Multi-Effects of Acute Salinity Stress on Osmoregulation, Physiological Metabolism, Antioxidant Capacity, Immunity, and Apoptosis in Macrobrachium rosenbergii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Design
2.2. Sample Collection
2.3. Detection of Hemolymph Osmolality
2.4. Assessment of Physiological Metabolism
2.5. Estimation of Anti-Oxidative Parameter and Immunity Index
2.6. Histological Analysis
2.7. TUNEL Assays
2.8. RNA Extraction and cDNA Synthesis
2.9. Real-Time Quantitative PCR (qPCR)
2.10. Correlation Analysis
2.11. Statistical Analysis
3. Results
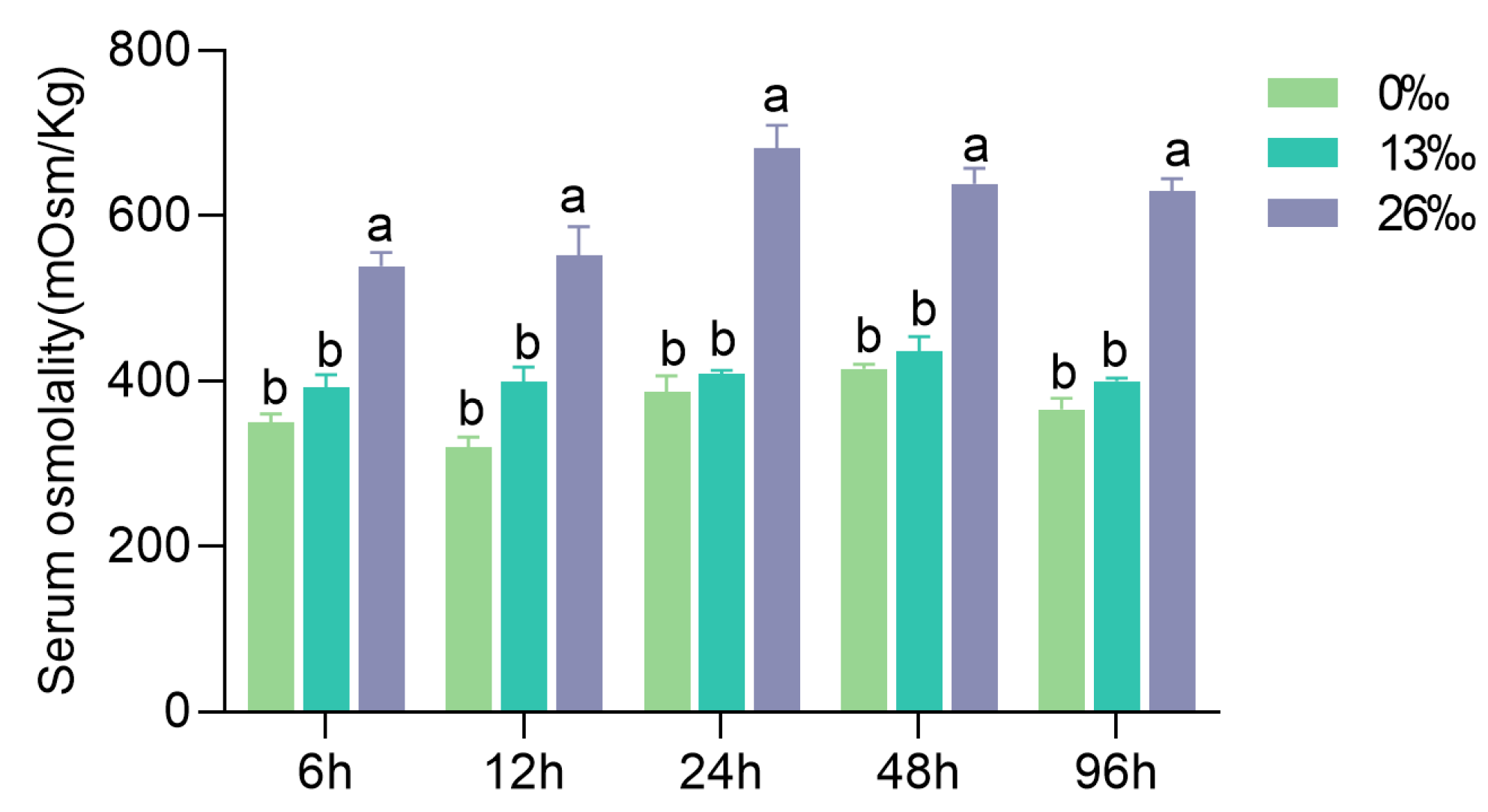
3.1. Hemolymph Osmolality
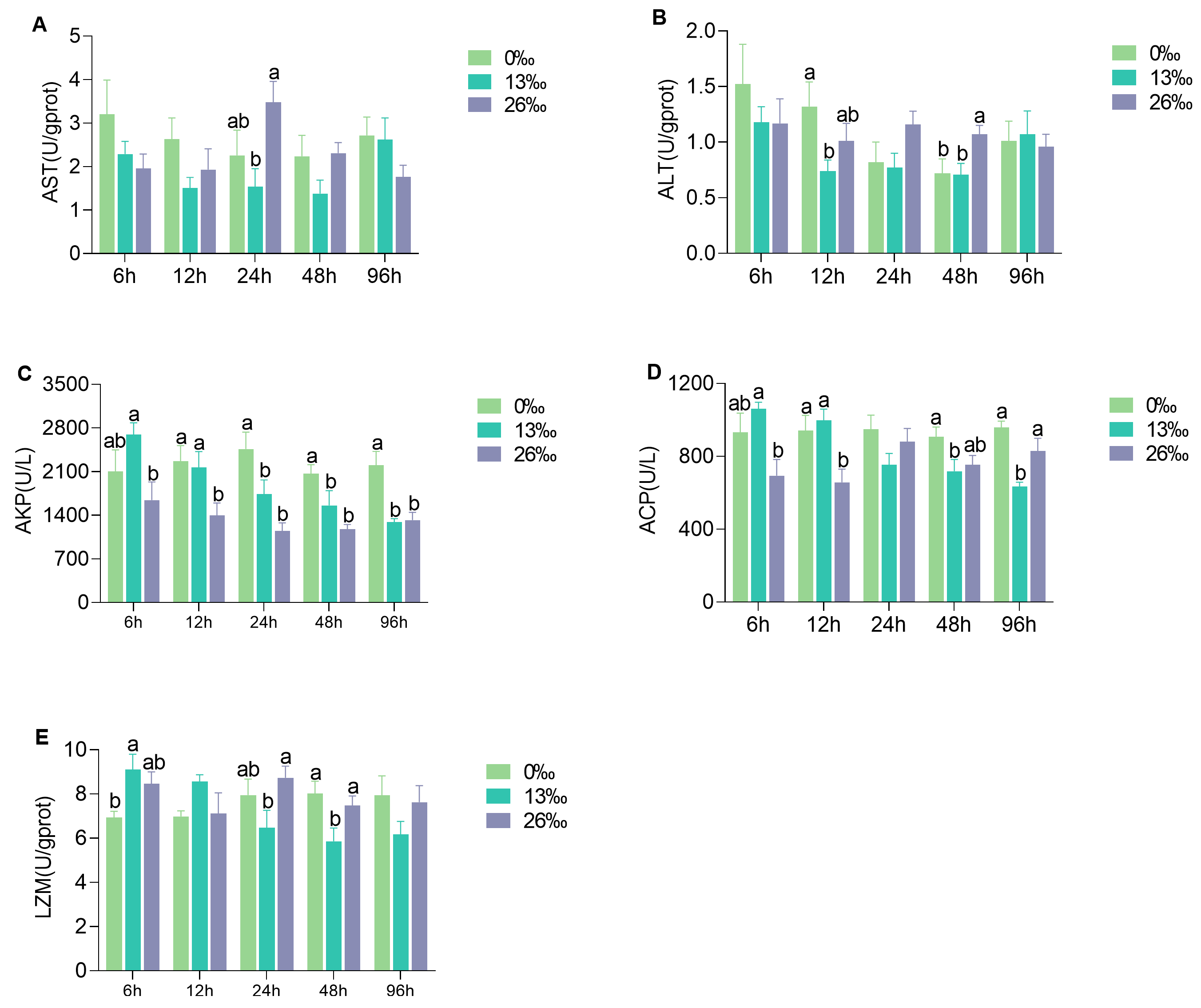
3.2. Physiological Metabolism Parameters in Hepatopancreas
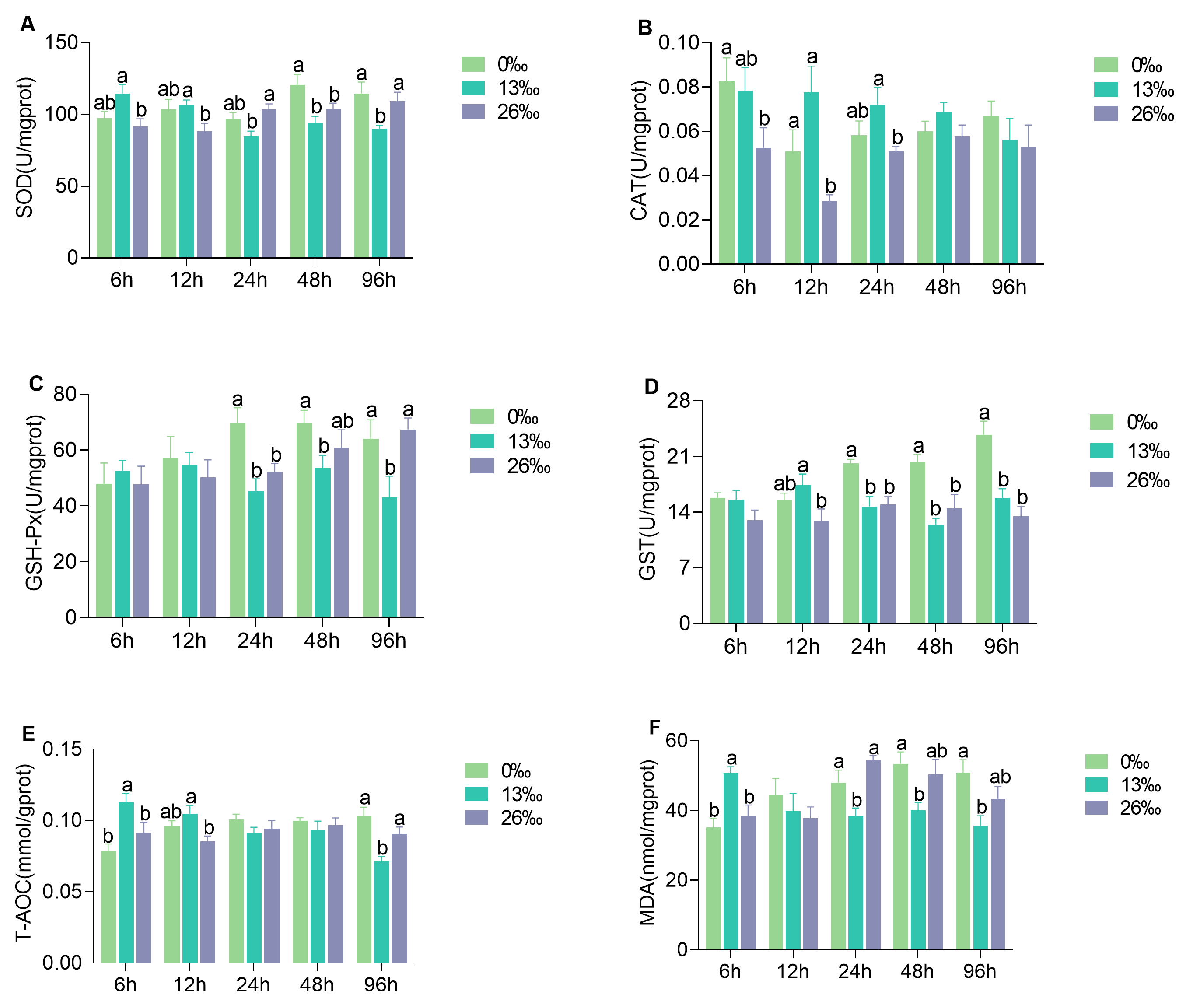
3.3. Antioxidant Enzyme Activities of Hepatopancreas
3.4. Immunocompetent Response to Salinity Stress
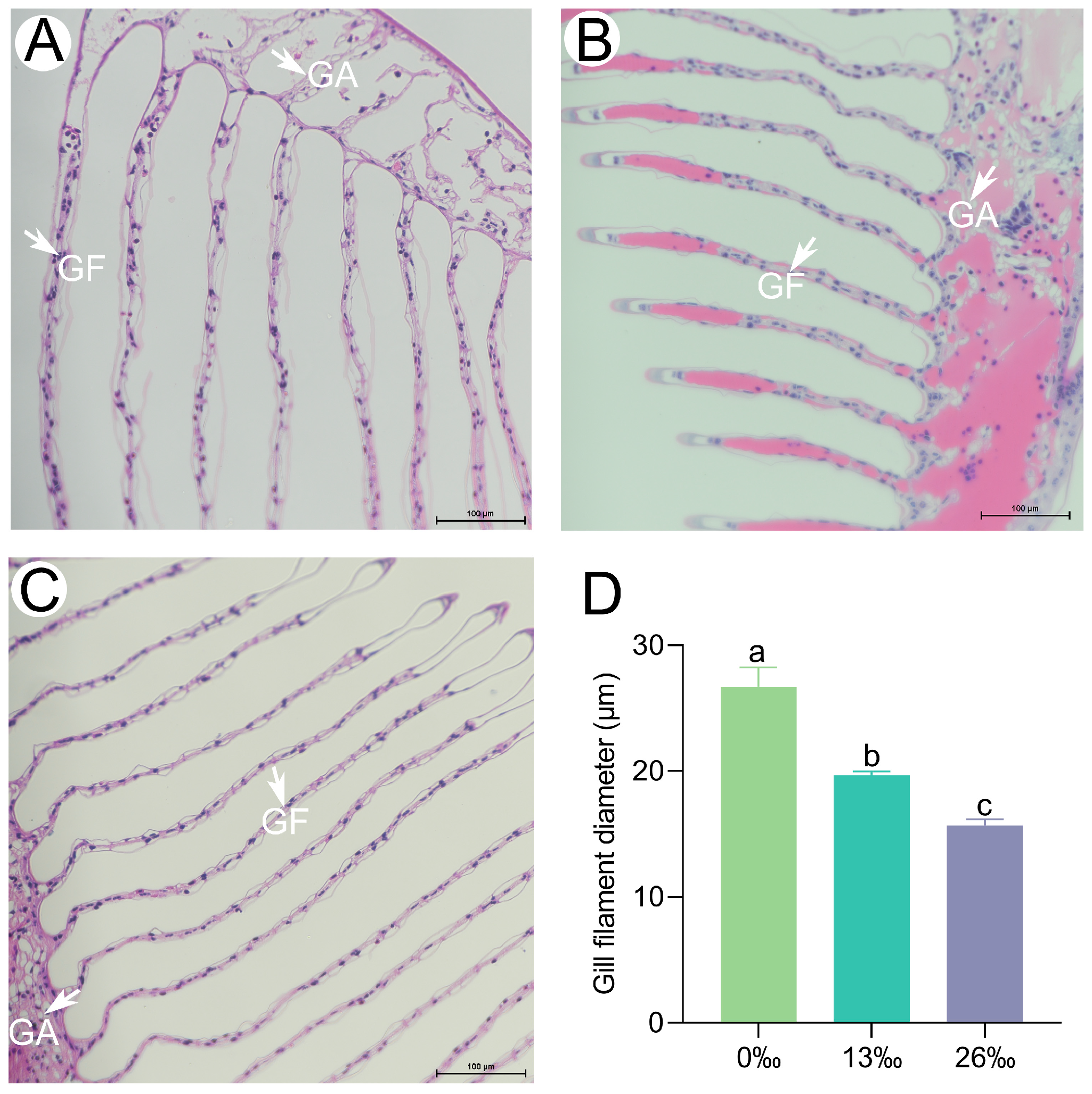
3.5. Histological Characteristics of Gills
3.6. Tissue Apoptosis Analysis
3.7. mRNA Expression of Apoptosis-Related Genes
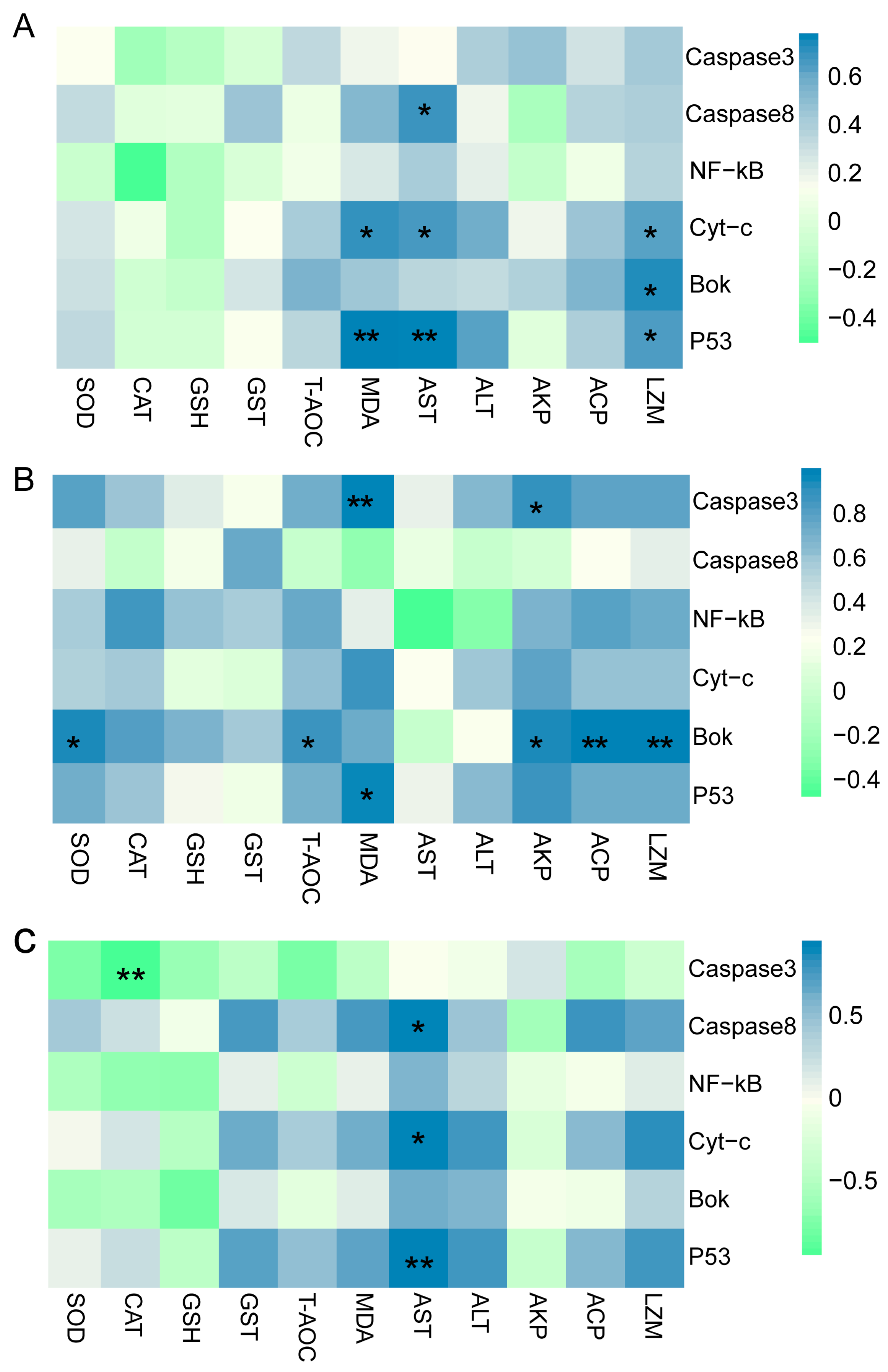
3.8. Correlation Analysis between Antioxidant Parameters and Apoptosis-Related Genes
4. Discussion
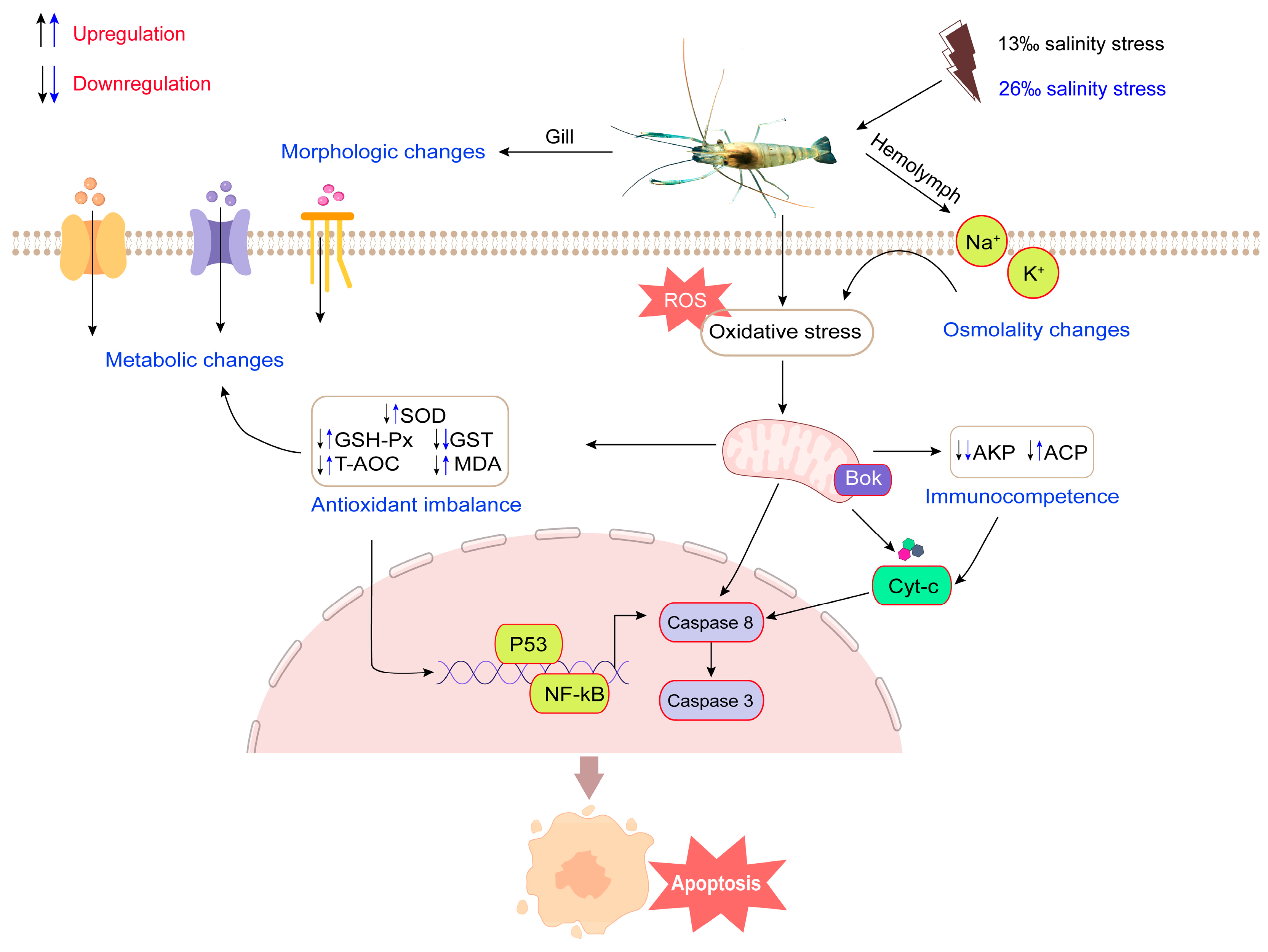
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, K.; Li, E.; Xu, C.; Wang, X.; Li, H.; Qin, J.G.; Chen, L. Growth and metabolomic responses of Pacific white shrimp (Litopenaeus vannamei) to different dietary fatty acid sources and salinity levels. Aquaculture 2019, 499, 329–340. [Google Scholar] [CrossRef]
- Yen, P.T.; Bart, A.N. Salinity effects on reproduction of giant freshwater prawn Macrobrachium rosenbergii (De Man). Aquaculture 2008, 280, 124–128. [Google Scholar] [CrossRef]
- Abou Anni, I.S.; Bianchini, A.; Barcarolli, I.F.; Varela, A.S.; Robaldo, R.B.; Tesser, M.B.; Sampaio, L.A. Salinity influence on growth, osmoregulation and energy turnover in juvenile pompano Trachinotus marginatus Cuvier 1832. Aquaculture 2016, 455, 63–72. [Google Scholar] [CrossRef]
- Herborg, L.M.; Rushton, S.P.; Clare, A.S.; Bentley, M.G. Spread of the Chinese mitten crab (Eriocheir sinensis H. Milne Edwards) in continental Europe: Analysis of a historical data set. Hydrobiologia 2003, 503, 21–28. [Google Scholar] [CrossRef]
- Dingle, H. Animal migration: Is there a common migratory syndrome? J. Ornithol. 2006, 147, 212–220. [Google Scholar] [CrossRef]
- Rotllant, G.; Simeó, C.G.; Macià, G.; Estévez, A. High environmental salinity reduces the reproductive potential of the spider crab (Maja brachydactyla) (Decapoda, Majidae). Mar. Ecol. 2014, 36, 496–505. [Google Scholar] [CrossRef]
