Thermo-Responsive Gel Containing Hydroxytyrosol-Chitosan Nanoparticles (Hyt@tgel) Counteracts the Increase of Osteoarthritis Biomarkers in Human Chondrocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Physico-Chemical Characterization of Hyt-Loading Nanoparticles (Hyt-NPs)
2.3. Hyt-Loaded Hydrogel (Hyt@tgel) Preparation
2.4. Hyt@tgel Characterization
2.4.1. Gelation Time and Syringeability
2.4.2. Mechanical Strength Test
2.4.3. Short-Term Stability Studies
2.4.4. In Vitro Hyt Release
2.5. In Vitro Cell Studies
2.5.1. Cell Culture and Treatment
2.5.2. Intracellular Oxidative Stress
2.5.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5.4. Quantitative Senescence-Associated Beta-Galactosidase Assay
2.5.5. RNA Isolation, Reverse Transcription, and Quantitative Real-Time PCR (qRT-PCR)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Physicochemical Characterization of Hyt-Loaded Nanoparticles (Hyt NPs)
3.2. In Vitro Hydrogel Formulation (Hyt@tgel) and Hyt Release
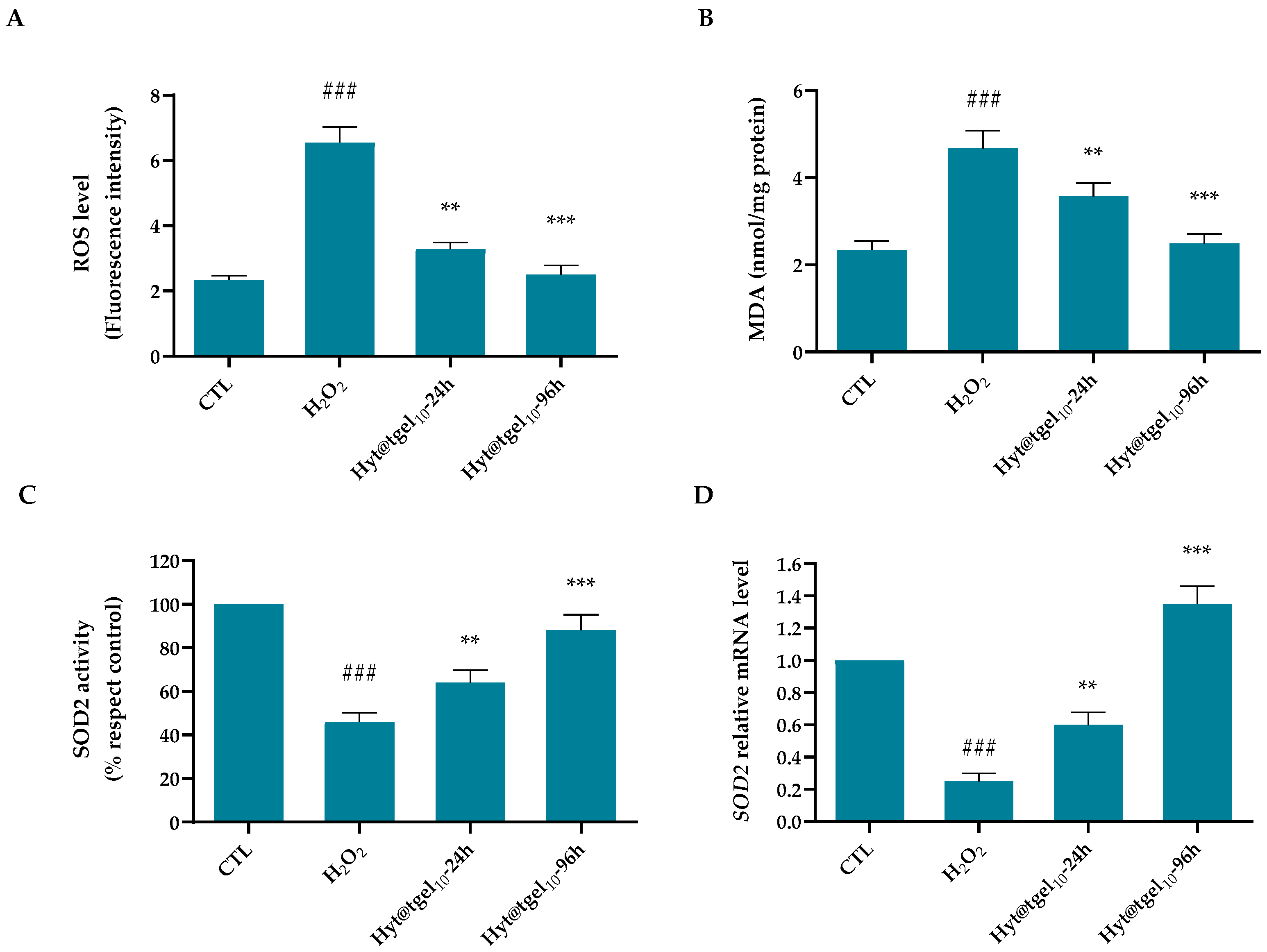
3.3. Oxidative Damage Protection of Hyt@tgel
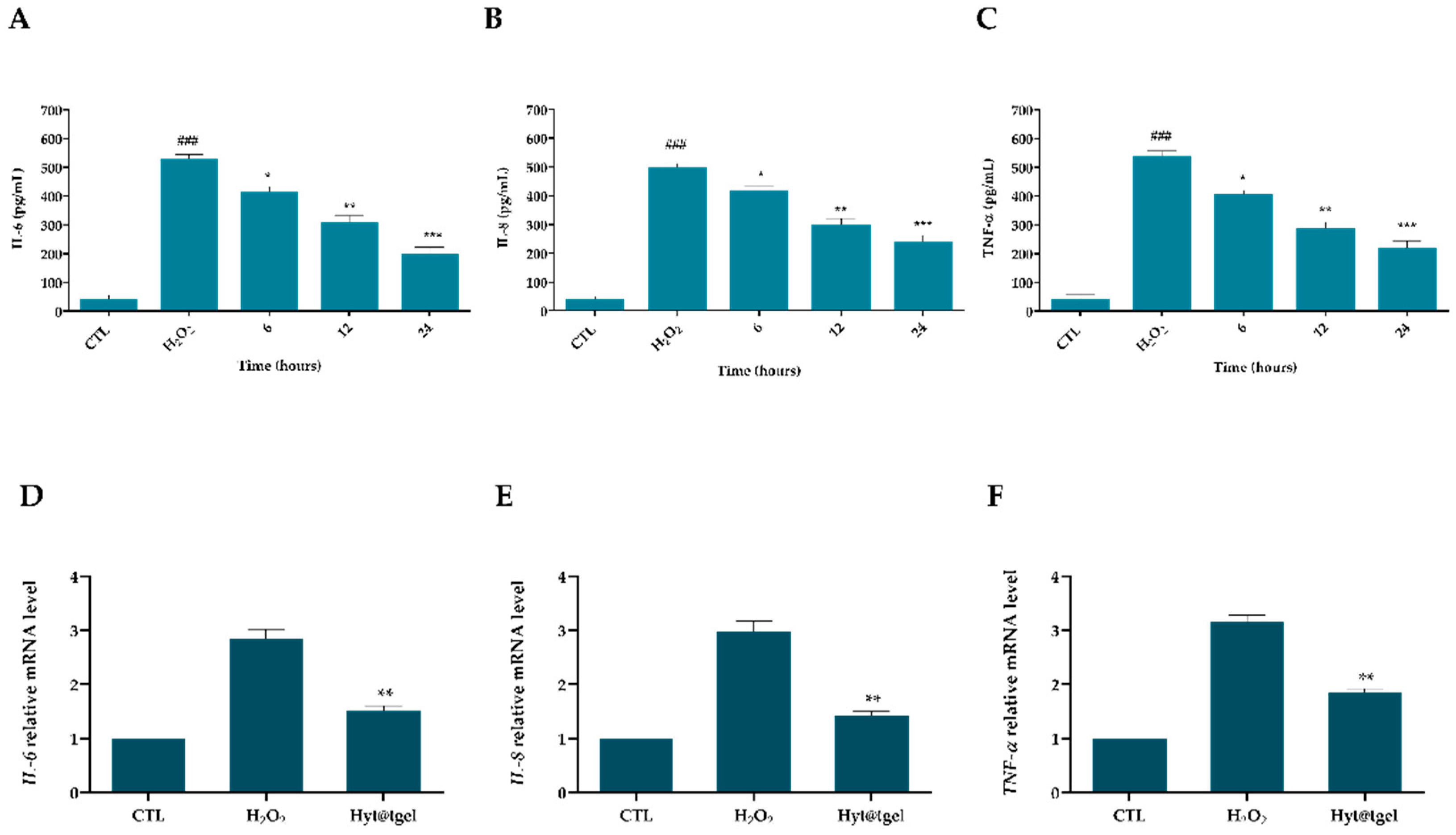
3.4. Hyt@tgel Suppresses Inflammatory Response in Chondrocytes
3.5. Hyt@tgel Protects against H2O2-Mediated Chondrocyte ECM Degradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roos, E.; Arden, N.K. Strategies for the prevention of knee osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 92–101. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, Z.; Sheng, P.; Mobasheri, A. The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res. Rev. 2021, 66, 101249. [Google Scholar] [CrossRef]
- Li, G.Y.; Yin, J.M.; Gao, J.J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.Q.; Zheng, M.H. Subchondral bone in osteoarthritis: Insight into risk factors and microstructural changes. Arthritis Res. Ther. 2013, 15, 223. [Google Scholar] [CrossRef] [Green Version]
- Martel-Pelletier, J.; Barr, A.; Cicuttini, F.; Conaghan, P.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.-P. Osteoarthritis. Nat. Rev. Dis. Prim. 2016, 2, 16072. [Google Scholar] [CrossRef] [Green Version]
- Ameye, L.G.; Chee, W.S. Osteoarthritis and nutrition. From nutraceuticals to functional foods: A systematic review of the scientific evidence. Arthritis Res. Ther. 2006, 8, R127. [Google Scholar] [CrossRef] [Green Version]
- Bijlsma, J.W.; Berenbaum, F.; Lafeber, F.P. Osteoarthritis: An update with relevance for clinical practice. Lancet 2011, 377, 2115–2126. [Google Scholar] [CrossRef]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 576–591. [Google Scholar] [CrossRef]
- Zahan, O.-M.; Serban, O.; Gherman, C.; Fodor, D. The evaluation of oxidative stress in osteoarthritis. Med. Pharm. Rep. 2020, 93, 12–22. [Google Scholar] [CrossRef]
- Bolduc, J.A.; Collins, J.A.; Loeser, R.F. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic. Biol. Med. 2019, 132, 73–82. [Google Scholar] [CrossRef]
