Effects of Inorganic and Organic Pollutants on the Biomarkers’ Response of Cerastoderma edule under Temperature Scenarios
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Site and Organisms’ Acclimation
2.2. Chemicals
2.3. Bioassays
2.4. Biochemical Analyses
2.4.1. Total Protein Concentration
2.4.2. Catalase (CAT)
2.4.3. Total Glutathione Peroxidase (tGPx)
2.4.4. Glutathione Reductase (GR)
2.4.5. Glutathione S-Transferases (GSTs)
2.4.6. Thiobarbituric Acid Reactive Species (TBARS)
2.4.7. Acetylcholinesterase (AChE)
2.5. Determination of the Integrated Biomarker Response (IBR)
2.6. Data Treatment and Statistical Analyses
2.7. Chemicals Quantification
3. Results and Discussion
3.1. Chemical Analyses of Medium and Tissues Used in Biochemical Analysis
3.2. Lethal Effects
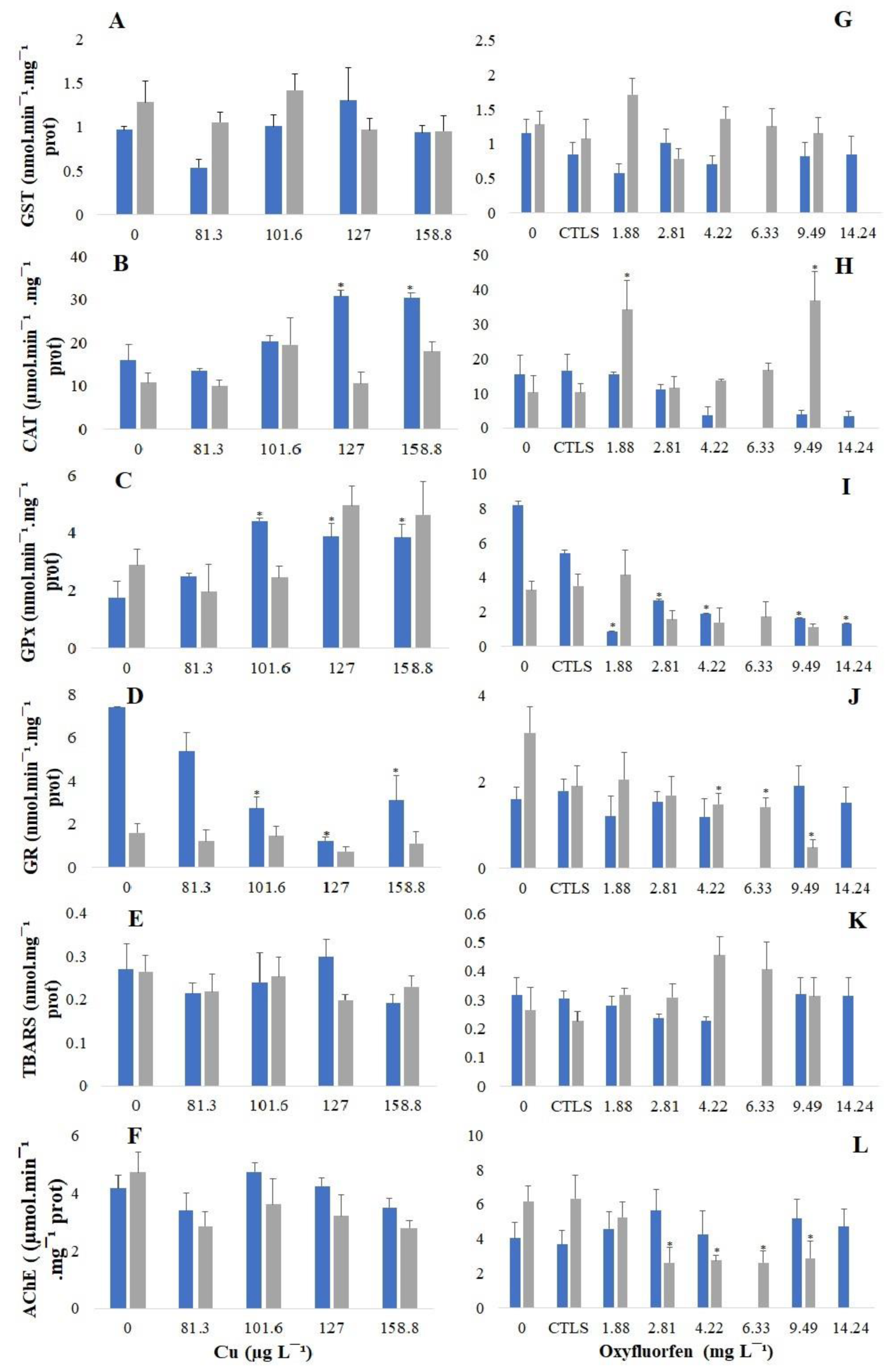