- Anger, K.; Charmantier, G. Ontogeny of osmoregulation and salinity tolerance in a mangrove crab, Sesarma curacaoense (Decapoda: Grapsidae). J. Exp. Mar. Biol. Ecol. 2000, 251, 265–274. [Google Scholar] [CrossRef]
- Qi, T.; Liu, J.; Zhao, P.; Ge, B.; Liu, Q.; Jiang, S.; Wang, Z.; Zhang, H.; Tang, B.; Ding, G.; et al. A novel modulation of physiological regulation in cultured Chinese mitten crab (Eriocheir japonica Sinensis) in response to consistent salinity changes. Gene 2020, 756, 144914. [Google Scholar] [CrossRef]
- Anand, P.S.S.; Aravind, R.; Balasubramanian, C.P.; Kumar, S.; Antony, J.; Biju, I.F.; Sangeetha, V.L.; Ambasankar, K.; Vijayan, K.K. Growth, survival, and osmo-ionic regulation in post larval and juvenile Indian white shrimp, Penaeus indicus, reared under three levels of salinity in a semifloc system. Aquaculture 2023, 564, 739042. [Google Scholar] [CrossRef]
- Huong, D.T.T.; Jasmani, S.; Jayasankar, V.; Wilder, M. Na+/K+-ATPase activity and osmo-ionic regulation in adult whiteleg shrimp Litopenaeus vannamei exposed to low salinities. Aquaculture 2010, 304, 88–94. [Google Scholar] [CrossRef]
- Furriel, R.P.M.; Firmino, K.C.S.; Masui, D.C.; Faleiros, R.O.; Torres, A.H.; McNamara, J.C. Structural and biochemical correlates of Na+,K+-Atpase driven ion uptake across the posterior gill epithelium of the true freshwater crab, Dilocarcinus pagei (Brachyura, Trichodactylidae). J. Exp. Zool. Part A 2010, 313, 508–523. [Google Scholar] [CrossRef]
- Leone, F.A.; Garçon, D.P.; Lucena, M.N.; Faleiros, R.O.; Azevedo, S.V.; Pinto, M.R.; McNamara, J.C. Gill-specific (Na+,K+)-ATPase activity and a-subunit mRNA expression during low-salinity acclimation of the ornate blue crab Callinectes ornatus (Decapoda, Brachyura). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2015, 186, 59–67. [Google Scholar] [CrossRef]
- Shui, C.; Shi, Y.; Hua, X.; Zhang, Z.; Zhang, H.; Lu, G.; Xie, Y. Serum osmolality and ions, and gill Na+/K+-ATPase of spottedtail goby Synechogobius ommaturus (R.) in response to acute salinity Changes. Aquac. Fish. 2018, 3, 79–83. [Google Scholar] [CrossRef]
- Rosas, C.; Bolongaro-Crevenna, A.; Sánchez, A.; Gaxiola, G.; Soto, L.; Escobar, E. Role of digestive gland in the energetic metabolism of Penaeus setiferus. Biol. Bull. 1995, 189, 168–174. [Google Scholar] [CrossRef]
- Li, E.; Wang, X.; Chen, K.; Xu, C.; Qin, J.G.; Chen, L. Physiological change and nutritional requirement of Pacific white shrimp Litopenaeus vannamei at low salinity. Rev. Fish. Sci. 2017, 9, 57–75. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, A.; Yuan, C.; Zhao, T.; Chang, H.; Zhang, J. Transcriptome analysis of liver lipid metabolism disorders of the turbot Scophthalmus maximus in response to low salinity stress. Aquaculture 2021, 534, 736273. [Google Scholar] [CrossRef]
