Intracellular Oxidative Stress Induced by Physical Exercise in Adults: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.1.1. Participants
2.1.2. Interventions
2.1.3. Comparators (C)
2.1.4. Outcomes (O)
2.1.5. Study Design (S)
2.1.6. Exclusion Criteria
2.2. Information Sources
2.3. Search Strategy
2.4. Selection Process
2.5. Data Collection Process
2.6. Data Items
2.7. Study Risk of Bias Assessment
2.8. Effect Measures
2.9. Synthesis Methods
2.9.1. Study Information Synthesis
2.9.2. Data Pre-Processing
2.9.3. Data Synthesis
2.10. Reporting Bias Assessment
2.11. Certainty Assessment
3. Results
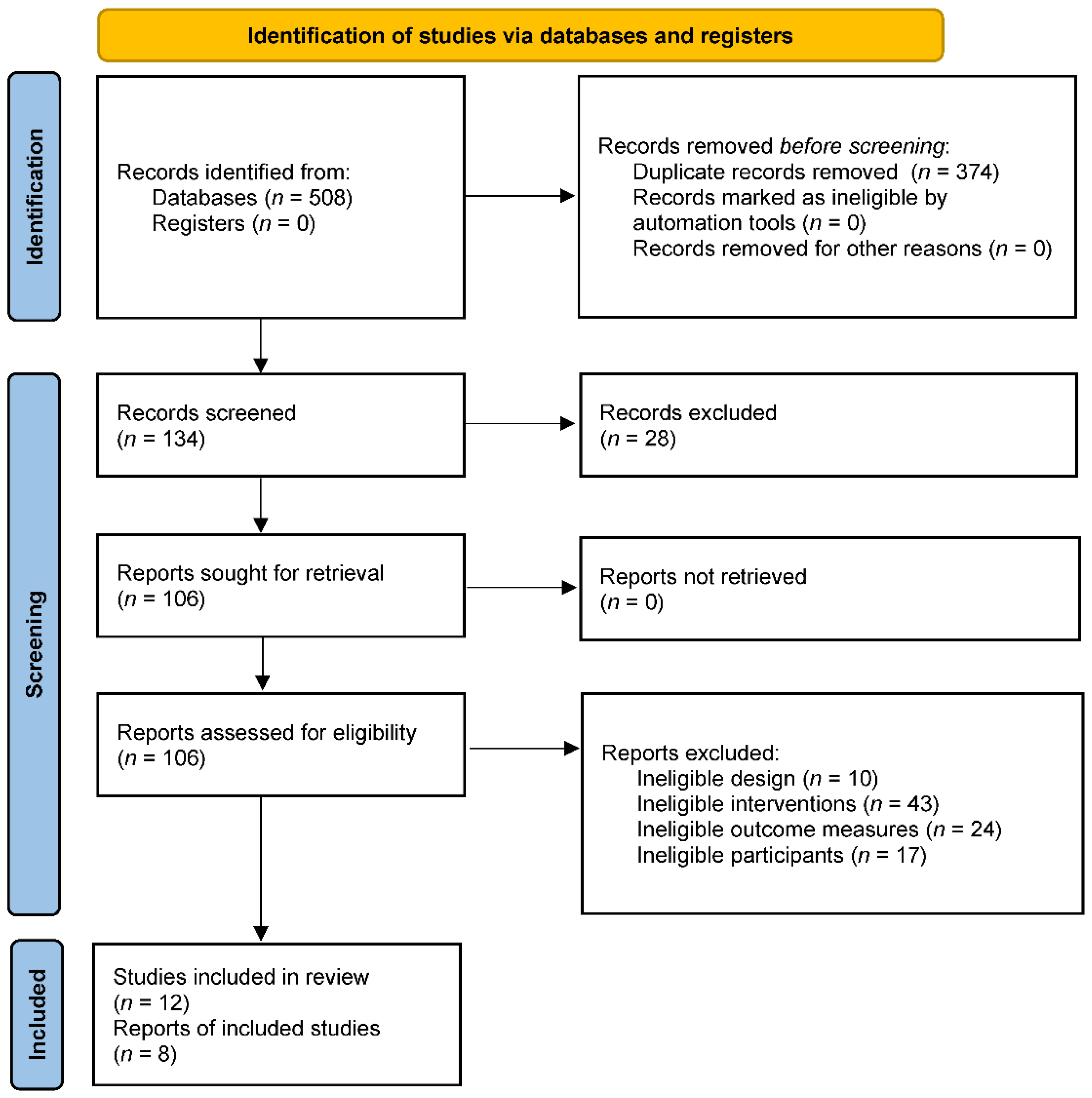
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias in Studies
3.4. Results of Individual Studies
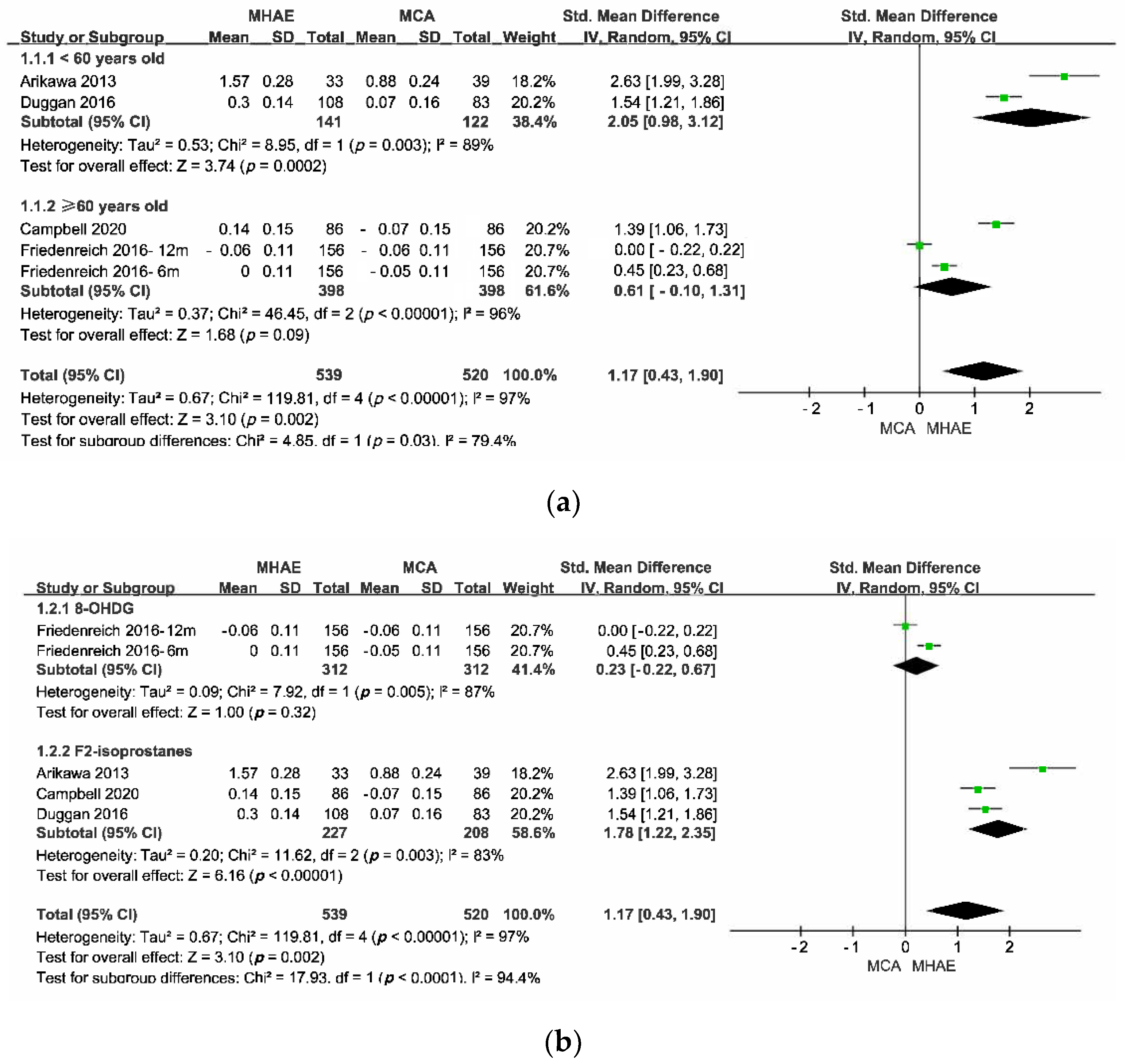
3.5. Results of Syntheses
3.5.1. Evidence Structure
3.5.2. Pair-Wise Meta-Analysis
3.5.3. Network Meta-Analysis
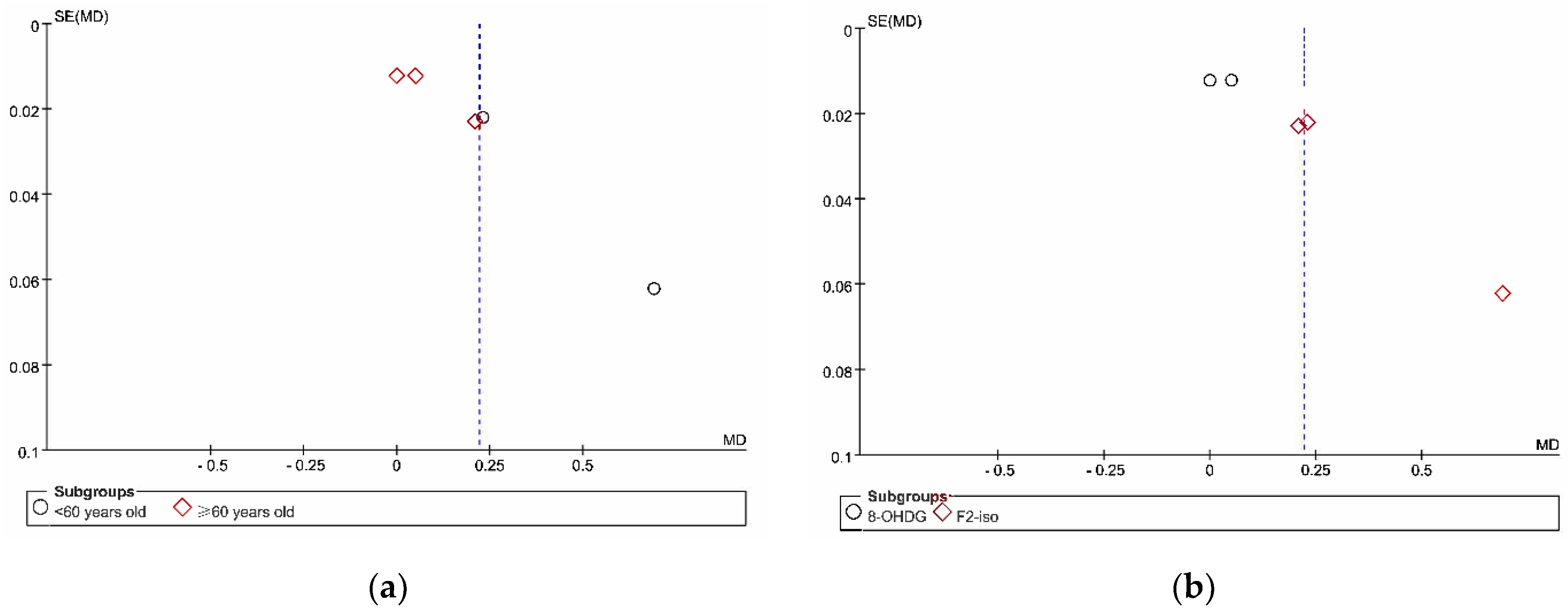
3.6. Reporting Bias
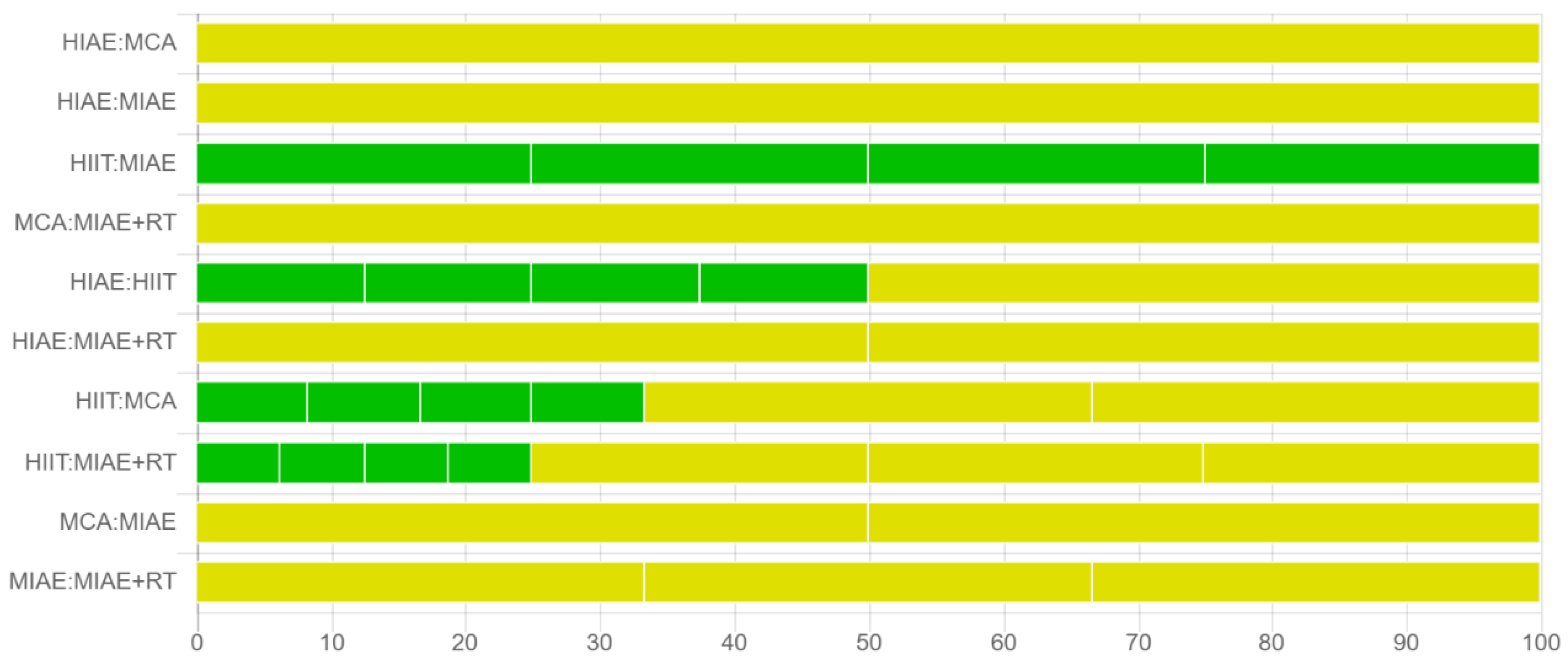
3.7. Certainty of Evidence
4. Discussion
5. Conclusions
6. Other Information
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Devasagayam, T.P.; Tilak, J.C.; Boloor, K.K.; Sane, K.S.; Ghaskadbi, S.S.; Lele, R.D. Free radicals and antioxidants in human health: Current status and future prospects. J. Assoc. Physicians India 2004, 52, 794–804. [Google Scholar]
- Huang, Z.; Rusanova, O.M. Cardiorespiratory System in the Context of Regular Exercise in Kayaking. Phys. Act. Health 2022, 6, 124–135. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- Reid, M.B. Invited Review: Redox modulation of skeletal muscle contraction: What we know and what we don’t. J. Appl. Physiol. 2001, 90, 724–731. [Google Scholar] [CrossRef]
- Xiang, L.; Mei, Q.; Wang, A.; Shim, V.; Fernandez, J.; Gu, Y. Evaluating function in the hallux valgus foot following a 12-week minimalist footwear intervention: A pilot computational analysis. J. Biomech. 2022, 132, 110941. [Google Scholar] [CrossRef] [PubMed]
- Opara, E.C. Oxidative stress, micronutrients, diabetes mellitus and its complications. J. R. Soc. Promot. Health 2002, 122, 28–34. [Google Scholar] [CrossRef]
- Brooker, R.J. Genetics: Analysis & Principles; Addison-Wesley: Reading, MA, USA, 1999. [Google Scholar]
- Giandonato, J.A.; Tringali, V.M.; Thoms, R.C. Improving mental health through physical activity: A narrative literature review. Phys. Act. Health 2021, 5, 146–153. [Google Scholar] [CrossRef]
- Simons, L.P.A.; Pijl, H.; Verhoef, J.; Lamb, H.J.; Ommen, B.V.; Gerritsen, B.; Bizino, M.B.; Snel, M.; Feenstra, R.; Jonker, C.M. E-health relationships diabetes: 50 weeks evaluation. Int. J. Biomed. Eng. Technol. 2022, 38, 81–98. [Google Scholar] [CrossRef]