- Ramasamy, T.S.; Yee, Y.M.; Khan, I.M. Chondrocyte Aging: The Molecular Determinants and Therapeutic Opportunities. Front. Cell Dev. Biol. 2021, 9, 625497. [Google Scholar] [CrossRef]
- Fernandes, L.; Hagen, K.B.; Bijlsma, J.W.J.; Andreassen, O.; Christensen, P.; Conaghan, P.; Doherty, M.; Geenen, R.; Hammond, A.; Kjeken, I.; et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann. Rheum. Dis. 2013, 72, 1125–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miles, E.A.; Zoubouli, P.; Calder, P. Differential anti-inflammatory effects of phenolic compounds from extra virgin olive oil identified in human whole blood cultures. Nutrition 2005, 21, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Finicelli, M.; Squillaro, T.; Di Cristo, F.; Di Salle, A.; Melone, M.A.B.; Galderisi, U.; Peluso, G. Metabolic syndrome, Mediterranean diet, and polyphenols: Evidence and perspectives. J. Cell. Physiol. 2019, 234, 5807–5826. [Google Scholar] [CrossRef] [PubMed]
- Finicelli, M.; Squillaro, T.; Galderisi, U.; Peluso, G. Polyphenols, the Healthy Brand of Olive Oil: Insights and Perspectives. Nutrients 2021, 13, 3831. [Google Scholar] [CrossRef]
- Valentino, A.; Di Cristo, F.; Bosetti, M.; Amaghnouje, A.; Bousta, D.; Conte, R.; Calarco, A. Bioactivity and Delivery Strategies of Phytochemical Compounds in Bone Tissue Regeneration. Appl. Sci. 2021, 11, 5122. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- De Luca, I.; Di Cristo, F.; Valentino, A.; Peluso, G.; Di Salle, A.; Calarco, A. Food-Derived Bioactive Molecules from Mediterranean Diet: Nanotechnological Approaches and Waste Valorization as Strategies to Improve Human Wellness. Polymers 2022, 14, 1726. [Google Scholar] [CrossRef]
- Visioli, F.; De La Lastra, C.A.; Andres-Lacueva, C.; Aviram, M.; Calhau, C.; Cassano, A.; D’Archivio, M.; Faria, A.; Favé, G.; Fogliano, V.; et al. Polyphenols and Human Health: A Prospectus. Crit. Rev. Food Sci. Nutr. 2011, 51, 524–546. [Google Scholar] [CrossRef]
- Silva, A.F.R.; Resende, D.; Monteiro, M.; Coimbra, M.A.; Silva, A.M.S.; Cardoso, S.M. Application of Hydroxytyrosol in the Functional Foods Field: From Ingredient to Dietary Supplements. Antioxidants 2020, 9, 1246. [Google Scholar] [CrossRef]
- Serra, A.; Rubió, L.; Borràs, X.; Macià, A.; Romero, M.-P.; Motilva, M.-J. Distribution of olive oil phenolic compounds in rat tissues after administration of a phenolic extract from olive cake. Mol. Nutr. Food Res. 2012, 56, 486–496. [Google Scholar] [CrossRef]
- Yonezawa, Y.; Miyashita, T.; Nejishima, H.; Takeda, Y.; Imai, K.; Ogawa, H. Anti-inflammatory effects of olive-derived hydroxytyrosol on lipopolysaccharide-induced inflammation in RAW264.7 cells. J. Veter. Med. Sci. 2018, 80, 1801–1807. [Google Scholar] [CrossRef] [Green Version]
