In Vivo Assessment of Antioxidant Potential of Human Milk Treated by Holder Pasteurization or High Hydrostatic Pressure Processing: A Preliminary Study on Intestinal and Hepatic Markers in Adult Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Milk Collection and HoP and HHP Processing
2.2. Quantification of H2O2 and Antioxidants in Milk Samples
2.3. Mice
2.4. Gene Expression
2.5. Statistics
3. Results
3.1. Concentrations of Antioxidants, H2O2 Levels and Total Antioxidant Capacities of Raw DM and HoP- and HHP-DM
3.1.1. Concentrations of Antioxidants
3.1.2. H2O2 Concentrations and Total Antioxidant Capacities of Raw-, HoP- and HHP-DM
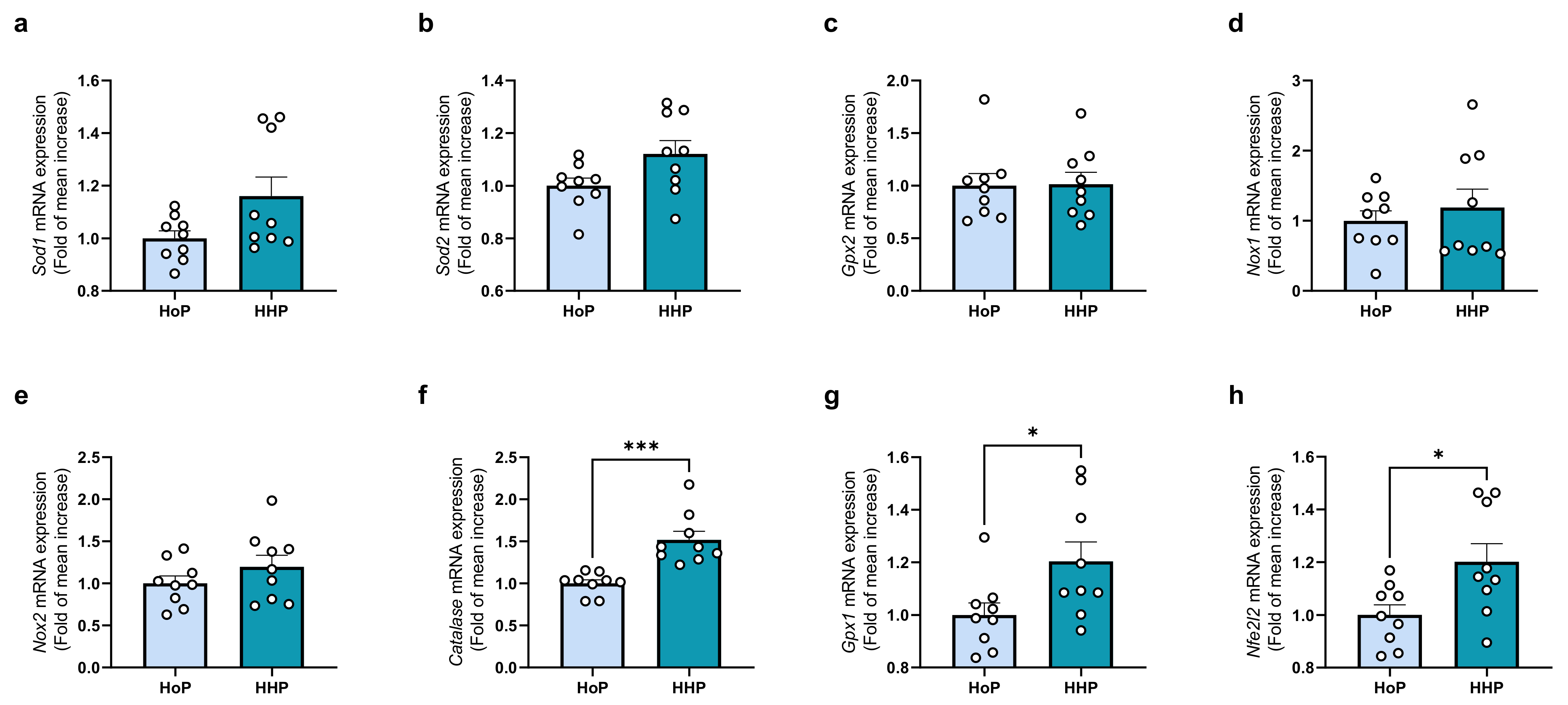
3.2. Effect of a Chronic Oral Treatment of Mice with HoP- and HHP-DM on the Gene Expression Level of Some Markers of OS in the Ileum
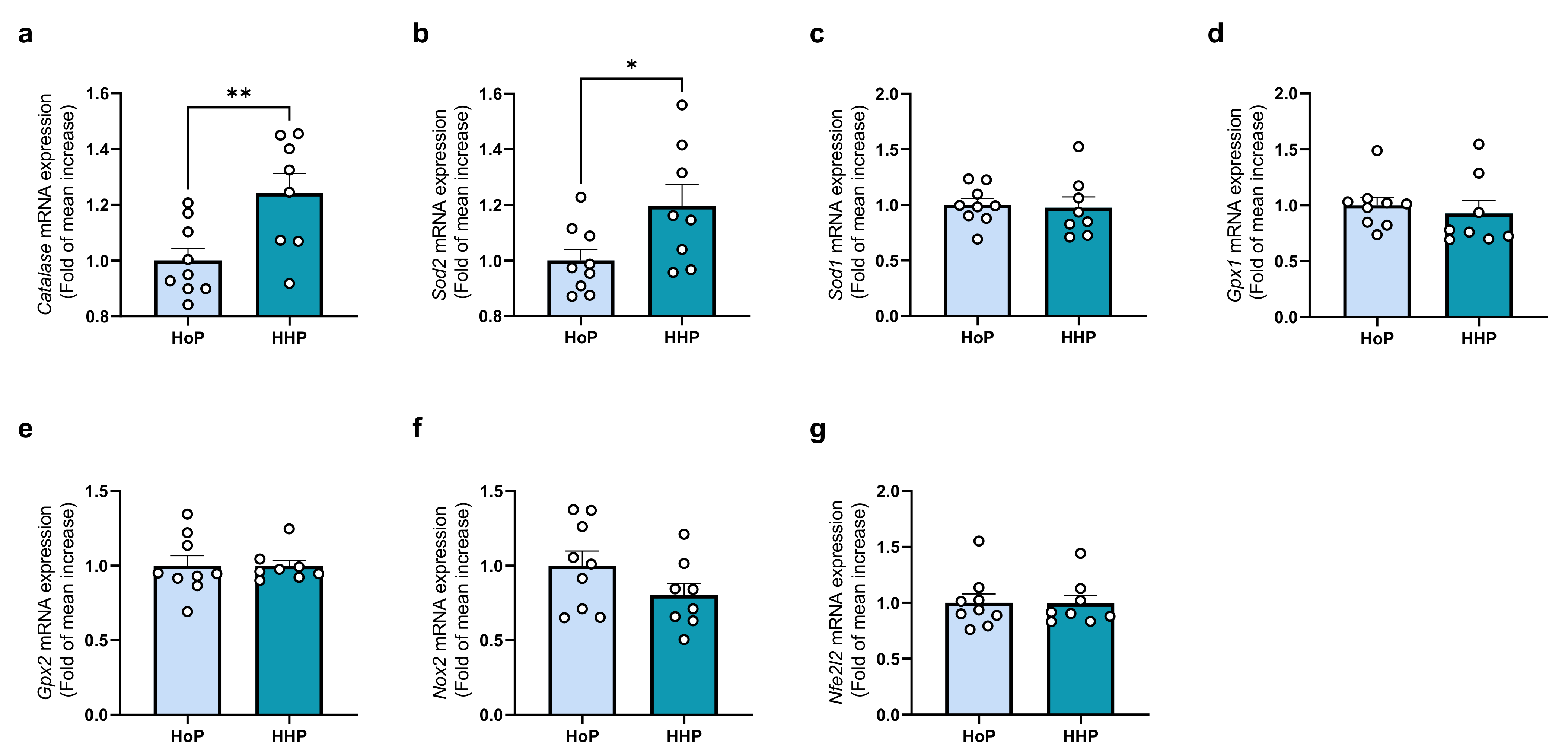
3.3. Effect of a Chronic Oral Treatment of Mice with HoP- and HHP-DM on the Gene-Expression Level of Some Markers of OS in the Liver
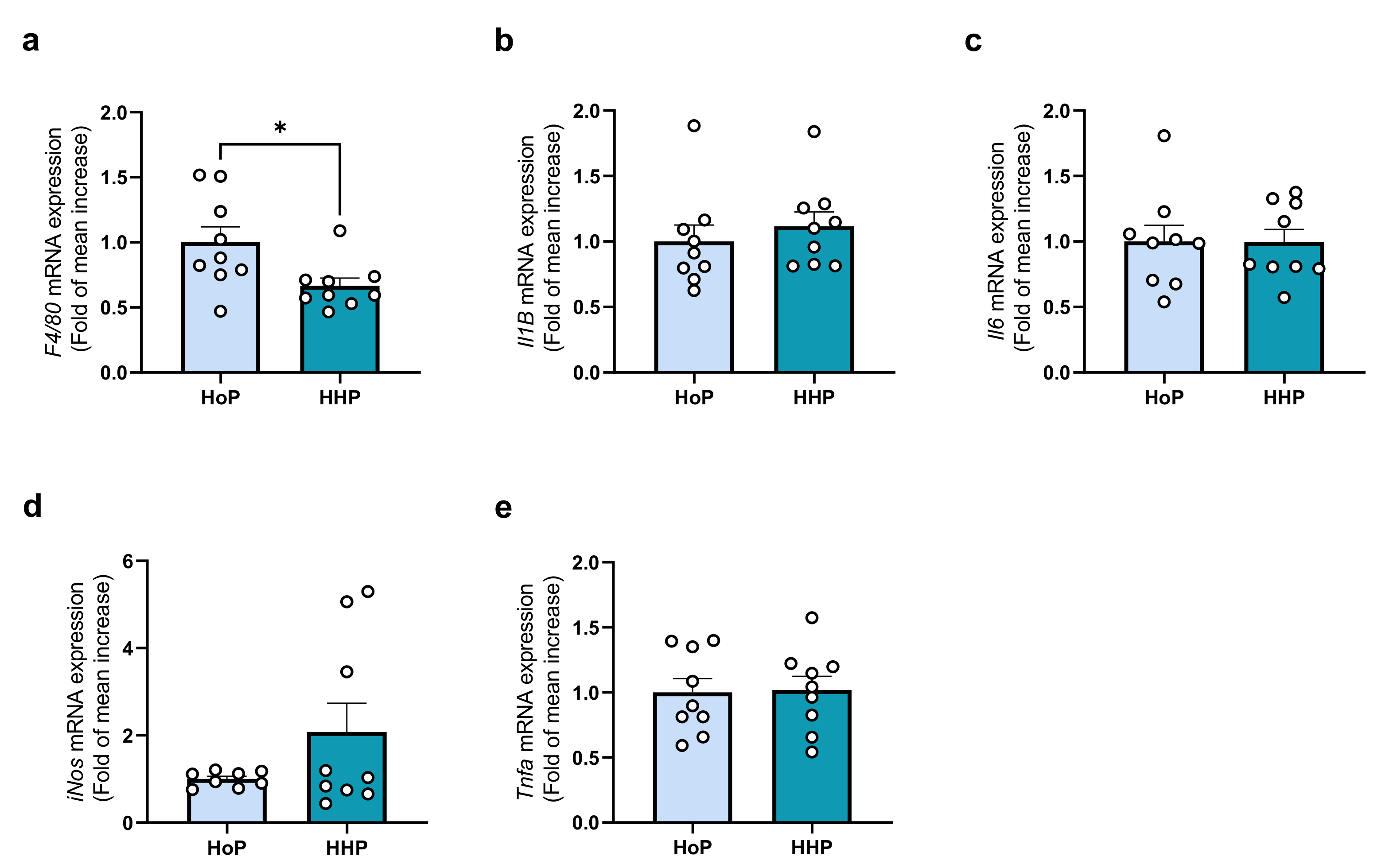
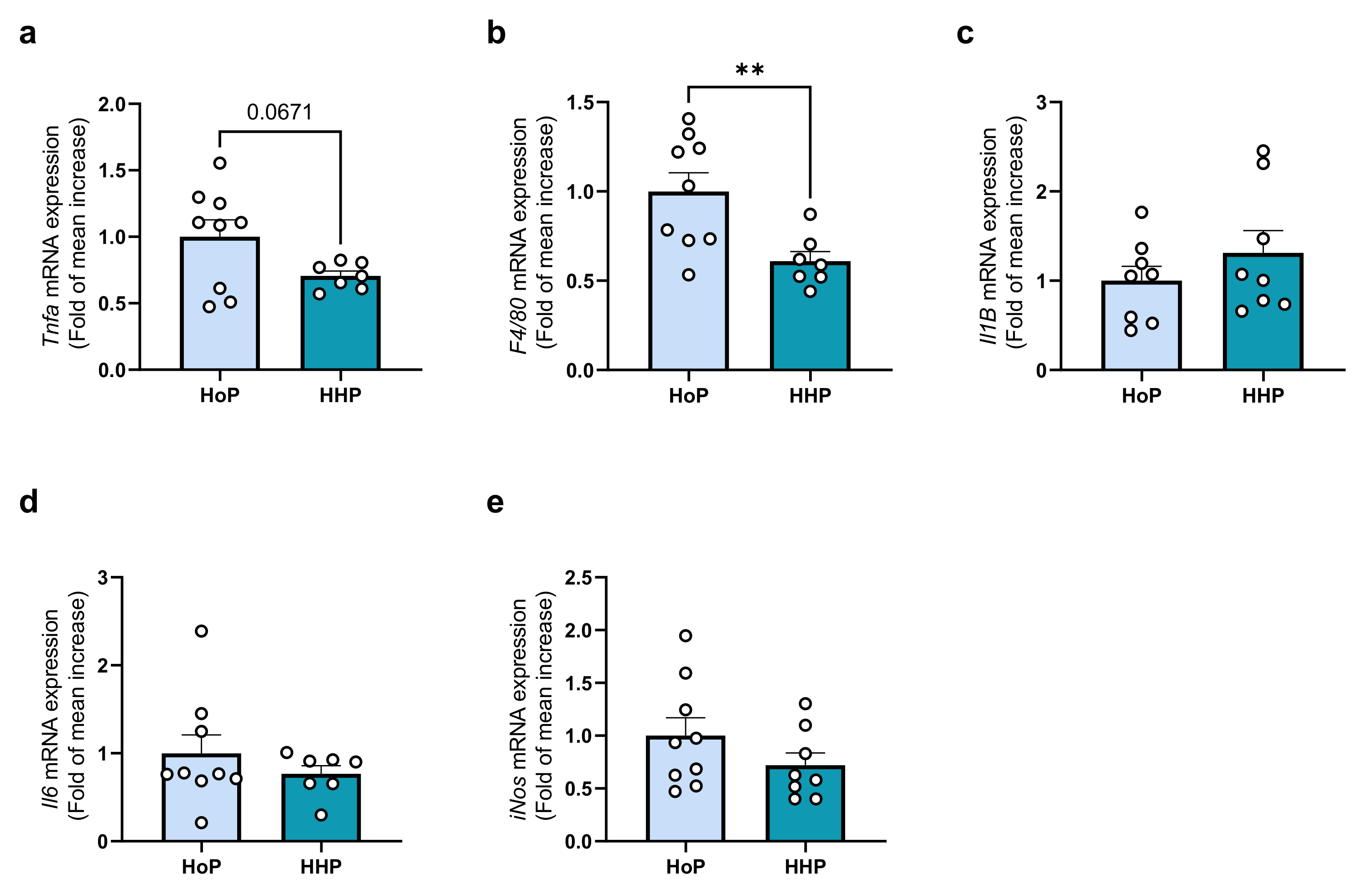
3.4. Effect of a Chronic Oral Treatment of Mice with HoP- and HHP-DM on the Gene-Expression Level of Some Markers of Inflammation in the Ileum and Liver
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cannavò, L.; Perrone, S.; Viola, V.; Marseglia, L.; di Rosa, G.; Gitto, E. Oxidative Stress and Respiratory Diseases in Preterm Newborns. Int. J. Mol. Sci. 2021, 22, 12504. [Google Scholar] [CrossRef] [PubMed]
