Abstract
The present experiment investigated the potential protective role of parsley (Petroselinum crispum) seed meal (PSM) in alleviating methomyl (MET)-adverse impacts on growth, whole-body composition, hematological indicators, hepatorenal function, immune response, oxidative status, and disease resistance to Pseudomonas aeruginosa. For this purpose, 225 healthy Nile tilapia (Oreochromis niloticus) were allotted into five groups (45 fish/group in triplicate). One group was reared in clean water and fed a non-supplemented basal diet, while the other groups were exposed to 20.39 μg L−1 MET and fed a non-fortified basal diet or basal diets supplemented with 0.5, 1.0, or 2.0% of PSM for 60 days. The obtained data revealed significantly lower weight gain, feed intake, and specific growth rate, but higher feed conversion ratio and decreases in crude protein, lipid, and ash contents in the MET-exposed fish. Anemia, leukopenia, lymphocytopenia, and esonipenia were also obvious. Furthermore, MET-exposed fish had significantly higher serum levels of hepatic enzymes and renal damage products. Nevertheless, there was a significant depletion of enzymatic and non-enzymatic antioxidants and increased malondialdehyde, myeloperoxidase, and tumor necrosis factor-α levels in MET-exposed fish. The MET exposure significantly depressed lysozyme activity, nitric oxide, complement3, acetylcholinesterase activity, total proteins, globulin, and albumin levels in O. niloticus serum. Furthermore, pathological alterations in the liver and kidney were noted. The relative percentage of survival rate in MET-exposed fish was dramatically reduced on day 14 post-challenge with P. aeruginosa. The inclusion of PSM, on the other hand, greatly alleviated most of the MET-related negative effects. Taken together, the dietary intervention with PSM has a promising role in alleviating MET-deleterious impacts, rendering parsley seeds a viable aqua feed additive for O. niloticus.
1. Introduction
Anthropogenic activities and modern lifestyles frequently result in the intentional or unintentional release of thousands of chemicals, including pesticides, into the environment. The aquatic ecosystem is one of the most polluted places, since it is the final resting place for most contaminants [1]. Fish are primarily affected by pesticide hazards due to their absorption through their skin and through gill uptake [2]. Methomyl (MET) is a carbamate pesticide widely used worldwide due to its high efficacy, high solubility, and wide biological effects [3]. Its use has contributed to pest management (controlling insects and nematodes), increasing agricultural productivity. Detectable levels of MET residue have been identified in various water bodies and foods due to its high water solubility, extensive use, and agricultural and industrial release into the environment [4]. MET residue levels in the aquatic environment have been documented to range from 0 to 55.3 g L−1 [5].
The World Health Organization (WHO) categorized MET as a very dangerous product (class 1B) [6]. Its exposure poses a serious health risk and causes toxicity and death as it has been found in humans and animals’ blood, liver, kidneys, and brain [7]. Several studies revealed that MET exposure causes serious harm to various biological and metabolic processes in fish organs. For instance, Islamy et al. [8] reported its genotoxic effects on Nile tilapia (Oreochromis niloticus). Additionally, Wang Yuqin [9] confirmed the increased oxidative stress in the liver and a decreased growth rate of O. niloticus due to MET exposure. Meng et al. [3] recorded the inhibition of the O. niloticus antioxidant system when exposed to 0.2–200 μg MET/L−1. Moreover, it reduced proinflammatory cytokine expression and abrogated defense against bacterial infections in tilapia [10].
The aquaculture industry must develop nutritional strategies capable of mitigating the risk of waterborne pollution while improving the immunological response and growth of different cultured fish species [11,12,13]. Parsley (Petroselinum crispum) is a culinary herb native to the Mediterranean region. Parsley is a member of the Umbelliferae family and is used in the pharmaceutical, cosmetic, and food industries [14]. It contains alpha-linolenic acid, a fatty acid important for growth and reproduction [15]. Flavonoids, terpenoids, carotenoids, myristicin, coumarins, ascorbic acid, and apiole have been identified as major components of parsley [16]. It boosts and promotes organ activity, enhancing their ability to absorb and utilize nutrients [17]. Moreover, it has been shown to have various potential medicinal properties, including antioxidant, antimicrobial, antihyperlipidemic, and hepatoprotective effects [18,19,20]. In modern medicine, parsley has been shown to have a wide range of pharmacological activity, including hepatoprotective, neuroprotective, antidiabetic, analgesic, immunostimulant, antioxidant, anti-platelet, cytoprotective, antibacterial, and antifungal properties [21,22]. However, there is little information about the effects of using PSM as a fish diet supplement.
Nile tilapia (Oreochromis niloticus) is a commonly produced fish for human consumption globally [23]. Toxic chemicals in the water, on the other hand, constitute a threat to O. niloticus health and growth [24]. To the best of our knowledge, no scientific published study has been conducted on the potential benefits of PSM dietary supplementation in mitigating the MET-induced adverse effects on the health of O. niloticus. Consequently, biochemical and histopathological endpoints were assessed, including hematological indices, hepatic enzymes, renal damage products, oxidative stress indices, acetylcholinesterase (AchE) activity, protein profile, and innate immune biomarkers. A comprehensive morphometric histological examination of hepatic and renal tissues in fish exposed to MET and fed a PSM-enriched diet was also carried out. In addition, the fish body’s chemical composition and growth performance were assessed. Moreover, susceptibility to Pseudomonas aeruginosa infection, one of the most prevalent bacteria that infect fish and causes significant damage of fish tissues and mortality [25,26], was also investigated.
2. Materials and Methods
2.1. Tested Compounds and Chemicals
Methomyl (97%) (methyl N-(methylcarbamoyloxy) ethanimidothioate was obtained from Sigma Aldrich (St. Louis, MO, USA). Parsley seeds were purchased in dry packages from the local market for herbs and medicinal plants in Zagazig, Egypt. The dried parsley seeds were washed first, dried, and then crushed and kept at 4 °C till use. All other analytical-grade chemicals were purchased from Sigma-Aldrich, St. Louis, MO, USA.
2.2. Experimental Fish
Apparently healthy fish, Oreochromis niloticus, were gathered from a commercial fish farm in Kafr El-Sheik governorate, Egypt (average body weight: 25.33 ± 0.26 g). Before the experiments, fish were stocked and acclimated for two weeks in 75 L glass aquaria (80 × 40 × 30 cm) with dechlorinated tap water and continual aeration from a central air compressor via an air stone. During the acclimatization period, fish were fed a basal diet. To eliminate excreta, approximately 30% of the water was emptied thrice weekly. The fish were subjected to a photoperiod of 12 h of light and 12 h of darkness. The physicochemical properties of the water used in the aquaria were investigated; the temperature was maintained at 26 ± 0.5 °C, and the pH and dissolved oxygen were kept at 7.5 ± 0.5 and 6.8 ± 0.23 mg L−1, respectively. The averages of ammonia, nitrite, and nitrate were recorded at 0.12 ± 0.02, 0.14 ± 0.04, and 2.30 ± 0.05 mg L−1, respectively.
The trial was conducted at the Fish Diseases and Management Department, Faculty of Veterinary Medicine, Zagazig University, Egypt. This institution’s Animal Use in Research Committee (IACUC) approved the experiment. The experiments were carried out in compliance with the National Institutes of Health’s (NIH) Ethical Guidelines for the Use and Care of Laboratory Animals in Scientific Investigations.
2.3. Diet Formulation and Experimental Plan
Two hundred and twenty-five healthy O. niloticus fish were randomly dispersed among five different groups. Each fish group was divided into three aquaria, with 15 fish each. In the control group (G1), fish were fed a basal diet without PSM supplementation and reared in aquaria containing clean water (Table 1), while G2 was exposed to 1/20 LC50 (20.39 µg L−1) MET [10] and fed a basal diet without PSM supplementation, and G3, G4, and G5 received basal diets enriched with 0.5%, 1.0%, and 2.0% PSM, respectively, with concomitant MET exposure at the same concentration. The experimental diet ingredients were well mixed before being mechanically pelleted, air-dried for 24 h at room temperature, and then stored at 4 °C until use. The basal diet was formulated to meet the optimal dietary needs of fish as specified by the Nutrient Requirements of Fish [27]. Each experimental diet was assessed for crude protein using the macro-Kjeldahl method, crude fat via the ether extraction technique, moisture by a forced-air oven, total ash using a muffle furnace, and crude fiber following AOAC [28] guidelines. Fish were fed until satiation thrice daily (8:00 a.m., 12:00 p.m., and 16:00 p.m.). The trial lasted 60 days. The total fish weight per aquarium was measured every two weeks to monitor fish growth. Every 48 h, the water was fully replaced by moving the fish to aquaria containing freshly prepared MET solutions.

Table 1.
Ingredients and chemical compositions of the experimental diets.
2.4. Growth Performance Assessment
To assess growth performance, each replicate’s fish was weighed at the start of the trial and every two weeks. The final body weight (FBW), weight gain (WG), specific growth rate (SGR), weight gain percentage, condition factor (K), feed intake (FI), and feed conversion ratio (FCR) were calculated as follows:
- FBW = total weight of fish, divided by the fish number in each replicate
- WG = total body weight minus initial body weight
- Weight gain percentage = [(final average BW − initial average BW)/initial average BW] × 100
- SGR = [(Ln final BW − Ln initial BW)/experiment duration (days)] × 100
- FI = feed consumed/number of survival fish
- FCR = dry feed fed (g)/wet weight gain (g)
- Condition factor (K) = (W/L3) × 100
where W = total fish weight (g) and L = fish length (cm), measured from the tip of the snout to the end of the middle caudal fin.