- Di Meo, F.; Valentino, A.; Petillo, O.; Peluso, G.; Filosa, S.; Crispi, S. Bioactive Polyphenols and Neuromodulation: Molecular Mechanisms in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 2564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuccelli, R.; Fabiani, R.; Rosignoli, P. Hydroxytyrosol Exerts Anti-Inflammatory and Anti-Oxidant Activities in a Mouse Model of Systemic Inflammation. Molecules 2018, 23, 3212. [Google Scholar] [CrossRef] [Green Version]
- Cetrullo, S.; D’Adamo, S.; Guidotti, S.; Borzì, R.M.; Flamigni, F. Hydroxytyrosol prevents chondrocyte death under oxidative stress by inducing autophagy through sirtuin 1-dependent and -independent mechanisms. Biochi. Biophys. Acta (BBA) 2016, 1860, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Facchini, A.; Cetrullo, S.; D’Adamo, S.; Guidotti, S.; Minguzzi, M.; Facchini, A.; Borzì, R.M.; Flamigni, F. Hydroxytyrosol Prevents Increase of Osteoarthritis Markers in Human Chondrocytes Treated with Hydrogen Peroxide or Growth-Related Oncogene α. PLoS ONE 2014, 9, e109724. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Detsi, A.; Kavetsou, E.; Kostopoulou, I.; Pitterou, I.; Pontillo, A.R.N.; Tzani, A.; Christodoulou, P.; Siliachli, A.; Zoumpoulakis, P. Nanosystems for the Encapsulation of Natural Products: The Case of Chitosan Biopolymer as a Matrix. Pharmaceutics 2020, 12, 669. [Google Scholar] [CrossRef]
- Le, H.; Xu, W.; Zhuang, X.; Chang, F.; Wang, Y.; Ding, J. Mesenchymal stem cells for cartilage regeneration. J. Tissue Eng. 2020, 11, 2041731420943839. [Google Scholar] [CrossRef]
- Zha, K.; Sun, Z.; Yang, Y.; Chen, M.; Gao, C.; Fu, L.; Li, H.; Sui, X.; Guo, Q.; Liu, S. Recent Developed Strategies for Enhancing Chondrogenic Differentiation of MSC: Impact on MSC-Based Therapy for Cartilage Regeneration. Stem Cells Int. 2021, 2021, 8830834. [Google Scholar] [CrossRef]
- Meng, F.G.; Zhang, Z.Q.; Huang, G.X.; Chen, W.S.; Zhang, Z.J.; He, A.S.; Liao, W.M. Chondrogenesis of mesenchymal stem cells in a novel hyaluronate-collagen-tricalcium phosphate scaffolds for knee repair. Eur. Cells Mater. 2016, 31, 79–94. [Google Scholar] [CrossRef]
- Arrich, J.; Piribauer, F.; Mad, P.; Schmid, D.; Klaushofer, K.; Müllner, M. Intra-articular hyaluronic acid for the treatment of osteoarthritis of the knee: Systematic review and meta-analysis. Can. Med. Assoc. J. 2005, 172, 1039–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichenbach, S.; Blank, S.; Rutjes, A.W.S.; Shang, A.; King, E.A.; Dieppe, P.A.; Jüni, P.; Trelle, S. Hylan versus hyaluronic acid for osteoarthritis of the knee: A systematic review and meta-analysis. Arthritis Rheum. 2007, 57, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.-S.; Park, W.; Park, H.; Lee, D.-K.; Na, K. Thermo-sensitive injectable hydrogel based on the physical mixing of hyaluronic acid and Pluronic F-127 for sustained NSAID delivery. Carbohydr. Polym. 2017, 156, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Conte, R.; Valentino, A.; Di Cristo, F.; Peluso, G.; Cerruti, P.; Di Salle, A.; Calarco, A. Cationic Polymer Nanoparticles-Mediated Delivery of miR-124 Impairs Tumorigenicity of Prostate Cancer Cells. Int. J. Mol. Sci. 2020, 21, 869. [Google Scholar] [CrossRef] [Green Version]
