Prinsepiae Nux Extract Activates NRF2 Activity and Protects UVB-Induced Damage in Keratinocyte
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Shen Nong Ben Cao Jing Top-Grade Drugs Library and the Extraction of Prinsepiae Nux
2.3. Cell Cultures
2.4. ARE-Luciferase Reporter Assay and Cell Viability Assay
2.5. NRF2 Knock-Down Experiment
2.6. NF-κB-Luciferase Reporter Assay
2.7. Immunoblot
2.8. Quantitative Real-Time PCR (QPCR)
2.9. UVB Irradiation
2.10. Intracellular ROS Detection
2.11. Tandem Mass Spectrometry (MS/MS) Non-Targeted Fragment Ions Collection Using Ultra-Performance Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry (UPLC-QTOF-MS)
2.12. Global Natural Product Social (GNPS)-Based Molecular Networking(MN) Analysis
2.13. Statistical Analysis
3. Results
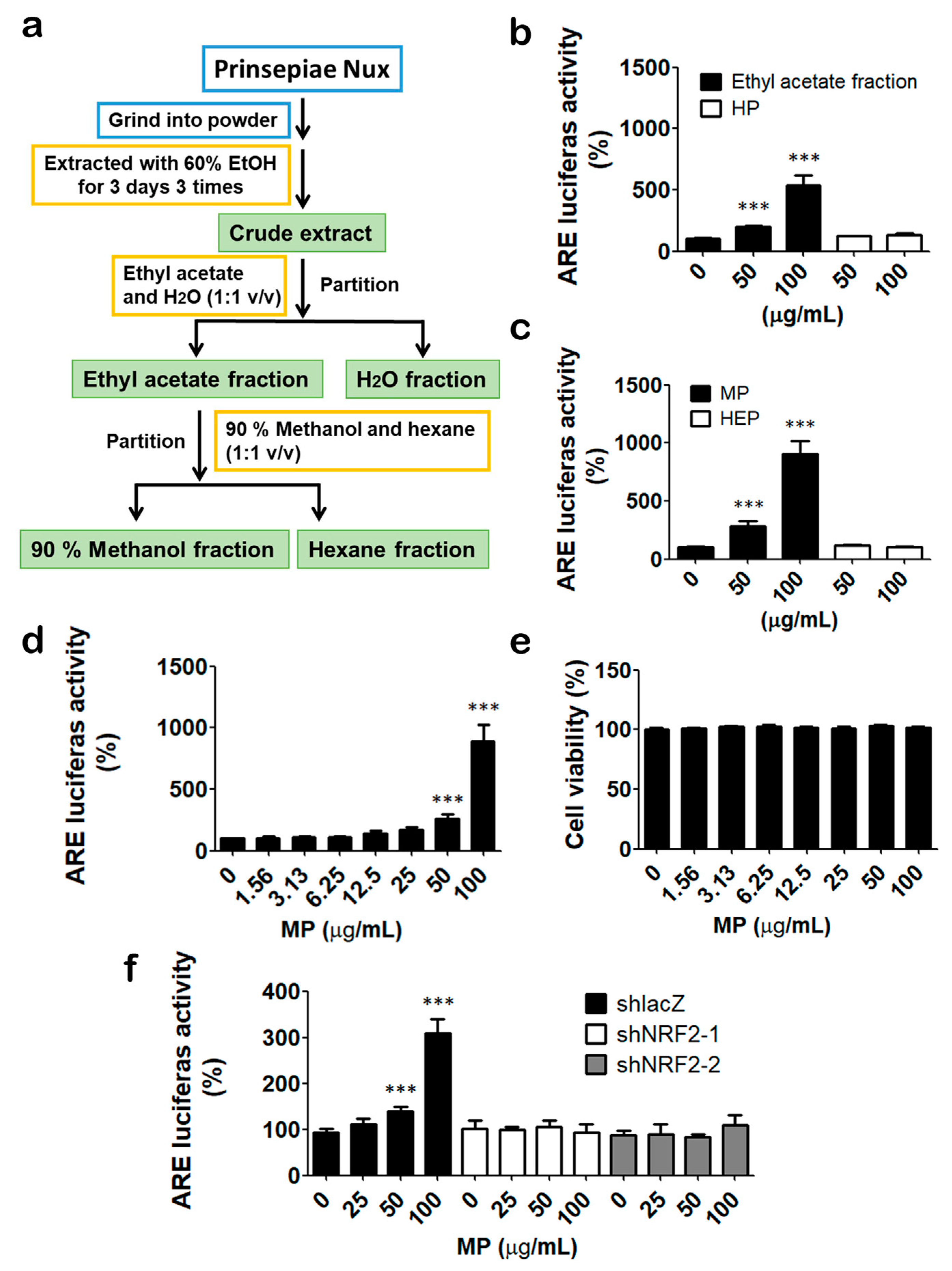
3.1. Identification of Prinsepiae Nux Extract as an NRF2 Activator
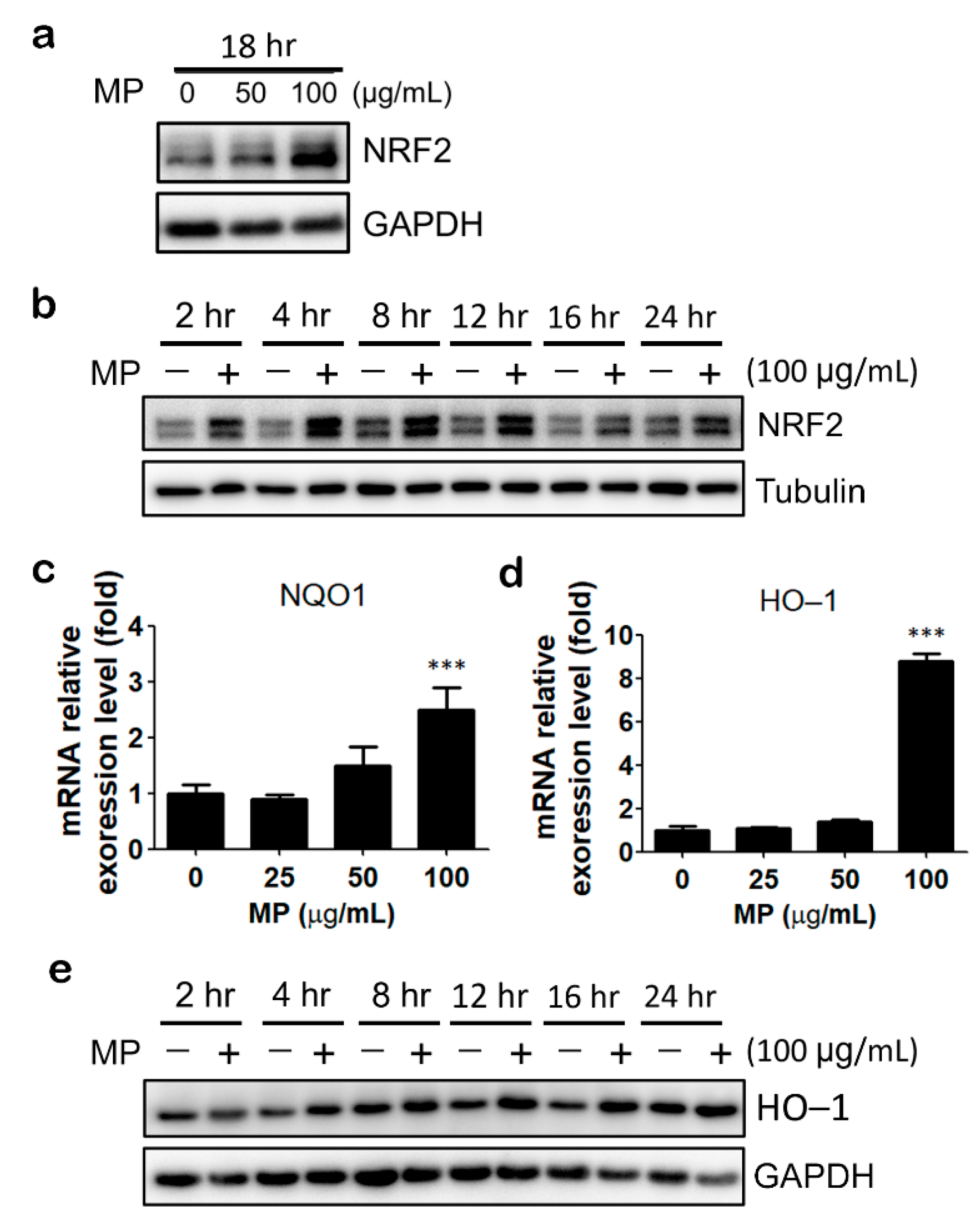
3.2. MP Fraction Activates NRF2 Signaling Pathway in HaCaT Cells
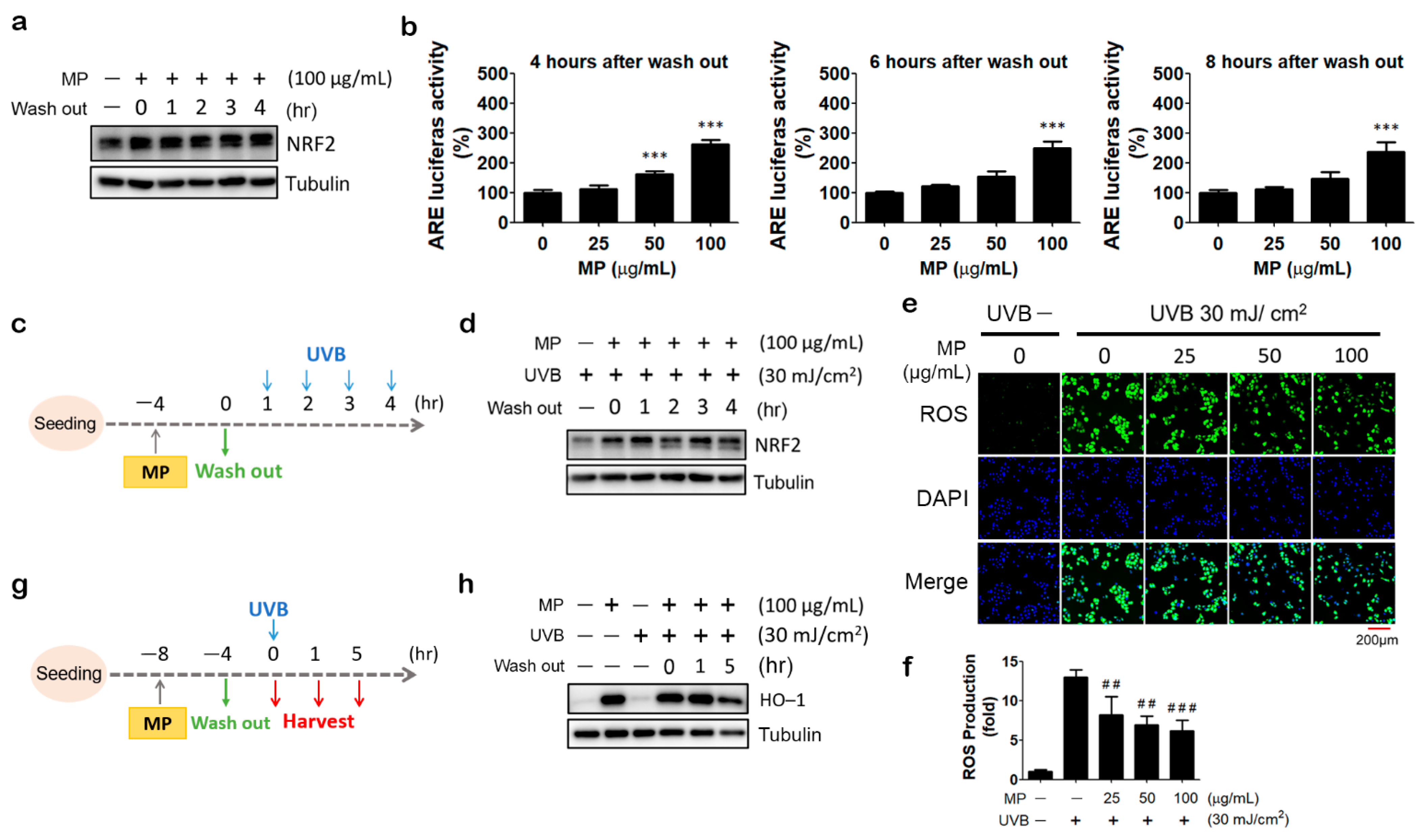
3.3. Pre-Treatment of MP Fraction Could Induce Prolonged Activation of NRF2
3.4. MP Fraction Pre-Treatment Abolished NRF2 Depletion and Reduced ROS Production in UVB-Irradiated HaCaT Cells
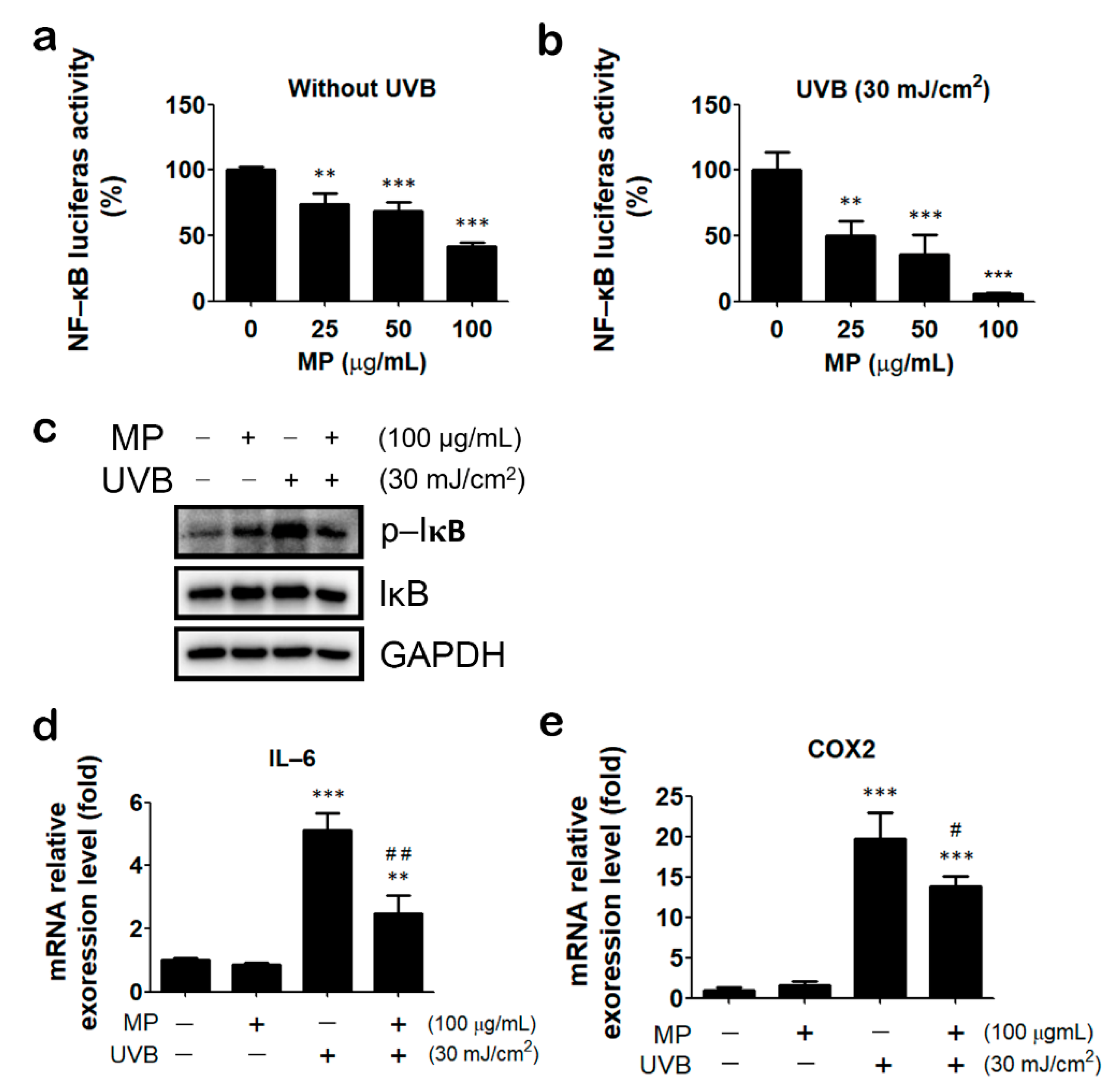
3.5. MP Fraction Ameliorated UVB-Induced Inflammatory Reaction
3.6. Characterizing the Constitutional Variation between Prinsepiae Nux Fractions Using Tandem Mass Spectroscopy and Molecular Networking Approach
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Schikowski, T.; Morita, A.; Berneburg, M. Environmentally-Induced (Extrinsic) Skin Aging: Exposomal Factors and Underlying Mechanisms. J. Investig. Dermatol. 