- Negro, S.; Boutsikou, T.; Briana, D.D.; Tataranno, M.L.; Longini, M.; Proietti, F.; Bazzini, F.; Dani, C.; Malamitsi-Puchner, A.; Buonocore, G.; et al. Maternal Obesity and Perinatal Oxidative Stress: The Strength of the Association. J. Biol. Regul. Homeost. Agents 2017, 31, 221–227. [Google Scholar] [PubMed]
- Gitto, E.; Reiter, R.J.; Karbownik, M.; Tan, D.X.; Gitto, P.; Barberi, S.; Barberi, I. Causes of Oxidative Stress in the Pre- and Perinatal Period. Biol. Neonate 2002, 81, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Robbins, M.E.; Revhaug, C.; Saugstad, O.D. Oxygen Radical Disease in the Newborn, Revisited: Oxidative Stress and Disease in the Newborn Period. Free. Radic. Biol. Med. 2019, 142, 61–72. [Google Scholar] [CrossRef]
- Hackam, D.J.; Sodhi, C.P. Bench to Bedside—New Insights into the Pathogenesis of Necrotizing Enterocolitis. Nat. Rev. Gastroenterol. Hepatol. 2022. [Google Scholar] [CrossRef]
- De Waard, M.; Li, Y.; Zhu, Y.; Ayede, A.I.; Berrington, J.; Bloomfield, F.H.; Busari, O.O.; Cormack, B.E.; Embleton, N.D.; van Goudoever, J.B.; et al. Time to Full Enteral Feeding for Very Low-Birth-Weight Infants Varies Markedly Among Hospitals Worldwide but may not be Associated with Incidence of Necrotizing Enterocolitis: The NEOMUNE-NeoNutriNet Cohort Study. J. Parenter. Enter. Nutr. 2019, 43, 658–667. [Google Scholar] [CrossRef]
- Simon-szabo, Z.; Fogarasi, E.; Nemes-Nagy, E.; Denes, L.; Croitoru, M.; Szabo, B. Oxidative Stress and Peripartum Outcomes (Review). Exp. Ther. Med. 2021, 22, 1–6. [Google Scholar] [CrossRef]
- Moreira-Monteagudo, M.; Leirós-Rodríguez, R.; Marqués-Sánchez, P. Effects of Formula Milk Feeding in Premature Infants: A Systematic Review. Children 2022, 9, 150. [Google Scholar] [CrossRef]