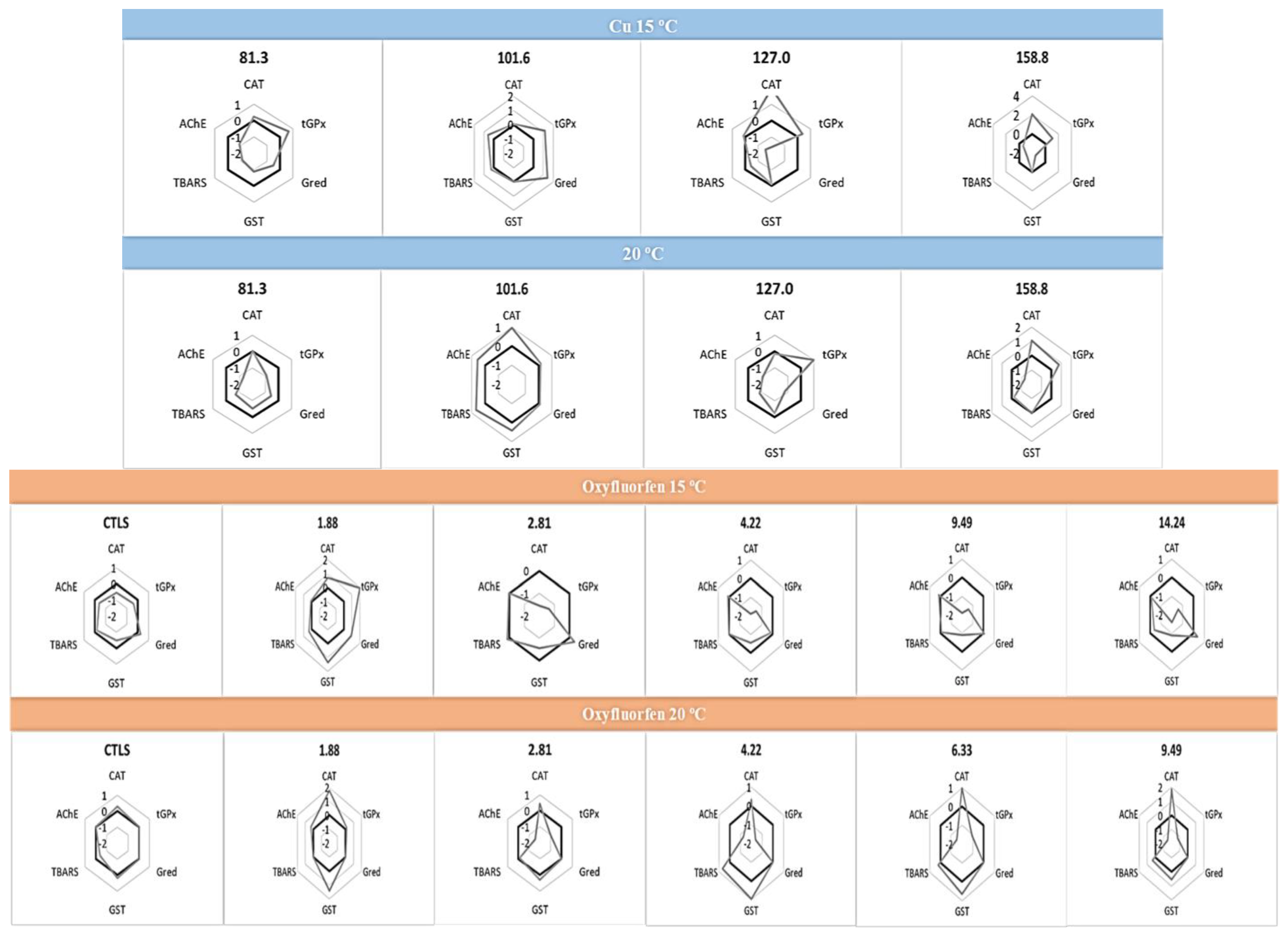
3.3. Biochemical Responses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fujiwara, T.; O’Hagan, D. Successful fluorine-containing herbicide agrochemicals. J. Fluor. Chem. 2014, 167, 16–29. [Google Scholar] [CrossRef]
- United Nations. World Population Prospects; United Nations: New York, NY, USA, 2022. [Google Scholar]
- FAO. Pesticides Use; FAO: Rome, Italy, 2020. [Google Scholar]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P.; Suttee, A.; Singh, G.; Barnwal, R.P.; Singla, N. Global Trends in pesticides: A looming threat and viable alternatives. Ecotoxicol. Environ. Saf. 2020, 201, 110812. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, H.; Roser, M.; Rosado, P. Pesticides. 2022. Available online: https://ourworldindata.org/pesticides (accessed on 24 January 2023).