- Liu, J.F.; Chang, W.Y.; Chan, K.H.; Tsai, W.Y.; Lin, C.L.; Hsu, M.C. Blood lipid peroxides and muscle damage increased following intensive resistance training of female weightlifters. Ann. N. Y. Acad. Sci. 2005, 1042, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Groussard, C.; Rannou-Bekono, F.; Machefer, G.; Chevanne, M.; Vincent, S.; Sergent, O.; Cillard, J.; Gratas-Delamarche, A. Changes in blood lipid peroxidation markers and antioxidants after a single sprint anaerobic exercise. Eur. J. Appl. Physiol. 2003, 89, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.B. Reactive Oxygen Species as Agents of Fatigue. Med. Sci. Sports Exerc. 2016, 48, 2239–2246. [Google Scholar] [CrossRef]
- Knez, W.L.; Jenkins, D.G.; Coombes, J.S. The effect of an increased training volume on oxidative stress. Int. J. Sports Med. 2014, 35, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Laitano, O.; Kalsi, K.K.; Pook, M.; Oliveira, A.R.; Gonzalez-Alonso, J. Separate and combined effects of heat stress and exercise on circulatory markers of oxidative stress in euhydrated humans. Eur. J. Appl. Physiol. 2010, 110, 953–960. [Google Scholar] [CrossRef]
- Dekany, M.; Nemeskeri, V.; Gyore, I.; Harbula, I.; Malomsoki, J.; Pucsok, J. Antioxidant status of interval-trained athletes in various sports. Int. J. Sports Med. 2006, 27, 112–116. [Google Scholar] [CrossRef]
- Hernandez, R.; Mahedero, G.; Caballero, M.J.; Rodriguez, J.; Manjon, I.; Rodriguez, I.; Maynar, M. Effects of physical exercise in pre-and postmenopausal women on lipid peroxidation and antioxidant systems. Endocr. Res. 1999, 25, 153–161. [Google Scholar] [CrossRef]
- Jemili, H.; Mejri, M.A.; Bouhlel, E.; Amri, M. Biochemical status, oxidative and antioxidant responses after 3-month specific training in elite karate athletes. Physiol. Int. 2017, 104, 344–354. [Google Scholar] [CrossRef][Green Version]
- Buettner, R.G. The pecking order of free radicals and antioxidants: Lipid peroxidation, α-tocopherol, and ascorbate. J. Arch. Biochem. Biophys. 1993, 300, 535–543. [Google Scholar] [CrossRef]
- Ye, Y.; Lin, H.; Wan, M.; Qiu, P.; Xia, R.; He, J.; Tao, J.; Chen, L.; Zheng, G. The Effects of Aerobic Exercise on Oxidative Stress in Older Adults: A Systematic Review and Meta-Analysis. Front. Physiol. 2021, 12, 701151. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Fisher, G.; Schwartz, D.D.; Quindry, J.; Barberio, M.D.; Foster, E.B.; Jones, K.W.; Pascoe, D.D. Lymphocyte enzymatic antioxidant responses to oxidative stress following high-intensity interval exercise. J. Appl. Physiol. (1985) 2011, 110, 730–737. [Google Scholar] [CrossRef]
- Powers, S.K.; Smuder, A.J.; Kavazis, A.N.; Hudson, M.B. Experimental guidelines for studies designed to investigate the impact of antioxidant supplementation on exercise performance. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Quindry, J.; Miller, L.; McGinnis, G.; Irwin, M.; Dumke, C.; Magal, M.; Triplett, N.T.; McBride, J.; Urbiztondo, Z. Muscle-fiber type and blood oxidative stress after eccentric exercise. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 462–470. [Google Scholar] [CrossRef]