- Huong, D.T.T.; Yang, W.J.; Okuno, A.; Wilder, M.N. Changes in free amino acids in the hemolymph of giant freshwater prawn Macrobrachium rosenbergii exposed to varying salinities: Relationship to osmoregulatory ability. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 128, 317–326. [Google Scholar] [CrossRef]
- Koyama, H.; Kuniyoshi, H.; Piyapattanakorn, S.; Watabe, S. Changes in metabolite concentrations in the abdominal muscle of the Kuruma shrimp Marsupenaeus japonicus in response to different salinities. Fish. Sci. 2021, 87, 383–401. [Google Scholar] [CrossRef]
- Paital, B.; Chainy, G.B.N. Antioxidant defenses and oxidative stress parameters in tissues of mud crab (Scylla serrata) with reference to changing salinity. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010, 151, 142–151. [Google Scholar] [CrossRef]
- Frías-Espericueta, M.G.; Bautista-Covarrubias, J.C.; Osuna-Martínez, C.C.; Delgado-Alvarez, C.; Bojórquez, C.; Aguilar-Juárez, M.; Roos-Muñoz, S.; Osuna-López, I.; Páez-Osuna, F. Metals and oxidative stress in aquatic decapod crustaceans: A review with special reference to shrimp and crabs. Aquat. Toxicol. 2022, 242, 106024. [Google Scholar] [CrossRef]
- Chu, P.; Luo, S.; Wang, H.; Zhang, K.; Wen, X.; Yin, S.; Wang, T. Interactive effects of temperature and salinity on the apoptosis, antioxidant enzymes, and mapk signaling pathway of juvenile pufferfish (Takifugu fasciatus). Aquac. Rep. 2023, 29, 101483. [Google Scholar] [CrossRef]
- Li, E.; Chen, L.; Zeng, C.; Yu, N.; Xiong, Z.; Chen, X.; Qin, J.G. Comparison of digestive and antioxidant enzymes activities, haemolymph oxyhemocyanin contents and hepatopancreas histology of white shrimp, Litopenaeus vannamei, at various salinities. Aquaculture 2008, 274, 80–86. [Google Scholar] [CrossRef]
- Intanai, I.; Taylor, E.W.; Whiteley, N.M. Effects of salinity on rates of protein synthesis and oxygen uptake in the post-larvae and juveniles of the tropical prawn Macrobrachium rosenbergii (De Man). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 152, 372–378. [Google Scholar] [CrossRef]
- Liu, B.; Gao, Q.; Liu, B.; Song, C.; Sun, C.; Liu, M.; Liu, X.; Liu, Y.; Li, Z.; Zhou, Q.; et al. Application of transcriptome analysis to understand the adverse effects of hypotonic stress on different development stages in the giant freshwater prawn Macrobrachium rosenbergii post-larvae. Antioxidants 2022, 11, 440. [Google Scholar] [CrossRef]
- Huong, D.T.T.; Wang, T.; Bayley, M.; Phuong, N.T. Osmoregulation, growth and moulting cycles of the giant freshwater prawn (Macrobrachium rosenbergii) at different salinities. Aquac. Res. 2010, 41, e135–e143. [Google Scholar] [CrossRef]