- Ali, I.; Alharbi, O.M.L.; ALOthman, Z.A.; Al-Mohaimeed, A.M.; Alwarthan, A. Modeling of fenuron pesticide adsorption on cnts for mechanistic insight and removal in water. Environ. Res. 2019, 170, 389–397. [Google Scholar] [CrossRef]
- Abreu-Villaça, Y.; Levin, E.D. Developmental neurotoxicity of succeeding generations of insecticides. Environ. Int. 2017, 99, 55–77. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, A.F.; Gonçalves, F.; Verdelhos, T.; Marques, J.C.; Gonçalves, A.M.M. Fatty acids profiles modifications in the bivalves Cerastoderma edule and Scrobicularia plana in response to copper sulphate. Ecol. Indic. 2018, 85, 318–328. [Google Scholar] [CrossRef]
- Filimonova, V.; Nys, C.; De Schamphelaere, K.A.C.; Gonçalves, F.; Marques, J.C.; Gonçalves, A.M.M.; De Troch, M. Ecotoxicological and biochemical mixture effects of an herbicide and a metal at the marine primary producer diatom Thalassiosira weissflogii and the primary consumer copepod Acartia tonsa. Environ. Sci. Pollut. Res. 2018, 25, 22180–22195. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, A.F.; Gonçalves, F.J.M.; Rocha, C.P.; Marques, J.C.; Ana, M.M. Biochemical effects of two pesticides in three different temperature scenarios on the diatom Thalassiosira weissflogii. Processes 2021, 9, 1247. [Google Scholar] [CrossRef]
- Mesquita, A.F.; Marques, S.M.; Marques, J.C.; Gonçalves, F.J.M.; Gonçalves, A.M.M. Copper sulphate impact on the antioxidant defence system of the marine bivalves Cerastoderma edule and Scrobicularia plana. Sci. Rep. 2019, 9, 16458. [Google Scholar] [CrossRef]
- Schmidt, F.F.; Lichtenstein, D.; Planatscher, H.; Mentz, A.; Kalinowski, J.; Steinhilber, A.E.; Joos, T.O.; Braeuning, A.; Pötz, O. Pesticide mixture effects on liver protein abundance in HepaRG cells. Toxicology 2021, 458. [Google Scholar] [CrossRef]
- Xie, Z.; Wong, N.; Qian, P.; Qiu, J. Responses of polychaete Hydroides elegans life stages to copper stress. Mar. Ecol. Prog. Ser. 2005, 285, 89–96. [Google Scholar] [CrossRef]
- Mebane, C.A.; Schmidt, T.S.; Miller, J.L.; Balistrieri, L.S. Bioaccumulation and toxicity of cadmium, copper, nickel, and zinc and their mixtures to aquatic insect communities. Environ. Toxicol. Chem. 2020, 39, 812–833. [Google Scholar] [CrossRef]
- Dos Santos, E.V.; Sáez, C.; Cañizares, P.; Martínez-Huitle, C.A.; Rodrigo, M.A. UV assisted electrochemical technologies for the removal of oxyfluorfen from soil washing wastes. Chem. Eng. J. 2017, 318, 2–9. [Google Scholar] [CrossRef]
- Fernández, P.; Alcántara, R.; Osuna, M.D.; Vila-Aiub, M.M.; De Prado, R. Forward selection for multiple resistance across the non-selective glyphosate, glufosinate and oxyfluorfen herbicides in Lolium weed species. Pest Manag. Sci. 2017, 73, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, H.M.A.; Banhawy, M.A.; Soliman, F.M.; Abdel-Rehim, S.A.; Müller, W.E.G.; Schröder, H.C. Induction of Hsp70 by the herbicide oxyfluorfen (Goal) in the egyptian nile fish, Oreochromis niloticus. Arch. Environ. Contam. Toxicol. 1999, 37, 78–84. [Google Scholar] [CrossRef]
- Geoffroy, L.; Teisseire, H.; Couderchet, M.; Vernet, G. Effect of oxyfluorfen and diuron alone and in mixture on antioxidative enzymes of Scenedesmus obliquus. Pestic. Biochem. Physiol. 2002, 72, 178–185. [Google Scholar] [CrossRef]
- DataBase, P.P.P. General Information for Oxyfluorfen. 2021. Available online: http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/502.html (accessed on 22 June 2021).