The mortality rate was calculated using the following formula: mortality rate = (number of dead fish/total initial fish number) × 100.
2.5. Whole-Body Chemical Analysis
At the end of the 60-day feeding trial, five fish from each replication were used to analyze the chemical composition of the total fish body. Following AOAC [28] protocols, crude protein levels were determined by a Kjeldahl distillation unit (UDK 129, Velp Scientifica, Usmate Velate, Italy). Moisture was determined by a natural convection oven (JSON-100, Gongju-City, Korea). The ash content was determined using muffle furnaces (Barnstead/Thermolyne Benchtop 47900, Thermo Scientific, Waltham, MA, USA). Soxhlet extractor glassware was also used to estimate crude lipids.
2.6. Blood and Tissue Sampling
After the 60-day feeding trial, blood was drawn from caudal vessels and split into two halves. The first half was obtained with EDTA tubes for a complete blood cell count. The second was collected without an anticoagulant until clotting happened; the tubes were kept at 4 °C overnight and centrifuged at 150× g for 5 min, followed by 350× g for 15 min. The serum was utilized to test immunological function, serum protein electrophoretic pattern, oxidant/antioxidant status, and liver and kidney functions. After collecting blood samples, the fish were dissected to obtain the liver and kidney, then fixed for 48 h in 10% neutral buffered formalin for histopathological investigations.
2.7. Estimation of Hematological Values
Hematological indices such as red blood cell counts (RBCs), packed cell volume (PCV), mean corpuscular volume (MCV), hemoglobin content (Hb), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) were determined using a Hema Screen 18 automatic hematology analyzer (Hospitex Diagnostics, Sesto Fiorentino, Italy). Dacie and Lewis’s manual technique was used for counting total leukocytes (WBCs), differential leukocyte counts (lymphocytes, neutrophils, and monocytes), and platelets [29].
2.8. Serum Biochemical Analysis
2.8.1. Hepatorenal Damage Products
Alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), creatinine, urea, ammonia, total bilirubin, and cholesterol were assessed in serum by Spinreact kits (Esteve De Bas, Girona, Spain) in accordance to the protocols described by Burtis and Ashwood [30], Wenger et al. [31], Murray and Kaplan [32], Pesce [33], Neely and Phillipson [34], Martinen [35], Naito [36], Fossati et al. [37], and Kaplan and Glucose [38], respectively.
2.8.2. Immune System Response
Serum complement3 (C3) levels were measured by fish-specific ELISA kits and the manufacturer’s instructions. Serum lysozyme activity was determined using spectrophotometry [39]. The concentration of nitric oxide (NO) was determined following the protocol of Dumock [40].
2.8.3. Stress, Inflammatory Status Assays, and Acetyl Choline Esterase Enzyme (AchE) Activity
Stress indicators were measured by commercial colorimetric kits of Biodiagnostic Co. (Cairo, Egypt). The catalase enzyme (CAT) level was determined using the Aebi [41] method. The superoxide dismutase (SOD) activity was measured by the Nishikimi et al. [42] protocol. Malondialdehyde (MDA) was measured according to the Uchiyama and Mihara [43] method. The EnzyChromTM Glutathione Peroxidase Assay Kit (EGPX-100) of Bio-Assay Systems (Hayward, CA, USA) was used to determine quantitative colorimetric glutathione peroxidase (GPx) [44].
Myeloperoxidase (MPO) activity in fish serum was determined based on Kumari and Sahoo [45]. Fish ELISA kits of My Biosource Co. for tumor necrosis factor-alpha (TNF-α) were used according to the instructions in the enclosed pamphlets of the kits. The acetylcholinesterase (AchE) activity was evaluated according to Ellman et al. [46]. According to Badawi [47], the electrophoretic pattern of serum proteins, comprising total proteins, albumin, and globulins, was analyzed.
2.9. Histopathological Study
At the end of the trial, the fish were necropsied using standard finfish necropsy procedures [48]. Liver and kidney tissue samples were obtained from nine randomly selected fish per group, rinsed in distilled water, and immediately fixed in a 10% neutral buffered formalin solution for 48 h. Post fixation, the specimens were processed using the paraffin technique, sectioned at four μm thick, and stained with hematoxylin and eosin following the protocol described by Suvarna et al. [49]. The stained slides were examined microscopically for morphological alterations. A multiparametric numerical histopathological assessment of the hepatic and renal tissues was performed with minor modifications to the protocol proposed by Bernet et al. [50]. In brief, five non-overlapped randomly chosen microscopic fields (10 objectives) per organ per fish were snapshotted, and the images were then analyzed to calculate lesion frequencies, liver and kidney indices (the higher the indices, the worse the histopathological alterations), and the total indices for a fish using the following formulas:
The following formula determined the frequency of lesions (FQ):
where (Nlesion) represents fish that exhibited a lesion, and (Ntotal) represents the total number of fish in the group.
FQ (%) = Nlesion × Ntotal−1 × 100
The liver and kidney indices were calculated by the following formula:
where (rp) is the reaction pattern, (alt) the histopathological alteration, (a) the score value (indicates the size of tissue affected by the alteration, and its value ranged between six (diffuse lesion) and zero (lack of the alteration)), and (w) the importance factor (indicates how much worse the alteration is, with its value ranging between three and one).
Organ index (Iorg) = Σrp Σalt (aorg rp alt × worg rp alt)
The total index for each individual fish was calculated by adding the indices for the liver and kidney according to the formula:
Total index (Tot-I) = Σorg Σrp Σalt (aorg rp alt × worg rp alt)
2.10. Challenge Test
Pseudomonas aeruginosa (previously isolated from naturally infected O. niloticus and previously identified and validated as pathogenic) was dispersed on tryptic soy agar (TSA) (Hi-media, Thane West, Maharashtra, India) and incubated for 48 h at 30 °C. The P. aeruginosa lethal dose (LD50) was evaluated. Fish were intraperitoneally (IP) injected with diverse doses of 24-h live bacteria; then, the infected fish mortality was reported for 3-days post-injection. The LD50, which resulted in 50% fish mortality, was 1 × 108 CFU/mL. Five fish per replicate (n = 15 fish/group) were intraperitoneally injected with 0.5 mL of the produced bacterial solution containing 1 × 108 CFU/mL after the feeding trial (60 days). The injected fish were monitored for two weeks to determine the percentage of mortality and post-mortem lesions.
2.11. Statistical Analysis
Using SPSS, the data were statistically investigated using one-way Analysis of Variance (ANOVA) (version 16.0, SPSS Inc., Chicago, IL, USA). A Tukey’s multiple comparisons post hoc test was used to compare means between groups, with statistical significance set at p ˂ 0.05. The results were displayed as means ± standard error (SE).
3. Results
3.1. Growth Performance
The growth performance indicators of fish fed graded levels of PSM and exposed to MET for 60 days are presented in Table 2. The acquired results verified that the FW, WG, WG WG%, DWG, SGR, FI, and K were significantly higher (p < 0.05) in MET-exposed fish and fed diets fortified with 0.5%, 1%, and 2% PSM (G3, G4, and G5) or basal diet (G1). Conversely, with increased PSM levels, the FCR decreased in fish exposed to MET and the fish fed the basal diet. The best performance was obtained in G4. Fish groups exposed to MET (G2) showed significantly (p < 0.05) lower FW, WG, WG%, DWG, SGR, FI, and K values compared with other groups. On the other hand, fish groups exposed to MET attained higher FCR than others.

Table 2.
Effect of parsley seed meal (PSM) supplementation on growth performance and whole body composition (% fresh weight basis) of O. niloticus exposed to methomyl (MET) 20.39 µg/L for 60 days.
3.2. Mortality Rate
The MET-exposed fish (G2) and those exposed to MET and fed 0.5% PSM (G3) had significantly (p < 0.05) higher mortality rates than fish fed the basal diet (G1) and those fed diets supplemented with 1.0% and 2.0% PSM (G4 and G5) (Table 2).
3.3. Whole-Body Composition
As shown in Table 2, the analysis of fish body composition revealed significant effects of dietary treatments. The fish fed a basal diet and exposed to MET (G2) displayed lower crude protein, lipids, and ash content than the other experimental groups. The fish exposed to MET and supplemented with PSM revealed significantly higher crude protein and lipid content than the G2 group (p < 0.05). The MET-exposed fish fed a supplemented diet with 2.0% PSM (G5) attained higher results than other MET-exposed groups.
3.4. Effects on Hematological Indices
The effects of MET exposure and dietary PSM supplementation in the diets of O. niloticus fish on hematological indices are displayed in Table 3. The erythrogram revealed that mean values for RBCs, Hb, PCV, and MCHC% were significantly lower in fish fed a basal diet and exposed to MET (G2) than those in control or supplemented groups with PSM. However, supplementing the MET-exposed fish with PSM significantly counteracted the MET-induced anemic condition. Fish that were fed with 1.0% of PSM had the best recovery. Regarding leukograms, fish exposed to MET showed a significant reduction in total WBCs, lymphocytes, eosinophils, heterophils, and monocyte levels compared to values in other groups. On the other hand, PSM supplementation significantly augmented the total WBCs, heterophils, lymphocytes, eosinophils, and monocytes counts relative to those in fish exposed to MET. Groups exposed to MET and supplemented with 1.0% PSM (G4) attained the best results, close to those of the controls.

Table 3.