- França, J.L.; Pinto, M.R.; Lucena, M.N.; Garçon, D.P.; Valenti, W.C.; McNamara, J.C.; Leone, F.A. Subcellular localization and kinetic characterization of a gill (Na+, K+)-ATPase from the giant freshwater prawn Macrobrachium rosenbergii. J. Membr. Biol. 2013, 246, 529–543. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, C.; Cheng, C.; Chen, J. Hemolymph oxyhemocyanin, protein, osmolality and electrolyte levels of Macrobrachium rosenbergii in relation to size and molt stage. Aquaculture 2001, 198, 387–400. [Google Scholar] [CrossRef]
- Chand, B.K.; Trivedi, R.K.; Dubey, S.K.; Rout, S.K.; Beg, M.M.; Das, U.K. Effect of salinity on survival and growth of giant freshwater prawn Macrobrachium rosenbergii (De Man). Aquac. Rep. 2015, 2, 26–33. [Google Scholar] [CrossRef]
- Lu, Z.; Yang, G.; Qin, Z.; Shen, H.; Zhang, M.; Shi, F.; Li, J.; Sarath Babu, V.; Lin, L. Glutamate related osmoregulation of guanine nucleotide-binding protein G (I) A2 from giant freshwater prawn (Macrobrachium rosenbergii) during molting and salinity stress. Aquaculture 2020, 521, 735000. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, J.; Hong, K.; Zhou, N.; Liu, X.; Hong, X.; Li, W.; Zhao, J.; Chen, C.; Wu, L.; et al. Transcriptome analysis reveals the molecular response to salinity challenge in larvae of the giant freshwater prawn Macrobrachium rosenbergii. Front. Physiol. 2022, 13, 885035. [Google Scholar] [CrossRef]
- Darzynkiewicz, Z.; Galkowski, D.; Zhao, H. Analysis of apoptosis by cytometry using TUNEL assay. Methods 2008, 44, 250–254. [Google Scholar] [CrossRef]
- Dong, X.; Liu, Q.; Zhao, W.; Ou, J.; Jiang, F.; Guo, H.; Lv, L. Effects of ammonia-N stress on the antioxidant enzymes, heat shock proteins, and apoptosis-related genes of Macrobrachium rosenbergii. Ital. J. Anim. Sci. 2021, 20, 453–464. [Google Scholar] [CrossRef]
- Zhan, F.; Zhou, S.; Shi, F.; Li, Q.; Lin, L.; Qin, Z. Transcriptome analysis of Macrobrachium rosenbergii hemocytes in response to Staphylococcus aureus infection. Fish Shellfish Immunol. 2023, 139, 108927. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.; Li, Y.; Shi, F.; Lu, Z.; Yang, M.; Li, Q.; Lin, L.; Qin, Z. Transcriptome analysis of Macrobrachium rosenbergii hemocytes reveals in-depth insights into the immune response to Vibrio parahaemolyticus infection. Fish Shellfish Immunol. 2023, 133, 108533. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wang, X.; Yao, Q.; Zhang, D.; Lei, X.; Wang, S.; Wan, J.; Liu, H.; Chen, Y.; Zhao, Y.; Wang, G.; et al. Effects of acute salinity stress on osmoregulation, antioxidant capacity and physiological metabolism of female Chinese mitten crabs (Eriocheir sinensis). Aquaculture 2022, 552, 737989. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, L.; Zhao, X.; Cheng, Y. Effects of salinity stress on osmotic pressure, free amino acids, and immune-associated parameters of the juvenile Chinese mitten crab, Eriocheir sinensis. Aquaculture 2022, 549, 737776. [Google Scholar] [CrossRef]