- Picaud, J.C.; Buffin, R. Human Milk—Treatment and Quality of Banked Human Milk. Clin. Perinatol. 2017, 44, 95–119. [Google Scholar] [CrossRef]
- Demazeau, G.; Plumecocq, A.; Lehours, P.; Martin, P.; Couëdelo, L.; Billeaud, C. A New High Hydrostatic Pressure Process to Assure the Microbial Safety of Human Milk While Preserving the Biological Activity of Its Main Components. Front. Public Health 2018, 6, 1–8. [Google Scholar] [CrossRef]
- Wesolowska, A.; Sinkiewicz-Darol, E.; Barbarska, O.; Strom, K.; Rutkowska, M.; Karzel, K.; Rosiak, E.; Oledzka, G.; Orczyk-Pawilowicz, M.; Rzoska, S.; et al. New Achievements in High-Pressure Processing to Preserve Human Milk Bioactivity. Front. Pediatrics 2018, 6, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marousez, L.; Tran, L.; Micours, E.; de Lamballerie, M.; Gottrand, F.; Pierrat, V.; Eberlé, D.; Ley, D.; Lesage, J. Metabolic Hormones in Human Breast Milk Are Preserved by High Hydrostatic Pressure Processing but Reduced by Holder Pasteurization. Food Chem. 2022, 377, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Marousez, L.; Sprenger, N.; de Lamballerie, M.; Jaramillo-Ortiz, S.; Tran, L.; Micours, E.; Gottrand, F.; Howsam, M.; Tessier, F.J.; Ley, D.; et al. High Hydrostatic Pressure Processing of Human Milk Preserves Milk Oligosaccharides and Avoids Formation of Maillard Reaction Products. Clin. Nutr. 2022, 41, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Juncker, H.G.; Ruhé, E.J.M.; Burchell, G.L.; van den Akker, C.H.P.; Korosi, A.; van Goudoever, J.B.; van Keulen, B.J. The Effect of Pasteurization on the Antioxidant Properties of Human Milk: A Literature Review. Antioxidants 2021, 10, 1737. [Google Scholar] [CrossRef]