- Armijo-Olivo, S.; Stiles, C.R.; Hagen, N.A.; Biondo, P.D.; Cummings, G.G. Assessment of study quality for systematic reviews: A comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: Methodological research. J. Eval. Clin. Pract. 2012, 18, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Gogtay, N.J.; Thatte, U.M. An Introduction to Meta-Analysis. J. Assoc. Physicians India 2017, 65, 78–85. [Google Scholar] [PubMed]
- Nikolakopoulou, A.; Higgins, J.P.T.; Papakonstantinou, T.; Chaimani, A.; Del Giovane, C.; Egger, M.; Salanti, G. CINeMA: An approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020, 17, e1003082. [Google Scholar] [CrossRef]
- Papakonstantinou, T.; Nikolakopoulou, A.; Higgins, J.P.T.; Egger, M.; Salanti, G. CINeMA: Software for semiautomated assessment of the confidence in the results of network meta-analysis. Campbell Syst. Rev. 2020, 16, e1080. [Google Scholar] [CrossRef]
- Allgayer, H.; Owen, R.W.; Nair, J.; Spiegelhalder, B.; Streit, J.; Reichel, C.; Bartsch, H. Short-term moderate exercise programs reduce oxidative DNA damage as determined by high-performance liquid chromatography-electrospray ionization-mass spectrometry in patients with colorectal carcinoma following primary treatment. Scand. J. Gastroenterol. 2008, 43, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Arikawa, A.Y.; Thomas, W.; Gross, M.; Smith, A.; Phipps, W.R.; Kurzer, M.S.; Schmitz, K.H. Aerobic training reduces systemic oxidative stress in young women with elevated levels of F2-isoprostanes. Contemp. Clin. Trials 2013, 34, 212–217. [Google Scholar] [CrossRef][Green Version]
- Campbell, P.T.; Gross, M.D.; Potter, J.D.; Schmitz, K.H.; Duggan, C.; McTiernan, A.; Ulrich, C.M. Effect of exercise on oxidative stress: A 12-month randomized, controlled trial. Med. Sci. Sports Exerc. 2010, 42, 1448–1453. [Google Scholar] [CrossRef]
- Correa, H.L.; Neves, R.V.P.; Deus, L.A.; Maia, B.C.H.; Maya, A.T.; Tzanno-Martins, C.; Souza, M.K.; Silva, J.A.B.; Haro, A.S.; Costa, F.; et al. Low-load resistance training with blood flow restriction prevent renal function decline: The role of the redox balance, angiotensin 1–7 and vasopressin. Physiol. Behav. 2021, 230, 113295. [Google Scholar] [CrossRef] [PubMed]
- Duggan, C.; Tapsoba, J.D.; Wang, C.Y.; Campbell, K.L.; Foster-Schubert, K.; Gross, M.D.; McTiernan, A. Dietary Weight Loss, Exercise, and Oxidative Stress in Postmenopausal Women: A Randomized Controlled Trial. Cancer Prev. Res. 2016, 9, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Friedenreich, C.M.; Pialoux, V.; Wang, Q.; Shaw, E.; Brenner, D.R.; Waltz, X.; Conroy, S.M.; Johnson, R.; Woolcott, C.G.; Poulin, M.J.; et al. Effects of exercise on markers of oxidative stress: An Ancillary analysis of the Alberta Physical Activity and Breast Cancer Prevention Trial. BMJ Open Sport Exerc. Med. 2016, 2, e000171. [Google Scholar] [CrossRef]
- Hudson, M.B.; Hosick, P.A.; McCaulley, G.O.; Schrieber, L.; Wrieden, J.; McAnulty, S.R.; Triplett, N.T.; McBride, J.M.; Quindry, J.C. The effect of resistance exercise on humoral markers of oxidative stress. Med. Sci. Sports Exerc. 2008, 40, 542–548. [Google Scholar] [CrossRef]
