Evaluation of the Protective and Regenerative Properties of Commercially Available Artichoke Leaf Powder Extract on Plasma and Liver Oxidative Stress Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. TLC Analysis
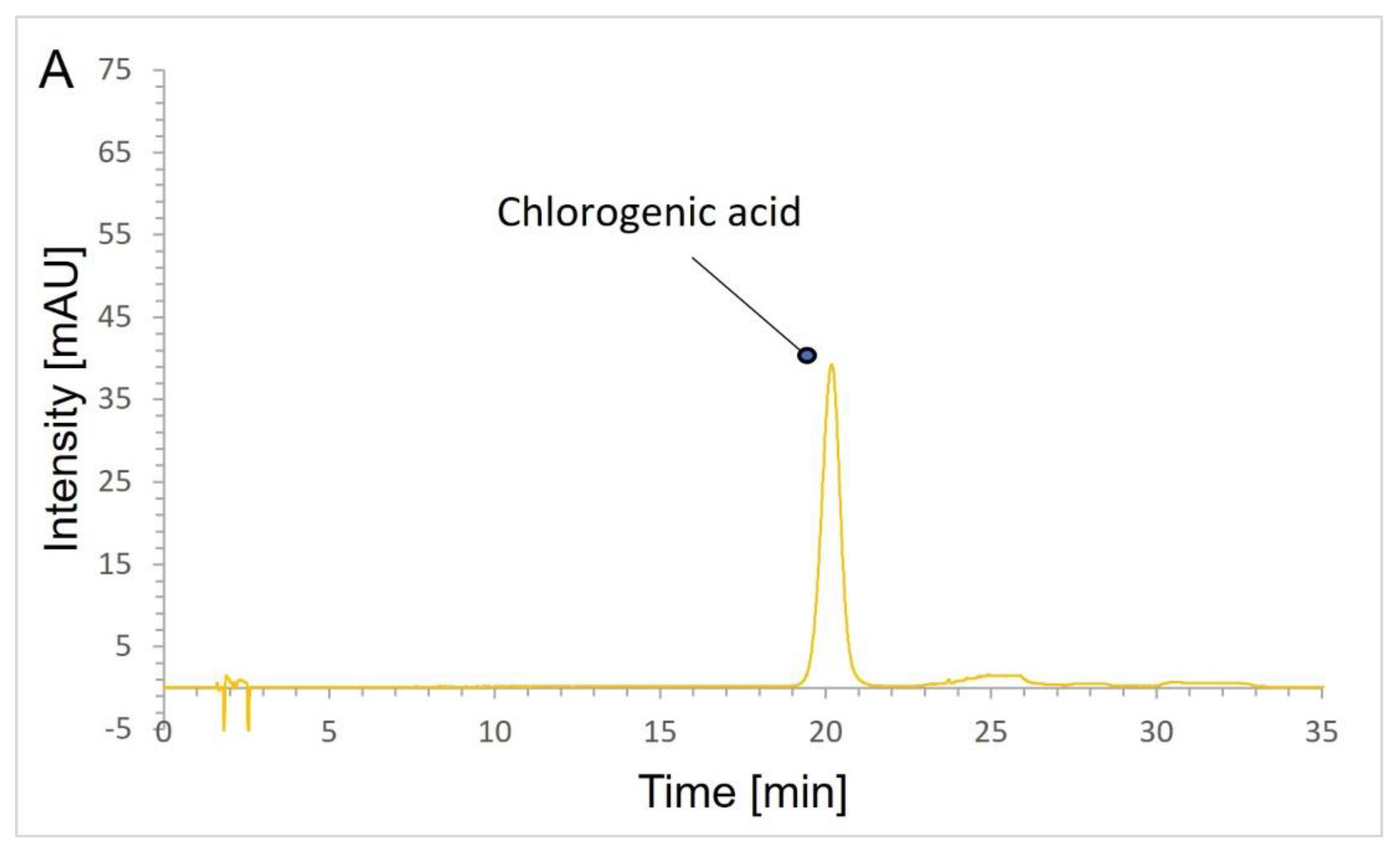
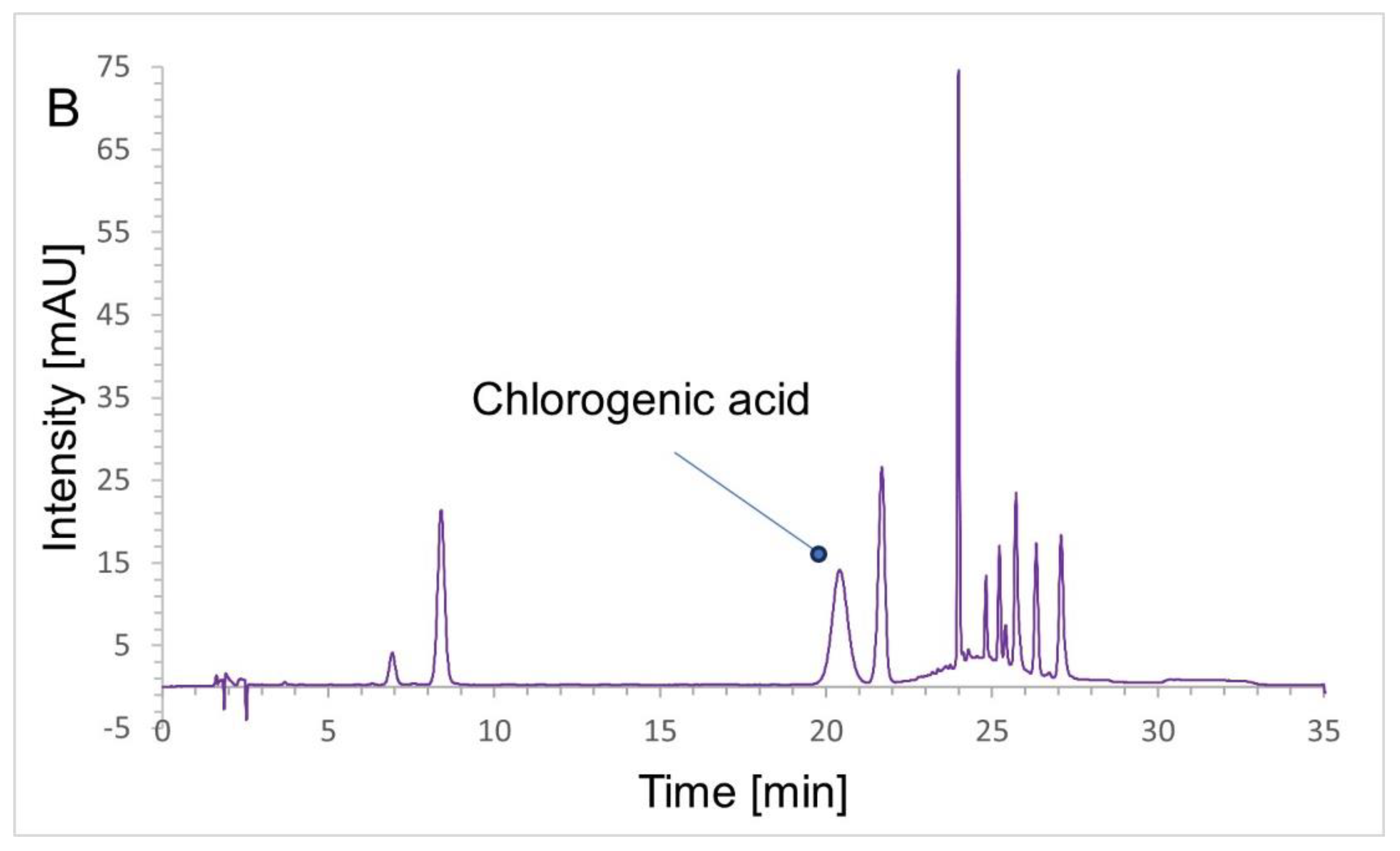
2.4. HPLC Analysis
2.4.1. Chromatographic Conditions
2.4.2. Validation Results
2.5. Ethics Committee Approval
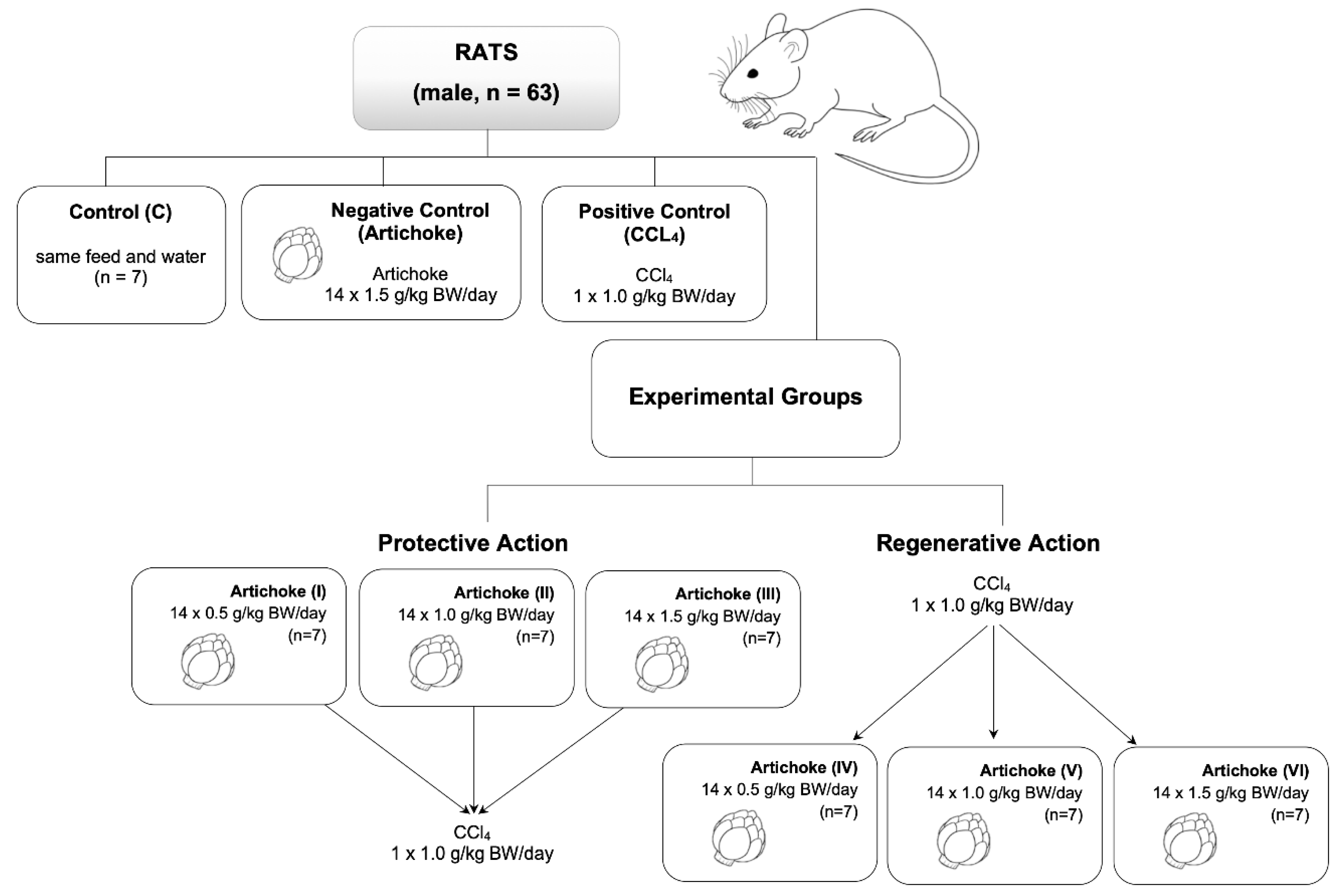
2.6. Animals and Experimental Treatments
2.7. Preparation of Tissue Samples
2.8. Biochemical Assays
2.9. Statistical Analysis
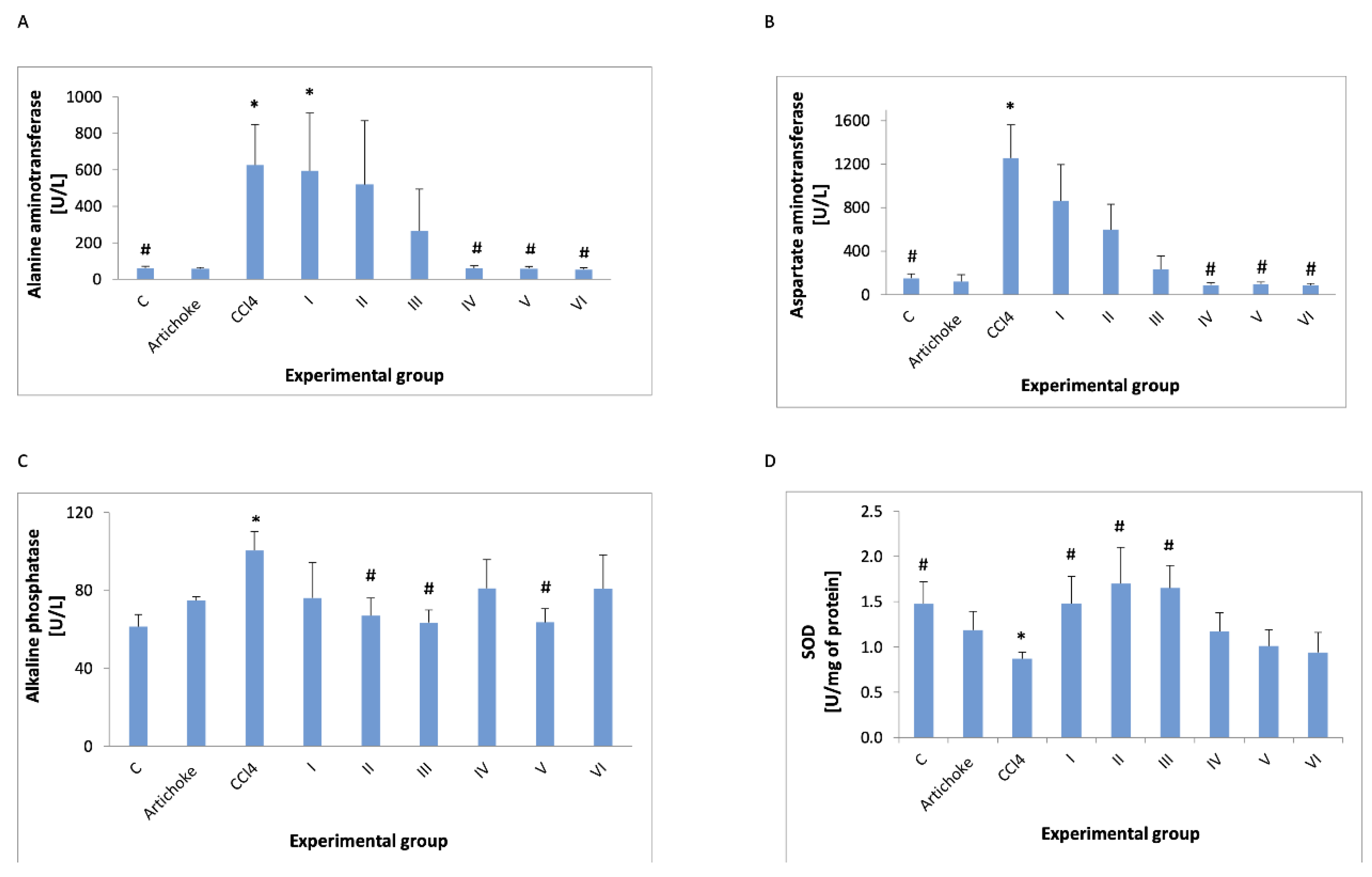
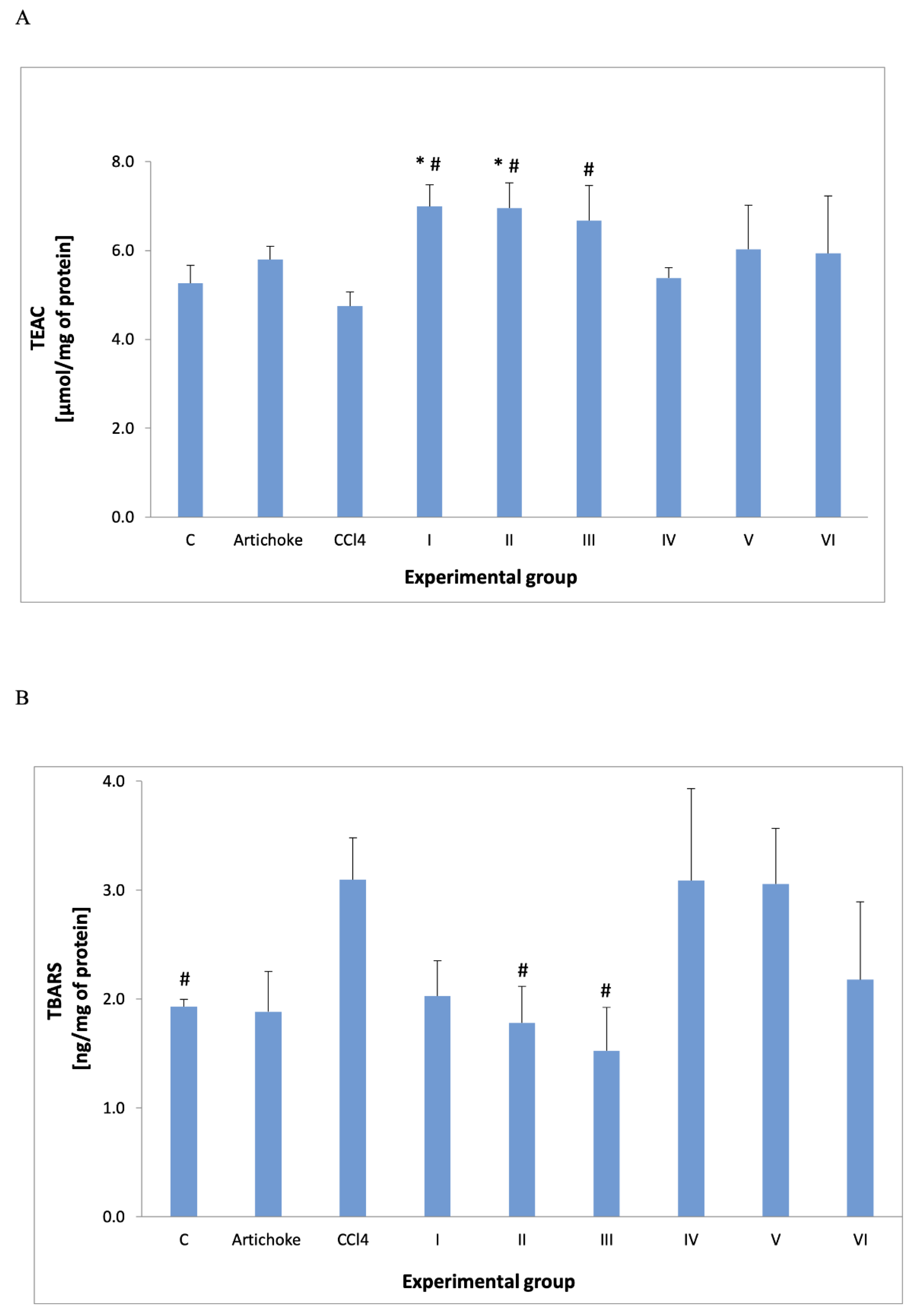
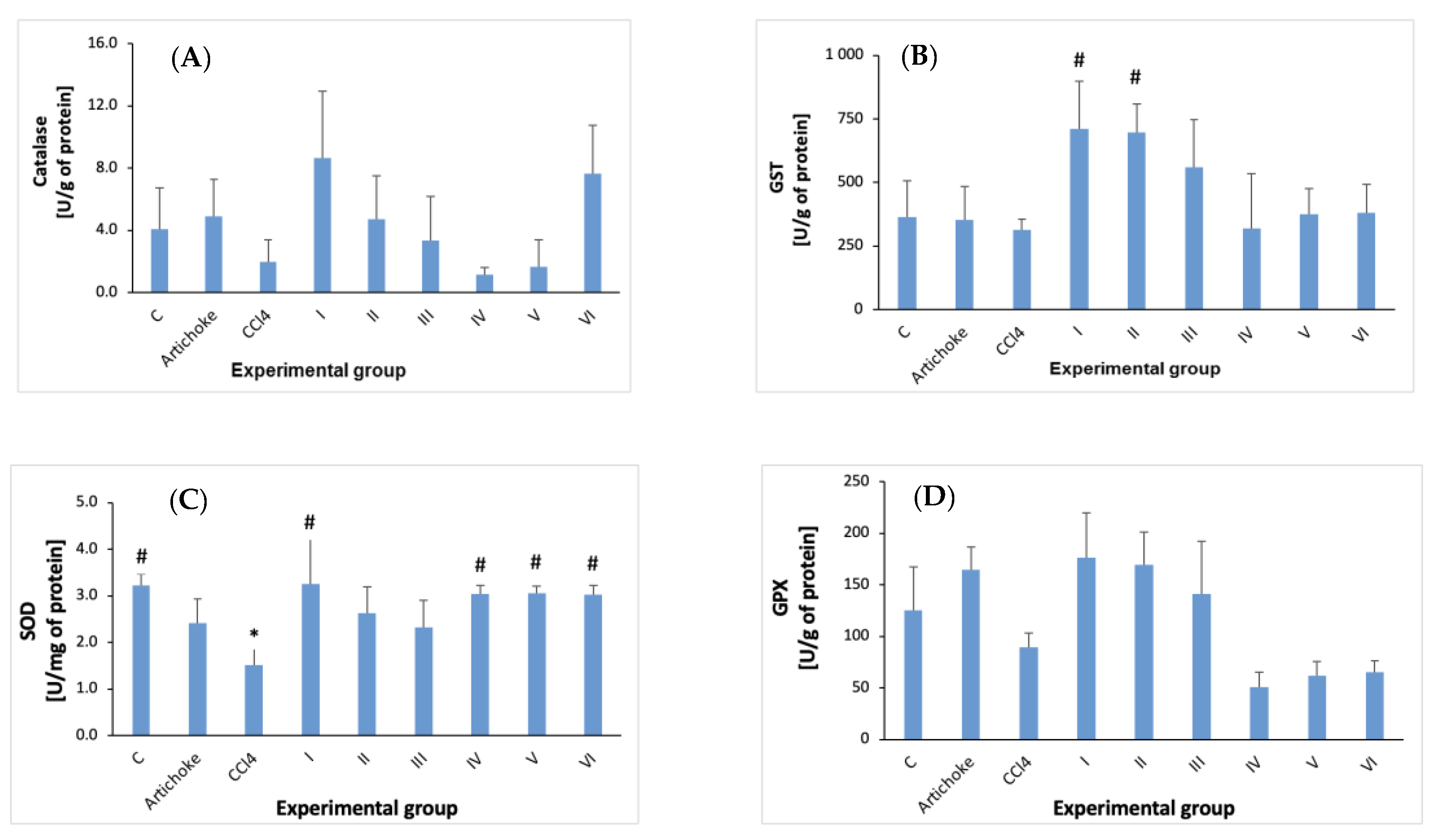
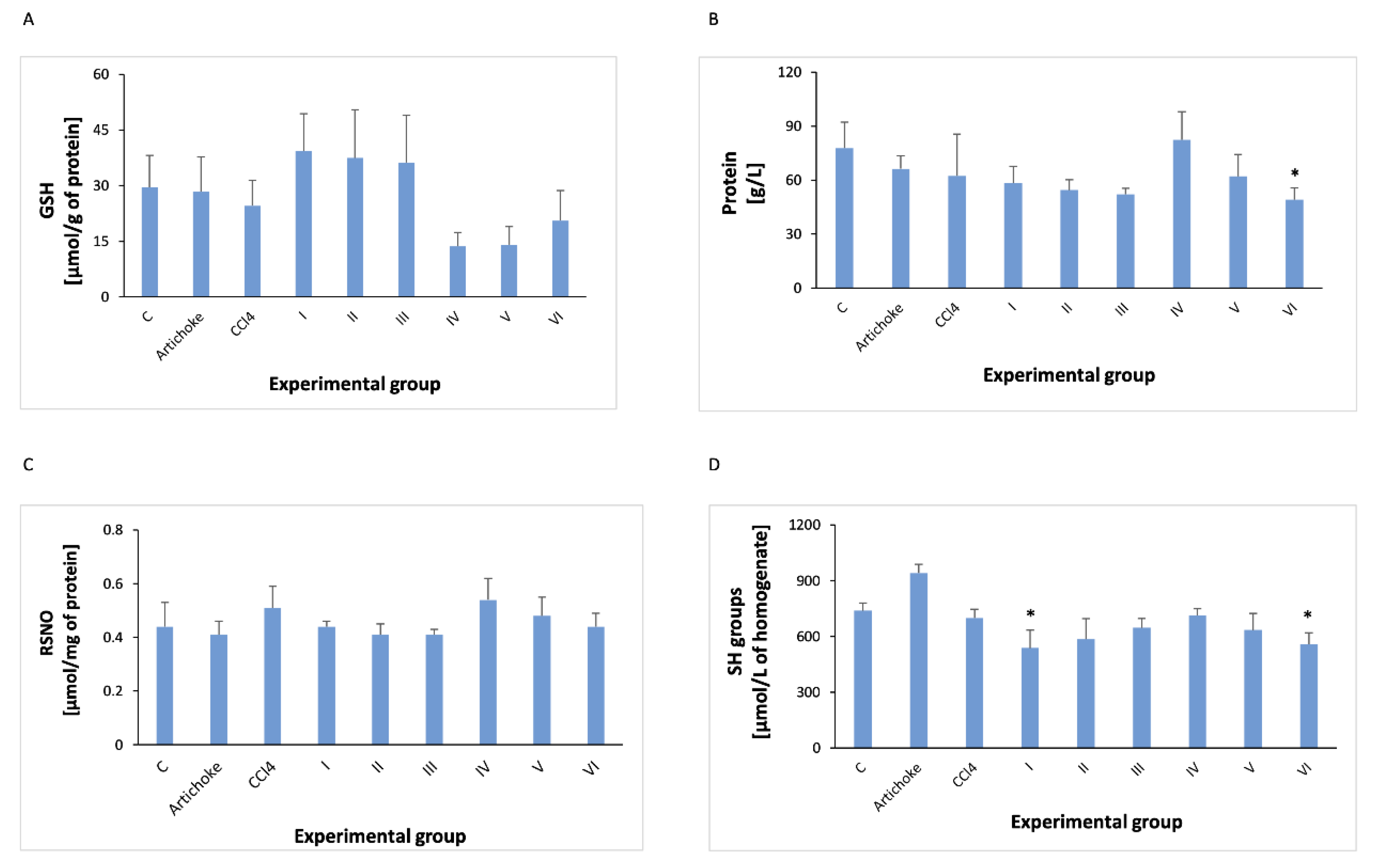
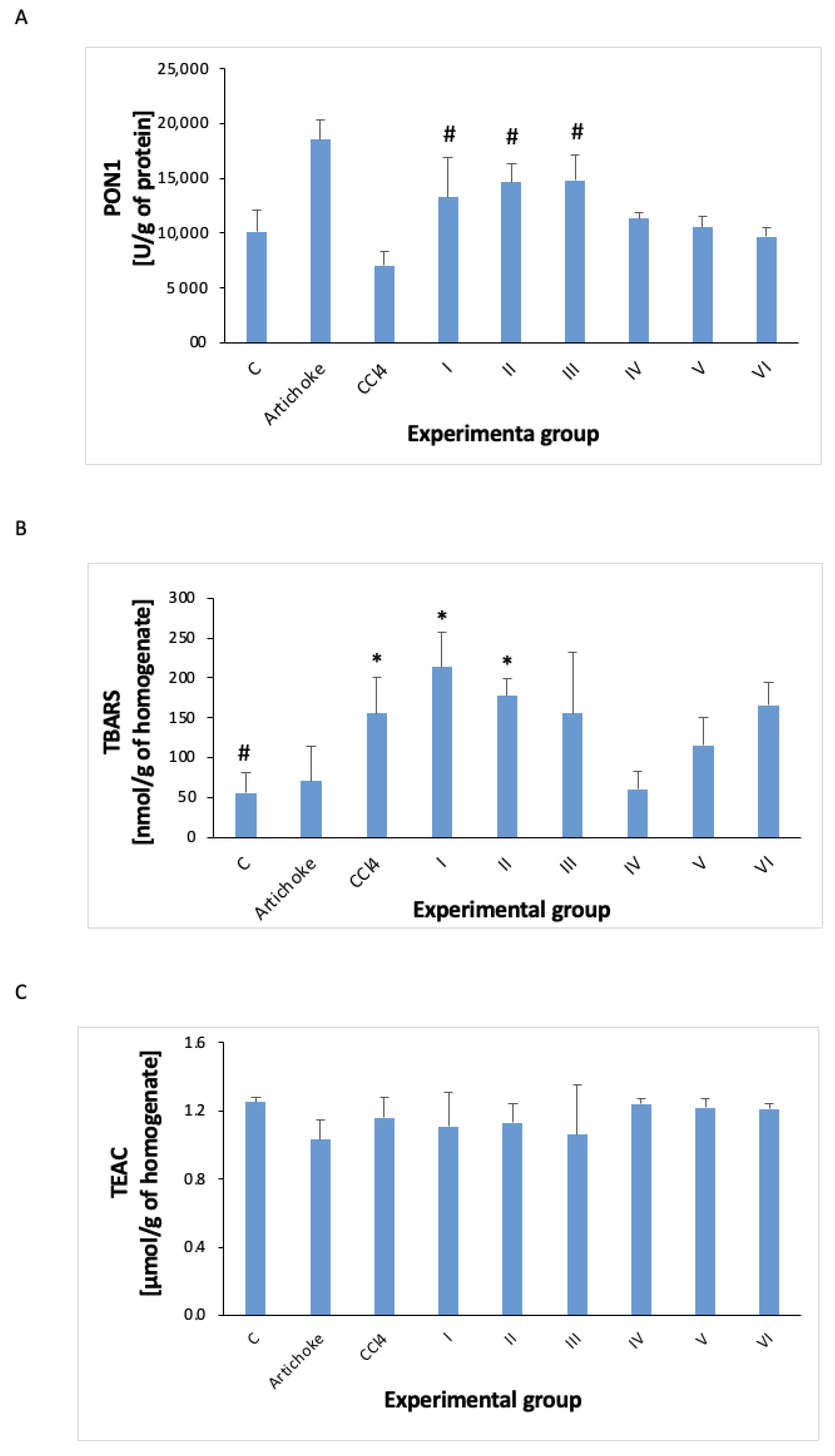
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramos-Tovar, E.; Muriel, P. Molecular Mechanisms that Link Oxidative Stress, Inflammation, and Fibrosis in the Liver. Antioxidants 2020, 9, 1279. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, M.; Burzynska-Pedziwiatr, I.; Wozniak, L. A Review of Natural and Synthetic Antioxidants Important for Health and Longevity. Curr. Med. Chem. 2010, 17, 3262–3288. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Roy, Z.; Bansal, R.; Siddiqui, L. Understanding the Role of Free Radicals and Antioxidant Enzymes in Human Diseases. Curr. Pharm. Biotechnol. 2023, 24, 1265–1276. [Google Scholar] [CrossRef]
- Orlovskaya, T.V.; Luneva, I.L.; Chelombit’Ko, V.A. Chemical composition of Cynara scolymus leaves. Chem. Nat. Compd. 2007, 43, 239–240. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Pereira, C.; Barros, L.; Ferreira, I.C.F.R. Leaf parts from Greek artichoke genotypes as a good source of bioactive compounds and antioxidants. Food Funct. 2017, 8, 2022–2029. [Google Scholar] [CrossRef]
- Goetz, P.; Le Jeune, R. Artichaut, Cynara scolymus. Phytothérapie 2007, 5, 219–222. [Google Scholar] [CrossRef]
- Santos, H.O.; Bueno, A.A.; Mota, J.F. The effect of artichoke on lipid profile: A review of possible mechanisms of action. Pharmacol. Res. 2018, 137, 170–178. [Google Scholar] [CrossRef]
- Gebhardt, R. Inhibition of cholesterol biosynthesis in primary cultured rat hepatocytes by artichoke (Cynara scolymus L.) extracts. J. Pharmacol. Exp. Ther. 1998, 286, 1122–1128. [Google Scholar]
