Characterization of Non-Invasively Induced Post-Traumatic Osteoarthritis in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Non-Invasive Post-Traumatic Osteoarthritis (PTOA) Mouse Model
2.3. Dynamic Mechanical Analysis (DMA) of the Knee Joints
2.4. Optical Image Scanning
2.5. Collection of Blood and Isolation of Cartilage Tissue
2.6. Total Nitrate/Nitrite (NO) Assay
2.7. Caspase3 Assay
2.8. Amplex Red Assay for H2O2
2.9. Prostaglandin E2 (PGE2) Assay
2.10. Gene Expression Analysis
2.11. Histopathology (Modified OA and Synovitis Scoring System)
2.12. Gait Analysis
2.13. Open-Field Analysis
2.14. Statistical Analysis
3. Results
3.1. Mechanical Loading Induced Oxidative Stress, Unmasks Cartilage Type II Collagen, and Increases MMP Activity in Knee Joints
3.2. Effect of Mechanical Loading on Biomechanical Properties
3.3. Biochemical Changes Accompanied by Mechanical Loading
3.4. Mechanically Induced Gene Expression Patterns
3.5. Mechanical Loading of the Knee Joints Increased OA and Synovitis Score
3.6. Altered Gait and Locomotory Behavior in Mechanically Loaded Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Punzi, L.; Galozzi, P.; Luisetto, R.; Favero, M.; Ramonda, R.; Oliviero, F.; Scanu, A. Post-traumatic arthritis: Overview on pathogenic mechanisms and role of inflammation. RMD Open 2016, 2, e000279. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.D.; Johnston, R.C.; Saltzman, C.L.; Marsh, J.L.; Buckwalter, J.A. Posttraumatic osteoarthritis: A first estimate of incidence, prevalence, and burden of disease. J. Orthop. Trauma 2006, 20, 739–744. [Google Scholar] [CrossRef]
- Lohmander, L.S.; Englund, P.M.; Dahl, L.L.; Roos, E.M. The long-term consequence of anterior cruciate ligament and meniscus injuries: Osteoarthritis. Am. J. Sports Med. 2007, 35, 1756–1769. [Google Scholar] [CrossRef] [PubMed]
