Abstract
The current study aimed to understand the synergistic impacts of silicon (Si; 1.0 mM) and boron (B; 10 µM) application on modulating physio-molecular responses of date palm to mitigate aluminum (Al3+; 2.0 mM) toxicity. Results revealed that compared to sole Si and B treatments, a combined application significantly improved plant growth, biomass, and photosynthetic pigments during Al toxicity. Interestingly, Si and B resulted in significantly higher exudation of organic acid (malic acids, citric acids, and acetic acid) in the plant’s rhizosphere. This is also correlated with the reduced accumulation and translocation of Al in roots (60%) and shoots (56%) in Si and B treatments during Al toxicity compared to in sole Al3+ treatment. The activation of organic acids by combined Si + B application has significantly regulated the ALMT1, ALMT2 and plasma membrane ATPase; PMMA1 and PMMA3 in roots and shoots. Further, the Si-related transporter Lsi2 gene was upregulated by Si + B application under Al toxicity. This was also validated by the higher uptake and translocation of Si in plants. Al-induced oxidative stress was significantly counteracted by exhibiting lower malondialdehyde and superoxide production in Si + B treatments. Experiencing less oxidative stress was evident from upregulation of CAT and Cyt-Cu/Zn SOD expression; hence, enzymatic activities such as polyphenol oxidase, catalase, peroxidase, and ascorbate peroxidase were significantly activated. In the case of endogenous phytohormones, Si + B application demonstrated the downregulation of the abscisic acid (ABA; NCED1 and NCED6) and salicylic acid (SA; PYL4, PYR1) biosynthesis-related genes. Consequently, we also noticed a lower accumulation of ABA and rising SA levels under Al-stress. The current findings illustrate that the synergistic Si + B application could be an effective strategy for date palm growth and productivity against Al stress and could be further extended in field trails in Al-contaminated fields.
1. Introduction
After silicon and oxygen, aluminum (Al) is considered as one of the most abundant elements on the earth’s crust, occurring in the form of silicates or other deposits [1,2]. The accumulation of Al in acidic soil (with a pH of 5.5 or lower) converts it into a phytotoxic form of Al3+, which negatively influences the plant root system and subsequently ruins the aerial parts of plants due to immediate root deterioration [3,4,5,6]. The toxicity of Al3+ in roots inhibits cell division and elongation, causing root apices to be swollen and leading to poor or no root-hair development, oxidative damage, and the impairment of several pathways responsible for signal transduction [7,8,9]. In parallel, the higher accumulation of Al3+ is also a major threat to humans and animals through the food chain [10]. For a higher rate of aluminum transportation from soil to plants and the food chain, there is an urgent need to control the higher uptake of aluminum in edible crops, which will lower possible health risks and problems in humans.
In recent decades, various mechanisms have been discussed, and employed by plants, to overcome Al3+ toxicity, for instance, the chelation of Al3+ by different organic acids such as citrate, oxalate, and malate, or via neutralizing the absorbed Al3+ inside the root and shoot symplasm. Moreover, plant roots secrete organic acids in response to Al3+ stress, which chelates Al3+ in the rhizosphere, protecting roots. The exudation of the organic acid is usually regulated by membrane-localized organic acid transporters such as the Al-activated malate transporter and multidrug and toxic compound extrusion [11]. However, several studies have described the regulation of organic acids by different exogenous signaling molecules and phytohormones such as abscisic acid (ABA), indole acetic acid (IAA), salicylic acid (SA), ethylene (ET), and nitric oxide (NO) [12,13,14]. Moreover, overcoming Al3+ toxicity, various proteins, phenolic compounds, and tannins have been reported that make complexes with the Al3+, which subsequently results in the compartmentalization of the Al3+ inside the vacuoles and thereby reduces Al3+ toxicity but leads to higher accumulation [15,16]. Various studies demonstrated the higher production of ROS in plants exposed to Al3+ stress, which subsequently induced oxidative stress, caused the peroxidation of the cell membrane, negatively influenced the structural integrity of cells, caused chromosomal abnormality, and led to cell death [17,18]. Furthermore, Al3+ toxicity is known for hampering the uptake and metabolism of different nutrients, such as Cu, N, Ca, Mn, Mg, P, and Fe [19]. It poses a higher affinity for reacting with the negatively charged plasma membrane.