- Ghada, Z.A.S.; Saad, T.M.M. Effect of Cynara scolymus L. (artichoke) extract on lipid profile of hyperlipidemic male rats. Egypt. J. Hosp. Med. 2009, 37, 733–741. [Google Scholar] [CrossRef]
- Küskü-Kiraz, Z.; Mehmetçik, G.; Doǧru-Abbasoǧlu, S.; Uysal, M. Artichoke leaf extract reduces oxidative stress and lipoprotein dyshomeostasis in rats fed on high cholesterol diet. Phytother. Res. 2010, 24, 565–570. [Google Scholar] [CrossRef]
- Kwon, E.-Y.; Kim, S.Y.; Choi, M.-S. Luteolin-Enriched Artichoke Leaf Extract Alleviates the Metabolic Syndrome in Mice with High-Fat Diet-Induced Obesity. Nutrients 2018, 10, 979. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, R.; Beckers, C.; Kirchhoff, G.; Trinczek-Gärtner, H.; Petrowicz, O.; Reimann, H. Increase in choleresis by means of artichoke extract. Phytomedicine 1994, 1, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Z.; Lee, S.-O.; Ye, Z.; Wu, X.; Hendrich, S. Artichoke Extract Lowered Plasma Cholesterol and Increased Fecal Bile Acids in Golden Syrian Hamsters. Phytother. Res. 2012, 26, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.-C.; Jhuang, J.-H.; Yao, H.-T. Artichoke leaf extract supplementation lowers hepatic oxidative stress and inflammation and increases multidrug resistance-associated protein 2 in mice fed a high-fat and high-cholesterol diet. Food Funct. 2021, 12, 7239–7249. [Google Scholar] [CrossRef]
- D’antuono, I.; Carola, A.; Sena, L.M.; Linsalata, V.; Cardinali, A.; Logrieco, A.F.; Colucci, M.G.; Apone, F. Artichoke Polyphenols Produce Skin Anti-Age Effects by Improving Endothelial Cell Integrity and Functionality. Molecules 2018, 23, 2729. [Google Scholar] [CrossRef]
- Li, H.; Xia, N.; Brausch, I.; Yao, Y.; Förstermann, U. Flavonoids from artichoke (Cynara scolymus L.) up-regulate endothelial-type nitric-oxide synthase gene expression in human endothelial cells. J. Pharmacol. Exp. Ther. 2004, 310, 926–932. [Google Scholar] [CrossRef]
- Lupattelli, G.; Marchesi, S.; Lombardini, R.; Roscini, A.R.; Trinca, F.; Gemelli, F.; Vaudo, G.; Mannarino, E. Artichoke juice improves endothelial function in hyperlipemia. Life Sci. 2004, 76, 775–7824. [Google Scholar] [CrossRef]
- Küçükgergin, C.; Aydın, A.F.; Özdemirler-Erata, G.; Mehmetçik, G.; Koçak-Toker, N.; Uysal, M. Effect of Artichoke Leaf Extract on Hepatic and Cardiac Oxidative Stress in Rats Fed on High Cholesterol Diet. Biol. Trace Elem. Res. 2010, 135, 264–274. [Google Scholar] [CrossRef]