- Guilak, F. Biomechanical factors in osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2011, 25, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A.; Anderson, D.D.; Brown, T.D.; Tochigi, Y.; Martin, J.A. The Roles of Mechanical Stresses in the Pathogenesis of Osteoarthritis: Implications for Treatment of Joint Injuries. Cartilage 2013, 4, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.D.; Chubinskaya, S.; Guilak, F.; Martin, J.A.; Oegema, T.R.; Olson, S.A.; Buckwalter, J.A. Post-traumatic osteoarthritis: Improved understanding and opportunities for early intervention. J. Orthop. Res. 2011, 29, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.; Barthold, S.; Davisson, M.; Newcomer, C.; Quimby, F.; Smith, A. The Mouse in Biomedical Research, 2nd ed.; Academic Press: Cambridge, MA, USA, 2007; Volume 2, p. 776. [Google Scholar]
- Poulet, B. Non-invasive Loading Model of Murine Osteoarthritis. Curr. Rheumatol. Rep. 2016, 18, 40. [Google Scholar] [CrossRef][Green Version]
- Christiansen, B.A.; Guilak, F.; Lockwood, K.A.; Olson, S.A.; Pitsillides, A.A.; Sandell, L.J.; Silva, M.J.; van der Meulen, M.C.H.; Haudenschild, D.R. Non-invasive mouse models of post-traumatic osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1627–1638. [Google Scholar] [CrossRef]
- Lieberthal, J.; Sambamurthy, N.; Scanzello, C.R. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1825–1834. [Google Scholar] [CrossRef]
- Olson, S.A.; Furman, B.; Guilak, F. Joint injury and post-traumatic arthritis. HSS J. 2012, 8, 23–25. [Google Scholar] [CrossRef]
- DiMicco, M.A.; Patwari, P.; Siparsky, P.N.; Kumar, S.; Pratta, M.A.; Lark, M.W.; Kim, Y.-J.; Grodzinsky, A.J. Mechanisms and kinetics of glycosaminoglycan release following in vitro cartilage injury. Arthritis Rheum. 2004, 50, 840–848. [Google Scholar] [CrossRef]
- Chen, C.T.; Bhargava, M.; Lin, P.M.; Torzilli, P.A. Time, stress, and location dependent chondrocyte death and collagen damage in cyclically loaded articular cartilage. J. Orthop. Res. 2003, 21, 888–898. [Google Scholar] [CrossRef]