2021, 141, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.L.; Lim, H.W.; Mohammad, T.F. Sunscreens and photoaging: A review of current literature. Am. J. Clin. Dermatol. 2021, 22, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Sample, A.; He, Y.Y. Mechanisms and prevention of UV-induced melanoma. Photodermatol. Photoimmunol. Photomed. 2018, 34, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Bagde, A.; Mondal, A.; Singh, M. Drug delivery strategies for chemoprevention of UVB-induced skin cancer: A review. Photodermatol. Photoimmunol. Photomed. 2018, 34, 60–68. [Google Scholar] [CrossRef] [PubMed]
- De Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet light induced generation of reactive oxygen species. Adv. Exp. Med. Biol. 2017, 996, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.C.; Liao, P.Y.; Hung, S.J.; Ge, J.S.; Chen, S.M.; Lai, J.C.; Hsiao, Y.P.; Yang, J.H. Topical application of glycolic acid suppresses the UVB induced IL-6, IL-8, MCP-1 and COX-2 inflammation by modulating NF-kappaB signaling pathway in keratinocytes and mice skin. J. Dermatol. Sci. 2017, 86, 238–248. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Levonen, A.L.; Landar, A.; Ramachandran, A.; Ceaser, E.K.; Dickinson, D.A.; Zanoni, G.; Morrow, J.D.; Darley-Usmar, V.M. Cellular mechanisms of redox cell signalling: Role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem. J. 2004, 378, 373–382. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta-Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Commission, T.H.P. Taiwan Herbal Pharmacopoeia, 3rd ed.; Ministry of Health and Welfare: Taiwan, China, 2018.
- Li, C.H.; Yen, C.H.; Chen, Y.F.; Lee, K.J.; Fang, C.C.; Zhang, X.; Lai, C.C.; Huang, S.F.; Lin, H.K.; Arthur Chen, Y.M. Characterization of the GNMT-HectH9-PREX2 tripartite relationship in the pathogenesis of hepatocellular carcinoma. Int. J. Cancer 2017, 140, 2284–2297. [Google Scholar] [CrossRef]