- Costabile, A.; Kolida, S.; Klinder, A.; Gietl, E.; Bäuerlein, M.; Frohberg, C.; Landschütze, V.; Gibson, G.R. A double-blind, placebo-controlled, cross-over study to establish the bifidogenic effect of a very-long-chain inulin extracted from globe artichoke (Cynara scolymus) in healthy human subjects. Br. J. Nutr. 2010, 104, 1007–1017. [Google Scholar] [CrossRef]
- Rahimi, R.; Abdollahi, M. Herbal medicines for the management of irritable bowel syndrome:acomprehensive review. World J. Gastroenterol. 2012, 18, 589–600. [Google Scholar] [CrossRef]
- Villani, A.; Tommasi, F.; Paciolla, C. The Arbuscular Mycorrhizal Fungus Glomus viscosum Improves the Tolerance to Verticillium Wilt in Artichoke by Modulating the Antioxidant Defense Systems. Cells 2021, 10, 1944. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.; Ahmed, O.M.; El-Twab, S.M.A.; Zaky, M.Y.; Bakry, L.N. Prophylactic effects of Cynara scolymus L. leaf and flower hydroethanolic extracts against diethylnitrosamine/acetylaminoflourene-induced lung cancer in Wistar rats. Environ. Sci. Pollut. Res. 2021, 28, 43515–43527. [Google Scholar] [CrossRef] [PubMed]
- Juzyszyn, Z.; Czerny, B.; Myśliwiec, Z.; Pawlik, A.; Droździk, M. The effect of artichoke (Cynara scolymus L.) extract on respiratory chain system activity in rat liver mitochondria. Phytother. Res. 2010, 24 (Suppl. S2), S123–S128. [Google Scholar] [CrossRef]
- Ben Salem, M.; Kolsi, R.B.A.; Dhouibi, R.; Ksouda, K.; Charfi, S.; Yaich, M.; Hammami, S.; Sahnoun, Z.; Zeghal, K.M.; Jamoussi, K.; et al. Protective effects of Cynara scolymus leaves extract on metabolic disorders and oxidative stress in alloxan-diabetic rats. BMC Complement. Altern. Med. 2017, 17, 328. [Google Scholar] [CrossRef] [PubMed]
- Florek, E.; Ignatowicz, E.; Piekoszewski, W. Effect of pregnancy and tobacco smoke on the antioxidant activity of rutin in an animal model. Pharmacol. Rep. 2009, 61, 935–940. [Google Scholar] [CrossRef]
- Wu, D.; Cederbaum, A. Oxidative Stress and Alcoholic Liver Disease. Semin. Liver Dis. 2009, 29, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Ignatowicz, E.; Woźniak, A.; Kulza, M.; Seńczuk-Przybyłowska, M.; Cimino, F.; Piekoszewski, W.; Chuchracki, M.; Florek, E. Exposure to alcohol and tobacco smoke causes oxidative stress in rats. Pharmacol. Rep. 2013, 65, 906–913. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Bartosz, G. Second Face of Oxygen, 2nd ed.; PWN: Warsaw, Poland, 2013. [Google Scholar]