- Peixoto, F.; Alves-Fernandes, D.; Santos, D.; Fontaínhas-Fernandes, A. Toxicological effects of oxyfluorfen on oxidative stress enzymes in tilapia Oreochromis niloticus. Pestic. Biochem. Physiol. 2006, 85, 91–96. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Sayed, D.A. Toxicological impact of oxyfluorfen 24% herbicide on the reproductive system, antioxidant enzymes, and endocrine disruption of Biomphalaria alexandrina (Ehrenberg, 1831) Snails. Environ. Sci. Pollut. Res. 2019, 26, 7960–7968. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013—The Physical Science Basis; Intergovernmental Panel on Climate Change, Ed.; Cambridge University Press: Geneva, Switzerland, 2014. [Google Scholar] [CrossRef]
- Tollefson, J. How hot will earth get by 2100? Nature 2020, 580, 443–445. [Google Scholar] [CrossRef]
- Gomez-Zavaglia, A.; Mejuto, J.C.; Simal-Gandara, J. Mitigation of emerging implications of climate change on food production systems. Food Res. Int. 2020, 134, 109256. [Google Scholar] [CrossRef]
- Quinn, B.K. Threshold temperatures for performance and survival of american lobster larvae: A review of current knowledge and implications to modeling impacts of climate change. Fish. Res. 2017, 186, 383–396. [Google Scholar] [CrossRef]
- IPCC. Global Warming of 1.5 °C; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Verdelhos, T.; Marques, J.C.; Anastácio, P. Behavioral and mortality responses of the bivalves Scrobicularia plana and Cerastoderma edule to temperature, as indicator of climate change’s potential impacts. Ecol. Indic. 2015, 58, 95–103. [Google Scholar] [CrossRef]
- Verdelhos, T.; Cardoso, P.G.; Dolbeth, M.; Pardal, M.A. Latitudinal gradients in Scrobicularia plana reproduction patterns, population dynamics, growth, and secondary production. Mar. Ecol. Prog. Ser. 2011, 442, 271–283. [Google Scholar] [CrossRef]
- Matoo, O.B.; Ivanina, A.V.; Ullstad, C.; Beniash, E.; Sokolova, I.I. Interactive effects of elevated temperature and CO2 levels on metabolism and oxidative stress in two common marine bivalves (Crassostrea virginica and Mercenaria mercenaria). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 164, 545–553. [Google Scholar] [CrossRef]
- Velez, C.; Figueira, E.; Soares, A.M.V.M.; Freitas, R. Effects of seawater temperature increase on economically relevant native and introduced clam species. Mar. Environ. Res. 2017, 123, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Boukadida, K.; Banni, M.; Gourves, P.Y.; Cachot, J. High sensitivity of embryo-larval stage of the mediterranean mussel, Mytilus galloprovincialis to metal pollution in combination with temperature increase. Mar. Environ. Res. 2016, 122, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Leite, C.; Coppola, F.; Monteiro, R.; Russo, T.; Polese, G.; Silva, M.R.F.; Lourenço, M.A.O.; Ferreira, P.; Soares, A.M.V.M.; Pereira, E.; et al. Toxic impacts of rutile titanium dioxide in Mytilus galloprovincialis exposed to warming conditions. Chemosphere 2020, 252, 126563. [Google Scholar] [CrossRef]
- Leite, C.; Coppola, F.; Queirós, V.; Russo, T.; Polese, G.; Pretti, C.; Pereira, E.; Freitas, R. Can temperature influence the impacts induced in Mytilus galloprovincialis by neodymium? comparison between exposure and recovery periods. Environ. Toxicol. Pharmacol. 2023, 97, 104029. [Google Scholar] [CrossRef]
- Morosetti, B.; Freitas, R.; Pereira, E.; Hamza, H.; Andrade, M.; Coppola, F.; Maggioni, D.; Della Torre, C. Will Temperature rise change the biochemical alterations induced in Mytilus galloprovincialis by cerium oxide nanoparticles and mercury? Environ. Res. 2020, 188, 109778. [Google Scholar] [CrossRef]
- Sicuro, B.; Castelar, B.; Mugetti, D.; Pastorino, P.; Chiarandon, A.; Menconi, V.; Galloni, M.; Prearo, M. Bioremediation with freshwater bivalves: A sustainable approach to reducing the environmental impact of inland trout farms. J. Environ. Manag. 2020, 276, 111327. [Google Scholar] [CrossRef]