- Jamurtas, A.Z.; Fatouros, I.G.; Deli, C.K.; Georgakouli, K.; Poulios, A.; Draganidis, D.; Papanikolaou, K.; Tsimeas, P.; Chatzinikolaou, A.; Avloniti, A.; et al. The Effects of Acute Low-Volume HIIT and Aerobic Exercise on Leukocyte Count and Redox Status. J. Sports Sci. Med. 2018, 17, 501–508. [Google Scholar]
- Mallard, A.R.; Hollekim-Strand, S.M.; Coombes, J.S.; Ingul, C.B. Exercise intensity, redox homeostasis and inflammation in type 2 diabetes mellitus. J. Sci. Med. Sport 2017, 20, 893–898. [Google Scholar] [CrossRef]
- Small, D.M.; Beetham, K.S.; Howden, E.J.; Briskey, D.R.; Johnson, D.W.; Isbel, N.M.; Gobe, G.C.; Coombes, J.S. Effects of exercise and lifestyle intervention on oxidative stress in chronic kidney disease. Redox Rep. 2017, 22, 127–136. [Google Scholar] [CrossRef]
- Vezzoli, A.; Mrakic-Sposta, S.; Montorsi, M.; Porcelli, S.; Vago, P.; Cereda, F.; Longo, S.; Maggio, M.; Narici, M. Moderate Intensity Resistive Training Reduces Oxidative Stress and Improves Muscle Mass and Function in Older Individuals. Antioxidants 2019, 8, 431. [Google Scholar] [CrossRef]
- Wadley, A.J.; Veldhuijzen van Zanten, J.J.; Stavropoulos-Kalinoglou, A.; Metsios, G.S.; Smith, J.P.; Kitas, G.D.; Aldred, S. Three months of moderate-intensity exercise reduced plasma 3-nitrotyrosine in rheumatoid arthritis patients. Eur. J. Appl. Physiol. 2014, 114, 1483–1492. [Google Scholar] [CrossRef]
- Varamenti, E.; Tod, D.; Pullinger, S.A. Redox Homeostasis and Inflammation Responses to Training in Adolescent Athletes: A Systematic Review and Meta-analysis. Sports Med. Open 2020, 6, 34. [Google Scholar] [CrossRef]
- Tryfidou, D.V.; McClean, C.; Nikolaidis, M.G.; Davison, G.W. DNA Damage Following Acute Aerobic Exercise: A Systematic Review and Meta-analysis. Sports Med. 2020, 50, 103–127. [Google Scholar] [CrossRef]
- Powers, S.K.; Jackson, M.J. Exercise-Induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle Force Production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J.; Fisher-Wellman, K.H.; Bell, H.K. The effect of long-term, high-volume aerobic exercise training on postprandial lipemia and oxidative stress. Phys. Sportsmed. 2010, 38, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Chaki, B.; Chattopadhyay, S.; Bandyopadhyay, A. High-Intensity Exercise Induced Oxidative Stress and Skeletal Muscle Damage in Postpubertal Boys and Girls: A Comparative Study. J. Strength Cond. Res. 2018, 32, 1045–1052. [Google Scholar] [CrossRef]
- Baker, J.S.; Bailey, D.M.; Hullin, D.; Young, I.; Davies, B. Metabolic implications of resistive force selection for oxidative stress and markers of muscle damage during 30 s of high-intensity exercise. Eur. J. Appl. Physiol. 2004, 92, 321–327. [Google Scholar] [CrossRef]
- Lu, Y.; Wiltshire, H.D.; Baker, J.S.; Wang, Q. Effects of High Intensity Exercise on Oxidative Stress and Antioxidant Status in Untrained Humans: A Systematic Review. Biology 2021, 10, 1272. [Google Scholar] [CrossRef]