- Tsukahara, H. Biomarkers for Oxidative Stress: Clinical Application in Pediatric Medicine. Curr. Med. Chem. 2007, 14, 339–351. [Google Scholar] [CrossRef]
- Bryan, N.S.; Grisham, M.B. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic. Biol. Med. 2007, 43, 645–657. [Google Scholar] [CrossRef]
- Saville, B. A scheme for the colorimetric determination of microgram amounts of thiols. Analyst 1958, 83, 670–672. [Google Scholar] [CrossRef]
- Konan, M.K.; Koffi, E.N.; Cisse, I.; Adima, A.A.; Bekro, Y.-A. Phytochemical, nutritional and antioxidant capacity of five Ivorian edible leaves aqueous extracts. J. Appl. Pharm. Sci. 2016, 6, 82–86. [Google Scholar] [CrossRef]
- Rael, L.T.; Thomas, G.W.; Craun, M.L.; Curtis, C.G.; Bar-Or, R.; Bar-Or, D. Lipid Peroxidation and the Thiobarbituric Acid Assay: Standardization of the Assay When Using Saturated and Unsaturated Fatty Acids. BMB Rep. 2004, 37, 749–752. [Google Scholar] [CrossRef]
- Nguyen, S.D.; Sok, D.-E. Preferable stimulation of PON1 arylesterase activity by phosphatidylcholines with unsaturated acyl chains or oxidized acyl chains at sn-2 position. Biochim. Biophys. Acta (BBA)—Biomembr. 2006, 1758, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; DELLA-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Debbarh, H.; Louanjli, N.; Aboulmaouahib, S.; Jamil, M.; Ahbbas, L.; Kaarouch, I.; Sefrioui, O.; Cadi, R. Antioxidant activities and lipid peroxidation status in human follicular fluid: Age-dependent change. Zygote 2021, 29, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Cichoz-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef]
- Unsal, V.; Cicek, M.; Sabancilar, I. Toxicity of carbon tetrachloride, free radicals and role of antioxidants. Rev. Environ. Health 2021, 36, 279–295. [Google Scholar] [CrossRef]
- A Hassan, S.; Salem, M.M.; Hamam, O. Antioxidative and antiapoptotic effects of vitamin A and vitamin C against carbon tetrachloride induced hepatotoxicity in mice. Egypt. J. Hosp. Med. 2003, 11, 30–40. [Google Scholar] [CrossRef]
- Ozdemir, A.; Tumkaya, L.; Kalcan, S.; Uyan, M.; Karakaya, A.; Demiral, G.; Samanci, T.C.; Mercantepe, T.; Cüre, M.C.; Cüre, E. The effects of TNF-α inhibitors on carbon tetrachloride-induced nephrotoxicity. Clin. Exp. Hypertens. 2022, 44, 291–296. [Google Scholar] [CrossRef]