- Huang, Y.H.; Zhang, M.; Li, Y.M.; Wu, D.L.; Liu, Z.Q.; Jiang, Q.C.; Zhao, Y.L. Effects of salinity acclimation on the growth performance, osmoregulation and energy metabolism of the oriental river prawn, Macrobrachium nipponense (De Haan). Aquac. Res. 2018, 50, 685–693. [Google Scholar] [CrossRef]
- Wang, W.N.; Wang, A.L.; Bao, L.; Wang, J.P.; Liu, Y.; Sun, R.Y. Changes of protein-bound and free amino acids in the muscle of the freshwater prawn Macrobrachium nipponense in different salinities. Aquaculture 2004, 233, 561–571. [Google Scholar] [CrossRef]
- Shinji, J.; Wilder, M.N. Dynamics of free amino acids in the hemolymph of Pacific whiteleg shrimp Litopenaeus vannamei exposed to different types of stress. Fish. Sci. 2012, 78, 1187–1194. [Google Scholar] [CrossRef]
- Wang, X.D.; Li, E.C.; Chen, K.; Wang, S.F.; Li, T.Y.; Xu, C.; Yu, N.; Qin, J.G.; Chen, L.Q. Response of facilitative glucose transporter 1 to salinity stress and dietary carbohydrate nutrition in white shrimp Litopenaeus vannamei. Aquac. Nutr. 2017, 23, 90–100. [Google Scholar] [CrossRef]
- Su, C.; Li, J.; Zhang, M.; Pan, L.; Wang, Y.; Ding, Y.; Chen, Z.; Lu, M. Dietary cholesterol enhances osmoregulation, antioxidant defenses and immune response of Litopenaeus vannamei to alleviate the macromolecular damage induced by salinity Stress. Aquaculture 2023, 563, 738861. [Google Scholar] [CrossRef]
- Chen, K.; Li, E.; Gan, L.; Wang, X.; Xu, C.; Lin, H.; Qin, J.G.; Chen, L. Growth and lipid metabolism of the Pacific white shrimp Litopenaeus vannamei at different salinities. J. Shellfish Res. 2014, 33, 825–832. [Google Scholar] [CrossRef]
- Lassoued, A.; Khalloufi, N.; Saidani, W.; Khazri, A.; Ghanem-Boughanmi, N.; Bouayed, J.; Ben-Attia, M. Effects of increased salinity on oxidative stress status in the freshwater mussel Unio ravoisieri. Chem. Ecol. 2023, 39, 256–267. [Google Scholar] [CrossRef]
- Bal, A.; Panda, F.; Pati, S.G.; Das, K.; Agrawal, P.K.; Paital, B. Modulation of physiological oxidative stress and antioxidant status by abiotic factors especially salinity in aquatic organisms. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 241, 108971. [Google Scholar] [CrossRef] [PubMed]