- Guo, D.; Ding, L.; Homandberg, G.A. Telopeptides of type II collagen upregulate proteinases and damage cartilage but are less effective than highly active fibronectin fragments. Inflamm. Res. 2009, 58, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta 2016, 1862, 576–591. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, L.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Pinkhassik, E.; David, V.; Stuart, J.M.; Hasty, K.A. Detection of early cartilage damage using targeted nanosomes in a post-traumatic osteoarthritis mouse model. Nanomedicine 2015, 11, 939–946. [Google Scholar] [CrossRef]
- Christiansen, B.A.; Anderson, M.J.; Lee, C.A.; Williams, J.C.; Yik, J.H.; Haudenschild, D.R. Musculoskeletal changes following non-invasive knee injury using a novel mouse model of post-traumatic osteoarthritis. Osteoarthr. Cartil. 2012, 20, 773–782. [Google Scholar] [CrossRef]
- Poulet, B.; Hamilton, R.W.; Shefelbine, S.; Pitsillides, A.A. Characterizing a novel and adjustable noninvasive murine joint loading model. Arthritis Rheum. 2011, 63, 137–147. [Google Scholar] [CrossRef]
- Cho, H.; Walker, A.; Williams, J.; Hasty, K.A. Study of osteoarthritis treatment with anti-inflammatory drugs: Cyclooxygenase-2 inhibitor and steroids. Biomed. Res. Int. 2015, 2015, 595273. [Google Scholar] [CrossRef]
- Amorosa, L.F.; Lee, C.H.; Aydemir, A.B.; Nizami, S.; Hsu, A.; Patel, N.R.; Gardner, T.R.; Navalgund, A.; Kim, D.-G.; Park, S.H.; et al. Physiologic load-bearing characteristics of autografts, allografts, and polymer-based scaffolds in a critical sized segmental defect of long bone: An experimental study. Int. J. Nanomed. 2013, 8, 1637–1643. [Google Scholar] [CrossRef]
- Kiefer, G.N.; Sundby, K.; McAllister, D.; Shrive, N.G.; Frank, C.B.; Lam, T.; Schachar, D.N.S. The effect of cryopreservation on the biomechanical behavior of bovine articular cartilage. J. Orthop. Res. 1989, 7, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.G.; Kwon, H.J.; Jeong, Y.H.; Chien, H.H.; Crance, S.; Agnew, A.M.; Battula, S.; Lee, J.W.; Wen, H.B. Associations of Resonance Frequency Analysis with Dynamic Mechanical Analysis of Dental Implant Systems. Clin. Implant. Dent. Relat. Res. 2016, 18, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.G.; Haghighi, A.; Kwon, H.J.; Coogan, J.S.; Nicolella, D.P.; Johnson, T.B.; Kim, H.D.; Kim, N.; Agnewc, A.M. Sex dependent mechanical properties of the human mandibular condyle. J. Mech. Behav. Biomed. Mater. 2017, 71, 184–191. [Google Scholar] [CrossRef]