- Weber, L.W.D.; Boll, M.; Stampfl, A. Hepatotoxicity and Mechanism of Action of Haloalkanes: Carbon Tetrachloride as a Toxicological Model. Crit. Rev. Toxicol. 2003, 33, 105–136. [Google Scholar] [CrossRef] [PubMed]
- Mehmetçik, G.; Özdemirler, G.; Koçak-Toker, N.; Çevikbaş, U.; Uysal, M. Effect of pretreatment with artichoke extract on carbon tetrachloride-induced liver injury and oxidative stress. Exp. Toxicol. Pathol. 2008, 60, 475–480. [Google Scholar] [CrossRef]
- Boll, M.; Weber, L.W.D.; Becker, E.; Stampfl, A. Mechanism of carbon tetrachloride-induced hepatotoxicity. Hepatocellular damage by reactive carbon tetrachloride metabolites. Z. Naturforschung—Sect. C J. Biosci. 2001, 56, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Islam, M.; Al Mamun, M.; Faruk; Rahman, M.; Alam, M.N.; Rahman, A.F.M.T.; Reza, H.M. Astaxanthin ameliorates hepatic damage and oxidative stress in carbon tetrachloride-administered rats. Pharmacogn. Res. 2017, 9 (Suppl. S1), S84–S91. [Google Scholar] [CrossRef] [PubMed]
- Bencheikh, N.; Bouhrim, M.; Kharchoufa, L.; Choukri, M.; Bnouham, M.; Elachouri, M. Protective Effect of Zizyphus lotus L. (Desf.) Fruit against CCl4-Induced Acute Liver Injury in Rat. Evid.-Based Complement. Altern. Med. 2019, 2019, 6161593. [Google Scholar] [CrossRef]
- Girish, C.; Koner, B.C.; Jayanthi, S.; Rao, K.R.; Rajesh, B.; Pradhan, S.C. Hepatoprotective activity of six polyherbal formulations in CCl4-induced liver toxicity in mice. Indian J. Exp. Biol. 2009, 47, 257–263. [Google Scholar]
- Agrawal, S.; Dhiman, R.K.; Limdi, J.K. Evaluation of abnormal liver function tests. Postgrad. Med. J. 2016, 92, 223–234. [Google Scholar] [CrossRef]
- Aubrecht, J.; Schomaker, S.J.; Amacher, D.E. Emerging hepatotoxicity biomarkers and their potential to improve understanding and management of drug-induced liver injury. Genome Med. 2013, 5, 85. [Google Scholar] [CrossRef]
- Chowdhury, M.R.H.; Sagor, M.A.T.; Tabassum, N.; Potol, M.A.; Hossain, H.; Alam, M.A. Supplementation of Citrus maxima peel powder prevented oxidative stress, fibrosis, and hepatic damage in carbon tetrachloride (CCl 4) treated rats. Evid.-Based Complement. Altern. Med. 2015, 2015, 598179. [Google Scholar] [CrossRef]
- Sharma, U.; Pal, D.; Prasad, R. Alkaline Phosphatase: An Overview. Indian J. Clin. Biochem. 2014, 29, 269–278. [Google Scholar] [CrossRef]
- Adedara, I.; Owumi, S.; Uwaifo, A.; Farombi, E. Aflatoxin B1 and ethanol co-exposure induces hepatic oxidative damage in mice. Toxicol. Ind. Health 2010, 26, 717–724. [Google Scholar] [CrossRef]