- Pati, S.G.; Panda, F.; Paital, B.; Sahoo, D.K.; Jena, S. Oxidative stress physiology in Scylla serrata for environmental health assessment. Front. Env. Sci. 2023, 11, 1142495. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, L.; Jiang, W.; Liu, Y.; Jiang, J.; Kuang, S.; Li, S.; Tang, L.; Tang, W.; Zhou, X.; et al. Dietary vitamin A improved the flesh quality of grass carp (Ctenopharyngodon idella) in relation to the enhanced antioxidant capacity through Nrf2/Keap 1a signaling pathway. Antioxidants 2022, 11, 148. [Google Scholar] [CrossRef]
- Wei, K.; Yang, J. Oxidative damage induced by copper and beta-cypermethrin in gill of the freshwater crayfish Procambarus clarkii. Ecotoxicol. Environ. Saf. 2015, 113, 446–453. [Google Scholar] [CrossRef]
- Yang, S.P.; Liu, H.L.; Wang, C.G.; Yang, P.; Sun, C.B.; Chan, S.M. Effect of oxidized fish oil on growth performance and oxidative stress of Litopenaeus vannamei. Aquac. Nutr. 2014, 21, 121–127. [Google Scholar] [CrossRef]
- Sinha, A.K.; AbdElgawad, H.; Zinta, G.; Dasan, A.F.; Rasoloniriana, R.; Asard, H.; Blust, R.; De Boeck, G. Nutritional status as the key modulator of antioxidant responses induced by high environmental ammonia and salinity stress in European sea bass (Dicentrarchus labrax). PLoS ONE 2015, 10, e0135091. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, J.A.; Lee, D.W.; Park, Y.S.; Kim, J.H.; Choi, C.Y. Oxidative stress and apoptosis in disk abalone (Haliotis discus Hannai) caused by water temperature and pH changes. Antioxidants 2023, 12, 1003. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Cui, Y.; Wang, R.; Chen, Y.; Zhao, N.; Wang, C.; Wang, Z.; Li, Y. Combined effects of high salinity and ammonia-N exposure on the energy metabolism, immune response, oxidative resistance and ammonia metabolism of the Pacific white shrimp Litopenaeus vannamei. Aquac. Rep. 2021, 20, 100648. [Google Scholar] [CrossRef]
- Chien, Y.H.; Pan, C.H.; Hunter, B. The resistance to physical stresses by Penaeus monodon juveniles fed diets supplemented with astaxanthin. Aquaculture 2003, 216, 177–191. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Noreldin, A.E.; Sewilam, H. Blood biochemical variables, antioxidative status, and histological features of intestinal, gill, and liver tissues of African catfish (Clarias gariepinus) exposed to high salinity and high-temperature stress. Environ. Sci. Pollut. Res. 2022, 29, 56357–56369. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, X.; Yang, Q.; Liu, H.; Nie, Q.; Hu, J.; Chi, S.; Tan, B. Effect of yeast hydrolysate supplementation on the resistance of Pacific white shrimp, Litopenaeus vannamei, challenged with low salinity stress. Aquac. Res. 2021, 53, 409–418. [Google Scholar] [CrossRef]