Effect of parsley seed meal (PSM) supplementation on hematological indices of O. niloticus exposed to methomyl (MET) 20.39 µg/L for 60 days.
3.5. Blood Biochemical Parameters
3.5.1. Hepatorenal Damage Products
In the current study, MET-exposed fish supplemented with PSM (G3, G4, and G5) showed enhanced liver and kidney function, as demonstrated by the reduced ALT, AST, LDH, urea, creatinine, ammonia, cholesterol, and total bilirubin concentrations relative to those in fish exposed to MET but without supplemented diet (G2). The supplementation of a 1.0% PSM (G4) based diet concomitantly with the MET exposure had the best result, which attained the control values (Table 4).

Table 4.
Immunological response as well as liver and kidney function tests of O. niloticus exposed to methomyl (MET) 20.39 µg/L and fed parsley seed meal (PSM) for 60 days.
3.5.2. Immune Response
The experimental treatments significantly influenced the innate immunity parameters (lysozyme, C3, and NO) in the current study (Table 4). The MET-exposed group had significantly lower serum humoral immunity variables such as lysozyme, C3, and NO than the other treatments. In contrast, in the groups fed a PSM-enriched diet and exposed to MET, the earlier immune indicators significantly improved compared to the MET-exposed group. Moreover, a significant enhancement in C3 concentration and lysozyme activity was noted, which attained the control level in the 1.0% PSM (G4) group but was still lower in the 0.5% and 2.0% PSM (G3 and G5) groups. In all PSM supplemented groups, however, the improvement in NO remained significantly lower than that observed in the control group.
3.5.3. Stress, Inflammatory Status Assays, and AChE Activity
Exposure to MET (G2) significantly (p < 0.05) decreased the serum concentrations of SOD, CAT, and GPX relative to those of the other fish groups (Table 5). Moreover, it significantly increased MDA, TNF-α, and MPO levels (p < 0.05). PSM supplementation in conjunction with MET exposure restored the normal range for these variables but did not achieve the control level. The best results were obtained when a 1.0% PSM (G4) supplement was added to a basal diet. MET-exposed fish had significantly lower serum amounts of total protein, globulin, and albumin than other groups. The addition of PSM alongside MET exposure reduced the decreases in protein profile metrics in all supplemented groups. Yet, the total protein, globulins, and albumin values were still significantly different from controls in groups supplemented with 0.5% and 2.0% PSM (G3 and G5). A significant (p < 0.05) decline in serum AChE was obvious in the MET-exposed group (G2), but the dietary addition of PSM significantly (p < 0.05) promoted AChE activity, which became not significant (p < 0.05) in the control group and groups supplemented with 1.0% and 2.0% PSM (G4 and G5).

Table 5.
Oxidative, inflammatory biomarkers, acetyl cholinesterase (AchE) activity, and protein profile of O. niloticus exposed to methomyl (MET) (20.39 µg/L) and fed parsley seed meal (PSM) for 60 days.
3.6. Histopathological Findings
3.6.1. Liver
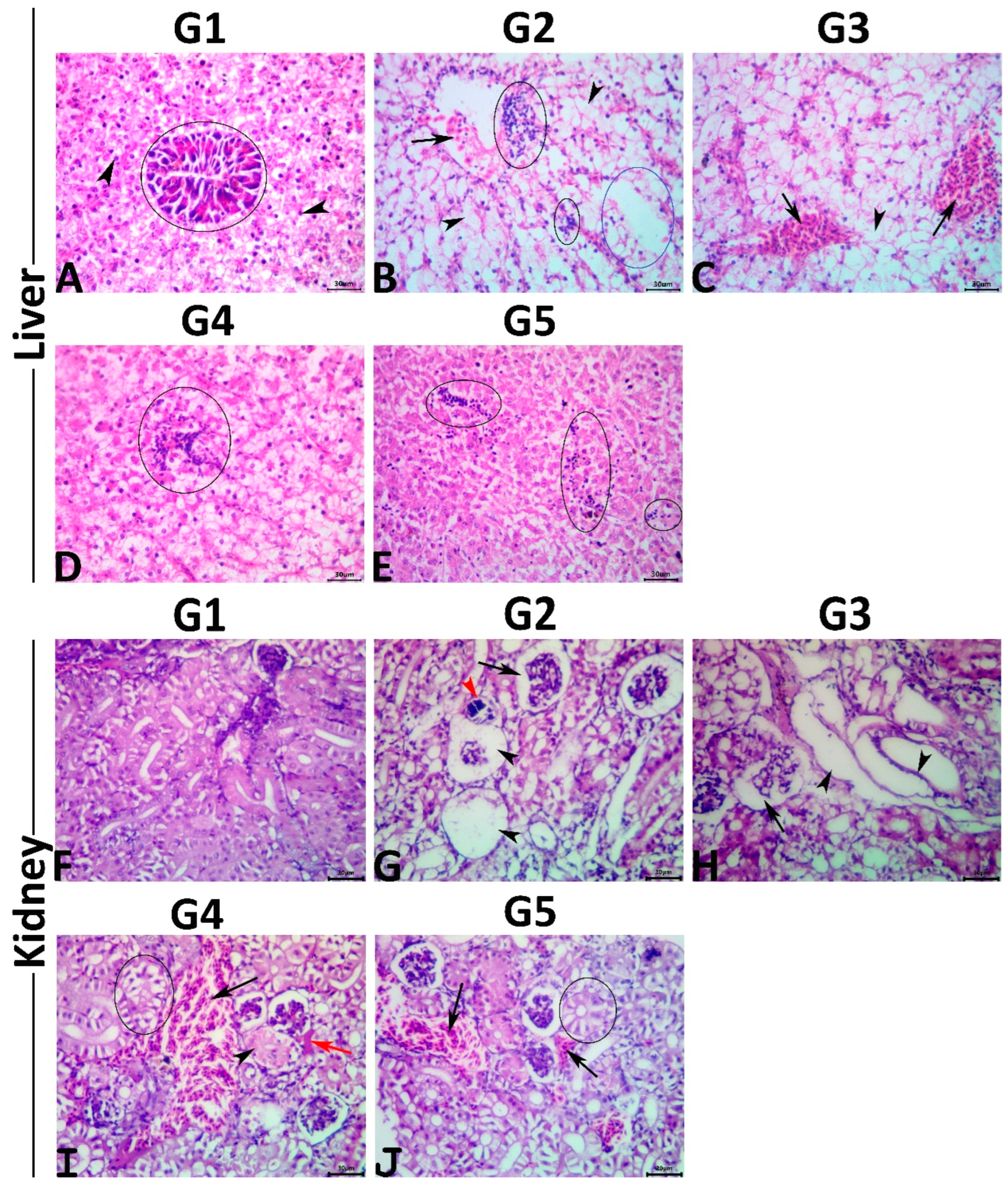
The hepatic tissue sections of the control fish revealed normal histological pictures of the hepatopancreas (Figure 1A). Typically, the hepatic parenchyma is surrounded by a very thin fibroconnective capsule and composed primarily of branched two-cell thick cords. These cords are composed of polyhedral hepatocytes with prominent spherical central nuclei containing one nucleolus. The cytoplasm of hepatocytes contained fairly large quantities of glycogen and/or lipid. Hepatic sinusoids were present between these cords and lined with fenestrated endothelium containing elongated nuclei protruding into the sinusoidal lumen. The Von Kupffer cells were not found. The branches of portal veins were surrounded by scattered exocrine acinar pancreatic tissue composed of clusters of pyramidal cells with clear basal nuclei and deep basophilic cytoplasm containing numerous prominent eosinophilic zymogen granules. Exposure to MET induced a diverse range of hepatopathic alterations, including inflammatory (focal and/or diffuse inflammatory cell infiltrate lymphocytes particularly), circulatory (congestions, sinusoidal dilatations, minute hemorrhages, and interstitial edema), regressive (cellular swelling with extensive vacuolations, single-cell necrosis, vacuolation foci, and coagulative necrotic foci), and progressive (focal areas of regenerated hepatocytes, and melanomacrophage aggregate hyperplasia) alterations (Figure 1B). No preneoplastic, dysplastic, or neoplastic alterations were seen in the hepatocytes or the cholangiocytes. Concurrent PSM supplementation with MET induced variable hepatoprotective effects against the MET-induced hepatopathy, as a moderate rescue effect was seen in the hepatic tissue sections of fish fed with 0.5% PSM. In comparison, notable rescue effects were seen in those treated with 1.0% and 2.0% PSM. Concisely, the livers of the fish exposed to MET and treated with 0.5% PSM revealed a significant reduction in the severities but not the frequencies of the circulatory and inflammatory changes associated with a non-significant decline in the retrogressive and progressive alterations. Most specimens of this group exhibited vascular congestions and cytoplasmic vacuolations (Figure 1C). The livers of the fish exposed to MET and treated with 1.0% and 2.0% PSM showed a notable decline in both the severities and frequencies of the MET-induced pathological alterations, yet none of the treatments (1.0% and 2.0%) regained the normal hepatic histology. Most tissue sections in both groups manifested mild degrees of cytoplasmic vacuolations, vascular congestions, and minute lymphocytic aggregations (Figure 1D,E). Multiparametric lesion scoring to the recorded hepatic histological alterations and the hepatic indices in all groups are summarized in Table 6.