- Paul-Pont, I.; Gonzalez, P.; Baudrimont, M.; Nili, H.; de Montaudouin, X. short-term metallothionein inductions in the edible cockle Cerastoderma edule after cadmium or mercury exposure: Discrepancy between mRNA and protein responses. Aquat. Toxicol. 2010, 97, 260–267. [Google Scholar] [CrossRef]
- Paul-Pont, I.; Gonzalez, P.; Baudrimont, M.; Jude, F.; Raymond, N.; Bourrasseau, L.; Le Goïc, N.; Haynes, F.; Legeay, A.; Paillard, C.; et al. Interactive effects of metal contamination and pathogenic organisms on the marine bivalve Cerastoderma edule. Mar. Pollut. Bull. 2010, 60, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, P.G.; Grilo, T.F.; Pereira, E.; Duarte, A.C.; Pardal, M.A. Mercury bioaccumulation and decontamination kinetics in the edible cockle Cerastoderma edule. Chemosphere 2013, 90, 1854–1859. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.; Martins, R.; Campino, B.; Figueira, E.; Soares, A.M.V.M.; Montaudouin, X. Trematode communities in cockles (Cerastoderma edule) of the Ria de Aveiro (Portugal): Influence of inorganic contamination. Mar. Pollut. Bull. 2014, 82, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Nilin, J.; Monteiro, M.; Domingues, I.; Loureiro, S.; Costa-Lotufo, L.V.; Soares, A.M.V.M. Bivalve esterases as biomarker: Identification and characterization in european cockles (Cerastoderma edule). Bull. Environ. Contam. Toxicol. 2012, 88, 707–711. [Google Scholar] [CrossRef]
- Gonçalves, A.M.M.; Mesquita, A.F.; Verdelhos, T.; Coutinho, J.A.P.; Marques, J.C.; Gonçalves, F. Fatty acids’ profiles as indicators of stress induced by of a common herbicide on two marine bivalves species: Cerastoderma edule (Linnaeus, 1758) and Scrobicularia plana (Da Costa, 1778). Ecol. Indic. 2016, 63, 209–218. [Google Scholar] [CrossRef]
- Jesus, F.; Mesquita, F.; Aldama, E.V.; Marques, A.; Gonçalves, A.M.M.; Magalhães, L.; Nogueira, A.J.A.; Ré, A.; Campos, I.; Pereira, J.L.; et al. Do freshwater and marine bivalves differ in their response to wildfire ash? effects on the antioxidant defense system and metal body burden. Int. J. Environ. Res. Public Health 2023, 20, 1326. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Claiborne, A. Catalase activity. In Handbook of Methods in Oxygen Radical Research; Ra, G., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar]
- Marques, A.; Piló, D.; Araújo, O.; Pereira, F.; Guilherme, S.; Carvalho, S.; Santos, M.A.; Pacheco, M.; Pereira, P. propensity to metal accumulation and oxidative stress responses of two benthic species (Cerastoderma edule and Nephtys hombergii): Are tolerance processes limiting their responsiveness? Ecotoxicology 2016, 25, 664–676. [Google Scholar] [CrossRef]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar]
- Carlberg, I.; Mannervik, B. Glutathione Reductase. Methods Enzymol. 1985, 113, 484–490. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Guilhermino, L.; Lopes, M.C.; Carvalho, A.P.; Soares, A.M.V.M. Inhibition of acetylcholinesterase activity as effect criterion in acute tests with juvenile Daphnia magna. Chemosphere 1996, 32, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, W.; Burgeot, T.; Porcher, J.M. A novel “integrated biomarker response” calculation based on reference deviation concept. Environ. Sci. Pollut. Res. 2013, 20, 2721–2725. [Google Scholar] [CrossRef]
- Peruzzi, M.; Bartolucci, G.; Cioni, F. Determination of phenoxyalkanoic acids and other herbicides at the Ng/Ml level in water by solid-phase extraction with poly(divinylbenzene-co-n-vinylpyrrolidone) sorbent and high-performance liquid chromatography-diode-array detection. J. Chromatogr. A 2000, 867, 169–175. [Google Scholar] [CrossRef]
- Grilo, T.F.; Cardoso, P.G.; Pato, P.; Duarte, A.C.; Pardal, M.A. Organochlorine accumulation on a highly consumed bivalve (Scrobicularia plana) and its main implications for human health. Sci. Total Environ. 2013, 461–462, 188–197. [Google Scholar] [CrossRef]