- Chen, Y.S.; Chang, H.S.; Hsiao, H.H.; Chen, Y.F.; Kuo, Y.P.; Yen, F.L.; Yen, C.H. Identification of Beilschmiedia tsangii root extract as a Liver cancer cell-normal keratinocyte dual-selective NRF2 regulator. Antioxidants 2021, 10, 544. [Google Scholar] [CrossRef]
- Wu, H.C.; Cheng, M.J.; Yen, C.H.; Chen, Y.A.; Chen, Y.S.; Chen, I.S.; Chang, H.S. Chemical Constituents with GNMT-Promoter-Enhancing and NRF2-Reduction Activities from Taiwan Agarwood Excoecaria formosana. Molecules 2020, 25, 1746. [Google Scholar] [CrossRef]
- Kim, M.; Park, Y.G.; Lee, H.J.; Lim, S.J.; Nho, C.W. Youngiasides A and C Isolated from Youngia denticulatum Inhibit UVB-Induced MMP Expression and Promote Type I Procollagen Production via Repression of MAPK/AP-1/NF-kappaB and Activation of AMPK/Nrf2 in HaCaT Cells and Human Dermal Fibroblasts. J. Agric. Food Chem. 2015, 63, 5428–5438. [Google Scholar] [CrossRef]
- Lee, J.K.; Ko, S.H.; Ye, S.K.; Chung, M.H. 8-Oxo-2′-deoxyguanosine ameliorates UVB-induced skin damage in hairless mice by scavenging reactive oxygen species and inhibiting MMP expression. J. Dermatol. Sci. 2013, 70, 49–57. [Google Scholar] [CrossRef]
- Huang, K.F.; Ma, K.H.; Jhap, T.Y.; Liu, P.S.; Chueh, S.H. Ultraviolet B irradiation induced Nrf2 degradation occurs via activation of TRPV1 channels in human dermal fibroblasts. Free Radic. Biol. Med. 2019, 141, 220–232. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Wen, X.; Hao, D.; Zhang, N.; He, G.; Jiang, X. NF-kappaB signaling in skin aging. Mech. Ageing Dev. 2019, 184, 111160. [Google Scholar] [CrossRef]