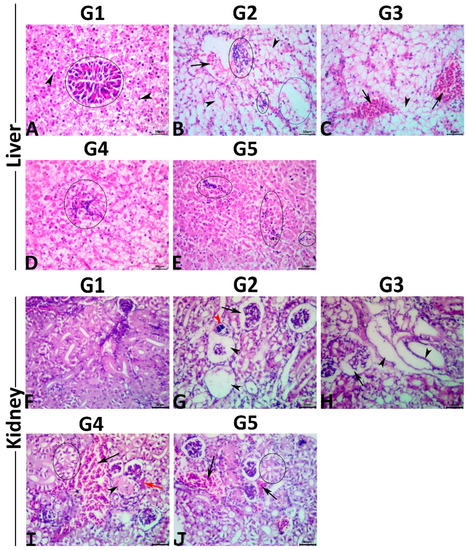
Figure 1.
(A–E); Representative photomicrograph of H&E-stained hepatic tissue sections showing a normal histological picture in the normal control fish (A); polyhedral hepatocytes (arrows), exocrine pancreas around portal vein (ellipse). MET-exposed fish showing vascular congestion (arrow), single-cell necrosis (arrowheads), multifocal mononuclear cell aggregations (black ellipse), and vacuolation foci (blue ellipse) (B). MET+ 0.5% PSM-treated fish showing vascular congestions (arrows), and single-cell necrosis (arrowhead) (C). MET+ 1% PSM-treated fish showing focal mononuclear cell aggregation (ellipse) (D). MET+ 0.5% PSM-treated fish showing multifocal minute mononuclear cell infiltrations (ellipses) (E). Scale bar 30 μm. (F–J); Representative photomicrograph of H&E-stained renal tissue sections showing a normal histological picture in the normal control fish (F). MET-exposed fish showing glomerular collapse (arrow), glomerular necrosis (black arrowheads), and calcified necrotic tubule (red arrowhead) (G). MET+ 0.5% PSM-treated fish showing collapsed glomerulus (arrow), and dilated tubules with flattened epithelial lining (arrowheads) (H). MET+ 1% PSM-treated fish showing vascular congestion (black arrow), interstitial edema (red arrow), tubular vacuolation (ellipse), and tubular coagulative necrosis (arrowhead) (I). MET+ 0.5% PSM-treated fish showing tubular vacuolation (ellipse), and vascular congestions (arrows) (J). Scale bar 30 μm.

Table 6.
Effect of parsley seed meal (PSM) supplementation on lesion scores of liver and kidney tissues of O. niloticus exposed to methomyl (MET) (20.39 µg/L) for 60 days.
3.6.2. Kidneys (Posterior Kidneys)
The renal tissue sections of the control fish showed the normal histological structure of the glomeruli, tubules, and interstitial tissue (Figure 1F). Exposure to MET induced notable nephropathic histological alterations, including inflammatory (interstitial mononuclear cell infiltrations), circulatory (glomerular and interstitial congestions, and interstitial edema), regressive (tubular vacuolations, tubular attenuation, tubular dilatation with flattening of the epithelial lining, single-cell necrosis, focal coagulative necrotic foci, cast formations, glomerular atrophy with widened Bowman’s space, and glomerular necrosis) (Figure 1G), and regressive (tubular regenerations, and melanomacrophage aggregate hyperplasia) alterations with an absence of the neoplastic changes. Concurrent PSM supplementation with MET had average nephroprotective effects. Concisely, the kidneys of the fish exposed to MET and treated with 0.5% PSM revealed a weak rescue effect on the renal histology, as most tissue specimens of this group showed similar but milder lesions to those seen in the MET-exposed fish (Figure 1H). The kidneys of the fish exposed to MET and treated with 1.0% and 2.0% PSM showed significant reductions in both the severities and frequencies of most MET-induced pathological alterations, particularly the retrogressive alterations, yet none of the treatments (1.0% and 2.0%) maintained the normal renal histology. Most tissue sections in both groups showed mild tubular vacuolations, attenuations, glomerular collapse, and vascular congestion (Figure 1I,J). The multiparametric lesion scores for the recorded renal histological alterations and renal indices in all groups are summarized in Table 6.
3.7. Effects on Fish Resistance against Challenge with P. aeruginosa
As presented in Table 7, loss of appetite, loss of reflexes, irregular swimming behavior, and postmortem changes were evident in all groups. Still, they were severe in the fish exposed to MET and fed a basal diet without supplementation (G2). This was also the highest mortality rate among P. aeruginosa-challenged fish. After the bacterial challenge, fish fed a basal diet without PSM supplementation and exposed to MET had the lowest RSP. In contrast to the MET-exposed group, all other groups evaluated significantly improved fish resistance to A. hydrophila infection. Notably, fish exposed to MET and fed a basal diet containing 1.0% PSM (G4) showed the highest RSP%.

Table 7.
Mortality rate, relative percent survival, clinical signs, and postmortem findings observed in survived O. niloticus fish in different experimental groups challenged with Pseudomonas aeruginosa.
4. Discussion
In the present study, MET exposure significantly reduced the O. niloticus growth performance in FBW, DWG, SGR, and feed utilization values. These findings followed the study of Mohamed et al. [10], which verified that chronic exposure to MET for 60 days resulted in low growth performance in O. niloticus. Additionally, Trachantong et al. [51] reported a decrease in the amphibian growth rate after MET exposure. The MET-induced growth retardation could be related to toxic stress-induced metabolic alterations in protein and carbohydrate metabolism, resulting in energy storage to repair tissue damage, leaving limited energy for growth. Moreover, MET could induce endocrine-disruption, resulting in smaller sizes, as previously reported in exposed tadpoles [51]. Remarkably, concurrent PSM administration in the O. niloticus diet improved growth even with MET exposure. It has been demonstrated that spices, herbs, and plant extracts can increase appetite and digestion in fish while also having antibacterial properties [12,52,53]. The PSM growth-augmenting effect could be attributed to the richness of parsley with active compounds (phenols, flavonoids, turbines, and glycosides) that serve as digestive system catalysts by enhancing the elasticity of the intestine’s vasculature [54], thus enhancing the secretion of enzymes (trypsin, chymotrypsin, amylase, lipase) that help in digestive function and excretion [55]. Moreover, the antimicrobial action of parsley is dominated by modulation of the gastrointestinal microbiota [21]. In line with our findings, improved feed conversion rate and growth were demonstrated in Cyprinus carpio, which were fed parsley-supplemented diets [56]. Previous studies have shown that parsley has a comparable protective activity against aflatoxins [57] or bifenthrin [58] in O. niloticus. The mortality of O. niloticus was increased by 13.33% after exposure to MET in the current investigation, indicating its significant toxicity. This could be due to MET-induced oxidative stress, immunotoxicity, genotoxicity, and neurobehavioral toxicity, which have previously been documented [10]. Moreover, MET is a potent neurotoxin as it inhibits the AChE enzyme activity, as recorded in the current study. Likely, Jablonski et al. [59] reported that MET affected embryo hatching and larva shape and behavior of zebrafish, resulting in smaller bodies and eyes, failure to inflate the swimming bladder, and hypolocomotor and highly neurotoxic activity. The PSM-enriched feed given concurrently with MET exposure, on the other hand, diminished mortality percent, perhaps via boosting growth performance and immunological response, as well as alleviation of oxidative damage [58]. Moreover, the mitigating effect of parsley was detected on brain neurons, neurotransmitter levels, and neurobehavioral performance in Cd-treated mice [60].
Tissue protein and lipid content are influenced by the dynamic equilibrium between their production and breakdown rates. In this study, the decrease in lipid content and crude protein was evident in the chemical composition analysis of fish exposed to MET. The former observation could be linked to cellular fraction disruption and the resulting impairment in protein synthesis machinery. The intense proteolysis and catabolic effects caused by MET toxicity release free amino acids to meet the increased energy needs caused by toxins [61]. In times of stress, active glycogenolysis and glycolytic pathways offer surplus energy, resulting in decreased crude lipid content [62]. Many studies reported a similar decrease in protein levels and carbohydrate levels [63]. In contrast, PSM dietary supplementation restored crude protein and lipid deposition in the O. niloticus muscles at the end of the feeding experiment. This could be due to parsley’s modulatory effect on the activity of fish digestive enzymes and intestinal microbiota, which improves the utilization of digestive products [58]. Increased expression of fatty acid synthesis-related markers and up-regulation of urea cycle components suggest a recovery in oxidative stress and glycolysis [64].
Blood biochemical indicators are crucial for assessing fish health, toxicity, and biomonitoring [65,66,67,68]. Herein, fish exposed to MET developed apparent anemia and a significant decrease in total leukocyte count with a parallel decline in heterophils, lymphocytes, monocytes, and eosinophils. Increased RBCs destruction, osmoregulatory impairment, or decreased erythropoiesis can cause a decline in RBC count [69]. As a compensatory reaction to maintaining gas exchange in damaged gills while decreasing oxygen-carrying capacity, decreases in Hb and PCV values were observed. The decreased leukocyte production could be attributed to depression of leukopoiesis, alteration of the cell membrane or disintegration of white blood cells, and a disruption in the innate immune response [70]. Additionally, lymphocytopenia may be connected to cortisol, a stress hormone that induces lymphocyte death or redistribution from the blood to the tissues [71]. In line with our findings, Mohamed et al. [10] found a decrease in erythrocytic, leukocytic, and Hb content in tilapia fish exposed to MET. MET also affected the erythrogram and total and differential leukocytic counts in rats’ blood [72]. Notably, PSM dietary supplementation significantly reversed the MET-induced hematological alterations. Similarly, parsley extract completely reversed the Hb decrease in O. niloticus with aflatoxin contamination [57]. Additionally, the hematological indices of O. niloticus fish exposed to MET and treated with taurine showed a significant improvement [10]. This could be due to PSM’s ability to protect the Hb molecule and the erythrocyte membrane from oxidative damage and the resumption of erythropoiesis. The increase in leukocyte and lymphocytic counts may be linked to preserving the leukocyte redox state and the immunostimulant impact of parsley, which boosts leukocyte proliferation.