- De La Colina, C.; Peña, A.; Mingorance, M.D.; Sánchez Rasero, F. Influence of the solid-phase extraction process on calibration and performance parameters for the determination of pesticide residues in water by gas chromatography. J. Chromatogr. A 1996, 733, 275–281. [Google Scholar] [CrossRef]
- Marques, S.M.; Antunes, S.C.; Nunes, B.; Gonçalves, F.; Pereira, R. Antioxidant response and metal accumulation in tissues of iberian green frogs (Pelophylax perezi) inhabiting a deactivated uranium mine. Ecotoxicology 2011, 20, 1315–1327. [Google Scholar] [CrossRef]
- Kibria, G.; Nugegoda, D.; Rose, G.; Haroon, A.K.Y. Climate change impacts on pollutants mobilization and interactive effects of climate change and pollutants on toxicity and bioaccumulation of pollutants in estuarine and marine biota and linkage to seafood security. Mar. Pollut. Bull. 2021, 167, 112364. [Google Scholar] [CrossRef]
- Pirone, G.; Coppola, F.; Pretti, C.; Soares, A.M.V.M.; Solé, M.; Freitas, R. The effect of temperature on triclosan and lead exposed mussels. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2019, 232, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, J.P.; Williams, R.J.; Gooddy, D.C.; Cape, J.N.; Guha, P. Impacts of climate change on the fate and behaviour of pesticides in surface and groundwater—A UK perspective. Sci. Total Environ. 2006, 369, 163–177. [Google Scholar] [CrossRef]
- Lamon, L.; Von Waldow, H.; Macleod, M.; Scheringer, M.; Marcomini, A.; Hungerbühler, K. Modeling the global levels and distribution of polychlorinated biphenyls in air under a climate change scenario. Environ. Sci. Technol. 2009, 43, 5818–5824. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, I.B.; Mesquita, A.F.; Gonçalves, F.J.M.; Marques, J.C.; Gonçalves, A.M.M. Biomarkers’ responses of the benthic clam Scrobicularia plana to the main active ingredients (S-metolachlor and terbuthylazine) of a common herbicide. Ecol. Indic. 2019, 96. [Google Scholar] [CrossRef]
- Esposito, G.; Pastorino, P.; Prearo, M.; Magara, G.; Cesarani, A.; Freitas, R.; Caldaroni, B.; Meloni, D.; Pais, A.; Dondo, A.; et al. Ecotoxicity of copper(I) chloride in grooved carpet shell (Ruditapes decussatus). Antioxidants 2022, 11, 2148. [Google Scholar] [CrossRef] [PubMed]
- Stara, A.; Pagano, M.; Capillo, G.; Fabrello, J.; Sandova, M.; Vazzana, I.; Zuskova, E.; Velisek, J.; Matozzo, V.; Faggio, C. assessing the effects of neonicotinoid insecticide on the bivalve mollusc Mytilus galloprovincialis. Sci. Total Environ. 2020, 700, 134914. [Google Scholar] [CrossRef]
- El-Rahman, G.I.A.; Ahmed, S.A.A.; Khalil, A.A.; Abd-Elhakim, Y.M. Assessment of hematological, hepato-renal, antioxidant, and hormonal responses of Clarias gariepinus exposed to sub-lethal concentrations of oxyfluorfen. Aquat. Toxicol. 2019, 217, 105329. [Google Scholar] [CrossRef]
- Li, Z.; Guo, J.; Jia, K.; Zheng, Z.; Chen, X.; Bai, Z.; Yang, Y.; Chen, B.; Yuan, W.; Chen, W.; et al. Oxyfluorfen Induces hepatotoxicity through lipo-sugar accumulation and inflammation in zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2022, 230, 113140. [Google Scholar] [CrossRef]
- Sea Temperature. Water Temperature in Aveiro by Month. 2023. Available online: https://seatemperature.net/current/portugal/aveiro-sea-temperature (accessed on 17 August 2023).
- Rodrigues, M.; Oliveira, A.; Queiroga, H.; Brotas, V.; Fortunato, A.B.; Manso, M.D. Relative Role of Climatic Factors and Anthropogenic Actions in the Waterquality and Ecological Dynamics of the Aveiro Lagoon (Portugal). Geophys. Res. 2013, 15, 184. [Google Scholar]
- Strehse, J.S.; Maser, E. Marine bivalves as bioindicators for environmental pollutants with focus on dumped munitions in the sea: A review. Mar. Environ. Res. 2020, 158, 105006. [Google Scholar] [CrossRef]