- Tramontina, R.; Galman, J.L.; Parmeggiani, F.; Derrington, S.R.; Bugg, T.D.; Turner, N.J.; Squina, F.M.; Dixon, N. Consolidated production of coniferol and other high-value aromatic alcohols directly from lignocellulosic biomass. Green Chem. 2020, 22, 144–152. [Google Scholar] [CrossRef]
- Napylov, A.; Reyes-Garces, N.; Gomez-Rios, G.; Olkowicz, M.; Lendor, S.; Monnin, C.; Bojko, B.; Hamani, C.; Pawliszyn, J.; Vuckovic, D. In vivo solid-phase Microextraction for sampling of oxylipins in brain of awake, moving rats. Angew. Chem. Int. Ed. Engl. 2020, 59, 2392–2398. [Google Scholar] [CrossRef]
- Ruiz-Torres, V.; Rodriguez-Perez, C.; Herranz-Lopez, M.; Martin-Garcia, B.; Gomez-Caravaca, A.M.; Arraez-Roman, D.; Segura-Carretero, A.; Barrajon-Catalan, E.; Micol, V. Marine Invertebrate Extracts Induce Colon Cancer Cell Death via ROS-Mediated DNA Oxidative Damage and Mitochondrial Impairment. Biomolecules 2019, 9, 771. [Google Scholar] [CrossRef] [Green Version]
- Jin, R.; Lin, Z.J.; Xue, C.M.; Zhang, B. An improved association-mining research for exploring Chinese herbal property theory: Based on data of the Shennong’s Classic of Materia Medica. J. Integr. Med. 2013, 11, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Review of dried fruits: Phytochemicals, antioxidant efficacies, and health benefits. J. Funct. Food 2016, 21, 113–132. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Wang, J.H. Chemical constituents of Nux Prinsepiae uniflorae. J. China Pharm. Univ. 2006, 23, 209–211. [Google Scholar]
- Li, N.; Li, H.-X.; Meng, D.-L.; Li, X. Chemical constituents of Nux Prinsepiae(II). J. China Pharm. Univ. 2009, 26, 871–873, 915. [Google Scholar]
