M.; Powers, S.K. Oxidative Stress, Antioxidant Status, and the Contracting Diaphragm. Can. J. Appl. Physiol. 1998, 23, 23–55. [Google Scholar] [CrossRef]
- Leeuwenburgh, C.; Heinecke, J.W. Oxidative Stress and Antioxidants in Exercise. CMC 2001, 8, 829–838. [Google Scholar] [CrossRef]
- Morrison, D.; Hughes, J.; Della Gatta, P.A.; Mason, S.; Lamon, S.; Russell, A.P.; Wadley, G.D. Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free. Radic. Biol. Med. 2015, 89, 852–862. [Google Scholar] [CrossRef]
- Chen, X.; Song, M.; Zhang, B.; Zhang, Y. Reactive Oxygen Species Regulate T Cell Immune Response in the Tumor Microenvironment. Oxidative Med. Cell. Longev. 2016, 2016, e1580967. [Google Scholar] [CrossRef]
- Bassoy, E.Y.; Walch, M.; Martinvalet, D. Reactive Oxygen Species: Do They Play a Role in Adaptive Immunity? Front. Immunol. 2021, 12, 755856. [Google Scholar]
- Chauhan, P.S.; Satti, N.K.; Sharma, P.; Sharma, V.K.; Suri, K.A.; Bani, S. Differential effects of chlorogenic acid on various immunological parameters relevant to rheumatoid arthritis. Phytother. Res. 2012, 26, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yu, Y.-H.; Wang, S.-T.; Ren, J.; Camer, D.; Hua, Y.-Z.; Zhang, Q.; Huang, J.; Xue, D.-L.; Zhang, X.-F.; et al. Chlorogenic acid protects d-galactose-induced liver and kidney injury via antioxidation and anti-inflammation effects in mice. Pharm. Biol. 2016, 54, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Ohkawara, T.; Takeda, H.; Nishihira, J. Protective effect of chlorogenic acid on the inflammatory damage of pancreas and lung in mice with l-arginine-induced pancreatitis. Life Sci. 2017, 190, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.; Vieira, P.; O’garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 2020, 217, e20190418. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, G.; De Carli, M.; Almerigogna, F.; Giudizi, M.G.; Biagiotti, R.; Romagnani, S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J. Immunol. 1993, 150, 353–360. [Google Scholar] [CrossRef]
- Patil, R. Chlorogenic acid may be a potent inhibitor of dimeric SARS-CoV-2 main protease 3CLpro: An in silico study. Tradit. Med. Res. 2021, 2, 135–148. [Google Scholar]
- Cua, D.J.; Tato, C.M. Innate IL-17-producing cells: The sentinels of the immune system. Nat. Rev. Immunol. 2010, 10, 479–489. [Google Scholar] [CrossRef]
- Millar, N.L.; Akbar, M.; Campbell, A.L.; Reilly, J.H.; Kerr, S.C.; McLean, M.; Frleta-Gilchrist, M.; Fazzi, U.G.; Leach, W.J.; Rooney, B.P.; et al. IL-17A mediates inflammatory and tissue remodelling events in early human tendinopathy. Sci. Rep. 2016, 6, 27149. [Google Scholar] [CrossRef]
- Ackermann, P.W.; Domeij-Arverud, E.; Leclerc, P.; Amoudrouz, P.; Nader, G.A. Anti-inflammatory cytokine profile in early human tendon repair. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1801–1806. [Google Scholar] [CrossRef]
| Antibody | Clone; Dilution | Source | Target Cell |
|---|---|---|---|
| CD4:PE | CVS4; 1:10 | BioRad, Berkeley, CA, USA | Lymphocytes |
| CD8:FITC | CVS21; 1:10 | BioRad, Berkeley, CA, USA | Lymphocytes |
| CD5:PE | CVS5; 1:10 | BioRad, Berkeley, CA, USA | Lymphocytes |
| CD14:AF405 | 433423; 1:10 | R&D Systems, Minneapolis, MI, USA | Monocytes |
| MHCII:FITC | CVS20; 1:20 | BioRad, Berkeley, CA, USA | Monocytes |
| FoxP3:APC | FJK-16s; 1:10 | Life Technologies, Bleiswijk, The Netherland | Lymphocytes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dąbrowska, I.; Grzędzicka, J.; Niedzielska, A.; Witkowska-Piłaszewicz, O. Impact of Chlorogenic Acid on Peripheral Blood Mononuclear Cell Proliferation, Oxidative Stress, and Inflammatory Responses in Racehorses during Exercise. Antioxidants 2023, 12, 1924. https://doi.org/10.3390/antiox12111924
Dąbrowska I, Grzędzicka J, Niedzielska A, Witkowska-Piłaszewicz O. Impact of Chlorogenic Acid on Peripheral Blood Mononuclear Cell Proliferation, Oxidative Stress, and Inflammatory Responses in Racehorses during Exercise. Antioxidants. 2023; 12(11):1924. https://doi.org/10.3390/antiox12111924
Chicago/Turabian StyleDąbrowska, Izabela, Jowita Grzędzicka, Adrianna Niedzielska, and Olga Witkowska-Piłaszewicz. 2023. "Impact of Chlorogenic Acid on Peripheral Blood Mononuclear Cell Proliferation, Oxidative Stress, and Inflammatory Responses in Racehorses during Exercise" Antioxidants 12, no. 11: 1924. https://doi.org/10.3390/antiox12111924
APA StyleDąbrowska, I., Grzędzicka, J., Niedzielska, A., & Witkowska-Piłaszewicz, O. (2023). Impact of Chlorogenic Acid on Peripheral Blood Mononuclear Cell Proliferation, Oxidative Stress, and Inflammatory Responses in Racehorses during Exercise. Antioxidants, 12(11), 1924. https://doi.org/10.3390/antiox12111924