- Lopes Kruger, R.; Costa Teixeira, B.; Boufleur Farinha, J.; Cauduro Oliveira Macedo, R.; Pinto Boeno, F.; Rech, A.; Lopez, P.; Silveira Pinto, R.; Reischak-Oliveira, A. Effect of exercise intensity on postprandial lipemia, markers of oxidative stress, and endothelial function after a high-fat meal. Appl. Physiol. Nutr. Metab. 2016, 41, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Bogdanis, G.C.; Stavrinou, P.; Fatouros, I.G.; Philippou, A.; Chatzinikolaou, A.; Draganidis, D.; Ermidis, G.; Maridaki, M. Short-term high-intensity interval exercise training attenuates oxidative stress responses and improves antioxidant status in healthy humans. Food Chem. Toxicol. 2013, 61, 171–177. [Google Scholar] [CrossRef]
- Tucker, P.S.; Briskey, D.R.; Scanlan, A.T.; Coombes, J.S.; Dalbo, V.J. High intensity interval training favourably affects antioxidant and inflammation mRNA expression in early-stage chronic kidney disease. Free Radic. Biol. Med. 2015, 89, 466–472. [Google Scholar] [CrossRef]
- Butcher, L.R.; Thomas, A.; Backx, K.; Roberts, A.; Webb, R.; Morris, K. Low-intensity exercise exerts beneficial effects on plasma lipids via PPARgamma. Med. Sci. Sports Exerc. 2008, 40, 1263–1270. [Google Scholar] [CrossRef]
- Davies, N.A.; Watkeys, L.; Butcher, L.; Potter, S.; Hughes, M.G.; Moir, H.; Morris, K.; Thomas, A.W.; Webb, R. The contributions of oxidative stress, oxidised lipoproteins and AMPK towards exercise-associated PPARgamma signalling within human monocytic cells. Free Radic. Res. 2015, 49, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K. Research on the Physiological Monitoring and Evaluation of Pre-Competition Altitude Training for Zhejiang Elite Swimmers. Phys. Act. Health 2021, 5, 64–70. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]



| Study | Participants | Interventions | ||||||
|---|---|---|---|---|---|---|---|---|
| Population | Average Age | Gender (F/M) | Duration | Group | Description | Protocol | Category | |
| Friedenreich 2016 [35] | Inactive, healthy, postmenopausal women | 60.9 | 320/0 | 12 months | Experimental Group | Moderate-to-vigorous aerobic exercise | 45 min/per time, from 50–60% HRmax to 70–80% HRmax, 225 min/week | MHAE |
| Control Group | Maintaining current active levels | Maintaining current active levels | MCA | |||||
| Vezzoli 2019 [40] | Elders with sarcopenia | 72.4 | 19/16 | 12 weeks | Experimental Group | Moderate-intensity resistive exercise training | 3/week, 6–8 min aerobic warm-up, 3 sets of 14–16 reps of chest presses, horizontal leg-presses, vertical rows, and shoulder exercises with free weights, 60% 1RM. | RT |
| Control Group | Maintaining current active levels | Maintaining regular activities of daily living | MCA | |||||
| Correa 2021 [33] | Hypertensive patients with stage II chronic kidney disease | 58.0 | 23/37 | 6 months | Experimental Group | Resistance training | 3/week, 3 sets of 12 reps at 50% 1RM, 3 sets of 10 reps at 60% 1RM, 3 sets of 8 reps at 70% 1RM. | RT |
| Control Group | Maintaining current active levels | Maintaining current active levels | MCA | |||||