- Yeap, S.K.; Beh, B.K.; Ali, N.M.; Yusof, H.M.; Ho, W.Y.; Koh, S.P.; Alitheen, N.B.; Long, K. Antistress and Antioxidant Effects of Virgin Coconut Oil in Vivo. Exp. Ther. Med. 2015, 9, 39–42. [Google Scholar] [CrossRef] [Green Version]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant Activity of Proteins and Peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef]
- Pincemail, J.; Kaci, M.-M.; Kevers, C.; Tabart, J.; Elle, R.E.; Meziane, S. PAOT-Liquid® Technology: An Easy Electrochemical Method for Evaluating Antioxidant Capacity of Wines. Diseases 2019, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Drougard, A.; Duparc, T.; Brenachot, X.; Carneiro, L.; Gouazé, A.; Fournel, A.; Geurts, L.; Cadoudal, T.; Prats, A.C.; Pénicaud, L.; et al. Hypothalamic Apelin/Reactive Oxygen Species Signaling Controls Hepatic Glucose Metabolism in the Onset of Diabetes. Antioxid. Redox Signal. 2014, 20, 557–573. [Google Scholar] [CrossRef] [Green Version]
- Abot, A.; Wemelle, E.; Laurens, C.; Paquot, A.; Pomie, N.; Carper, D.; Bessac, A.; Mas Orea, X.; Fremez, C.; Fontanie, M.; et al. Identification of New Enterosynes Using Prebiotics: Roles of Bioactive Lipids and Mu-Opioid Receptor Signalling in Humans and Mice. Gut 2021, 70, 1078–1087. [Google Scholar] [CrossRef]
- Chen, H.J.; Hsu, C.H.; Chiang, B.L. Serum Retinol Levels and Neonatal Outcomes in Preterm Infants. J. Formos. Med. Assoc. 2017, 116, 626–633. [Google Scholar] [CrossRef]