- Zhou, H.; Zhao, R.; Yang, J. Two new alkaloid galactosides from the kernel of Prinsepia uniflora. Nat. Prod. Res. 2013, 27, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zheng, X.; Hu, H.; Zhao, J. Study on extracts constituents of Nux Prinsepiae uniflorae. In Medicine Sciences and Bioengineering; Wang, M., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 741–744. [Google Scholar]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Gondal, T.A.; Saeed, F.; Imran, A.; Shahbaz, M.; Fokou, P.V.T.; Umair Arshad, M.; Khan, H. Kaempferol: A key emphasis to its anticancer potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Zwolak, I. Protective effects of dietary antioxidants against vanadium-induced toxicity: A review. Oxidative Med. Cell. Longev. 2020, 2020, 1490316. [Google Scholar] [CrossRef]
- Li, W.; Sun, K.; Hu, F.; Chen, L.; Zhang, X.; Wang, F.; Yan, B. Protective effects of natural compounds against oxidative stress in ischemic diseases and cancers via activating the Nrf2 signaling pathway: A mini review. J. Biochem. Mol. Toxicol. 2021, 35, e22658. [Google Scholar] [CrossRef]
- Dong, Y.; Stewart, T.; Bai, L.; Li, X.; Xu, T.; Iliff, J.; Shi, M.; Zheng, D.; Yuan, L.; Wei, T.; et al. Coniferaldehyde attenuates Alzheimer’s pathology via activation of Nrf2 and its targets. Theranostics 2020, 10, 179–200. [Google Scholar] [CrossRef]
- Ma, W.F.; Duan, X.C.; Han, L.; Zhang, L.L.; Meng, X.M.; Li, Y.L.; Wang, M. Vanillic acid alleviates palmitic acid-induced oxidative stress in human umbilical vein endothelial cells via Adenosine Monophosphate-Activated Protein Kinase signaling pathway. J. Food Biochem. 2019, 43, e12893. [Google Scholar] [CrossRef]
- Varì, R.; Scazzocchio, B.; Santangelo, C.; Filesi, C.; Galvano, F.; D’Archivio, M.; Masella, R.; Giovannini, C. Protocatechuic acid prevents oxLDL-induced apoptosis by activating JNK/Nrf2 survival signals in macrophages. Oxidative Med. Cell. Longev. 2015, 2015, 351827. [Google Scholar] [CrossRef] [Green Version]