The presence of silicon (Si) in the soil is considered a beneficial mineral element for plant growth and development and for mitigating biotic and abiotic stress conditions. Therefore, the stress-alleviating effects of Si vary with plant species, and generally a significant stress-mitigating role is reported in plants with a higher accumulation of Si in different tissues [20]. Similarly, Si enhances Al3+ tolerance in plants by the activation of antioxidant activities [21,22], the formation of the Si-Al3+ complex [23], pH increase [24], the enhancement of organic acid anions, mineral uptake, and the exudation of phenolic compounds [25], as well as hormonal signaling [21,26,27,28]. While Si’s involvement in ameliorating Al-induced stress could be due to altering Al transporter activities, conclusive evidence regarding the effect of Si on regulating Al transporters is not available. Moreover, the formation of complexes of boron and silicon in soil under certain environmental conditions due to sharing considerable chemical similarity has been reported to alter heavy metal phytotoxic effects, uptake, and transportation [25]. However, there is also a shortage of reports regarding the stress-alleviating role of Si under Al3+ toxicity in the presence of boron in date palm.
Boron has been placed on the list of essential micronutrients and is considered an essential element for plant growth and the development of higher crop plants [18,29,30,31]. The deficiency of B in plants leads to many deformities in plants, such as root growth inhibition; the induction of chlorotic disorders in leaves; a reduction in yield; and the impairment of the cellular components, structure, and composition of the cell wall [31,32,33]. Similarly, the alleviation of Al3+-induced stress by the exogenous treatment of B in different plants has been reported previously by many researchers in different plants [29,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55]. Moreover, these studies have suggested that B possibly overcomes the consequences of Al3+ toxicity by restricting Al binding sites. B is reported to enhance alkalization in pea roots and thereby reduce Al toxicity [5], whereas high rainfall may wash out the alkalinity and alkalinity-related earth elements from the soil, thus causing acidic conditions, the accumulation of Al contamination, and B deficiency problems [29].
In Oman, date palm (Phoenix dactylifera) is an important fruit crop and makes Oman the eighth largest producer globally, with an average of 260,000 mt per annum production. Date palm is reported to be grown in 50% of Oman’s cultivated land and comprises 82% of all the cultivated fruit crops in Oman [36,37]. Metals contamination due to rapid urbanization and anthropogenic activities can negatively affect date palm growth and the quality of fruit [38,39]. During germination and the initial growth stages, date palm plants are reported to be fragile and tender and to adopt different sensing mechanisms to achieve germination under favorable environmental conditions [39]. Hence, the germination and early growth stages of date palm are adversely impacted by heavy metal pollution. Previously, Awad et al. [40] reported that the toxic effect of Al significantly inhibited the embryo production of date palm callus and negatively affected biochemical activities, including soluble carbohydrates, total proteins, antioxidative activities, free amino acids, and molecular responses. The adverse impacts of aluminum toxicity on date palm seedlings growing in Al-contaminated soil at biochemical and molecular levels have not been explored. Limited numbers of scientific reports provide enough information regarding the interaction of Si and B for influencing Al toxicity in date palm. Therefore, the current study aimed to unveil the ameliorative effect of Si and B and their combination on the Al-induced inhibition of growth attributes, and oxidative damage at physiological, biochemical, and molecular levels. The synergism of Si and B under Al-toxicity in date palm was elucidated by stress-related hormonal modulation, ROS-homeostasis, and stress-responsive gene alteration. Moreover, the effect of Si and B on modulating organic acid exudation and their subsequent impact on Al-uptake and Al-translocation from roots to shoots and their responsive genes expression were also explored.
2. Material and Methods
2.1. Plant Growth and Experimental Design
Oman Botanical Garden provided seedlings of date palm (Khalas cultivar). Uniform seedlings in length and number of leaves were transplanted into pots containing sphagnum peat moss (electrical conductivity (EC) 2.0 (dS m−1), moisture content (38.5%), pH 4.5–5.5, organic matter 91.1% (sodium chloride 850 mg kg−1, nitrogen 800–2500 mg kg−1, and phosphorus 150–850 mg kg−1). The seedlings were treated with distilled water (50 mL to each pot) in the growth chamber for three months to equilibrate the seedlings growth. The growth chamber conditions were adjusted to 12 h light (30 °C; relative humidity 60%) and 12 h dark (25 °C; humidity 60 %) conditions. Thereafter, date palm seedlings were subjected to Si (Na2SiO3; 1.0 mM), B (H3BO3; 10 µM), and Al3+ (AlCl3; 2.0 mM). Si, B, and Al3+ concentrations were selected through a pre-experiment on various plants [29,41,42,43]. For the experiment, date palm seedlings were treated with 50 mL solution four times a week. Seedlings were subjected to one of eight treatments: (1) Ct (without Si, B and Al3+), (2) Si (1.0 mM), (3) B (20 µM), (4) Si + B (1mM of Si; 20 µM B), (5) Al3+ (2.0 mM), (6) Si + Al3+ (1.0 mM of Si; 2.0 mM of Al3+), (7) B + Al3+ (10 µm of B; 2.0 mM of Al3+), and (8) Si + B + Al3+ (1.0 mM of Si, 10 µm of B, and 2.0 mM Al3+). All the solutions were prepared in distilled water. The pH of all nutritional solutions was adjusted to 6.0. The pots were put in the greenhouse in a randomized arrangement with five replications. For 10 weeks, 50 mL of Si, B, and Al3+ was administered simultaneously to the plant’s root zone. After 10 weeks of treatment, the growth characteristics were recorded and plants were collected in liquid nitrogen and kept at −80 °C. The experiment was done twice, each time with 10 replications.
