Copper-Induced Interactions of Caffeic Acid and Sinapic Acid to Generate New Compounds in Artificial Biological Fluid Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Analytical Conditions of Cinnamic Acids
2.3. Sample Preparation of Hydroxycinnamic Acids and Metal Ions for the Assessment of Stability
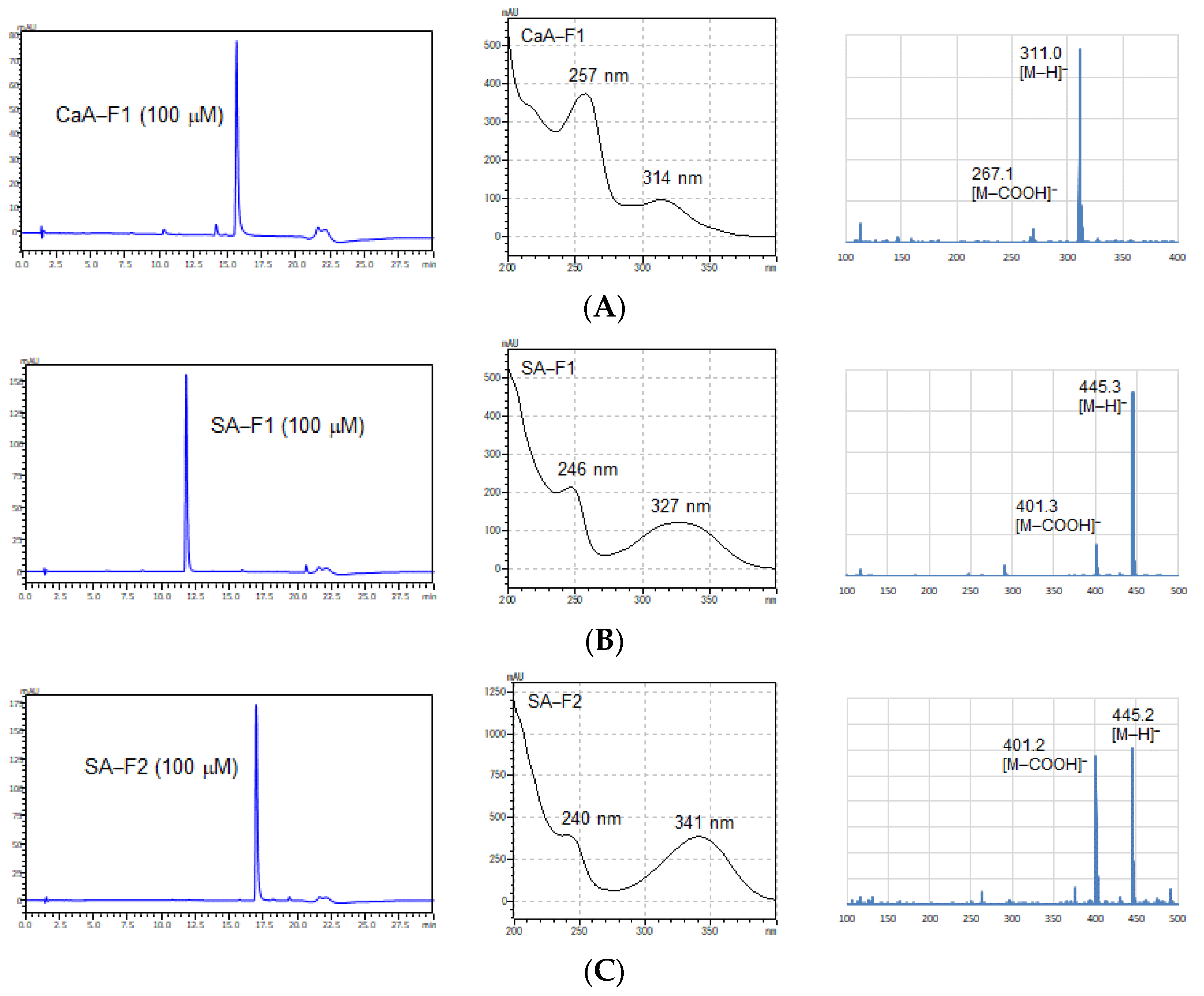
2.4. Extraction and Purification of CaA and SA Derivatives for the Identification of Their Structure
2.5. Determination of Antioxidant Activities
2.6. Electron Spin Resonance (ESR) Condition for Measurement of Hydroxyl Radical
2.7. Statistical Analysis
3. Results and Discussion
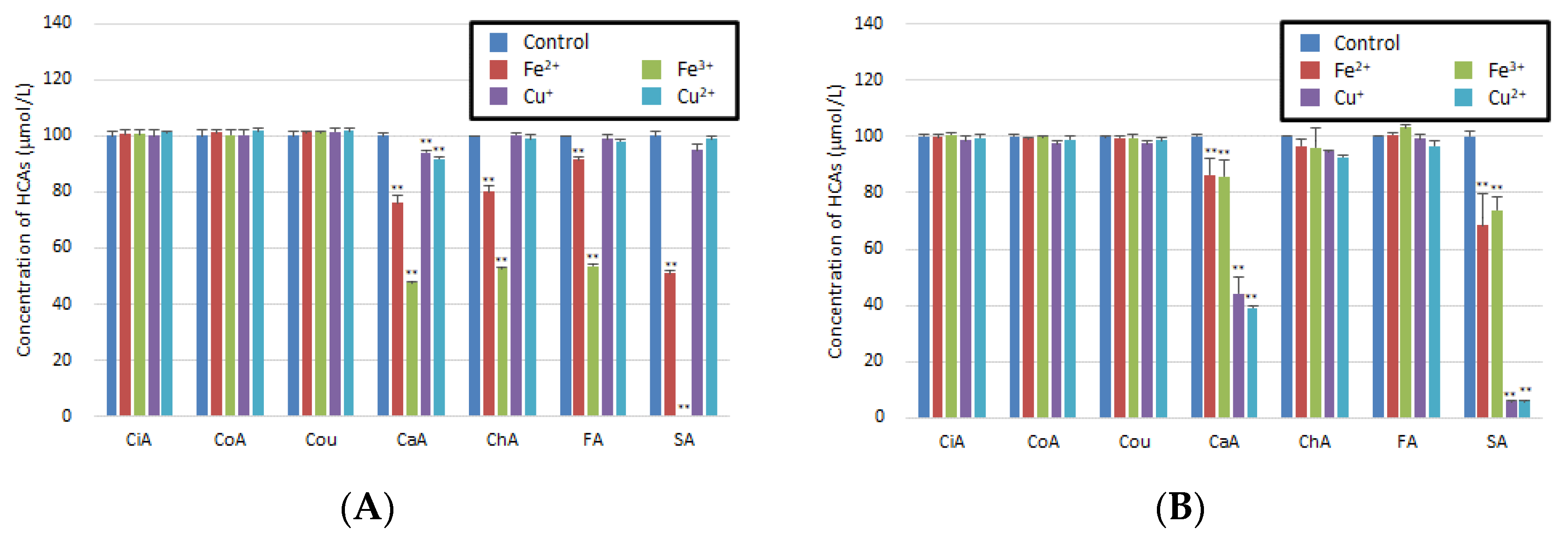
3.1. Reaction of HCAs with Metal Ions in Artificial Gastric Juice or Artificial Intestinal Fluid
3.2. Identification of New Compounds by Nuclear Magnetic Resonance (NMR) Spectroscopy and MS
3.3. Comparison of Reaction Conditions for CaA or SA with Copper Ion
3.4. Co-Reaction of CaA or SA with Metal Ions
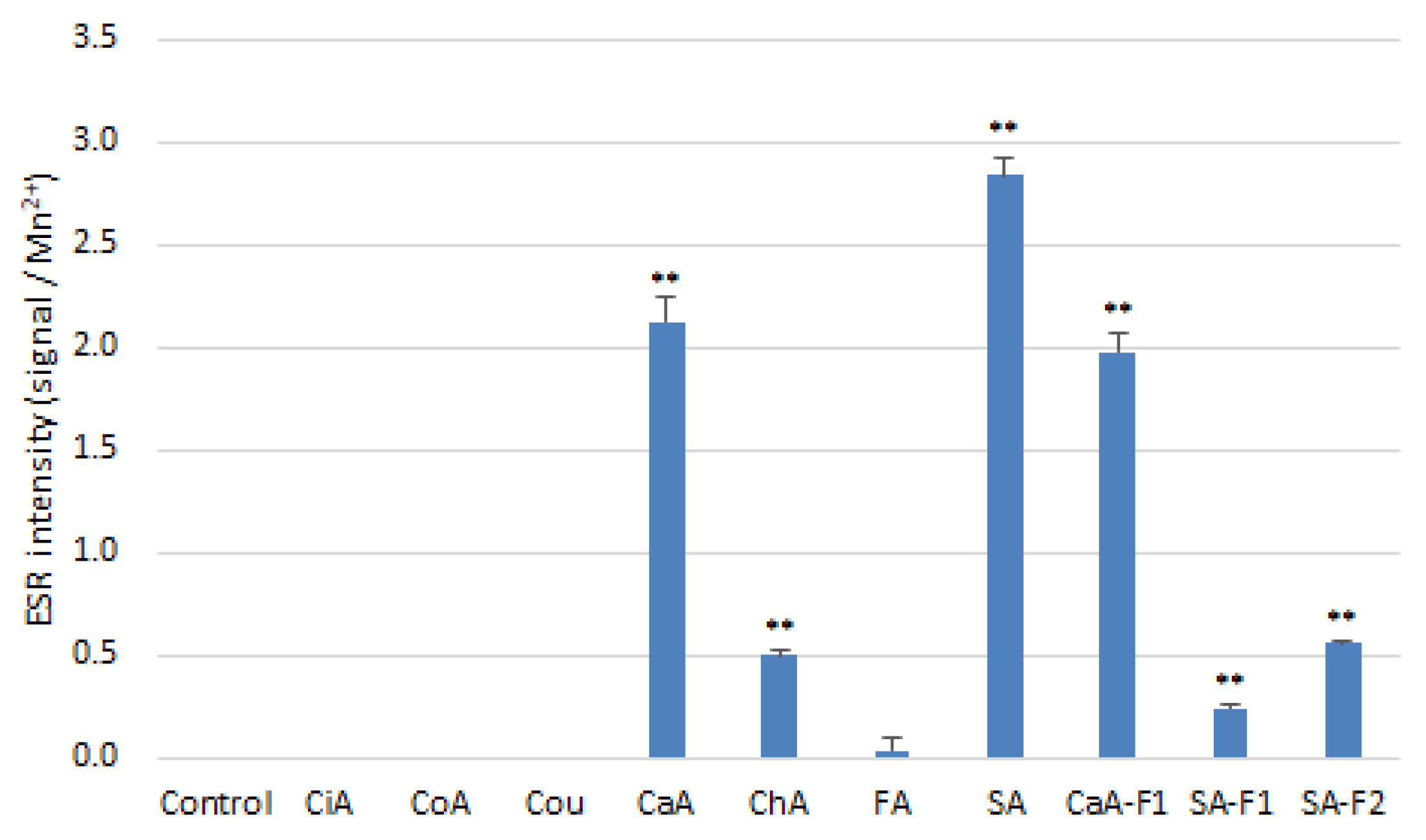
3.5. Antioxidant and Prooxindat Activity of HCAs and Related Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimer Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ying, J.F.; Lu, Z.B.; Fu, L.Q.; Tong, Y.; Wang, Z.; Li, W.F.; Mou, X.Z. The role of iron homeostasis and iron-mediated ROS in cancer. Am. J. Cancer Res. 2021, 11, 1895–1912. [Google Scholar] [PubMed]
- Pulido, R.; Hernández-García, M.; Saura-Calixto, F. Contribution of beverages to the intake of lipophilic and hydrophilic antioxidants in the Spanish diet. Eur. J. Clin. Nutr. 2003, 57, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Goñi, I.; Hernández-Galiot, A. Intake of Nutrient and Non-Nutrient Dietary Antioxidants. Contribution of Macromolecular Antioxidant Polyphenols in an Elderly Mediterranean Population. Nutrients 2019, 11, 2165. [Google Scholar] [CrossRef] [Green Version]
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and their applications: An approach in food chemistry and innovation potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi-Asl, N.; Garrido, J.; Khazraei, H.; Borges, F.; Firuzi, O. Antioxidant properties of hydroxycinnamic acids: A review of structure- activity relationships. Curr. Med. Chem. 2013, 20, 4436–4450. [Google Scholar] [CrossRef] [Green Version]