- Meeran, M.F.N.; Laham, F.; Azimullah, S.; Sharma, C.; Al Kaabi, A.J.; Tariq, S.; Adeghate, E.; Goyal, S.N.; Ojha, S. beta-Caryophyllene, a natural bicyclic sesquiterpene attenuates beta-adrenergic agonist-induced myocardial injury in a cannabinoid receptor-2 dependent and independent manner. Free Radic. Biol. Med. 2021, 167, 348–366. [Google Scholar] [CrossRef]
- Yu, X.; Cui, L.; Zhang, Z.; Zhao, Q.; Li, S. alpha-Linolenic acid attenuates doxorubicin-induced cardiotoxicity in rats through suppression of oxidative stress and apoptosis. Acta Biochim. Biophys. Sin. 2013, 45, 817–826. [Google Scholar] [CrossRef]
- Vigliante, I.; Mannino, G.; Maffei, M.E. OxiCyan®, a phytocomplex of bilberry (Vaccinium myrtillus) and spirulina (Spirulina platensis), exerts both direct antioxidant activity and modulation of ARE/Nrf2 pathway in HepG2 cells. J. Funct. Foods 2019, 61, 103508. [Google Scholar] [CrossRef]
- Alam, S.-I.; Kim, M.-W.; Shah, F.A.; Saeed, K.; Ullah, R.; Kim, M.-O. Alpha-linolenic acid impedes cadmium-induced oxidative stress, neuroinflammation, and neurodegeneration in mouse brain. Cells 2021, 10, 2274. [Google Scholar] [CrossRef]
- Duangjan, C.; Rangsinth, P.; Zhang, S.; Wink, M.; Tencomnao, T. Anacardium Occidentale L. leaf extracts protect against glutamate/H2O2-induced oxidative toxicity and induce neurite outgrowth: The involvement of SIRT1/Nrf2 signaling pathway and teneurin 4 transmembrane protein. Front. Pharmacol. 2021, 12, 627738. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, L.; Shang, Y.; Yang, X.; Li, J.; He, J.; Gao, X.; Chang, Y.X. A strategy for intelligent chemical profiling-guided precise quantitation of multi-components in traditional Chinese medicine formulae-QiangHuoShengShi decoction. J. Chromatogr. A 2021, 1649, 462178. [Google Scholar] [CrossRef]