When a fish is exposed to various water toxicants, its innate immune system, the first line of pathogen defense, is compromised [73]. The innate immune response system relies heavily on various cellular and humoral components [74]. Lysozymes hydrolyze the peptidoglycan layer of the bacterial cell wall by acting as opsonins, causing the complement system and phagocytes to activate [39]. In fish, complement C3 performs various immunological tasks, including removing invading pathogens, initiating inflammatory responses, and clearing other homeostatic cells [75]. NO, which boosts macrophages’ ability to eliminate infections while also playing a role in the inflammatory response, is another key sign of innate immunity [76]. Herein, immunological responses (lysozyme activity, NO, and C3) were significantly reduced in fish exposed to MET, indicating the innate immune response suppression. The decrease in lysozyme after MET exposure in fish could be attributed to decreased leukocyte count and function, including neutrophils and macrophages [77]. Moreover, the noted reduction in NO amount may reveal declined phagocytosis. The immunosuppressive impact of MET could be due to a toxic insult that lowers total leukocytic and lymphocytic counts. It could also result from uncontrolled inflammatory cytokine production and increased lymphoid organ and immune cell apoptosis. Furthermore, MET has been implicated as an endocrine disruptor [51]. In agreement with our findings, the activity of serum lysozyme, C3, and NO was reduced in O. niloticus after 60 days of exposure to MET [10].
Serum protein, albumin, and globulin levels are also linked to innate immunity [78]. Following 60 days of exposure to MET in O. niloticus, a significant fall in total serum protein, albumin, and globulin was detected, indicating immunoglobulin underproduction [79]. Hypoproteinemia was triggered due to catabolism of protein and/or malfunction of the liver [80]. As a result, the depleted immune components and the hypoproteinemic condition were restored when PSM was supplemented. This immunostimulant effect corresponds to an improvement in total leukocytic and lymphocytic counts. Previous research has shown that parsley boosts humoral and cellular immunity [81,82].
During normal metabolism, reactive oxygen species (ROS) such as superoxide anions and hydroxyl radicals are generated in aerobic cells, mostly through mitochondrial oxidative metabolism, and some of these intermediates are thought to be harmful to cells, causing oxidative stress and oxidative damage [83]. Nonetheless, aerobic organisms have evolved mechanisms to activate antioxidant defense systems in different organs and tissues, which include antioxidant enzymes like CAT, SOD, GR, GPx, and GST, as well as antioxidant scavengers to protect against damage caused by high levels of ROS [84]. In the current study, noticeable exhaustion of the GPx, CAT, and SOD activities but increased inflammatory markers (MPO and TNF-α) and MDA concentrations were recorded in fish exposed to MET. It is possible to hypothesize that under conditions of chronic exposure to MET, it could not be metabolized and was not entirely eliminated from the tilapia body by the cells. As a result, the MET accumulated in the body and destroyed the protein structures of the antioxidant enzymes, resulting in decreased antioxidant enzyme activity, according to Jagetia and Aggarwal [85]. The conjugation of MET or its degradation products to polyunsaturated fatty acids may be blamed for the MET-induced elevation in MDA levels [86]. In this regard, Mohamed et al. [10] proved that the main toxic effects of MET in O. niloticus take place via oxidative stress, resulting in oxidative damage through reducing antioxidant enzyme activity and inducing DNA damage and lipid peroxidation. As proven by the fact that MET can cause oxidative damage, LPO levels are raised, and several antioxidant enzymes are disrupted [87]. In contrast, PSM significantly suppresses the oxidative and lipid peroxidative damage induced by MET by stimulating the production of antioxidant enzymes and thereby boosting cellular antioxidant defenses. Parsley is abundant in polyphenolic flavonoid anti-oxidants such as apiin, apigenin, and luteolin [88]. Along with luteolin and vitamin C, parsley acts as an anti-inflammatory agent, enhancing catalase and glutathione activity and lowering lipid peroxidation [89]. Previous reports have evidenced parsley efficiency in heavy metal excretion from the body [60,90].
The liver is the first organ to be exposed to any foreign molecule delivered by portal circulation, and it sustains the most damage. The release of intracellular enzymes such as AST, ALT, LDH, and ALP into the circulation is one of the most sensitive and dramatic indications of hepatocyte injury in response to toxicity [12,91]. The current findings revealed a significant increase in the activities of AST, ALT, LDH, and ALP in the serum of fish together with metabolic products such as cholesterol and bilirubin, implying that MET could cause liver damage. This changed biochemical profile was linked to a variety of hepatic lesions, including lymphocytic infiltration, coagulative necrotic foci, congestion, sinusoidal dilatations, and melanomacrophage aggregate hyperplasia. Changes in membrane permeability and loss of functional integrity of cell membranes in the liver also cause cellular leakage, resulting in the pervasive release of these enzymes from the cell [92]. Furthermore, MET-induced hypercholesterolemia may be due to organisms’ requirements for additional energy reserves to cope with stressors’ effects [93]. Our observed increase in these parameters due to MET intoxication is in accordance with the findings of Hashish and Elgaml [94]. Restoration of the disturbed enzyme activities and hepatic architecture with the correction of metabolic product concentrations after PSM supplementation is the primary explanation for their hepatoprotective effects. Indeed, the hepatoprotective effect of parsley arises from its capacity to scavenge ROS, as reported in numerous assays [95,96]. The decreased LDH and hypocholesterolemia due to the concurrent treatment with 20% parsley seed methanol extract was documented in rats [97]. The stabilization of serum bilirubin levels by PSM is a clear indication of the improvement in the functional status of the liver cells. This activity could be attributed to the phytochemical analysis’s bioactive components, including flavonoids, lignans, alkaloids, bisbenzyl, coumarins, and terpenes [98].
Fish kidneys gather the most post-branchial blood, so renal diseases could be effective indicators of environmental contamination [99]. MET caused a considerable increase in serum ammonia, urea, and creatinine activity in the present study. The elevated levels of ammonia, urea, and creatinine may be linked to the effects of MET on muscle metabolism and its limited impact on purine metabolism and renal tubule injury. These findings matched those of Hashish and Elgaml [94]. Conversely, the present study found that PSM supplementation might reverse MET-induced nephrotoxicity in O. niloticus fish, as indicated by restoration of the disturbed metabolites and renal architecture. Apigenin and its glucosidal flavonoids, which are abundant in parsley, have been discovered to have anti-inflammatory, antioxidant, and anticancer properties, particularly in the case of renal inflammation [100]. A similar nephroprotective effect of PSM has been previously recognized [101].
In the current study, O. niloticus was challenged with P. aeruginosa after MET exposure for 60 days. The findings demonstrated that the RPS was dropped noticeably in MET-exposed and fed basal diet following the challenge. Moreover, severe abnormal behavior and clinical signs were observed. The loss of cellular and humoral immune components caused by MET could be a major contributor to lowering the RPS and, as a result, compromising the fish response to P. aeruginosa in the challenge. Importantly, when PSM was added to the fish diet with MET exposure, it improved the outcomes of suppressed innate immunity against P. aeruginosa, which could be strongly linked to the immune-stimulatory activity observed in this trial. Correspondingly, Farag et al. [58] demonstrated that dietary parsley essential oil supplementation in the O. niloticus diet alleviated the adverse effects of bifenthrin and enhanced disease resistance against Aeromonas hydrophila.
Importantly, the effects of new feed ingredients or additives on fish health indicators and safety should be carefully considered for an appropriate assessment of any nutritional strategy [13]. In this regard, the liver and kidney function indicators are important determinants in assessing feed ingredient safety in fish diets because of their roles in detoxification, biotransformation, and excretion of xenobiotics [102,103]. Of note, in the current study, supplementing fish with diets fortified with up to 2% of PSM, even with MET exposure, did not negatively impacted the liver and kidney function indicators, indicating a wide safety margin of PSM as a feed supplement in fish.
5. Conclusions
Finally, the current study verified for the first time that dietary supplementation of PSM can positively affect health against MET-induced growth retardation, anemia, leukopenia, hepato-renal damage, immunosuppression, AchE inhibition, and weakened disease resistance in O. niloticus. The antioxidant and anti-inflammatory activities could be the probable underlying mechanisms of PSM. Additionally, it has the potential to recover the histopathological architecture of the liver and kidney. The prevailing study’s findings may point to PSM as an eco-friendly aqua-feed supplement that enhances fish health even with waterborne contaminants. Future mechanistic studies are needed to elucidate PSM’s other probable underlying mechanisms of action, focusing on the genome transcriptome and proteome. Moreover, further studies assessing the effects of PSM on MET accumulation in fish tissues after different periods of exposure and recoveries are required.