- Nićiforović, N.; Abramovič, H. Sinapic Acid and Its Derivatives: Natural Sources and Bioactivity. Compr. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Meinhart, A.D.; Damin, F.M.; Caldeirão, L.; de Jesus Filho, M.; da Silva, L.C.; da Silva Constant, L.; Filho, J.T.; Wagner, R.; Godoy, H.T. Chlorogenic and caffeic acids in 64 fruits consumed in Brazil. Food Chem. 2019, 286, 51–63. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Hossain, H.; Hossain, M.; Reza, H.M.; Rahman, M.M.; Ullah, M.O. Hydroxycinnamic acid derivatives: A potential class of natural compounds for the management of lipid metabolism and obesity. Nutr. Metab. 2016, 13, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neelam; Khatkar, A.; Sharma, K.K. Phenylpropanoids and its derivatives: Biological activities and its role in food, pharmaceutical and cosmetic industries. Crit. Rev. Food Sci. Nutr. 2020, 60, 2655–2675. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Bamunuarachchi, N.I.; Tabassum, N.; Kim, Y.M. Caffeic Acid and Its Derivatives: Antimicrobial Drugs toward Microbial Pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef] [PubMed]
- Govindaraju, M.; Shekar, H.S.; Sateesha, S.B.; Vasudeva Raju, P.; Sambasiva Rao, K.R.; Rao, K.S.J.; Rajamma, A.J. Copper interactions with DNA of chromatin and its role in neurodegenerative disorders. J. Pharm. Anal. 2013, 3, 354–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron homeostasis and oxidative stress: An intimate relationship. Biochim. Biophys. Acta. Mol. Cell Res. 2019, 1866, 118535. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, P.; Zhang, J.; Zhang, Y.; Zhang, G.; Liu, Y.; Cheng, X. Generation of reactive oxygen species by promoting the Cu(II)/Cu(I) redox cycle with reducing agents in aerobic aqueous solution. Water Sci. Technol. 2018, 78, 1390–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Zhao, P.; Pan, Y.; Wagner, C. Predictive Performance of Physiologically Based Pharmacokinetic Models for the Effect of Food on Oral Drug Absorption: Current Status. CPT Pharmacomet. Syst. Pharmacol. 