- Lin, T.K.; Zhong, L.; Santiago, J.L. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef]
- Letawe, C.; Boone, M.; Pierard, G.E. Digital image analysis of the effect of topically applied linoleic acid on acne microcomedones. Clin. Exp. Dermatol. 1998, 23, 56–58. [Google Scholar] [CrossRef]
- Darmstadt, G.L.; Mao-Qiang, M.; Chi, E.; Saha, S.K.; Ziboh, V.A.; Black, R.E.; Santosham, M.; Elias, P.M. Impact of topical oils on the skin barrier: Possible implications for neonatal health in developing countries. Acta Paediatr. 2002, 91, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Ryu, A.; Hashimoto, A.; Oka, M.; Ichihashi, M. Linoleic acid and alpha-linolenic acid lightens ultraviolet-induced hyperpigmentation of the skin. Arch. Dermatol. Res. 1998, 290, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Takemura, N.; Takahashi, K.; Tanaka, H.; Ihara, Y.; Ikemoto, A.; Fujii, Y.; Okuyama, H. Dietary, but not topical, alpha-linolenic acid suppresses UVB-induced skin injury in hairless mice when compared with linoleic acids. Photochem. Photobiol. 2002, 76, 657–663. [Google Scholar] [CrossRef]
- Moore, E.M.; Wagner, C.; Komarnytsky, S. The Enigma of Bioactivity and Toxicity of Botanical Oils for Skin Care. Front. Pharmacol. 2020, 11, 785. [Google Scholar] [CrossRef]
- Ispiryan, A.; Viskelis, J.; Viskelis, P. Red Raspberry (Rubus idaeus L.) Seed Oil: A Review. Plants 2021, 10, 944. [Google Scholar] [CrossRef]
- Huang, T.H.; Wang, P.W.; Yang, S.C.; Chou, W.L.; Fang, J.Y. Cosmetic and Therapeutic Applications of Fish Oil’s Fatty Acids on the Skin. Mar. Drugs 2018, 16, 256. [Google Scholar] [CrossRef]