- Rahman, A.; Yamazaki, D.; Sufiun, A.; Kitada, K.; Hitomi, H.; Nakano, D.; Nishiyama, A. A novel approach to adenine-induced chronic kidney disease associated anemia in rodents. PLoS ONE 2018, 7, e0192531. [Google Scholar] [CrossRef]
- Bafana, A.; Dutt, S.; Kumar, A.; Kumar, S.; Ahuja, P.S. The basic and applied aspects of superoxide dismutase. J. Mol. Catal. B Enzym. 2011, 68, 129–138. [Google Scholar] [CrossRef]
- Napierala, M.; Olszewski, J.; Miechowicz, I.; Jablecka, A.; Czarnywojtek, A.; Malinger, S.; Florek, E. The influence of tobacco smoke exposure on selected markers of oxidative stress, kidneys and liver function in the serum of rats with streptozotocin-induced diabetes. Pharmacol. Rep. 2019, 71, 1293–1298. [Google Scholar] [CrossRef]
- Napierala, M.; Merritt, T.; Mazela, J.; Jablecka, K.; Miechowicz, I.; Marszalek, A.; Florek, E. The effect of tobacco smoke on oxytocin concentrations and selected oxidative stress parameters in plasma during pregnancy and post-partum—An experimental model. Hum. Exp. Toxicol. 2017, 36, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.D.; Sadda, M.R.; Mendler, M.H.; Bottiglieri, T.; Kanel, G.; Mato, J.M.; Lu, S.C. Abnormal Hepatic Methionine and Glutathione Metabolism in Patients With Alcoholic Hepatitis. Alcohol. Clin. Exp. Res. 2004, 28, 173–181. [Google Scholar] [CrossRef]
- Pastore, A.; Panera, N.; Mosca, A.; Caccamo, R.; Camanni, D.; Crudele, A.; De Stefanis, C.; Alterio, A.; Di Giovamberardino, G.; De Vito, R.; et al. Changes in total homocysteine and glutathione levels after laparoscopic sleeve gastrectomy in children with metabolic-associated fatty liver disease. Obes. Surg. 2022, 32, 82–89. [Google Scholar] [CrossRef]
- Metwally, N.S.; Kholeif, T.E.; Ghanem, K.Z.; Farrag, A.R.H.; Ammar, N.M.; Abdel-Hamid, A.H.Z. The protective effects of fish oil and artichoke on hepatocellular carcinoma in rats. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 1429–1444. [Google Scholar] [PubMed]
- El Morsy, E.M.; Kamel, R. Protective effect of artichoke leaf extract against paracetamol-induced hepatotoxicity in rats. Pharm. Biol. 2015, 53, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, Y.; Fernandez, B.O.; Steinbicker, A.U.; Spagnolli, E.; Malhotra, R.; Bloch, D.B.; Bloch, K.D.; Zapol, W.M.; Feelisch, M. Pharmacological preconditioning with inhaled nitric oxide (NO): Organ-specific differences in the lifetime of blood and tissue NO metabolites. Nitric Oxide 2018, 80, 52–60. [Google Scholar] [CrossRef] [PubMed]