2018, 7, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.J.; Jin, X.L.; Qian, Y.P.; Wang, Q.; Yang, R.T.; Dai, F.; Tang, J.J.; Shang, Y.J.; Cheng, L.X.; Yang, J.; et al. Hydroxycinnamic acids as DNA-cleaving agents in the presence of Cu(II) ions: Mechanism, structure-activity relationship, and biological implications. Chemistry 2009, 15, 12889–12899. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; He, Y.; Jia, L.; Yang, C.S.; Reiter, R.J.; Zhang, J. Antioxidant and Pro-Oxidant Activities of Melatonin in the Presence of Copper and Polyphenols In Vitro and In Vivo. Cells 2019, 8, 903. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, Y.; Nomoto, M.; Oda, M.; Mochizuki, K.; Nakano, Y.; Ishii, Y.; Ito, R.; Saito, K.; Umemura, T.; Nishikawa, A.; et al. Characterization of nitrated phenolic compounds for their anti-oxidant, pro-oxidant, and nitration activities. Arch. Biochem. Biophys. 2011, 513, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Jauregi, P.; Guo, Y.; Adeloye, J.B. Whey proteins-polyphenols interactions can be exploited to reduce astringency or increase solubility and stability of bioactives in foods. Food Res. Int. 2021, 141, 110019. [Google Scholar] [CrossRef] [PubMed]
- Bunzel, M.; Ralph, J.; Kim, H.; Lu, F.; Ralph, S.A.; Marita, J.M.; Hatfield, R.D.; Steinhart, H. Sinapate dehydrodimers and sinapate-ferulate heterodimers in cereal dietary fiber. J. Agric. Food Chem. 2003, 51, 1427–1434. [Google Scholar] [CrossRef]
- Grúz, J.; Pospíšil, J.; Kozubíková, H.; Pospíšil, T.; Doležal, K.; Bunzel, M.; Strnad, M. Determination of free diferulic, disinapic and dicoumaric acids in plants and foods. Food Chem. 2015, 171, 280–286. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A. Naturally occurring naphthalenes: Chemistry, biosynthesis, structural elucidation, and biological activities. Phytochem. Rev. 2016, 15, 279–295. [Google Scholar] [CrossRef]