| Hudson 2008 [36] | Adults with resistance training experience | 21.8 | Unclear | 1 time | Experimental Group | Hypertrophy resistance training | 4 sets, 10 reps with 90 s of rest at 75% 1RM | RT |
| Control Group | Strength training | 11 sets, 3 reps with 5 min of rest at 90% 1RM | ST | |||||
| Jamurtas 2018 [37] | Healthy young men | 22.4 | 0/12 | 1 time | Experimental Group | High-intensity interval training | 4 sets of 30 s sprints on a cycle-ergometer with 4 min of recovery against the resistance of 0.375 kg/kg of body mass | HIIT |
| Control Group | Traditional continuous aerobic exercise | 30 min cycling on a cycle-ergometer at 70% of VO2max | HIAE | |||||
| Arikawa 2013 [31] | Sedentary young women | 25.3 | 319/0 | 16 weeks | Experimental Group | Moderate-to-vigorous aerobic exercise | 30 min/per, 5/week, from moderate to 80–85% HRmax | MHAE |
| Control Group | Maintaining current active levels | Maintaining current active levels | MCA | |||||
| Campbell 2020 [32] | Overweight or obese, postmenopausal, sedentary women | 60.7 | 173/0 | 12 months | Experimental Group | Moderate-intensity aerobic exercise | 60–75% HRmax, ≥ 45 min/day, 5 days/week | MHAE |
| Control Group | Stretching and relaxation | 1/week, 45 min/per, stretching and relaxation; asked to not change exercise habits otherwise | MCA | |||||
| Duggan 2016 [34] | Overweight or obese, postmenopausal women | 57.8 | 204/0 | 12 months | Experimental Group | Moderate-to-vigorous aerobic exercise | 45 min/day, 5/week, moderate to 70–85% HRmax of aerobic exercise, 2/week of home exercise, 3/week supervised sessions | MHAE |
| Control Group | Maintaining current active levels | No change in their diet or exercise habits | MCA | |||||
| Mallard 2017 [38] | Participants with type 2 diabetes | 57.0 | 14/22 | 12 weeks | Experimental Group | High-intensity interval training | 10 min warm-up at 70% HRmax, 4 × 4 min HIIT at 90–95% HRmax, interspersed with 3 min active recovery at 70% HRmax in RI, ending with 5 min cool-down, 40 min total, 3/week. | HIIT |
| Control Group | Moderate-intensity continuous training | 210 min/week unsupervised moderate-intensity at 70% HRmax exercise | MIAE | |||||
| Small 2017 [39] | Patients with stages 3–4 of chronic kidney disease | 61.9 | 59/78 | 12 months | Experimental Group | Standard nephrology care | A review by a nephrologist recommended lifestyle modification but no specific information or education | MCA |
| Control Group | Exercise training and lifestyle intervention | Gym session (20–30 min aerobic training, 20 min resistance training), home-based whole-body resistance training exercises, 2–3/week. | MIAE + RT | |||||
| Allgayer 2008 [30] | Patients with colorectal cancer | 58.6 | 17/44 | 2 weeks | Experimental Group | Moderate-intensity exercise program | 0.3–0.4 maximal individual aerobic exercise performance, 30–40 min/per | MIAE |
| Control Group | High-intensity exercise program | 0.5–0.6 maximal individual aerobic exercise performance, 30–40 min/per | HIAE | |||||