- Freitas, R.; Costa, E.; Velez, C.; Santos, J.; Lima, A.; Oliveira, C.; Maria Rodrigues, A.; Quintino, V.; Figueira, E. Looking for suitable biomarkers in benthic macroinvertebrates inhabiting coastal areas with low metal contamination: Comparison between the bivalve Cerastoderma edule and the polychaete Diopatra neapolitana. Ecotoxicol. Environ. Saf. 2012, 75, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Kourdali, S.; Boudjema, K.; Meknachi, A.; Bounakous, N.; Jaouadi, B.; Mechri, S.; Badis, A. An ecotoxicological approach for assessing marine pollution: Comparative study of multi-responses of marine mussels, Mytilus galloprovincialis and Perna perna, exposed to pollutant heavy metals (copper and lead). Reg. Stud. Mar. Sci. 2022, 52, 102334. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, J.; Ingole, B.S.; Singh, N. Glutathione S-transferase, catalase, superoxide dismutase, glutathione peroxidase, and lipid peroxidation as biomarkers of oxidative stress in snails: A review. Invertebr. Surviv. J. 2016, 13, 336–349. [Google Scholar]
- Freitas, R.; Silvestro, S.; Coppola, F.; Meucci, V.; Battaglia, F.; Intorre, L.; Soares, A.M.V.M.; Pretti, C.; Faggio, C. Biochemical and physiological responses induced in Mytilus galloprovincialis after a chronic exposure to salicylic acid. Aquat. Toxicol. 2019, 214, 105258. [Google Scholar] [CrossRef]
- Sillero-Ríos, J.; Sureda, A.; Capó, X.; Oliver-Codorniú, M.; Arechavala-Lopez, P. Biomarkers of physiological responses of Octopus vulgaris to different coastal environments in the western Mediterranean sea. Mar. Pollut. Bull. 2018, 128, 240–247. [Google Scholar] [CrossRef]
- Vieira, C.E.D.; Pérez, M.R.; Acayaba, R.D.A.; Raimundo, C.C.M.; dos Reis Martinez, C.B. DNA damage and oxidative stress induced by imidacloprid exposure in different tissues of the neotropical fish Prochilodus lineatus. Chemosphere 2018, 195, 125–134. [Google Scholar] [CrossRef]
- Dias, R.; D’Costa, A.; Praveen Kumar, M.K.; Shyama, S.K. DNA damage and biochemical responses in estuarine bivalve Donax incarnatus (Gmelin, 1791) exposed to sub-lethal concentrations of an organophosphate pesticide monocrotophos. Environ. Monit. Assess. 2021, 193, 1–12. [Google Scholar] [CrossRef]
- Matozzo, V.; Fabrello, J.; Masiero, L.; Ferraccioli, F.; Finos, L.; Pastore, P.; Di Gangi, I.M.; Bogialli, S. Ecotoxicological risk assessment for the herbicide glyphosate to non-target aquatic species: A case study with the mussel Mytilus galloprovincialis. Environ. Pollut. 2018, 233, 623–632. [Google Scholar] [CrossRef]
- Bowen, L.; Miles, A.K.; Kolden, C.A.; Saarinen, J.A.; Bodkin, J.L.; Murray, M.J.; Tinker, M.T. Effects of wildfire on sea otter (Enhydra lutris) gene transcript profiles. Mar. Mammal Sci. 2015, 31, 191–210. [Google Scholar] [CrossRef]
- Regoli, F.; Giuliani, M.E. Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res. 2014, 93, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Mnkandla, S.M.; Basopo, N.; Siwela, A.H. The effect of persistent heavy metal exposure on some antioxidant enzyme activities and lipid peroxidation of the freshwater snail, Lymnaea natalensis. Bull. Environ. Contam. Toxicol. 2019, 103, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Martínez, G.V.; DelValls, T.A.; Martín-Díaz, M.L. General stress, detoxification pathways, neurotoxicity and genotoxicity evaluated in Ruditapes philippinarum exposed to human pharmaceuticals. Ecotoxicol. Environ. Saf. 2016, 124, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Juhel, G.; Bayen, S.; Goh, C.; Lee, W.K.; Kelly, B.C. Use of a suite of biomarkers to assess the effects of carbamazepine, bisphenol a, atrazine, and their mixtures on green mussels, Perna viridis. Environ. Toxicol. Chem. 2017, 36, 429–441. [Google Scholar] [CrossRef]