- Wei, H.; Chen, M.; Deng, Z.; Zhao, W.; Li, Y.; Fang, W.; Ma, Z.; Wang, Y.; Yu, G. Immune and antioxidant responses of pearl oyster Pinctada maxima exposed to acute salinity stress. Aquac. Res. 2022, 53, 2439–2447. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Li, L.; Zhang, W.; Lu, W. Effects of salinity on the physiological responses of the large yellow croaker Pseudosciaena crocea under indoor culture conditions. Aquac. Res. 2015, 47, 3410–3420. [Google Scholar] [CrossRef]
- Chang, C.; Wang, Y.; Lee, T. Hypothermal stress-induced salinity-dependent oxidative stress and apoptosis in the livers of euryhaline milkfish, Chanos chanos. Aquaculture 2021, 534, 736280. [Google Scholar] [CrossRef]
- Xue, C.; Xu, K.; Jin, Y.; Bian, C.; Sun, S. Transcriptome analysis to study the molecular response in the gill and hepatopancreas tissues of Macrobrachium nipponense to salinity acclimation. Front. Physiol. 2022, 13, 926885. [Google Scholar] [CrossRef]
- Wang, H.; Wei, H.; Tang, L.; Lu, J.; Mu, C.; Wang, C. A proteomics of gills approach to understanding salinity adaptation of Scylla paramamosain. Gene 2018, 677, 119–131. [Google Scholar] [CrossRef]
- Xu, W.B.; Zhang, Y.M.; Li, B.Z.; Lin, C.Y.; Chen, D.Y.; Cheng, Y.X.; Guo, X.L.; Dong, W.R.; Shu, M.A. Effects of low salinity stress on osmoregulation and gill transcriptome in different populations of mud crab Scylla paramamosain. Sci. Total Environ. 2023, 867, 161522. [Google Scholar] [CrossRef]
- Yin, X.; Zhuang, X.; Liao, M.; Cui, Q.; Yan, C.; Huang, J.; Jiang, Z.; Huang, L.; Luo, W.; Liu, Y.; et al. Andrographis paniculata improves growth and non-specific immunity of shrimp Litopenaeus vannamei, and protects it from Vibrio alginolyticus by reducing oxidative stress and apoptosis. Dev. Comp. Immunol. 2023, 139, 104542. [Google Scholar] [CrossRef]
- Fritsch, M.; Günther, S.D.; Schwarzer, R.; Albert, M.-C.; Schorn, F.; Werthenbach, J.P.; Schiffmann, L.M.; Stair, N.; Stocks, H.; Seeger, J.M.; et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 2019, 575, 683–687. [Google Scholar] [CrossRef]
- Jackson, R.; McNeil, B.; Taylor, C.; Holl, G.; Ruff, D.; Gwebu, E.T. Effect of aged garlic extract on caspase-3 activity, in vitro. Nutr. Neurosci. 2002, 5, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Iwai, K.; Kondo, T.; Watanabe, M.; Yabu, T.; Kitano, T.; Taguchi, Y.; Umehara, H.; Takahashi, A.; Uchiyama, T.; Okazaki, T. Ceramide increases oxidative damage due to inhibition of catalase by caspase-3-dependent proteolysis in HL-60 cell apoptosis. J. Biol. Chem. 2003, 278, 9813–9822. [Google Scholar] [CrossRef] [PubMed]
- Kalpage, H.A.; Wan, J.; Morse, P.T.; Zurek, M.P.; Turner, A.A.; Khobeir, A.; Yazdi, N.; Hakim, L.; Liu, J.; Vaishnav, A.; et al. Cytochrome c phosphorylation: Control of mitochondrial electron transport chain flux and apoptosis. Int. J. Biochem. Cell Biol. 2020, 121, 105704. [Google Scholar] [CrossRef] [PubMed]