Author Contributions
Conceptualization, W.E.-H., S.A.A., E.A.A.M., M.M.M.M., Y.K.M., Y.S.A., M.M.S., Y.M.A.-E. and A.E.E.-M.; methodology, W.E.-H., S.A.A., E.A.A.M., M.M.M.M., Y.K.M. and A.E.E.-M.; software, W.E.-H., M.M.M.M. and Y.M.A.-E.; validation, W.E.-H., S.A.A. and M.M.M.M.; formal analysis, W.E.-H., M.M.M.M. and Y.M.A.-E.; investigation, W.E.-H., S.A.A., E.A.A.M., M.M.M.M., Y.K.M. and A.E.E.-M.; resources, Y.S.A. and M.M.S.; data curation, W.E.-H., M.M.M.M. and Y.M.A.-E.; writing—original draft preparation, W.E.-H.; writing—review and editing, S.A.A., E.A.A.M., M.M.M.M., Y.K.M., Y.S.A., M.M.S., Y.M.A.-E. and A.E.E.-M.; visualization, W.E.-H., M.M.M.M. and Y.M.A.-E.; project administration, Y.S.A. and M.M.S.; funding acquisition, Y.S.A. and M.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Taif University Researchers Supporting Project (TURSP-2020-258), Taif University, Taif, Saudi Arabia.
Institutional Review Board Statement
All experiment procedures were approved by the Institutional Animal Care and Use Committee (ZU-IACUC/2022) of Zagazig University.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We appreciate and thank Taif University for the financial support for Taif University Researchers Supporting Project (TURSP-2020-258), Taif University, Taif, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Loro, V.L.; Clasen, B.E. Chapter Five—Agrochemicals: Ecotoxicology and management in aquaculture. In Aquaculture Toxicology; Kibenge, F.S.B., Baldisserotto, B., Chong, R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 79–106. [Google Scholar]
- Rani, R.; Sharma, P.; Kumar, R.; Hajam, Y.A. Chapter 3—Effects of heavy metals and pesticides on fish. In Bacterial Fish Diseases; Dar, G.H., Bhat, R.A., Qadri, H., Al-Ghamdy, K.M., Hakeem, K.R., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 59–86. [Google Scholar]
- Meng, S.L.; Chen, J.Z.; Hu, G.D.; Song, C.; Fan, L.M.; Qiu, L.P.; Xu, P. Effects of chronic exposure of methomyl on the antioxidant system in liver of Nile tilapia (Oreochromis niloticus). Ecotoxicol. Environ. Saf. 2014, 101, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kongphonprom, K.; Burakham, R. Determination of carbamate insecticides in water, fruit, and vegetables by ultrasound-assisted dispersive liquid–liquid microextraction and high-performance liquid chromatography. Anal. Lett. 2016, 49, 753–767. [Google Scholar] [CrossRef]
- Van Scoy, A.R.; Yue, M.; Deng, X.; Tjeerdema, R.S. Environmental Fate and Toxicology of Methomyl. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2013; pp. 93–109. [Google Scholar]
- Mansour, S.A.; Abbassy, M.A.; Shaldam, H.A. Zinc ameliorate oxidative stress and hormonal disturbance induced by methomyl, abamectin, and their mixture in male rats. Toxics 2017, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-M. Methomyl poisoning presenting with decorticate posture and cortical blindness. Neurol. Int. 2014, 6, 4–5. [Google Scholar] [CrossRef]
- Islamy, R.A.; Yanuhar, U.; Hertika, A.M.S. Assessing the genotoxic potentials of methomyl-based pesticide in Tilapia (Oreochromis niloticus) using micronucleus assay. J. Exp. Life Sci. 2017, 7, 88–93. [Google Scholar] [CrossRef]
- Wang, Y.J.P.M.; Meng, S.; Zheng, Y.; Chen, J.; Wu, W. Ipomoea aquatica: Purification of Aquaculture Water Contaminated by Methomyl and Effect on Fish Growth. Chin. Agric. Sci. Bull. 2020, 36, 147–153. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Abdel Rahman, A.N.; Salem, G.A.; Deib, M.M.E.; Nassan, M.A.; Rhouma, N.R.; Khater, S.I. The Antioxidant Role of a Taurine-Enriched Diet in Combating the Immunotoxic and Inflammatory Effects of Pyrethroids and/or Carbamates in Oreochromis niloticus. Animals 2021, 11, 1318. [Google Scholar] [CrossRef]
- El-Houseiny, W.; Abd El-Hakim, Y.M.; Metwally, M.M.M.; Abdel Ghfar, S.S.; Khalil, A.A. The single or combined Silybum marianum and co-enzyme Q10 role in alleviating fluoride-induced impaired growth, immune suppression, oxidative stress, histological alterations, and reduced resistance to Aeromonas sobria in African catfish (Clarias gariepinus). Aquaculture 2022, 548, 737693. [Google Scholar] [CrossRef]
- El-Houseiny, W.; Khalil, A.A.; Abd-Elhakim, Y.M.; Badr, H.A. The potential role of turmeric and black pepper powder diet supplements in reversing cadmium-induced growth retardation, ATP depletion, hepatorenal damage, and testicular toxicity in Clarias gariepinus. Aquaculture 2019, 510, 109–121. [Google Scholar] [CrossRef]
- Ayyat, M.S.; Abdel-Rahman, G.A.; Ayyat, A.M.N.; Abdel-Rahman, M.S.; Al-Sagheer, A.A. Evaluation of leaf protein concentrate from Beta vulgaris and Daucus carota as a substitute for soybean meal in Oreochromis niloticus fingerlings diets. Aquac. Res. 2021, 52, 3256–3269. [Google Scholar] [CrossRef]
- Lopez, M.; Sanchez-Mendoza, I.; Ochoa-Alejo, N. Compartive study of volatile components and fatty acids of plants and in vitro cultures of parsley (Petroselinum crispum (Mill) nym ex hill). J. Agric. Food Chem. 1999, 47, 3292–3296. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P. Parsley herb. Br. Herb. Compend. 1992, 1, 230–239. [Google Scholar]
- Tunali, T.; Yarat, A.; Yanardag, R.; Özçelik, F.; Özsoy, Ö.; Ergenekon, G.; Emekli, N. Effect of parsley (Petroselinum crispum) on the skin of STZ induced diabetic rats. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 1999, 13, 138–141. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Khalil, S.R.; Abd El-Aziz, R.M.; Zaglool, A.W.; Moselhy, A.A.A.; Abou-Zeid, S.M. Effect of parsley essential oil on digestive enzymes, intestinal morphometry, blood chemistry and stress-related genes in liver of Nile tilapia fish exposed to Bifenthrin. Aquaculture 2022, 546, 737322. [Google Scholar] [CrossRef]
- Nielsen, S.E.; Young, J.F.; Daneshvar, B.; Lauridsen, S.T.; Knuthsen, P.; Sandström, B.; Dragsted, L.O. Effect of parsley (Petroselinum crispum) intake on urinary apigenin excretion, blood antioxidant enzymes and biomarkers for oxidative stress in human subjects. Br. J. Nutr. 1999, 81, 447–455. [Google Scholar] [CrossRef]
- Wong, P.Y.Y.; Kitts, D.D. Studies on the dual antioxidant and antibacterial properties of parsley (Petroselinum crispum) and cilantro (Coriandrum sativum) extracts. Food Chem. 2006, 97, 505–515. [Google Scholar] [CrossRef]
- Fejes, S.; Blazovics, A.; Lemberkovics, E.; Petri, G.; Szöke, E.; Kery, A. Free radical scavenging and membrane protective effects of methanol extracts from Anthriscus cerefolium L.(Hoffm.) and Petroselinum crispum (Mill.) Nym. ex AW Hill. Phytother. Res. 2000, 14, 362–365. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Abbasabadi, Z.; Ardekani, M.R.S.; Rahimi, R.; Farzaei, F. Parsley: A review of ethnopharmacology, phytochemistry and biological activities. J. Tradit. Chin. Med. 2013, 33, 815–826. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Taha, H.S.A.; Ismail, T.A.; Khalil, S.R.; Abou-Zeid, S.M. Immune response and susceptibility of Nile tilapia fish to Aeromonas hydrophila infection following the exposure to Bifenthrin and/or supplementation with Petroselinum crispum essential oil. Ecotoxicol. Environ. Saf. 2021, 216, 112205. [Google Scholar] [CrossRef]
- Teletchea, F. Fish Domestication: An Overview. Anim. Domest. 2019, 69–90. [Google Scholar]
- Khalil, A.A.; Abd-Elhakim, Y.M.; Said, E.N.; Moselhy, A.A.A.; Abu-Elsaoud, A.M.; El-Houseiny, W. Milk thistle and co-enzyme Q10 fortified diets lessen the nickel chloride-induced neurotoxic and neurobehavioral impairments in Oreochromis niloticus via regulating the oxidative stress response, acetylcholinesterase activity, and brain nickel content. Aquaculture 2022, 553, 738102. [Google Scholar] [CrossRef]