- Khattab, A.; Marzok, S.; Ibrahim, M. Development of optimized mucoadhesive thermosensitive pluronic based in situ gel for controlled delivery of Latanoprost: Antiglaucoma efficacy and stability approaches. J. Drug Deliv. Sci. Technol. 2019, 53, 101134. [Google Scholar] [CrossRef]
- Amaghnouje, A.; Mechchate, H.; Es-Safi, I.; Boukhira, S.; Aliqahtani, A.S.; Noman, O.M.; Nasr, F.A.; Conte, R.; Calarco, A.; Bousta, D. Subacute Assessment of the Toxicity and Antidepressant-Like Effects of Origanum majorana L. Polyphenols in Swiss Albino Mice. Molecules 2020, 25, 5653. [Google Scholar] [CrossRef]
- Scuruchi, M.; D’Ascola, A.; Avenoso, A.; Mandraffino, G.; Campo, S.; Campo, G.M. Endocan, a novel inflammatory marker, is upregulated in human chondrocytes stimulated with IL-1 beta. Mol. Cell. Biochem. 2021, 476, 1589–1597. [Google Scholar] [CrossRef]
- Di Cristo, F.; Valentino, A.; De Luca, I.; Peluso, G.; Bonadies, I.; Calarco, A.; Di Salle, A. PLA Nanofibers for Microenvironmental-Responsive Quercetin Release in Local Periodontal Treatment. Molecules 2022, 27, 2205. [Google Scholar] [CrossRef]
- Musto, P.; Calarco, A.; Pannico, M.; La Manna, P.; Margarucci, S.; Tafuri, A.; Peluso, G. Hyperspectral Raman imaging of human prostatic cells: An attempt to differentiate normal and malignant cell lines by univariate and multivariate data analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 476–488. [Google Scholar] [CrossRef]
- Calarco, A.; Di Salle, A.; Tammaro, L.; De Luca, I.; Mucerino, S.; Petillo, O.; Riccitiello, F.; Vittoria, V.; Peluso, G. Long-Term Fluoride Release from Dental Resins Affects STRO-1+ Cell Behavior. J. Dent. Res. 2015, 94, 1099–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, B.; Chen, D.; Zhang, J.; Hu, S.; Jin, H.; Tong, P. Osteoarthritis Pathogenesis: A Review of Molecular Mechanisms. Calcif. Tissue Res. 2014, 95, 495–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anandacoomarasamy, A.; March, L. Current evidence for osteoarthritis treatments. Ther. Adv. Musculoskelet. Dis. 2010, 2, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Nowaczyk, A.; Szwedowski, D.; Dallo, I.; Nowaczyk, J. Overview of First-Line and Second-Line Pharmacotherapies for Osteoarthritis with Special Focus on Intra-Articular Treatment. Int. J. Mol. Sci. 2022, 23, 1566. [Google Scholar] [CrossRef]
- Menazea, A.; Ahmed, M. Wound healing activity of Chitosan/Polyvinyl Alcohol embedded by gold nanoparticles prepared by nanosecond laser ablation. J. Mol. Struct. 2020, 1217, 128401. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Rathore, P.; Mahor, A.; Jain, S.; Haque, A.; Kesharwani, P. Formulation development, in vitro and in vivo evaluation of chitosan engineered nanoparticles for ocular delivery of insulin. RSC Adv. 2020, 10, 43629–43639. [Google Scholar] [CrossRef]