- El-Sherif, A.M.; Abou-Shady, M.A.; Al-Bahrawy, A.M.; Bakr, R.M.; Hosny, A.-M.M. Nitric oxide levels in chronic liver disease patients with and without oesophageal varices. Hepatol. Int. 2008, 2, 341–345. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bakdemir, M.; Çetin, E. Hepatoprotective effects of ethyl pyruvate against carbon tetrachloride-induced oxidative stress, biochemical and histological alterations in rats. Arch. Physiol. Biochem. 2021, 127, 359–366. [Google Scholar] [CrossRef]
- Taysi, S.; Umudum, Z.; Sari, R.A.; Kuskay, S.; Bakan, N. Nitric oxide level and superoxide dismutase activity in serum of patients with rheumatoid arthritis. Pain Clin. 2003, 15, 429–434. [Google Scholar] [CrossRef]
- Kujawska, M.; Ignatowicz, E.; Murias, M.; Ewertowska, M.; Mikołajczyk, K.; Jodynis-Liebert, J. Protective Effect of Red Beetroot against Carbon Tetrachloride- and N-Nitrosodiethylamine-Induced Oxidative Stress in Rats. J. Agric. Food Chem. 2009, 57, 2570–2575. [Google Scholar] [CrossRef]
- Betancor-Ferna, A.; Pe, A.; Sies, H.; Stahl, W. Screening pharmaceutical preparations containing extracts of turmeric rhizome, artichoke leaf, devil’s claw root and garlic or salmon oil for antioxidant capacity. J. Pharm. Pharmacol. 2003, 55, 981–986. [Google Scholar] [CrossRef]
- Ras, A.; Milanovic, I.; Pavlovic, N.; Cebović, T.; Vukmirovic, S.; Mikov, M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement. Altern. Med. 2014, 14, 225. [Google Scholar] [CrossRef]
- Lv, D.; Zhu, C.-Q.; Liu, L. Sesamin ameliorates oxidative liver injury induced by carbon tetrachloride in rat. Int. J. Clin. Exp. Pathol. 2015, 8, 5733–5738. [Google Scholar]
- Al-Sayed, E.; El-Lakkany, N.M.; El-Din, S.H.S.; Sabra, A.-N.A.; Hammam, O.A. Hepatoprotective and antioxidant activity of Melaleuca styphelioideson carbon tetrachloride-induced hepatotoxicity in mice. Pharm. Biol. 2014, 52, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Bi, L.; Jin, L.; Wang, Y.; Li, Y.; Li, Z.; He, W.; Cui, H.; Miao, J.; Wang, L. Geniposide Ameliorates Liver Fibrosis through Reducing Oxidative Stressand Inflammatory Respose, Inhibiting Apoptosis and Modulating Overall Metabolism. Front. Pharmacol. 2021, 12, 772635. [Google Scholar] [CrossRef]