- Thompson, M.D.; Cooney, R.V. The Potential Physiological Role of γ-Tocopherol in Human Health: A Qualitative Review. Nutr. Cancer 2020, 72, 808–825. [Google Scholar] [CrossRef] [PubMed]
- Cieslak, M.; Ferreira, C.H.F.; Shifrin, Y.; Pan, J.; Belik, J. Human Milk H2O2 Content: Does It Benefit Preterm Infants? Pediatric Res. 2018, 83, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Wesolowska, A.; Sinkiewicz-Darol, E.; Barbarska, O.; Bernatowicz-Lojko, U.; Borszewska-Kornacka, M.K.; van Goudoever, J.B. Innovative Techniques of Processing Human Milk to Preserve Key Components. Nutrients 2019, 11, 1169. [Google Scholar] [CrossRef] [Green Version]
- Malinowska-Pańczyk, E.; Matusiak-Żyrowska, D.; Puta, M.; Kiełbratowska, B. The Effect of High Pressure and Subzero Temperature on the Microflora and Selected Components of Human Milk. In Proceedings of the 55th EHPRG Meeting: High Pressure Science and Technology, Poznan, Poland, 3 September 2017. [Google Scholar]
- Al-Shehri, S.S.; Knox, C.L.; Liley, H.G.; Cowley, D.M.; Wright, J.R.; Henman, M.G.; Hewavitharana, A.K.; Charles, B.G.; Shaw, P.N.; Sweeney, E.L.; et al. Breastmilk-Saliva Interactions Boost Innate Immunity by Regulating the Oral Microbiome in Early Infancy. PLoS ONE 2015, 10, e0135047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Hernández, S.; Esteban-Muñoz, A.; Samaniego-Sánchez, C.; Giménez-Martínez, R.; Miralles, B.; Olalla-Herrera, M. Study of the Phenolic Compound Profile and Antioxidant Activity of Human Milk from Spanish Women at Different Stages of Lactation: A Comparison with Infant Formulas. Food Res. Int. 2021, 141, 110149. [Google Scholar] [CrossRef] [PubMed]