- Campos, E.V.R.; Proença, P.L.F.; da Costa, T.G.; de Lima, R.; Fraceto, L.F.; de Araujo, D.R. Using Chitosan-Coated Polymeric Nanoparticles-Thermosensitive Hydrogels in association with Limonene as Skin Drug Delivery Strategy. BioMed Res. Int. 2022, 2022, 9165443. [Google Scholar] [CrossRef]
- Conte, R.; De Luise, A.; Valentino, A.; Di Cristo, F.; Petillo, O.; Riccitiello, F.; Di Salle, A.; Calarco, A.; Peluso, G. Chapter 10—Hydrogel Nanocomposite Systems: Characterization and Application in Drug-Delivery Systems. In Nanocarriers for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Chen, Z.-P.; Liu, W.; Liu, D.; Xiao, Y.-Y.; Chen, H.-X.; Chen, J.; Li, W.; Cai, H.; Li, W.; Cai, B.-C.; et al. Development of brucine-loaded microsphere/thermally responsive hydrogel combination system for intra-articular administration. J. Control. Release 2012, 162, 628–635. [Google Scholar] [CrossRef]
- Wang, S.; Wei, X.; Sun, X.; Chen, C.; Zhou, J.; Zhang, G.; Wu, H.; Guo, B.; Wei, L. A novel therapeutic strategy for cartilage diseases based on lipid nanoparticle-RNAi delivery system. Int. J. Nanomed. 2018, 13, 617–631. [Google Scholar] [CrossRef] [Green Version]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef] [PubMed]
- Amrati, F.E.-Z.; Bourhia, M.; Slighoua, M.; Ibnemoussa, S.; Bari, A.; Ullah, R.; Amaghnouje, A.; Di Cristo, F.; El Mzibri, M.; Calarco, A.; et al. Phytochemical Study on Antioxidant and Antiproliferative Activities of Moroccan Caralluma europaea Extract and Its Bioactive Compound Classes. Evid. Based Complement. Altern. Med. 2020, 2020, 8409718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuo, Q.; Yang, W.; Chen, J.; Wang, Y. Metabolic syndrome meets osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Sarsour, E.H.; Kumar, M.G.; Kalen, A.L.; Goswami, M.; Buettner, G.R.; Goswami, P.C. MnSOD activity regulates hydroxytyrosol-induced extension of chronological lifespan. AGE 2011, 34, 95–109. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Choi, Y.; Park, T. Hepatoprotective effect of oleuropein in mice: Mechanisms uncovered by gene expression profiling. Biotechnol. J. 2010, 5, 950–960. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Khan, N.M.; Ahmad, I.; Haqqi, T.M. Parkin clearance of dysfunctional mitochondria regulates ROS levels and increases survival of human chondrocytes. Osteoarthr. Cartil. 2018, 26, 1087–1097. [Google Scholar] [CrossRef]
- Salgado, C.; Jordan, O.; Allémann, E. Osteoarthritis In Vitro Models: Applications and Implications in Development of Intra-Articular Drug Delivery Systems. Pharmaceutics 2021, 13, 60. [Google Scholar] [CrossRef]
- Shi, Q.; Vaillancourt, F.; Côté, V.; Fahmi, H.; Lavigne, P.; Afif, H.; Di Battista, J.A.; Fernandes, J.C.; Benderdour, M. Alterations of metabolic activity in human osteoarthritic osteoblasts by lipid peroxidation end product 4-hydroxynonenal. Arthritis Res. Ther. 2006, 8, R159. [Google Scholar] [CrossRef] [Green Version]