| Wadley 2014 [41] | Rheumatoid arthritis patients | 56.0 | 13/6 | 12 weeks | Experimental Group | High-intensity aerobic exercise | 3/week, 70% VO2max aerobic exercise, 3 sets, 3–4 intervals, 30–40 min total | HIAE |
| Control Group | Maintaining current activity | Be advised on the benefits of exercise throughout the same period | MCA | |||||
| Study | Duration | Reporting Time | Indicator | Unit | Main Results | ||
|---|---|---|---|---|---|---|---|
| Experimental Group | Control Group | Between Groups | |||||
| Friedenreich 2016 [35] | 12 months | Pre-program 6 months 12 months | Plasma 8-OHDG | ng/L | Almost unchanged in all recording times | Increased insignificantly in all recording times | Without significant difference in all recording times |
| Vezzoli 2019 [40] | 12 weeks | Pre-program Post-program | Plasma PCs * | nmol/mg· protein | Decreased significantly | Almost unchanged | With significant difference |
| Urine 8-OHDG * | ng/mg· creatinine | Decreased significantly | Increased insignificantly | With significant difference | |||
| Correa 2021 [33] | 6 months | Pre-program Post-program | Plasma F2-isoprostanes * | mg/mL | Decreased significantly | Increased insignificantly | With significant difference |
| Hudson 2008 [36] | 1 time | Pre-exercise Post-exercise 60 min post-exercise | Plasma PCs * | umol/mg protein | Increased significantly only 60 min post-exercise | Increased significantly both post-exercise and 60 min post-exercise | With significant difference only post-exercise |
| Jamurtas 2018 [37] | 1 time | Pre-exercise Post-exercise 24 h post-exercise 48 h post-exercise 72 h post-exercise | Plasma PCs * | nmol/mg· protein | Increased significantly only post-exercise | Almost unchanged in all recording times | With significant difference only post-exercise |
| Arikawa 2013 [31] | 4 months | Pre-program Post-program | Plasma F2-isoprostanes | pg/mL | Decreased significantly | Decreased significantly | Without significant difference |
| Campbell 2020 [32] | 12 months | Pre-program Post-program | Urinary F2-isoprostane | mg/mg creatinine | Increased insignificantly | Decreased insignificantly | Without significant difference |
| Duggan 2016 [34] | 12 months | Pre-program Post-program | Plasma F2-isoprostanes | mg/mL | Decreased significantly | Decreased insignificantly | With significant difference |
| Mallard 2017 [38] | 12 weeks | Pre-program Post-program 12 months post-program | Plasma PCs | U/mg protein | Decreased insignificantly in all recording times | Increased insignificantly in all recording times | Without significant difference in all recording times |
| Plasma F2-isoprostanes | pg/mL | Increased insignificantly in all recording times | Decreased insignificantly in all recording times | Without significant difference in all recording times | |||
| Small 2017 [39] | 12 months | Pre-program Post-program | Plasma F2-isoprostanes | pg/mL | Increased insignificantly | Increased insignificantly | Without significant difference |