- Sandrini, J.Z.; Rola, R.C.; Lopes, F.M.; Buffon, H.F.; Freitas, M.M.; Martins, C. de M. G.; da Rosa, C.E. Effects of glyphosate on cholinesterase activity of the mussel Perna perna and the fish Danio rerio and Jenynsia multidentata: In vitro studies. Aquat. Toxicol. 2013, 130–131, 171–173. [Google Scholar] [CrossRef]
- Telahigue, K.; Rabeh, I.; Mhadhbi, L.; Nechi, S.; Chelbi, E.; Ben Ali, M.; Hedfi, A.; AL-Harbi, M.S.; Hajji, T. Glyphosate exposure modulates lipid composition, histo-architecture and oxidative stress status and induces neurotoxicity in the smooth scallop Flexopecten glaber. Pestic. Biochem. Physiol. 2022, 184, 105099. [Google Scholar] [CrossRef]
- Fadhlaoui, M.; Lavoie, I. Effects of temperature and glyphosate on fatty acid composition, antioxidant capacity, and lipid peroxidation in the gastropod Lymneae sp. Water 2021, 13, 1039. [Google Scholar] [CrossRef]
- Abele, D.; Heise, K.; Pörtner, H.O.; Puntarulo, S. Temperature-dependence of mitochondrial function and production of reactive oxygen species in the intertidal mud clam Mya arenaria. J. Exp. Biol. 2002, 205 Pt 13, 1831–1841. [Google Scholar] [CrossRef]
- Vaughn, C.C.; Hoellein, T.J. Bivalve impacts in freshwater and marine ecosystems. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 183–208. [Google Scholar] [CrossRef]

| Nominal | Measured | ||||
|---|---|---|---|---|---|
| 15 °C | 20 °C | 25 °C | |||
| Water | Tissue | Tissue | Tissue | ||
| Cu (µg L−1) | Field | <LOQ | <LOQ | <LOQ | <LOQ |
| Depuration | <LOQ | <LOQ | <LOQ | <LOQ | |
| CTL | <LOQ | <LOQ | <LOQ | <LOQ | |
| 81.28 | 78.33 (±0.0018) | 34.34 (±0.0017) | 51.63 (±0.0008) | ||
| 101.6 | 100.89 (±0.0310) | 35.43 (±0.0026) | 56.36 (±0.0038) | ||
| 127.0 | 124.50 (±0.0230) | 46.43 (±0.0028) | 78.30 (±0.0163) | ||
| 158.8 | 156.87 (±0.0215) | 55.03 (±0.0101) | 102.25 (±0.0108) | ||
| Oxyfluorfen (mg L−1) | Field | <LOQ | <LOQ | <LOQ | <LOQ |
| Depuration | <LOQ | <LOQ | <LOQ | <LOQ | |
| CTL | <LOQ | <LOQ | <LOQ | <LOQ | |
| CTLS | <LOQ | <LOQ | <LOQ | <LOQ | |
| 1.88 | 1.92 (±0.0620) | 0.69 (±0.0022) | 1.00 (±0.0039) | ||
| 2.81 | 2.49 (±0.0873) | 0.87 (±0.0031) | 1.29 (±0.0055) | ||
| 4.22 | 4.49 (±0.0952) | 1.62 (±0.0034) | 2.30 (±0.0081) | ||
| 6.33 | 6.25 (±0.1286) | 2.22 (±0.0013) | 3.87 (±0.0060) | ||
| 9.49 | 8.16 (±0.3647) | 2.83 (±0.0046) | 5.10 (±0.0022) | ||
| 14.24 | 14.27 (±0.2999) | 5.15 (±0.0011) | 7.60 (±0.0019) | ||
| Cu (mg L−1) | Oxyfluorfen (mg L−1) | |||
|---|---|---|---|---|
| 15 °C | 20 °C | 15 °C | 20 °C | |
| NOEC | 0.081 | - | 2.813 | - |
| LOEC | 0.102 | 0.081 | 4.219 | 1.875 |
| LC10 | 0.119 (0.061–0.153) | 0.075 (0.002–0.116) | 6.781 (0.000–6.580) | 1.371 (0.00–6.580) |
| LC20 | 0.157 (0.114–0.187) | 0.121 (0.068–0.155) | 12.258 (7.087–20.989) | 7.098 (6.856–24.041) |
| LC50 | 0.229 (0.199–0.265) | 0.209 (0.176–0.248) | 22.735 (16.324–48.510) | 18.52 (11.238–44.868) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesquita, A.F.; Gonçalves, F.J.M.; Gonçalves, A.M.M. Effects of Inorganic and Organic Pollutants on the Biomarkers’ Response of Cerastoderma edule under Temperature Scenarios. Antioxidants 2023, 12, 1756. https://doi.org/10.3390/antiox12091756
Mesquita AF, Gonçalves FJM, Gonçalves AMM. Effects of Inorganic and Organic Pollutants on the Biomarkers’ Response of Cerastoderma edule under Temperature Scenarios. Antioxidants. 2023; 12(9):1756. https://doi.org/10.3390/antiox12091756
Chicago/Turabian StyleMesquita, Andreia F., Fernando J. M. Gonçalves, and Ana M. M. Gonçalves. 2023. "Effects of Inorganic and Organic Pollutants on the Biomarkers’ Response of Cerastoderma edule under Temperature Scenarios" Antioxidants 12, no. 9: 1756. https://doi.org/10.3390/antiox12091756
APA StyleMesquita, A. F., Gonçalves, F. J. M., & Gonçalves, A. M. M. (2023). Effects of Inorganic and Organic Pollutants on the Biomarkers’ Response of Cerastoderma edule under Temperature Scenarios. Antioxidants, 12(9), 1756. https://doi.org/10.3390/antiox12091756