- Bedingfield, S.K.; Colazo, J.M.; Yu, F.; Liu, D.D.; Jackson, M.A.; Himmel, L.E.; Cho, H.; Crofford, L.J.; Hasty, K.A.; Duvall, C.L. Amelioration of post-traumatic osteoarthritis via nanoparticle depots delivering small interfering RNA to damaged cartilage. Nat. Biomed. Eng. 2021, 5, 1069–1083. [Google Scholar] [CrossRef]
- Terato, K.; Shimozuru, Y.; Katayama, K.; Takemitsu, Y.; Yamashita, I.; Miyatsu, M.; Fujii, K.; Sagara, M.; Kobayashi, S.; Goto, M.; et al. Specificity of antibodies to type II collagen in rheumatoid arthritis. Arthritis Rheum. 1990, 33, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Kielland, A.; Blom, T.; Nandakumar, K.S.; Holmdahl, R.; Blomhoff, R.; Carlsen, H. In vivo imaging of reactive oxygen and nitrogen species in inflammation using the luminescent probe L-012. Free Radic. Biol. Med. 2009, 47, 760–766. [Google Scholar] [CrossRef]
- Glasson, S.S.; Chambers, M.G.; Van Den Berg, W.B.; Little, C.B. The OARSI histopathology initiative-recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr. Cartil. 2010, 18 (Suppl. 3), S17–S23. [Google Scholar] [CrossRef]
- Lewis, J.S.; Hembree, W.C.; Furman, B.D.; Tippets, L.; Cattel, D.; Huebner, J.L.; Little, D.; DeFrate, L.E.; Kraus, V.B.; Guilak, F.; et al. Acute joint pathology and synovial inflammation is associated with increased intra-articular fracture severity in the mouse knee. Osteoarthr. Cartil. 2011, 19, 864–873. [Google Scholar] [CrossRef]
- Kemp, K.C.; Cerminara, N.; Hares, K.; Redondo, J.; Cook, A.J.; Haynes, H.R.; Burton, B.R.; Pook, M.; Apps, R.; Scolding, N.J.; et al. Cytokine therapy-mediated neuroprotection in a Friedreich’s ataxia mouse model. Ann. Neurol. 2017, 81, 212–226. [Google Scholar] [CrossRef]
- Seibenhener, M.L.; Wooten, M.C. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J. Vis. Exp. 2015, 96, e52434. [Google Scholar] [CrossRef]