| Allgayer 2008 [30] | 2 weeks | Pre-program Post-program | Urinary 8-OHDG | ng/mg | Decreased significantly | Increased insignificantly | With significant difference |
| Wadley 2014 [41] | 12 weeks | Pre-program Post-program | Plasma PCs | mmol/mg protein | Decreased insignificantly | Increased insignificantly | Without significant difference |
| Intervention | Rank 1 | Rank 2 | Rank 3 | Rank 4 | Rank 5 |
|---|---|---|---|---|---|
| HIAE | 0.01 | 0.04 | 0.41 | 0.23 | 0.31 |
| HIAE + RT | 0.07 | 0.05 | 0.23 | 0.26 | 0.39 |
| HIIT | 0.59 | 0.28 | 0.05 | 0.04 | 0.04 |
| MCA | 0.03 | 0.05 | 0.24 | 0.44 | 0.24 |
| MIAE | 0.31 | 0.58 | 0.07 | 0.04 | 0.01 |
| Comparison | Structure | Number of Studies | Within-Study Bias | Reporting Bias | Indirectness | Imprecision | Heterogeneity | Incoherence | Confidence Rating | Reason(s) for Downgrading |
|---|---|---|---|---|---|---|---|---|---|---|
| AE + RT:MCA | Mixed | 1 | SC | SC | NC | MC | NC | MC | Very low | Reporting bias & Imprecision & Incoherence |
| HIAE:MCA | 1 | SC | SC | NC | MC | NC | MC | Very low | Reporting bias & Imprecision & Incoherence | |
| HIAE:MIAE | 1 | SC | SC | NC | SC | MC | MC | Very low | Reporting bias & Heterogeneity & Incoherence | |
| HIIT:MIAE | 4 | NC | SC | NC | MC | NC | MC | Very low | Reporting bias & Imprecision & Incoherence | |
| AE + RT:HIAE | Indirect | 0 | SC | SC | NC | MC | NC | MC | Very low | Reporting bias & Imprecision & Incoherence |
| AE + RT:HIIT | 0 | SC | SC | NC | SC | MC | MC | Very low | Reporting bias & Heterogeneity & Incoherence | |
| AE + RT:MIAE | 0 | SC | SC | NC | SC | MC | MC | Very low | Reporting bias & Heterogeneity & Incoherence | |
| HIAE:HIIT | 0 | SC | SC | NC | SC | MC | MC | Very low | Reporting bias & Heterogeneity & Incoherence | |
| HIIT:MCA | 0 | SC | SC | NC | SC | MC | MC | Very low | Reporting bias & Heterogeneity & Incoherence | |
| MCA:MIAE | 0 | SC | SC | NC | SC | MC | MC | Very low | Reporting bias & Heterogeneity & Incoherence |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Chen, C.; Teo, E.-C.; Zhang, Y.; Huang, J.; Xu, Y.; Gu, Y. Intracellular Oxidative Stress Induced by Physical Exercise in Adults: Systematic Review and Meta-Analysis. Antioxidants 2022, 11, 1751. https://doi.org/10.3390/antiox11091751
Zhou Z, Chen C, Teo E-C, Zhang Y, Huang J, Xu Y, Gu Y. Intracellular Oxidative Stress Induced by Physical Exercise in Adults: Systematic Review and Meta-Analysis. Antioxidants. 2022; 11(9):1751. https://doi.org/10.3390/antiox11091751
Chicago/Turabian StyleZhou, Zhanyi, Chaoyi Chen, Ee-Chon Teo, Yan Zhang, Jialu Huang, Yining Xu, and Yaodong Gu. 2022. "Intracellular Oxidative Stress Induced by Physical Exercise in Adults: Systematic Review and Meta-Analysis" Antioxidants 11, no. 9: 1751. https://doi.org/10.3390/antiox11091751
APA StyleZhou, Z., Chen, C., Teo, E.-C., Zhang, Y., Huang, J., Xu, Y., & Gu, Y. (2022). Intracellular Oxidative Stress Induced by Physical Exercise in Adults: Systematic Review and Meta-Analysis. Antioxidants, 11(9), 1751. https://doi.org/10.3390/antiox11091751