- Chabert, P.; Auger, C.; Pincemail, J.; Schini-Kerth, V.B. Overview of Plant-Derived Antioxidants. In Systems Biology of Free Radicals and Antioxidants; Springer: Berlin/Heidelberg, Germany, 2012; pp. 4005–4022. [Google Scholar]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Wemelle, E.; Marousez, L.; de Lamballerie, M.; Knauf, C.; Lesage, J. High Hydrostatic Pressure Processing of Human Milk Increases Apelin and GLP-1 Contents to Modulate Gut Contraction and Glucose Metabolism in Mice Compared to Holder Pasteurization. Nutrients 2022, 14, 219. [Google Scholar] [CrossRef]
- Noyan-Ashraf, M.H.; Abdul Momen, M.; Ban, K.; Sadi, A.M.; Zhou, Y.Q.; Riazi, A.M.; Baggio, L.L.; Henkelman, R.M.; Husain, M.; Drucker, D.J. GLP-1R Agonist Liraglutide Activates Cytoprotective Pathways and Improves Outcomes after Experimental Myocardial Infarction in Mice. Diabetes 2009, 58, 975–983. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.M.; Gao, H.L.; Wang, Y.L.; Xu, Q.; Guo, C.Y. Attenuation of High Glucose-Induced Rat Cardiomyocyte Apoptosis by Exendin-4 via Intervention of HO-1/Nrf-2 and the PI3K/AKT Signaling Pathway. Chin. J. Physiol. 2017, 60, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Shi, J.; Zhao, L.; Guan, J.; Liu, F.; Huo, G.; Li, B. Lactobacillus Plantarum KLDS1.0344 and Lactobacillus Acidophilus KLDS1.0901 Mixture Prevents Chronic Alcoholic Liver Injury in Mice by Protecting the Intestinal Barrier and Regulating Gut Microbiota and Liver-Related Pathways. J. Agric. Food Chem. 2021, 69, 183–197. [Google Scholar] [CrossRef]