- Hanninen, L.; Pastell, M. CowLog: Open-source software for coding behaviors from digital video. Behav. Res. Methods 2009, 41, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Attur, M.; Al-Mussawir, H.E.; Patel, J.; Kitay, A.; Dave, M.; Palmer, G.; Pillinger, M.H.; Abramson, S.B. Prostaglandin E2 exerts catabolic effects in osteoarthritis cartilage: Evidence for signaling via the EP4 receptor. J. Immunol. 2008, 181, 5082–5098. [Google Scholar] [CrossRef]
- Poulet, B.; Ulici, V.; Stone, T.C.; Pead, M.; Gburcik, V.; Constantinou, E.; Palmer, D.B.; Beier, F.; Timmons, J.A.; Pitsillides, A.A. Time-series transcriptional profiling yields new perspectives on susceptibility to murine osteoarthritis. Arthritis Rheum. 2012, 64, 3256–3266. [Google Scholar] [CrossRef] [PubMed]
- Yorimitsu, M.; Nishida, K.; Shimizu, A.; Doi, H.; Miyazawa, S.; Komiyama, T.; Nasu, Y.; Yoshida, A.; Watanabe, S.; Ozaki, T. Intra-articular injection of interleukin-4 decreases nitric oxide production by chondrocytes and ameliorates subsequent destruction of cartilage in instability-induced osteoarthritis in rat knee joints. Osteoarthr. Cartil. 2008, 16, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Ziskoven, C.; Jäger, M.; Zilkens, C.; Bloch, W.; Brixius, K.; Krauspe, R. Oxidative stress in secondary osteoarthritis: From cartilage destruction to clinical presentation? Orthop. Rev. 2010, 2, e23. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Bhatti, F.U.; Yoon, T.W.; Hasty, K.A.; Stuart, J.M.; Yi, A.K. Non-invasive dual fluorescence in vivo imaging for detection of macrophage infiltration and matrix metalloproteinase (MMP) activity in inflammatory arthritic joints. Biomed. Opt. Express 2016, 7, 1842–1852. [Google Scholar] [CrossRef]
- Malfait, A.M. Osteoarthritis year in review 2015: Biology. Osteoarthr. Cartil. 2016, 24, 21–26. [Google Scholar] [CrossRef]
- Loeser, R.F. Osteoarthritis year in review 2013: Biology. Osteoarthr. Cartil. 2013, 21, 1436–1442. [Google Scholar] [CrossRef]