- Hong, S.; Pangloli, P.; Perumal, R.; Cox, S.; Noronha, L.E.; Dia, V.P.; Smolensky, D. A Comparative Study on Phenolic Content, Antioxidant Activity and Anti-Inflammatory Capacity of Aqueous and Ethanolic Extracts of Sorghum in Lipopolysaccharide-Induced RAW 264.7 Macrophages. Antioxidants 2020, 9, 1297. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Poyatos, M.D.P.; Ruiz-Medina, A.; Zengin, G.; Llorent-Martínez, E.J. Phenolic Characterization, Antioxidant Activity, and Enzyme Inhibitory Properties of Berberis thunbergii DC. Leaves: A Valuable Source of Phenolic Acids. Molecules 2019, 24, 4171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Hirasawa, T.; Maruyama, Y.; Ishii, Y.; Ito, R.; Saito, K.; Umemura, T.; Nishikawa, A.; Nakazawa, H. Effect of interaction between phenolic compounds and copper ion on antioxidant and pro-oxidant activities. Toxicol. Vitro 2011, 25, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Gritti, F.; Tanaka, N.; Guiochon, G. Comparison of the fast gradient performance of new prototype silica monolithic columns and columns packed with fully porous and core-shell particles. J. Chromatogr. A 2012, 1236, 28–41. [Google Scholar] [CrossRef]
- Radwan, M.A.; Salama, A.K. Market basket survey for some heavy metals in Egyptian fruits and vegetables. Food Chem. Toxicol. 2006, 44, 1273–1278. [Google Scholar] [CrossRef]
- Tsuda, T.; Inoue, T.; Kojima, M.; Aoki, S. Market basket and duplicate portion estimation of dietary intakes of cadmium, mercury, arsenic, copper, manganese, and zinc by Japanese adults. J. AOAC Int. 1995, 78, 1363–1368. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Båmstedt, U.; Martinussen, M.B. Estimating digestion rate and the problem of individual variability, exemplified by a scyphozoan jellyfish. J. Exp. Mar. Biol. Ecol. 2000, 251, 1–15. [Google Scholar] [CrossRef]
- Hayes, A.M.R.; Swackhamer, C.; Mennah-Govela, Y.A.; Martinez, M.M.; Diatta, A.; Bornhorst, G.M.; Hamaker, B.R. Pearl millet (Pennisetum glaucum) couscous breaks down faster than wheat couscous in the Human Gastric Simulator, though has slower starch hydrolysis. Food Funct. 2020, 11, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Nea, F.; Bitchi, M.B.; Genva, M.; Ledoux, A.; Tchinda, A.T.; Damblon, C.; Frederich, M.; Tonzibo, Z.F.; Fauconnier, M.L. Phytochemical Investigation and Biological Activities of Lantana rhodesiensis. Molecules 2021, 26, 846. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Dong, L.; Zhang, R.; Chi, J.; Liu, L.; Zhang, M.; Jia, X. Structural elucidation, distribution and antioxidant activity of bound phenolics from whole grain brown rice. Food Chem. 2021, 358, 129872. [Google Scholar] [CrossRef] [PubMed]




| HCAs | DPPH | CUPRAC | FRAP |
|---|---|---|---|
| CiA | N.D. | N.D. | N.D. |
| CoA | 0.22 ± 0.003 | 0.53 ± 0.01 | 0.19 ± 0.002 |
| Cou | N.D. | N.D. | N.D. |
| CaA | 2.96 ± 0.05 | 3.31 ± 0.10 | 1.90 ± 0.04 |
| ChA | 3.83 ± 0.62 | 3.52 ± 0.03 | 1.59 ± 0.08 |
| FA | 1.87 ± 0.44 | 1.25 ± 0.02 | 0.99 ± 0.01 |
| SA | 1.87 ± 0.13 | 1.60 ± 0.03 | 1.71 ± 0.02 |
| CaA-F1 | 5.00 ± 0.58 | 4.90 ± 0.09 | 2.70 ± 0.05 |
| SA-F1 | 2.42 ± 0.14 | 3.17 ± 0.04 | 3.01 ± 0.05 |
| SA-F2 | 2.42 ± 0.50 | 2.74 ± 0.05 | 2.64 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwasaki, Y.; Manabe, R.; Kimoto, M.; Fukuda, M.; Mase, N.; Miyazawa, M.; Hosokawa, K.; Kamei, J. Copper-Induced Interactions of Caffeic Acid and Sinapic Acid to Generate New Compounds in Artificial Biological Fluid Conditions. Antioxidants 2022, 11, 1307. https://doi.org/10.3390/antiox11071307
Iwasaki Y, Manabe R, Kimoto M, Fukuda M, Mase N, Miyazawa M, Hosokawa K, Kamei J. Copper-Induced Interactions of Caffeic Acid and Sinapic Acid to Generate New Compounds in Artificial Biological Fluid Conditions. Antioxidants. 2022; 11(7):1307. https://doi.org/10.3390/antiox11071307
Chicago/Turabian StyleIwasaki, Yusuke, Rie Manabe, Mika Kimoto, Mao Fukuda, Narumi Mase, Mako Miyazawa, Kotomi Hosokawa, and Junzo Kamei. 2022. "Copper-Induced Interactions of Caffeic Acid and Sinapic Acid to Generate New Compounds in Artificial Biological Fluid Conditions" Antioxidants 11, no. 7: 1307. https://doi.org/10.3390/antiox11071307
APA StyleIwasaki, Y., Manabe, R., Kimoto, M., Fukuda, M., Mase, N., Miyazawa, M., Hosokawa, K., & Kamei, J. (2022). Copper-Induced Interactions of Caffeic Acid and Sinapic Acid to Generate New Compounds in Artificial Biological Fluid Conditions. Antioxidants, 11(7), 1307. https://doi.org/10.3390/antiox11071307