- Choi, H.J.; Song, B.R.; Kim, J.E.; Bae, S.J.; Choi, Y.J.; Lee, S.J.; Gong, J.E.; Lee, H.S.; Lee, C.Y.; Kim, B.H.; et al. Therapeutic Effects of Cold-Pressed Perilla Oil Mainly Consisting of Linolenic acid, Oleic Acid and Linoleic Acid on UV-Induced Photoaging in NHDF Cells and SKH-1 Hairless Mice. Molecules 2020, 25, 989. [Google Scholar] [CrossRef]
- Hwang, E.; Lee, D.G.; Park, S.H.; Oh, M.S.; Kim, S.Y. Coriander leaf extract exerts antioxidant activity and protects against UVB-induced photoaging of skin by regulation of procollagen type I and MMP-1 expression. J. Med. Food 2014, 17, 985–995. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-H.; Chen, Y.-S.; Lai, K.-H.; Lu, C.-K.; Chang, H.-S.; Wu, H.-C.; Yen, F.-L.; Chen, L.-Y.; Lee, J.-C.; Yen, C.-H. Prinsepiae Nux Extract Activates NRF2 Activity and Protects UVB-Induced Damage in Keratinocyte. Antioxidants 2022, 11, 1755. https://doi.org/10.3390/antiox11091755
Wang S-H, Chen Y-S, Lai K-H, Lu C-K, Chang H-S, Wu H-C, Yen F-L, Chen L-Y, Lee J-C, Yen C-H. Prinsepiae Nux Extract Activates NRF2 Activity and Protects UVB-Induced Damage in Keratinocyte. Antioxidants. 2022; 11(9):1755. https://doi.org/10.3390/antiox11091755
Chicago/Turabian StyleWang, Shih-Han, Yi-Siao Chen, Kuei-Hung Lai, Chung-Kuang Lu, Hsun-Shuo Chang, Ho-Cheng Wu, Feng-Lin Yen, Lo-Yun Chen, Jin-Ching Lee, and Chia-Hung Yen. 2022. "Prinsepiae Nux Extract Activates NRF2 Activity and Protects UVB-Induced Damage in Keratinocyte" Antioxidants 11, no. 9: 1755. https://doi.org/10.3390/antiox11091755
APA StyleWang, S.-H., Chen, Y.-S., Lai, K.-H., Lu, C.-K., Chang, H.-S., Wu, H.-C., Yen, F.-L., Chen, L.-Y., Lee, J.-C., & Yen, C.-H. (2022). Prinsepiae Nux Extract Activates NRF2 Activity and Protects UVB-Induced Damage in Keratinocyte. Antioxidants, 11(9), 1755. https://doi.org/10.3390/antiox11091755