- Saarakkala, S.; Julkunen, P.; Kiviranta, P.; Mäkitalo, J.; Jurvelin, J.S.; Korhonen, R.K. Depth-wise progression of osteoarthritis in human articular cartilage: Investigation of composition, structure and biomechanics. Osteoarthr. Cartil. 2010, 18, 73–81. [Google Scholar] [CrossRef]
- Setton, L.A.; Elliott, D.M.; Mow, V.C. Altered mechanics of cartilage with osteoarthritis: Human osteoarthritis and an experimental model of joint degeneration. Osteoarthr. Cartil. 1999, 7, 2–14. [Google Scholar] [CrossRef]
- Doyran, B.; Tong, W.; Li, Q.; Jia, H.; Zhang, X.; Chen, C.; Enomoto-Iwamoto, M.; Lu, X.L.; Qin, L.; Han, L. Nanoindentation modulus of murine cartilage: A sensitive indicator of the initiation and progression of post-traumatic osteoarthritis. Osteoarthr. Cartil. 2017, 25, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.P.; Martel-Pelletier, J.; Abramson, S.B. Osteoarthritis, an inflammatory disease: Potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001, 44, 1237–1247. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Kamrava, S.K.; Joghataei, M.T.; Darabi, R.; Shakeri-Zadeh, A.; Shahriari, M.; Reiter, R.J.; Ghaznavi, H.; Mehrzadi, S. Apoptosis signaling pathways in osteoarthritis and possible protective role of melatonin. J. Pineal. Res. 2016, 61, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Gui, J.; Wang, L.; Xu, Y.; Jiang, Y.; Xiong, M.; Cui, Y. Aquaporin 1 contributes to chondrocyte apoptosis in a rat model of osteoarthritis. Int. J. Mol. Med. 2016, 38, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Gosset, M.; Berenbaum, F.; Levy, A.; Pigenet, A.; Thirion, S.; Cavadias, S.; Claire, J. Mechanical stress and prostaglandin E2 synthesis in cartilage. Biorheology 2008, 45, 301–320. [Google Scholar] [CrossRef]
- Hashimoto, S.; Rai, M.F.; Janiszak, K.L.; Cheverud, J.M.; Sandell, L.J. Cartilage and bone changes during development of post-traumatic osteoarthritis in selected LGXSM recombinant inbred mice. Osteoarthr. Cartil. 2012, 20, 562–571. [Google Scholar] [CrossRef][Green Version]