- Zan, M.A.; Ferraz, A.B.F.; Richter, M.F.; Picada, J.N.; de Andrade, H.H.R.; Lehmann, M.; Dihl, R.R.; Nunes, E.; Semedo, J.; Da Silva, J. In vivo genotoxicity evaluation of an artichoke (Cynara scolymus L.) aqueous extract. J. Food Sci. 2013, 78, T367–T371. [Google Scholar] [CrossRef] [PubMed]
- Speroni, E.; Cervellati, R.; Govoni, P.; Guizzardi, S.; Renzulli, C.; Guerra, M.C. Efficacy of different Cynara scolymus preparations on liver complaints. J. Ethnopharmacol. 2003, 86, 203–211. [Google Scholar] [CrossRef] [PubMed]


| Time (min) | Phase A (%) | Phase B (%) |
|---|---|---|
| 0 | 93 | 7 |
| 18 | 93 | 7 |
| 22 | 75 | 25 |
| 33 | 75 | 25 |
| 35 | 0 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florek, E.; Szukalska, M.; Markiewicz, K.; Miechowicz, I.; Gornowicz-Porowska, J.; Jelińska, A.; Kasprzyk-Pochopień, J.; Nawrot, J.; Sobczak, A.; Horoszkiewicz, M.; et al. Evaluation of the Protective and Regenerative Properties of Commercially Available Artichoke Leaf Powder Extract on Plasma and Liver Oxidative Stress Parameters. Antioxidants 2023, 12, 1846. https://doi.org/10.3390/antiox12101846
Florek E, Szukalska M, Markiewicz K, Miechowicz I, Gornowicz-Porowska J, Jelińska A, Kasprzyk-Pochopień J, Nawrot J, Sobczak A, Horoszkiewicz M, et al. Evaluation of the Protective and Regenerative Properties of Commercially Available Artichoke Leaf Powder Extract on Plasma and Liver Oxidative Stress Parameters. Antioxidants. 2023; 12(10):1846. https://doi.org/10.3390/antiox12101846
Chicago/Turabian StyleFlorek, Ewa, Marta Szukalska, Katarzyna Markiewicz, Izabela Miechowicz, Justyna Gornowicz-Porowska, Anna Jelińska, Joanna Kasprzyk-Pochopień, Joanna Nawrot, Agnieszka Sobczak, Małgorzata Horoszkiewicz, and et al. 2023. "Evaluation of the Protective and Regenerative Properties of Commercially Available Artichoke Leaf Powder Extract on Plasma and Liver Oxidative Stress Parameters" Antioxidants 12, no. 10: 1846. https://doi.org/10.3390/antiox12101846
APA StyleFlorek, E., Szukalska, M., Markiewicz, K., Miechowicz, I., Gornowicz-Porowska, J., Jelińska, A., Kasprzyk-Pochopień, J., Nawrot, J., Sobczak, A., Horoszkiewicz, M., Piekoszewski, W., & Nowak, G. (2023). Evaluation of the Protective and Regenerative Properties of Commercially Available Artichoke Leaf Powder Extract on Plasma and Liver Oxidative Stress Parameters. Antioxidants, 12(10), 1846. https://doi.org/10.3390/antiox12101846