- Rastelli, M.; Cani, P.D.; Knauf, C. The Gut Microbiome Influences Host Endocrine Functions. Endocr. Rev. 2019, 40, 1271–1284. [Google Scholar] [CrossRef] [PubMed]

| Targeted Gene (Accession Number) | Forward Primer | Reverse Primer |
|---|---|---|
| Catalase (NM_009804.2) | TGAGAAGCCTAAGAACGCAATTC | CCCTTCGCAGCCATGTG |
| Sod1 (NM_011434.2) | GTGATTGGGATTGCGCAGTA | TGGTTTGAGGGTAGCAGATGAGT |
| Sod2 (NM_013671.3) | TTAACGCGCAGATCATGCA | GGTGGCGTTGAGATTGTTCA |
| Gpx1 (NM_001329528.1) | ATCAGTTCGGACACCAGGAGA | GTAAAGAGCGGGTGAGCCTTCT |
| Gpx2 (NM_030677.2) | TTCCCTTGCAACCAGTTCGGA | AGGATGCTCGTTCTGCCCATT |
| Nox1 (NM_172203.2) | TGCAGGCATCCTCATTTTGCG | TGGGTGCATGACAACCTTGG |
| Nox2 (NM_007807.5) | GCCAGTGTGTCGAAATCTGCT | AATTGTGTGGATGGCGGTGT |
| Nfe2l2 (NM_001399226.1) | GGTTGCCCACATTCCCAAACA | ATATCCAGGGCAAGCGACTCA |
| Tnfα (NM_013693.3) | GGGACAGTGACCTGGACTGT | TTCGGAAAGCCCATTTGAGT |
| Il1β (NM_008361.4) | ACCTTCCAGGATGAGGACATGAG | CATCCCATGAGTCACAGAGGATG |
| Il6 (DQ788722.1) | GCCCACCAAGAACGATAGTCA | CAAGAAGGCAACTGGATGGAA |
| F4/80 (NM_001355722.1) | TGACAACCAGACGGCTTGTG | GCAGGCGAGGAAAAGATAGTGT |
| iNos (NM_001313922.1) | CTCCACAAGCTGGCTCGCTT | TTCAAGCACCTCCAGGAACGT |
| Antioxidant Compounds | Raw DM | HoP-DM | HHP-DM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | % RM | Mean ± SEM | % RM | |||||||
| Vitamin A (mg/L) | 0.32 | ± | 0.04 | 0.29 | ± | 0.03 | −8 | 0.30 | ± | 0.04 | −7 |
| α-tocopherol (mg/L) | 8.75 | ± | 0.80 | 8.79 | ± | 0.98 | 0 | 9.17 | ± | 1.60 | 5 |