- Henrotin, Y.; Kurz, B.; Aigner, T. Oxygen and reactive oxygen species in cartilage degradation: Friends or foes? Osteoarthr. Cartil. 2005, 13, 643–654. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Romero, C.; Calamia, V.; Mateos, J.; Carreira, V.; Martínez-Gomariz, M.; Fernández, M.; Blanco, F.J. Mitochondrial dysregulation of osteoarthritic human articular chondrocytes analyzed by proteomics: A decrease in mitochondrial superoxide dismutase points to a redox imbalance. Mol. Cell. Proteom. 2009, 8, 172–189. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.-P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, F.; Della Bella, E.; Cepollaro, S.; Brogini, S.; Martini, L.; Fini, M. Novel therapeutic targets in osteoarthritis: Narrative review on knock-out genes involved in disease development in mouse animal models. Cytotherapy 2016, 18, 593–612. [Google Scholar] [CrossRef] [PubMed]
- Ravalli, S.M.; Szychlinska, M.A.; Leonardi, R.M.; Musumeci, G. Recently highlighted nutraceuticals for preventive management of osteoarthritis. World J. Orthop. 2018, 9, 255–261. [Google Scholar] [CrossRef]
- Castrogiovanni, P.; Trovato, F.M.; Loreto, C.; Nsir, H.; Szychlinska, M.A.; Musumeci, G. Nutraceutical Supplements in the Management and Prevention of Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 2042. [Google Scholar] [CrossRef]
- Rosillo, M.; De La Lastra, C.A.; Castejón, M.L.; Montoya, T.; Cejudo-Guillén, M.; Sánchez-Hidalgo, M. Polyphenolic extract from extra virgin olive oil inhibits the inflammatory response in IL-1β-activated synovial fibroblasts. Br. J. Nutr. 2019, 121, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.-Y.; Pang, K.-L. Therapeutic Effects of Olive and Its Derivatives on Osteoarthritis: From Bench to Bedside. Nutrients 2017, 9, 1060. [Google Scholar] [CrossRef] [PubMed]
- Richard, N.; Arnold, S.; Hoeller, U.; Kilpert, C.; Wertz, K.; Schwager, J. Hydroxytyrosol Is the Major Anti-Inflammatory Compound in Aqueous Olive Extracts and Impairs Cytokine and Chemokine Production in Macrophages. Planta Med. 2011, 77, 1890–1897. [Google Scholar] [CrossRef] [Green Version]
- Mével, E.; Merceron, C.; Vinatier, C.; Krisa, S.; Richard, T.; Masson, M.; Lesoeur, J.; Hivernaud, V.; Gauthier, O.; Abadie, J.; et al. Olive and grape seed extract prevents post-traumatic osteoarthritis damages and exhibits in vitro anti IL-1β activities before and after oral consumption. Sci. Rep. 2016, 6, 33527. [Google Scholar] [CrossRef] [Green Version]
- Charlier, E.; Deroyer, C.; Ciregia, F.; Malaise, O.; Neuville, S.; Plener, Z.; Malaise, M.; de Seny, D. Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem. Pharmacol. 2019, 165, 49–65. [Google Scholar] [CrossRef]