- Polyak, K.; Xia, Y.; Zweier, J.L.; Kinzler, K.W.; Vogelstein, B. A model for p53-induced apoptosis. Nature 1997, 389, 300–305. [Google Scholar] [CrossRef]
- Ren, X.; Wang, L.; Xu, Y.; Wang, Q.; Lv, J.; Liu, P.; Li, J. Characterization of p53 from the marine crab Portunus trituberculatus and its functions under low salinity conditions. Front. Physiol. 2021, 12, 724693. [Google Scholar] [CrossRef]
- Donner, A.J.; Hoover, J.M.; Szostek, S.A.; Espinosa, J.M. Stimulus-specific transcriptional regulation within the p53 network. Cell Cycle 2007, 6, 2594–2598. [Google Scholar] [CrossRef]
- Belardo, G.; Piva, R.; Santoro, M.G. Heat stress triggers apoptosis by impairing NF-kB survival signaling in malignant B cells. Leukemia 2010, 24, 187–196. [Google Scholar] [CrossRef]
- Chui, A.P.Y.; Wong, Y.H.; Sun, J.; Chang, T.K.T.; Qiu, J.W.; Qian, P.Y.; Ang, P. Effects of heat and hyposalinity on the gene expression in Acropora pruinosa larvae. Front. Mar. Sci. 2023, 10, 1096407. [Google Scholar]
- Zhang, F.; Ren, L.; Zhou, S.; Duan, P.; Xue, J.; Chen, H.; Feng, Y.; Yue, X.; Yuan, P.; Liu, Q.; et al. Role of B-cell lymphoma 2 ovarian killer (Bok) in acute toxicity of human lung epithelial cells caused by cadmium chloride. Med. Sci. Monit. 2019, 25, 5356–5368. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, H.; Wei, J.; Hong, K.; Zhou, Q.; Liu, X.; Hong, X.; Li, W.; Liu, C.; Zhu, X.; et al. Multi-Effects of Acute Salinity Stress on Osmoregulation, Physiological Metabolism, Antioxidant Capacity, Immunity, and Apoptosis in Macrobrachium rosenbergii. Antioxidants 2023, 12, 1836. https://doi.org/10.3390/antiox12101836
Wang Y, Li H, Wei J, Hong K, Zhou Q, Liu X, Hong X, Li W, Liu C, Zhu X, et al. Multi-Effects of Acute Salinity Stress on Osmoregulation, Physiological Metabolism, Antioxidant Capacity, Immunity, and Apoptosis in Macrobrachium rosenbergii. Antioxidants. 2023; 12(10):1836. https://doi.org/10.3390/antiox12101836
Chicago/Turabian StyleWang, Yakun, Huarong Li, Jie Wei, Kunhao Hong, Qiaoyan Zhou, Xiaoli Liu, Xiaoyou Hong, Wei Li, Chao Liu, Xinping Zhu, and et al. 2023. "Multi-Effects of Acute Salinity Stress on Osmoregulation, Physiological Metabolism, Antioxidant Capacity, Immunity, and Apoptosis in Macrobrachium rosenbergii" Antioxidants 12, no. 10: 1836. https://doi.org/10.3390/antiox12101836
APA StyleWang, Y., Li, H., Wei, J., Hong, K., Zhou, Q., Liu, X., Hong, X., Li, W., Liu, C., Zhu, X., & Yu, L. (2023). Multi-Effects of Acute Salinity Stress on Osmoregulation, Physiological Metabolism, Antioxidant Capacity, Immunity, and Apoptosis in Macrobrachium rosenbergii. Antioxidants, 12(10), 1836. https://doi.org/10.3390/antiox12101836