- Thomas, J.; Thanigaivel, S.; Vijayakumar, S.; Acharya, K.; Shinge, D.; Seelan, T.S.; Mukherjee, A.; Chandrasekaran, N. Pathogenecity of Pseudomonas aeruginosa in Oreochromis mossambicus and treatment using lime oil nanoemulsion. Colloids Surf. B Biointerfaces 2014, 116, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, S.; Kumar, N.; Mohanty, S.; Samanta, M.; Mandal, R.N.; Maiti, N.K. Characterisation of Pseudomonas aeruginosa isolated from freshwater culture systems. Microbiol. Res. 2007, 162, 391–396. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Nutrient Requirements of Fish and Shrimp; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC: Washington, DC, USA, 2006. [Google Scholar]
- Bain, B.J.; Bates, I.; Laffan, M.A.; Lewis, S.M. Dacie and Lewis Practical Hamatology: Expert Consult: Online and Print; Elsevier Health Sciences: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Burtis, C.A.; Ashwood, E.R. Tietz Textbook of Clinical Chemistry; American Association for Clinical Chemistry: Washington, DC, USA, 1994. [Google Scholar]
- Wenger, C.; Kaplan, A.; Rubaltelli, F.; Hammerman, C. Alkaline phosphatase. In Clinical Chemistry; The C.V. Mosby Co.: St Louis, MO, USA; Toronto, ON, Canada; Princeton, NJ, USA, 1984; pp. 1094–1098. [Google Scholar]
- Murray, R.; Kaplan, A. Aspartate aminotransferase. In Clinical Chemistry. Theory, Analysis and Correlation; Kaplan, L.A., Pesce, A.J., Eds.; CV Mosby Company: Maryland Heights, MI, USA, 1984; pp. 1105–1108. [Google Scholar]
- Pesce, A. Lactate dehydrogenase. In Clinical Chemistry; Kaplan, A., Pesce, A., Eds.; CV Mosby Company: Maryland Heights, MI, USA, 1984; Volume 438, pp. 1117–1124. [Google Scholar]
- Neely, W.; Phillipson, J. Automated enzymatic method for determining ammonia in plasma with 14 day stability. Clin. Chem. 1988, 34, 1868–1869. [Google Scholar] [CrossRef]
- Martinen, K. Improved micro-method for determination of serum bilirubin. Clin. Chim. Acta 1966, 13, 161–170. [Google Scholar] [CrossRef]
- Naito, H.K.; David, J.A. Laboratory considerations: Determination of cholesterol, triglyceride, phospholipid, and other lipids in blood and tissues. Lab. Res. Methods Biol. Med. 1984, 10, 1–76. [Google Scholar]
- Fossati, P.; Prencipe, L.; Berti, G. Enzymic creatinine assay: A new colorimetric method based on hydrogen peroxide measurement. Clin. Chem. 1983, 29, 1494–1496. [Google Scholar] [CrossRef]
- Kaplan, A.; Glucose, K.A. Clinical Chemistry; CV Mosby Company: Maryland Heights, MI, USA, 1984; Volume 436, pp. 1032–1036. [Google Scholar]
- Ellis, A.E. Lysozyme assays. Tech. Fish Immunol. 1990, 1, 101–103. [Google Scholar]
- Dumock, J. The determination of nitrite in water. Analyst 1961, 86, 414–416. [Google Scholar]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Nishikimi, M.; Appaji Rao, N.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Kumari, J.; Sahoo, P.K. Effects of cyclophosphamide on the immune system and disease resistance of Asian catfish Clarias batrachus. Fish Shellfish Immunol. 2005, 19, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Badawi, H.K. Electrophoretic studies of serum proteins of four Tilapia species (Pisces). Mar. Biol. 1971, 8, 96–98. [Google Scholar] [CrossRef]
- Meyers, T.R. Standard necropsy procedures for finfish. In NWFHS Laboratory Procedures Manual, 5th ed.; US Fish and Wildlife Service: Washingron, DC, USA, 2009; pp. 64–74. [Google Scholar]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Bernet, D.; Schmidt, H.; Meier, W.; Burkhardt-Holm, P.; Wahli, T. Histopathology in fish: Proposal for a protocol to assess aquatic pollution. J. Fish Dis. 1999, 22, 25–34. [Google Scholar] [CrossRef]
- Trachantong, W.; Saenphet, S.; Saenphet, K.; Chaiyapo, M. Lethal and sublethal effects of a methomyl-based insecticide in Hoplobatrachus rugulosus. J. Toxicol. Pathol. 2017, 30, 15–24. [Google Scholar] [CrossRef]
- Ibrahim, R.E.; El-Houseiny, W.; Behairy, A.; Mansour, M.F.; Abd-Elhakim, Y.M. Ameliorative effects of Moringa oleifera seeds and leaves on chlorpyrifos-induced growth retardation, immune suppression, oxidative stress, and DNA damage in Oreochromis niloticus. Aquaculture 2019, 505, 225–234. [Google Scholar] [CrossRef]
- Naiel, M.A.E.; Ismael, N.E.M.; Negm, S.S.; Ayyat, M.S.; Al-Sagheer, A.A. Rosemary leaf powder–supplemented diet enhances performance, antioxidant properties, immune status, and resistance against bacterial diseases in Nile Tilapia (Oreochromis niloticus). Aquaculture 2020, 526, 735370. [Google Scholar] [CrossRef]
- Safamehr, A.; Fallah, F.; Nobakht, A. Growth Performance and Biochemical Parametersof Broiler Chickens on Diets Consist of Chicory (Cichorium intybus) and Nettle (Urtica dioica) with or without Multi-Enzyme. Iran. J. Appl. Anim. Sci. 2013, 3, 131–137. [Google Scholar]
- Bahnas, M.; Ragab, M.; Asker, N.; Emam, R. Effects of using parsley or its by-product with or without enzyme supplementation on performance of growing Japanese quails. Egypt. Poult. Sci 2009, 29, 241–262. [Google Scholar]
- Mooraki, N.; Dadgar, S.; Sadegh, N.M. The effect of using parsley (petroselinum sativum) on growth performance of koi fish (Cyprinus carpio). J. Aquac. Dev. 2014, 8, 63–72. [Google Scholar]
- El-Barbary, M.I.; Mehrim, A.I. Protective effect of antioxidant medicinal herbs, rosemary and parsley, on subacute aflatoxicosis in Oreochromis niloticus. J. Fish. Aquat. Sci. 2009, 4, 178–190. [Google Scholar] [CrossRef][Green Version]
- Farag, M.R.; Mahmoud, H.K.; El-Sayed, S.A.A.; Ahmed, S.Y.A.; Alagawany, M.; Abou-Zeid, S.M. Neurobehavioral, physiological and inflammatory impairments in response to bifenthrin intoxication in Oreochromis niloticus fish: Role of dietary supplementation with Petroselinum crispum essential oil. Aquat. Toxicol. 2021, 231, 105715. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, C.A.; Pereira, T.C.B.; Teodoro, L.D.S.; Altenhofen, S.; Rübensam, G.; Bonan, C.D.; Bogo, M.R. Acute toxicity of methomyl commercial formulation induces morphological and behavioral changes in larval zebrafish (Danio rerio). Neurotoxicol. Teratol. 2022, 89, 107058. [Google Scholar] [CrossRef] [PubMed]
- Maodaa, S.N.; Allam, A.A.; Ajarem, J.; Abdel-Maksoud, M.A.; Al-Basher, G.I.; Wang, Z.Y. Effect of parsley (Petroselinum crispum, Apiaceae) juice against cadmium neurotoxicity in albino mice (Mus musculus). Behav. Brain Funct. 2016, 12, 1–16. [Google Scholar] [CrossRef]
- Naqvi, G.-E.-Z.; Shoaib, N.; Ali, A.M. Pesticides impact on protein in fish (Oreochromis mossambicus) tissues. Indian J. Mar. Sci. 2017, 46, 1864–1868. [Google Scholar]
- Fahmy, G.H. Malathion toxicity: Effect on some metabolic activities in Oreochromis niloticus, the Tilapia Fish. Int. J. Biosci. Biochem. Bioinform. 2012, 2, 52–55. [Google Scholar] [CrossRef]
- Thenmozhi, C.; Vignesh, V.; Thirumurugan, R.; Arun, S. Impacts of malathion on mortality and biochemical changes of freshwater fish Labeo rohita. Iran. J. Environ. Health Sci. Eng. 2011, 8, 325–332. [Google Scholar]
- Jia, H.; Aw, W.; Hanate, M.; Takahashi, S.; Saito, K.; Tanaka, H.; Tomita, M.; Kato, H. Multi-faceted integrated omics analysis revealed parsley (Petroselinum crispum) as a novel dietary intervention in dextran sodium sulphate induced colitic mice. J. Funct. Foods 2014, 11, 438–448. [Google Scholar] [CrossRef]
- Abd El-Hakim, Y.M.; El-Houseiny, W.; Abd Elhakeem, E.-M.; Ebraheim, L.L.; Moustafa, A.A.; Mohamed, A.A.R. Melamine and curcumin enriched diets modulate the haemato-immune response, growth performance, oxidative stress, disease resistance, and cytokine production in oreochromis niloticus. Aquat. Toxicol. 2020, 220, 105406. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.-R.; El-Houseiny, W.; Abd Elhakeem, E.-M.; Ebraheim, L.L.; Ahmed, A.I.; Abd El-Hakim, Y.M. Effect of hexavalent chromium exposure on the liver and kidney tissues related to the expression of CYP450 and GST genes of Oreochromis niloticus fish: Role of curcumin supplemented diet. Ecotoxicol. Environ. Saf. 2020, 188, 109890. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, W.A.; El-Houseiny, W.; Ibrahim, R.E.; Abd-Elhakim, Y.M. Palliative effects of zinc sulfate against the immunosuppressive, hepato- and nephrotoxic impacts of nonylphenol in Nile tilapia (Oreochromis niloticus). Aquaculture 2019, 504, 227–238. [Google Scholar] [CrossRef]