- Hwang, H.S.; Kim, H.A. Chondrocyte Apoptosis in the Pathogenesis of Osteoarthritis. Int. J. Mol. Sci. 2015, 16, 26035–26054. [Google Scholar] [CrossRef]
- Khan, I.M.; Gilbert, S.J.; Caterson, B.; Sandell, L.J.; Archer, C.W. Oxidative stress induces expression of osteoarthritis markers procollagen IIA and 3B3(-) in adult bovine articular cartilage. Osteoarthr. Cartil. 2008, 16, 698–707. [Google Scholar] [CrossRef]
- Mueller, M.B.; Tuan, R.S. Anabolic/Catabolic balance in pathogenesis of osteoarthritis: Identifying molecular targets. PMR 2011, 3 (Suppl. 1), S3–S11. [Google Scholar] [CrossRef]
- Rai, M.F.; Duan, X.; Quirk, J.D.; Holguin, N.; Schmidt, E.J.; Chinzei, N.; Silva, M.J.; Sandell, L.J. Post-Traumatic Osteoarthritis in Mice Following Mechanical Injury to the Synovial Joint. Sci. Rep. 2017, 7, 45223. [Google Scholar] [CrossRef]
- Cook, J.L.; Kuroki, K.; Visco, D.; Pelletier, J.P.; Schulz, L.; Lafeber, F.P. The OARSI histopathology initiative-recommendations for histological assessments of osteoarthritis in the dog. Osteoarthr. Cartil. 2010, 18 (Suppl. 3), S66–S79. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, T.; Mitchell, R.; Bhasin, P.; Schon, L.; Zhang, Z. A Surgical Model of Posttraumatic Osteoarthritis with Histological and Gait Validation. Orthop. J. Sports Med. 2016, 4, 2325967116658874. [Google Scholar] [CrossRef] [PubMed]
- Ter Heegde, F.; Luiz, A.P.; Santana-Varela, S.; Chessell, I.P.; Welsh, F.; Wood, J.N.; Chenu, C. Noninvasive Mechanical Joint Loading as an Alternative Model for Osteoarthritic Pain. Arthritis Rheumatol. 2019, 71, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, C.; Chanalaris, A.; Knights, C.; Ismail, H.; Sacitharan, P.K.; Gentry, C.; Bevan, S.; Vincent, T.L. Nociceptive sensitizers are regulated in damaged joint tissues, including articular cartilage, when osteoarthritic mice display pain behavior. Arthritis Rheumatol. 2016, 68, 857–867. [Google Scholar] [CrossRef]
- Poulet, B.; de Souza, R.; Kent, A.V.; Saxon, L.; Barker, O.; Wilson, A.; Chang, Y.-M.; Cake, M.; Pitsillides, A.A. Intermittent applied mechanical loading induces subchondral bone thickening that may be intensified locally by contiguous articular cartilage lesions. Osteoarthr. Cartil. 2015, 23, 940–948. [Google Scholar] [CrossRef]