- Musumeci, G.; Castrogiovanni, P.; Trovato, F.M.; Weinberg, A.M.; Al-Wasiyah, M.K.; Alqahtani, M.H.; Mobasheri, A. Biomarkers of Chondrocyte Apoptosis and Autophagy in Osteoarthritis. Int. J. Mol. Sci. 2015, 16, 20560–20575. [Google Scholar] [CrossRef] [Green Version]
- Musumeci, G.; Aiello, F.C.; Szychlinska, M.A.; Di Rosa, M.; Castrogiovanni, P.; Mobasheri, A. Osteoarthritis in the XXIst Century: Risk Factors and Behaviours that Influence Disease Onset and Progression. Int. J. Mol. Sci. 2015, 16, 6093–6112. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sampson, E.R.; Jin, H.; Li, J.; Ke, Q.H.; Im, H.-J.; Chen, D. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res. Ther. 2013, 15, R5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, J.; Zhang, G.; Li, X.; Qiu, X.; Ouyang, J.; Dai, J.; Min, S. Matrix Metalloproteinase 3: A Promoting and Destabilizing Factor in the Pathogenesis of Disease and Cell Differentiation. Front. Physiol. 2021, 12, 663978. [Google Scholar] [CrossRef]
- Ryu, J.-H.; Yang, S.; Shin, Y.; Rhee, J.; Chun, C.-H.; Chun, J.-S. Interleukin-6 plays an essential role in hypoxia-inducible factor 2α-induced experimental osteoarthritic cartilage destruction in mice. Arthritis Rheum. 2011, 63, 2732–2743. [Google Scholar] [CrossRef]
- Benya, P.; Padilla, S.R.; Nimni, M.E. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell 1978, 15, 1313–1321. [Google Scholar] [CrossRef]
- Kawaguchi, J.; Mee, P.; Smith, A. Osteogenic and chondrogenic differentiation of embryonic stem cells in response to specific growth factors. Bone 2005, 36, 758–769. [Google Scholar] [CrossRef]
- Setzu, A.; Lathia, J.D.; Zhao, C.; Wells, K.; Rao, M.S.; Ffrench-Constant, C.; Franklin, R.J.M. Inflammation stimulates myelination by transplanted oligodendrocyte precursor cells. Glia 2006, 54, 297–303. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, K.; Litherland, G.J.; Rai, T.S. Cellular senescence in osteoarthritis pathology. Aging Cell 2017, 16, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Brandl, A.; Hartmann, A.; Bechmann, V.; Graf, B.; Nerlich, M.; Angele, P. Oxidative stress induces senescence in chondrocytes. J. Orthop. Res. 2011, 29, 1114–1120. [Google Scholar] [CrossRef]
- Rim, Y.A.; Nam, Y.; Ju, J.H. The Role of Chondrocyte Hypertrophy and Senescence in Osteoarthritis Initiation and Progression. Int. J. Mol. Sci. 2020, 21, 2358. [Google Scholar] [CrossRef] [Green Version]
- Vinatier, C.; Dominguez, E.; Guicheux, J.; Caramés, B. Role of the Inflammation-Autophagy-Senescence Integrative Network in Osteoarthritis. Front. Physiol. 2018, 9, 706. [Google Scholar] [CrossRef] [PubMed]
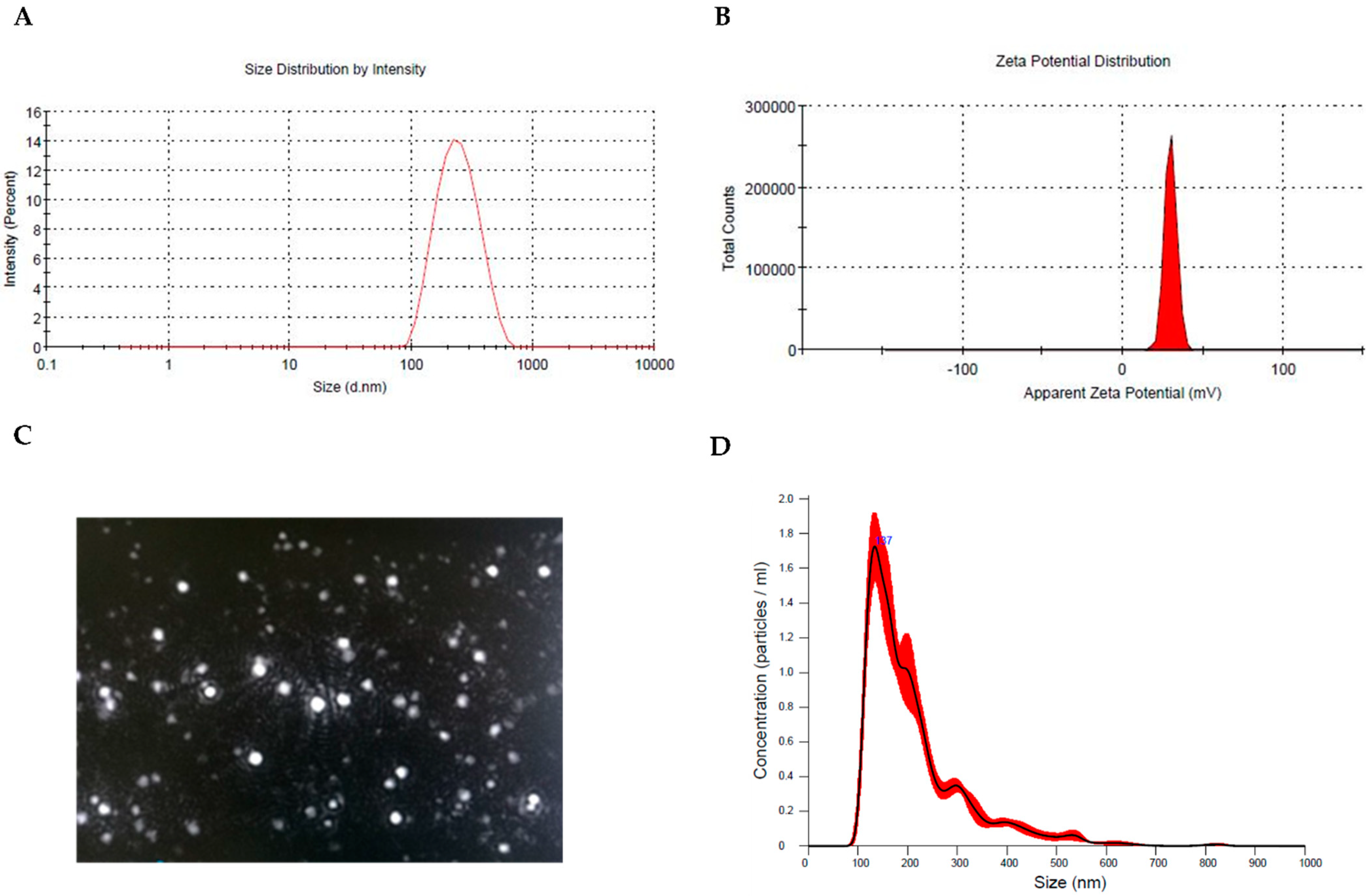

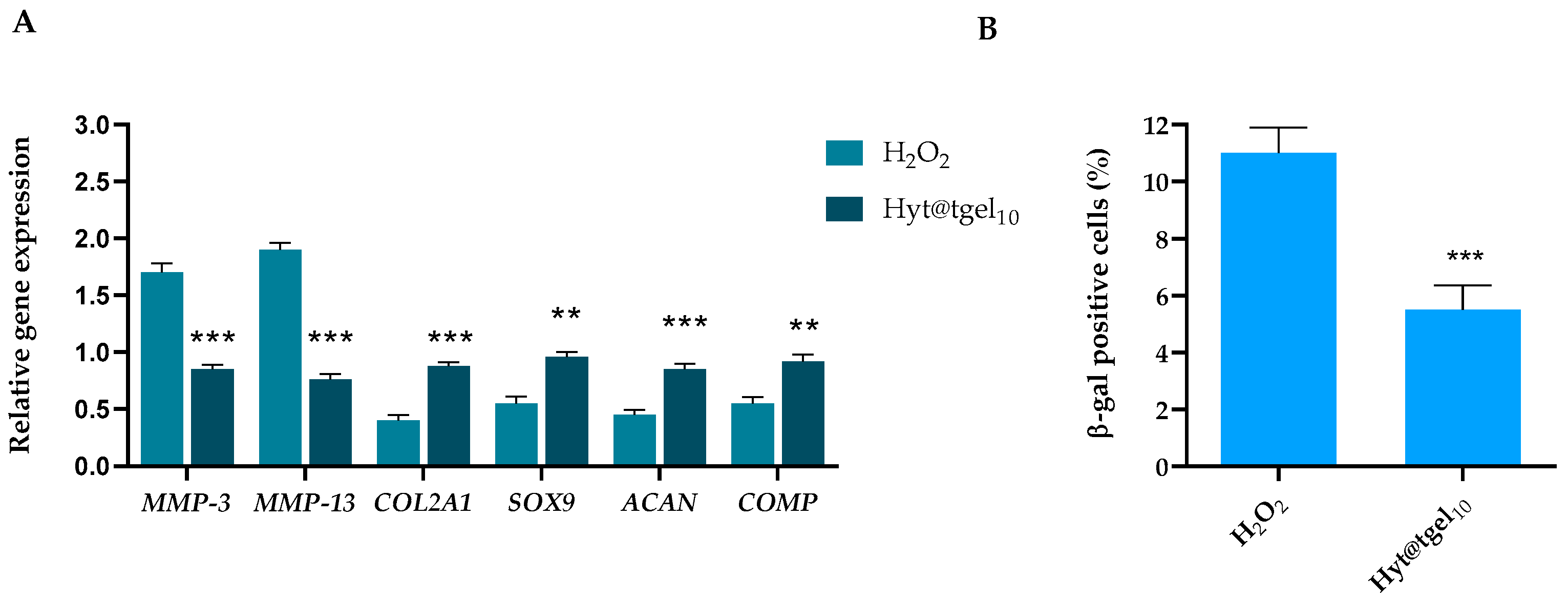
| Gene | Accession Number | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|---|
| COL2A1 | NM_001844.5 | CTGGTGTGAAGGGTGAGAGT | AGTCCGTCCTCTTTCACCAG |
| ACAN | NM_001135.4 | TCCCCAACAGATGCTTCCAT | GTACTTGTTCCAGCCCTCCT |
| SOX9 | NM_000346.4 | CCGCTCACAGTACGACTACA | GTGAAGGTGGAGTAGAGGCC |
| COMP | NM_000095.4 | CCTTCAATGGCGTGGACTTC | TGACCACGTAGAAGCTGGAG |