- Ayyat, M.S.; Ayyat, A.M.N.; Abd El-Latif, K.M.; Hessein, A.A.A.; Al-Sagheer, A.A. Inorganic mercury and dietary safe feed additives enriched diet impacts on growth, immunity, tissue bioaccumulation, and disease resistance in Nile tilapia (Oreochromis niloticus). Aquat. Toxicol. 2020, 224, 105494. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Li, Z.; Zuberi, A.; Arifeen, M.Z.U.; Baig, M.M.F.A. Biomarkers of pyrethroid toxicity in fish. Environ. Chem. Lett. 2019, 17, 945–973. [Google Scholar] [CrossRef]
- Zaahkouk, S.A.M.; Helal, E.; Abd-Rabo, T.E.I.; Rashed, S.Z.A. Carbamate Toxicity and Protective effect ofvit. A and vit. E on some biochemicalaspects of male albino rats. Egypt. J. Hosp. Med. 2000, 1, 60–77. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Z.; Yao, H.; Liang, Y.; Xing, H.; Xu, S. Effects of atrazine and chlorpyrifos on oxidative stress-induced autophagy in the immune organs of common carp (Cyprinus carpio L.). Fish Shellfish Immunol. 2015, 44, 12–20. [Google Scholar] [CrossRef]
- Mossa, A.-T.H.; Abbassy, M.A. Adverse Haematological and biochemical effects of certain. Res. J. Environ. Toxicol. 2012, 6, 160–168. [Google Scholar] [CrossRef]
- Saurabh, S.; Sahoo, P. Lysozyme: An important defence molecule of fish innate immune system. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Magnadóttir, B. Innate immunity of fish (overview). Fish Shellfish Immunol. 2006, 20, 137–151. [Google Scholar] [CrossRef]
- Holland, M.C.H.; Lambris, J.D. The complement system in teleosts. Fish Shellfish Immunol. 2002, 12, 399–420. [Google Scholar] [CrossRef] [PubMed]
- Villamil, L.a.; Tafalla, C.; Figueras, A.; Novoa, B. Evaluation of immunomodulatory effects of lactic acid bacteria in turbot (Scophthalmus maximus). Clin. Diagn. Lab. Immunol. 2002, 9, 1318–1323. [Google Scholar] [CrossRef] [PubMed]
- Ghiasi, F.; Mirzargar, S.; Badakhshan, H.; Shamsi, S. Effects of low concentration of cadmium on the level of lysozyme in serum, leukocyte count and phagocytic index in Cyprinus carpio under the wintering conditions. J. Fish. Aquat. Sci. 2010, 5, 113–119. [Google Scholar] [CrossRef]
- Wiegertjes, G.F.; Stet, R.J.; Parmentier, H.K.; van Muiswinkel, W.B. Immunogenetics of disease resistance in fish: A comparative approach. Dev. Comp. Immunol. 1996, 20, 365–381. [Google Scholar] [CrossRef]
- Graeter, L.; Hertenstein, E.; Accurso, C.; Labiner, G. Elsevier’s Medical Laboratory Science Examination Review; Elsevier Health Sciences: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Kaliwal, B.; Ksheerasagar, R. Histological and biochemical changes in the liver of albino mice on exposure to insecticide, carbosulfan. Casp. J. Environ. Sci. 2006, 4, 67–76. [Google Scholar]
- Osman, M.; Amber, K.H.; Mahmoud, M.A. Response of broiler chicks performance to partial dietary inclusion of Radish, Rocket and Parsley cakes. Egypt. Poult. Sci. J. 2004, 24, 429–446. [Google Scholar]
- Hassanien, H.; El-Karim, A. Effects of dietary ginseng or parsley supplementation in diets of growing rabbits raised under two different caged design systems. Egypt. J. Rabbit Sci. 2013, 23, 179–202. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.; Shen, H.; Wang, X.; Wu, J.; Xue, Y. Effects of chronic exposure of 2,4-dichlorophenol on the antioxidant system in liver of freshwater fish Carassius auratus. Chemosphere 2004, 55, 167–174. [Google Scholar] [CrossRef]
- Meng, S.L.; Hu, G.D.; Qiu, L.P.; Song, C.; Fan, L.M.; Chen, J.Z.; Xu, P. Effects of chronic exposure of methomyl on the antioxidant system in kidney of Nile tilapia (Oreochromis niloticus) and recovery pattern. J. Toxicol. Environ. Health Part A 2013, 76, 937–943. [Google Scholar] [CrossRef]
- Jagetia, G.C.; Aggarwal, B.B. “Spicing up” of the immune system by curcumin. J. Clin. Immunol. 2007, 27, 19–35. [Google Scholar] [CrossRef]
- Gutteridge, J.M.; Halliwell, B. Free radicals and antioxidants in the year 2000: A historical look to the future. Ann. N. Y. Acad. Sci. 2000, 899, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.A.; Mossa, A.-T.H.; Heikal, T.M. Effects of methomyl on lipid peroxidation and antioxidant enzymes in rat erythrocytes: In vitro studies. Toxicol. Ind. Health 2009, 25, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.A.; Nascimento, M.A.; Bozzo, T.A.; Cintra, A.; da Silva, S.M.; Dalboni, M.A.; Mouro, M.G.; Higa, E.M.S. Ascorbic acid reduces gentamicin-induced nephrotoxicity in rats through the control of reactive oxygen species. Clin. Nutr. 2014, 33, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-C.; Wang, C.-J.; Chen, Y.-H.; Hsu, J.-D.; Cheng, S.-Y.; Chen, H.-C.; Lee, H.-J. Polyphenol extracts from Hibiscus sabdariffa Linnaeus attenuate nephropathy in experimental type 1 diabetes. J. Agric. Food Chem. 2009, 57, 2206–2210. [Google Scholar] [CrossRef]
- Darias, V.; Martin-Herrera, D.; Abdala, S.; De la Fuente, D. Plants used in urinary pathologies in the Canary Islands. Pharm. Biol. 2001, 39, 170–180. [Google Scholar] [CrossRef]
- Mahboub, H.H.; Beheiry, R.R.; Shahin, S.E.; Behairy, A.; Khedr, M.H.E.; Ibrahim, S.M.; Elshopakey, G.E.; Daoush, W.M.; Altohamy, D.E.; Ismail, T.A.; et al. Adsorptivity of mercury on magnetite nano-particles and their influences on growth, economical, hemato-biochemical, histological parameters and bioaccumulation in Nile tilapia (Oreochromis niloticus). Aquat. Toxicol. 2021, 235, 105828. [Google Scholar] [CrossRef]
- Patil, J.A.; Patil, A.J.; Sontakke, A.V.; Govindwar, S.P. Effect of methomyl on hepatic mixed function oxidases in rats. Indian J. Pharm. 2008, 40, 158–163. [Google Scholar] [CrossRef]
- Lee, Y.C.; Townsend, R.; Hardy, M.R.; Lönngren, J.; Arnarp, J.; Haraldsson, M.; Lönn, H. Binding of synthetic oligosaccharides to the hepatic Gal/GalNAc lectin. Dependence on fine structural features. J. Biol. Chem. 1983, 258, 199–202. [Google Scholar] [CrossRef]
- Hashish, E.; Elgaml, S. Role of nicotinic acid in mitigating methomyl induced acute toxicity in albino rats. J. Clin. Exp. Pathol. 2016, 6, 268. [Google Scholar]
- Kolarovic, J.; Popovic, M.; Zlinská, J.; Trivic, S.; Vojnovic, M. Antioxidant activities of celery and parsley juices in rats treated with doxorubicin. Molecules 2010, 15, 6193–6204. [Google Scholar] [CrossRef]
- Jafar, S.; Mehri, L.; Hadi, B.; Jamshid, M. The antiurolithiasic and hepatocurative activities of aqueous extracts of Petroselinum sativum on ethylene glycol-induced kidney calculi in rats. Sci. Res. Essays 2012, 7, 1577–1583. [Google Scholar]
- El Rabey, H.A.; Al-Seeni, M.N.; Al-Ghamdi, H.B. Comparison between the hypolipidemic activity of parsley and carob in hypercholesterolemic male rats. BioMed Res. Int. 2017, 2017, 3098745. [Google Scholar] [CrossRef] [PubMed]
- Soliman, H.A.; El-Desouky, M.A.; Hozayen, W.G.; Ahmed, R.R.; Khaliefa, A.K. Hepatoprotective effects of parsley, basil, and chicory aqueous extracts against dexamethasone-induced in experimental rats. J. Intercult. Ethnopharmacol. 2016, 5, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, E.I. Gill and kidney histopathology in the freshwater fish Cyprinus carpio after acute exposure to deltamethrin. Environ. Toxicol. Pharmacol. 2006, 22, 200–204. [Google Scholar] [CrossRef]
- Pápay, Z.E.; Kósa, A.; Boldizsár, I.; Ruszkai, A.; Balogh, E.; Klebovich, I.; Antal, I. Pharmaceutical and formulation aspects of Petroselinum crispum extract. Acta Pharm. Hung. 2012, 82, 3–14. [Google Scholar]
- Salama, A.; Abd El-Wahed, A.; Mostafa, A. Protective effect of some plants against the toxicity of kidneys caused by gentamicin. J. Med. Sci. Res. 2020, 3, 5–11. [Google Scholar] [CrossRef]
- Yadav, M.; Khati, A.; Chauhan, R.; Arya, P.; Semwal, A. A Review on Feed Additives used in Fish Diet. Int. J. Environ. Agric. Biotechnol. 2021, 6, 2. [Google Scholar] [CrossRef]
- Maage, A.; Hove, H.; Julshamn, K. 22—Monitoring and surveillance to improve farmed fish safety. In Improving Farmed Fish Quality and Safety; Lie, Ø., Ed.; Woodhead Publishing: Sawston, UK, 2008; pp. 547–564. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).