- O’Grady, K.; Kavanaugh, T.; Cho, H.; Ye, H.; Gupta, M.; Madonna, M.; Lee, J.; O’Brien, C.; Skala, M.; Hasty, K. Drug Free ROS Sponge Polymeric Microspheres Reduce Tissue Damage from Ischemic and Mechanical Injury. ACS Biomater. Sci. Eng. 2018, 4, 1251–1264. [Google Scholar] [CrossRef]

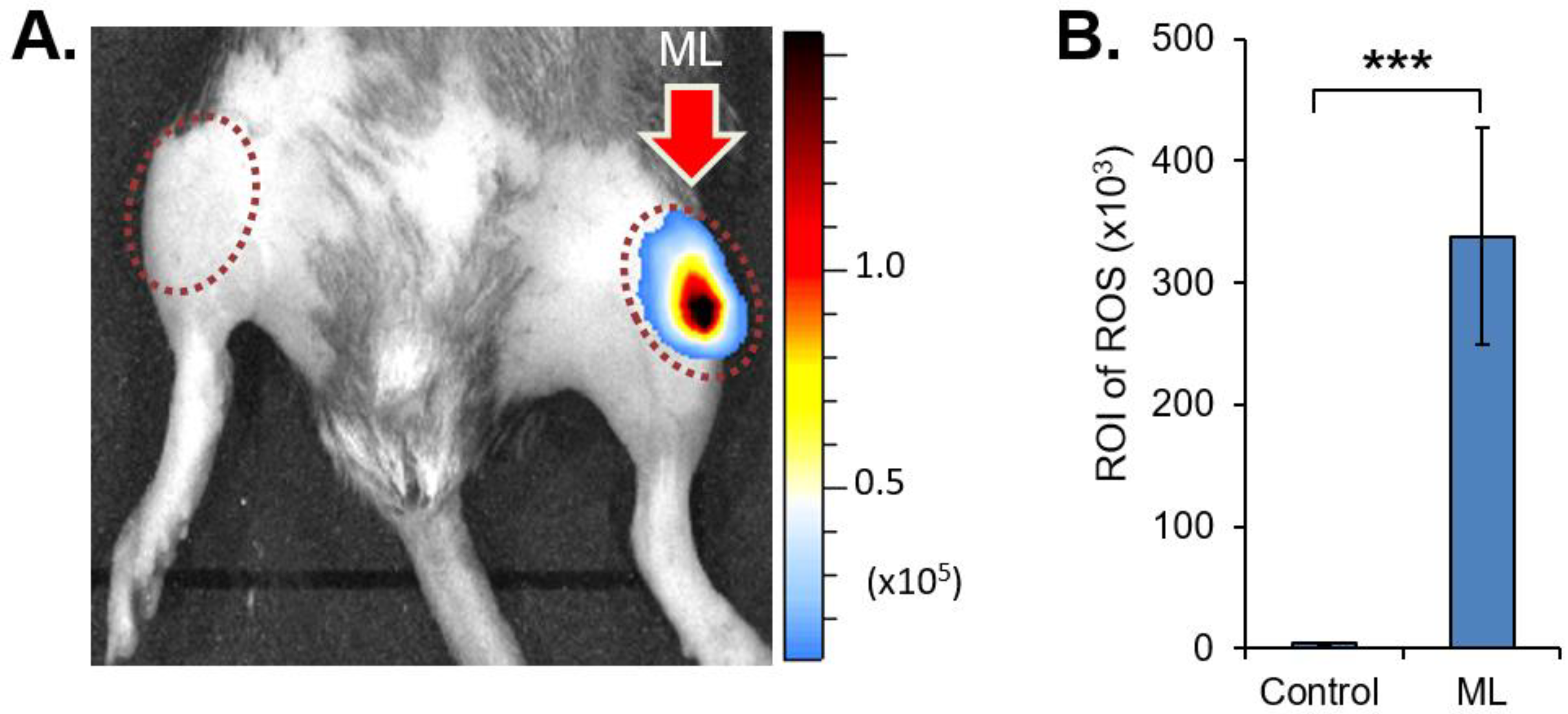
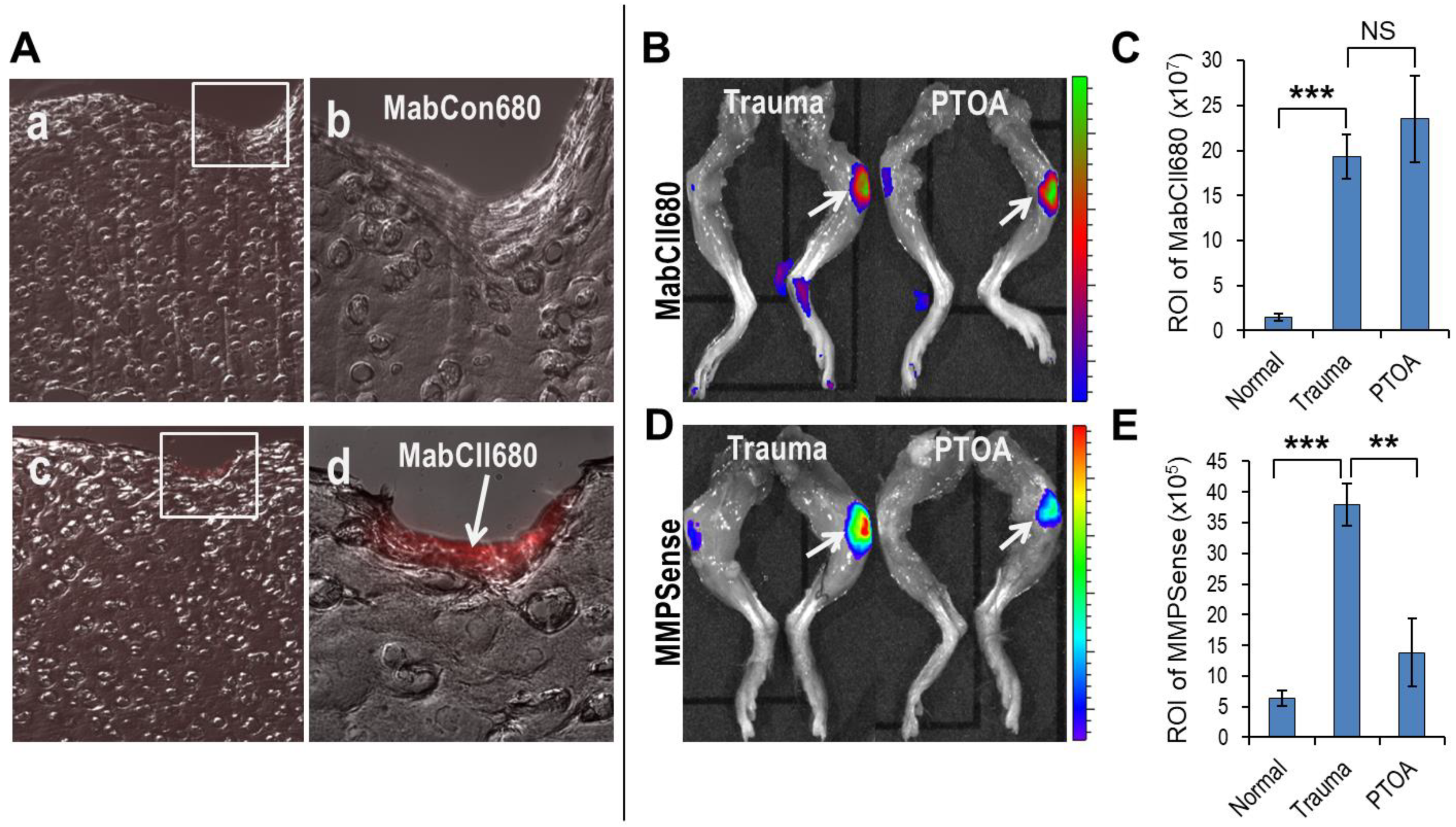



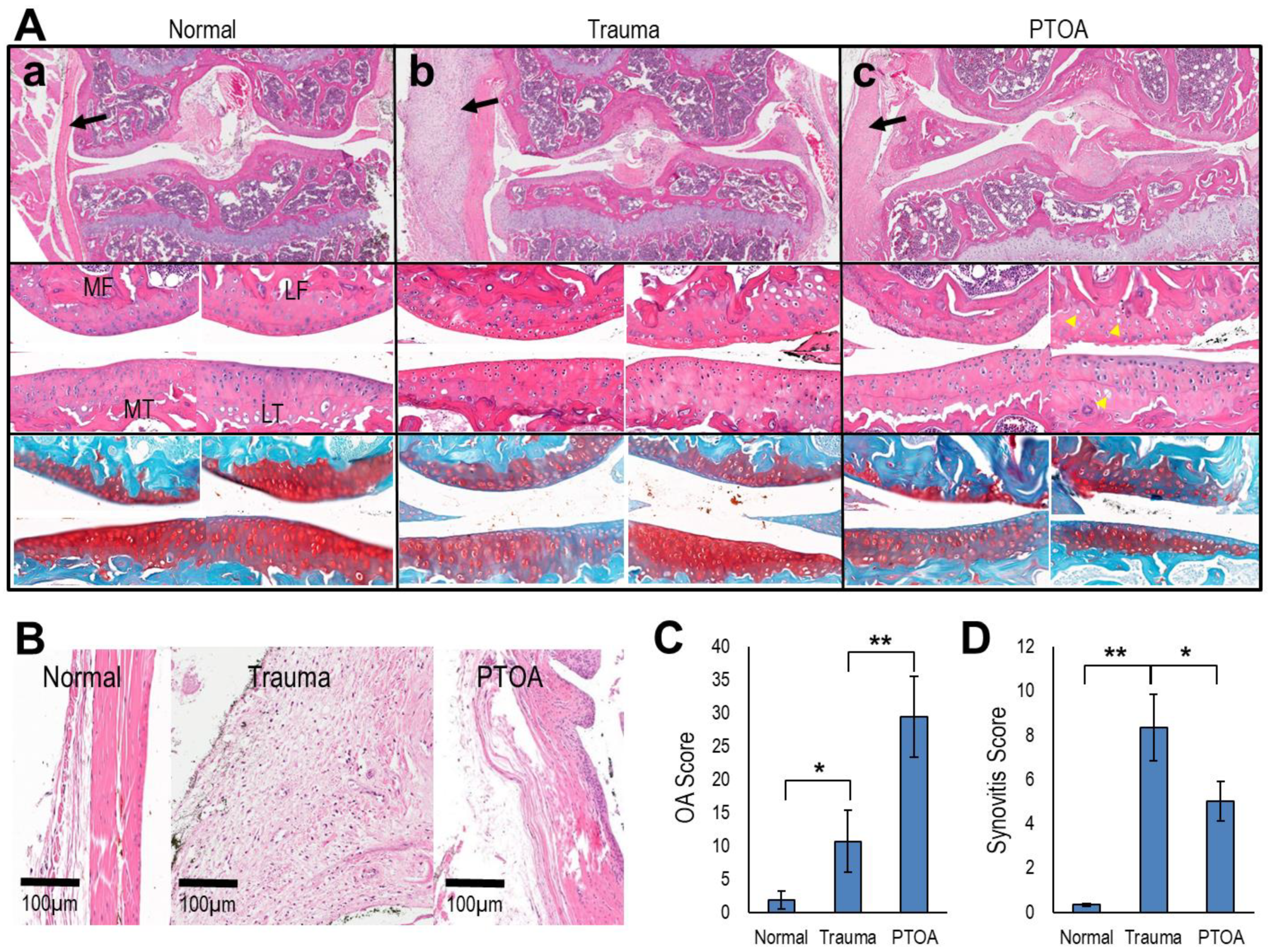
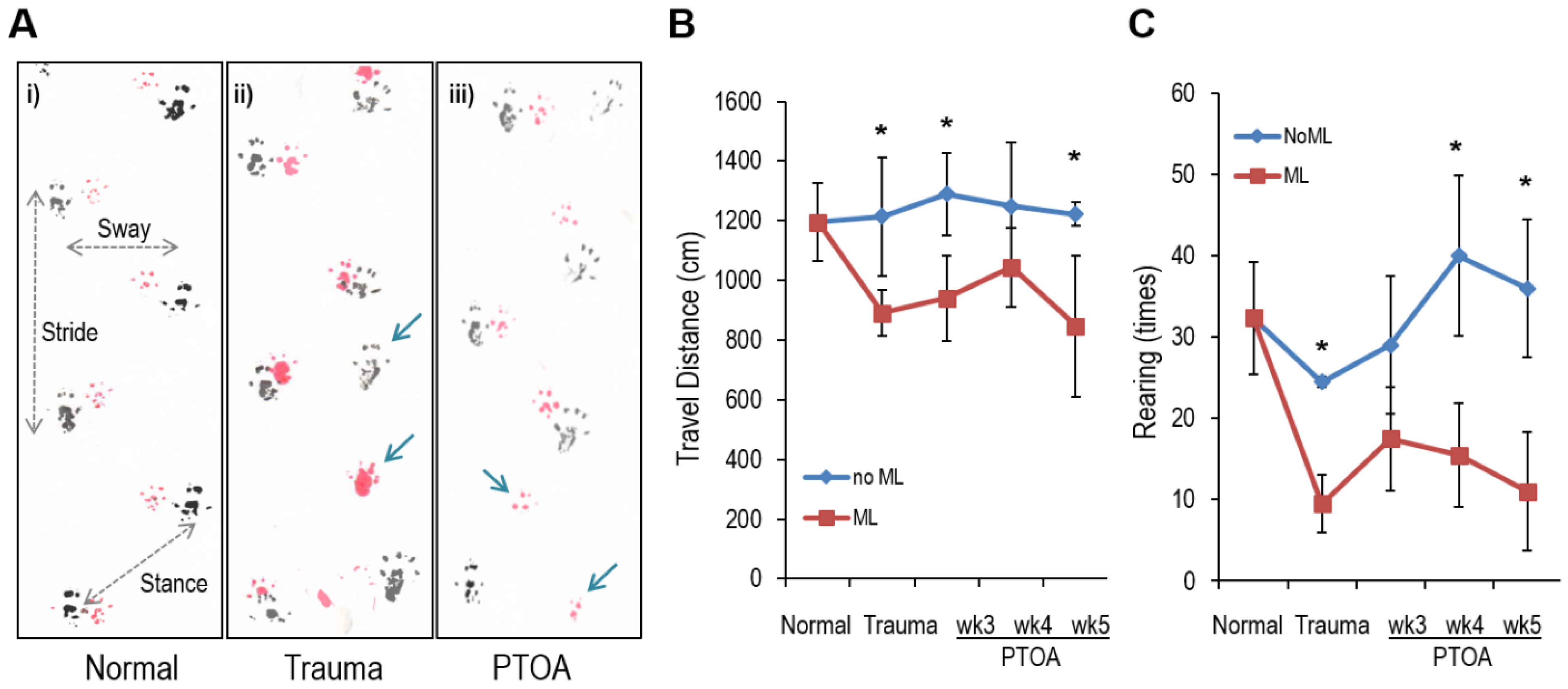
| Normal (N) | Trauma | PTOA | N v Trauma | N v PTOA | |
|---|---|---|---|---|---|
| Stride (cm) | 7.066 ± 0.163 | 6.400 ± 0.167 | 6.717 ± 0.264 | p = 0.001 | p = 0.031 |
| Sway (cm) | 3.533 ± 0.103 | 3.317 ± 0.194 | 3.483 ± 0.223 | p = 0.035 | p = 0.245 |
| Stance (cm) | 4.183 ± 0.160 | 3.667 ± 0.175 | 4.117 ± 0.204 | p = 0.003 | p = 0.142 |
| Missing step (%) | 0.35 | 9.36 | 4.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatti, F.-U.-R.; Jeong, Y.-H.; Kim, D.-G.; Yi, A.-K.; Brand, D.D.; Hasty, K.A.; Cho, H. Characterization of Non-Invasively Induced Post-Traumatic Osteoarthritis in Mice. Antioxidants 2022, 11, 1783. https://doi.org/10.3390/antiox11091783
Bhatti F-U-R, Jeong Y-H, Kim D-G, Yi A-K, Brand DD, Hasty KA, Cho H. Characterization of Non-Invasively Induced Post-Traumatic Osteoarthritis in Mice. Antioxidants. 2022; 11(9):1783. https://doi.org/10.3390/antiox11091783
Chicago/Turabian StyleBhatti, Fazal-Ur-Rehman, Yong-Hoon Jeong, Do-Gyoon Kim, Ae-Kyung Yi, David D. Brand, Karen A. Hasty, and Hongsik Cho. 2022. "Characterization of Non-Invasively Induced Post-Traumatic Osteoarthritis in Mice" Antioxidants 11, no. 9: 1783. https://doi.org/10.3390/antiox11091783
APA StyleBhatti, F.-U.-R., Jeong, Y.-H., Kim, D.-G., Yi, A.-K., Brand, D. D., Hasty, K. A., & Cho, H. (2022). Characterization of Non-Invasively Induced Post-Traumatic Osteoarthritis in Mice. Antioxidants, 11(9), 1783. https://doi.org/10.3390/antiox11091783