| MMP-3 | NM_002422.5 | CCTCTGATGGCCCAGAATTGA | GAAATTGGCCACTCCCTGGGT |
| MMP-13 | NM_002427.4 | GTCCAGGAGATGAAGACCCC | CTCGGAGACTGGTAATGGCA |
| SOD2 | NM_000636.4 | CTGGACAAACCTCAGCCCTA | TGATGGCTTCCAGCAACTC |
| IL-6 | NM_000600.5 | CGCCTTCGGTCCAGTTGCC | GCCAGTGCCTCTTTGCTGCTTT |
| IL-8 | NM_000584.4 | CTCTTGGCAGCCTTCCTGATTTC | TTTTCCTTGGGGTCCAGACAGAG |
| TNF-α | NM_000594.4 | AACATCCAACCTTCCCAAACGC | TGGTCTCCAGATTCCAGATGTCAGG |
| ACTB | NM_001101.5 | ACTCTTCCAGCCTTCCTTCC | CGTACAGGTCTTTGCGGATG |
| Chitosan (mg/mL) | CS:TPP Mass Ratio | Size (nm ± SD) | PDI (mV ± SD) | EE (% ± SD) |
|---|---|---|---|---|
| 0.1 | 1:1 | 510.14 ± 13.21 | 0.28 ± 0.03 | 18.31 ± 1.23 |
| 0.1 | 5:1 | 365.23 ± 9.01 | 0.15 ± 0.04 | 36.71 ± 1.26 |
| 0.1 | 10:1 | 298.73 ± 9.06 | 0.26 ± 0.02 | 39.27 ± 2.14 |
| 0.5 | 1:1 | 330.33 ± 7.52 | 0.17 ± 0.01 | 28.96 ± 1.34 |
| 0.5 | 5:1 | 279.61 ± 0.72 | 0.23 ± 0.04 | 41.25 ± 2.41 |
| 0.5 | 10:1 | 219.63 ± 0.46 | 0.21 ± 0.03 | 53.37 ± 2.62 |
| 1 | 1:1 | 348.16 ±12.64 | 0.14 ± 0.02 | 29.81 ± 1.29 |
| 1 | 5:1 | 224.43 ± 0.46 | 0.25 ± 0.01 | 48.57 ± 1.85 |
| 1 | 10:1 | 137.56 ± 3.13 | 0.16 ± 0.03 | 74.18 ± 3.16 |
| Hyt@tgel10 Before Storage | Hyt@tgel10 after 14-Day Storage at 4 ± 1 °C | Hyt@tgel10 after 14-Day Storage at 25 ± 1 °C | |
|---|---|---|---|
| Average particle size (nm) | 137.56 ± 3.13 | 135.32 ± 2.56 nm | 139.00 ± 6.53 nm |
| PDI | 0.16 ± 0.03 | 0.15 ± 0.02 | 0.18 ± 0.05 |
| Entrapment efficiency (% EE) | 74.18 ± 3.16 | 75.88 ± 4.13 | 77.22 ± 5.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentino, A.; Conte, R.; De Luca, I.; Di Cristo, F.; Peluso, G.; Bosetti, M.; Calarco, A. Thermo-Responsive Gel Containing Hydroxytyrosol-Chitosan Nanoparticles (Hyt@tgel) Counteracts the Increase of Osteoarthritis Biomarkers in Human Chondrocytes. Antioxidants 2022, 11, 1210. https://doi.org/10.3390/antiox11061210
Valentino A, Conte R, De Luca I, Di Cristo F, Peluso G, Bosetti M, Calarco A. Thermo-Responsive Gel Containing Hydroxytyrosol-Chitosan Nanoparticles (Hyt@tgel) Counteracts the Increase of Osteoarthritis Biomarkers in Human Chondrocytes. Antioxidants. 2022; 11(6):1210. https://doi.org/10.3390/antiox11061210
Chicago/Turabian StyleValentino, Anna, Raffaele Conte, Ilenia De Luca, Francesca Di Cristo, Gianfranco Peluso, Michela Bosetti, and Anna Calarco. 2022. "Thermo-Responsive Gel Containing Hydroxytyrosol-Chitosan Nanoparticles (Hyt@tgel) Counteracts the Increase of Osteoarthritis Biomarkers in Human Chondrocytes" Antioxidants 11, no. 6: 1210. https://doi.org/10.3390/antiox11061210
APA StyleValentino, A., Conte, R., De Luca, I., Di Cristo, F., Peluso, G., Bosetti, M., & Calarco, A. (2022). Thermo-Responsive Gel Containing Hydroxytyrosol-Chitosan Nanoparticles (Hyt@tgel) Counteracts the Increase of Osteoarthritis Biomarkers in Human Chondrocytes. Antioxidants, 11(6), 1210. https://doi.org/10.3390/antiox11061210