| γ-tocopherol (mg/L) | 0.80 | ± | 0.07 | 0.70 | ± | 0.06 | −12 * | 0.76 | ± | 0.07 | −5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wemelle, E.; Marousez, L.; Lesage, J.; De Lamballerie, M.; Knauf, C.; Carneiro, L. In Vivo Assessment of Antioxidant Potential of Human Milk Treated by Holder Pasteurization or High Hydrostatic Pressure Processing: A Preliminary Study on Intestinal and Hepatic Markers in Adult Mice. Antioxidants 2022, 11, 1091. https://doi.org/10.3390/antiox11061091
Wemelle E, Marousez L, Lesage J, De Lamballerie M, Knauf C, Carneiro L. In Vivo Assessment of Antioxidant Potential of Human Milk Treated by Holder Pasteurization or High Hydrostatic Pressure Processing: A Preliminary Study on Intestinal and Hepatic Markers in Adult Mice. Antioxidants. 2022; 11(6):1091. https://doi.org/10.3390/antiox11061091
Chicago/Turabian StyleWemelle, Eve, Lucie Marousez, Jean Lesage, Marie De Lamballerie, Claude Knauf, and Lionel Carneiro. 2022. "In Vivo Assessment of Antioxidant Potential of Human Milk Treated by Holder Pasteurization or High Hydrostatic Pressure Processing: A Preliminary Study on Intestinal and Hepatic Markers in Adult Mice" Antioxidants 11, no. 6: 1091. https://doi.org/10.3390/antiox11061091
APA StyleWemelle, E., Marousez, L., Lesage, J., De Lamballerie, M., Knauf, C., & Carneiro, L. (2022). In Vivo Assessment of Antioxidant Potential of Human Milk Treated by Holder Pasteurization or High Hydrostatic Pressure Processing: A Preliminary Study on Intestinal and Hepatic Markers in Adult Mice. Antioxidants, 11(6), 1091. https://doi.org/10.3390/antiox11061091