2.2. Chlorophyll a and Chlorophyll b
Leaf samples (200 mg) of date palm seedlings were ground in mortar using 80% acetone to analyze photosynthetic pigments (Chl a, Chl b, and carotenoids). To estimate the Chl a and Chl b content, the methodology of Sumanta et al. [44] was employed. The absorbance values for Chl a, Chl b, and carotenoids were recorded at 663, 645, and 470 nm for Chl a, Chl b, and carotenoid, respectively.
2.3. Quantification of Malondialdehyde (MDA)
The extent of lipid peroxidation or formation of MDA was estimated using the methodology reported by Bilal et al. [45] and Khan et al. [46]. Briefly, 10 mM phosphate buffer extracted tissue homogenates (pH 7.0). In a reaction tube, 0.2 mL of tissue homogenate was mixed with 0.2 mL of 8.1 percent sodium dodecyl sulfate (SDS), 1.5 mL of 20 percent acetic acid (pH 3.5), and 1.5 mL of 0.81 percent thiobarbituric aqueous acid (TBA) solution for the measurement of MDA. After that, the mixture was heated for 60 min in boiling water followed by cooling to room temperature and the addition of 5.0 mL butanol:pyridine (15:1 v/v) solution. A spectrophotometer was used to measure the optical density of the resultant pink solution at 532 nm after the top organic layer was removed.
2.4. Determination of Superoxide (O2•−)
The measurements of O2•− were done according to the method reported by Gajewska and Skłodowska [47] and Khan et al. [42]. Briefly, 1.0 g of fresh plant material was immersed in phosphate buffer (pH 7.0) containing sodium phosphate (10 mM), nitrobluetetrazolium (NBT) (0.05 percent; w/v), and sodium azide (NaN3), to prepare the homogenate for the reaction (10 mM). For 1 h, the mixture was left at room temperature. Afterward, 5 mL of the mixture was put in a new tube and heated at 85 °C for fifteen min. The mixture was then chilled and vacuum filtered. A spectrophotometer was employed to determine the sample’s absorbance at 580 nm. The experiment was appropriately conducted three times.
2.5. Antioxidant Enzyme Assay
The antioxidant enzymes ascorbate peroxidase (APX), polyphenol oxidase (PPO), peroxidase (POD), and catalase (CAT) were analyzed as described by Bilal et al. [45], with minor modifications. Briefly, date palm leaves and roots (100 mg) were crushed via liquid nitrogen. Phosphate buffer (100 mM) was added to the sample to obtain a homogenous pH 7.0. The resulting homogenate was centrifuged for 30 min at 10,000 rpm and 4 °C L.
The reaction mixture for POD analysis was made up of 0.1 M potassium phosphate buffer (pH 6.8), 50 µL H2O2 (50 M), 50 µL pyrogallol (50 M), and 100 µL crude extract sample. This mixture was incubated at 25 °C for 5 min; then, 5% H2SO4 (v/v) was added to cease the enzymatic activity. An optical density of 420 nm was used to determine the quantity of purpurogallin produced. The reaction mixture for determining polyphenol oxidase (PPO) activity used comparable components to the POD assay; however, without H2O2, the resultant activity was measured at 420 nm. An increase of 0.1 units of absorbance was used to quantify a single unit of PPO and POD. The CAT activity was investigated as described by Aebi [48]. Briefly, the crude enzyme extract was added to 0.2 M H2O2 in 10 mM phosphate buffer (pH 7.0), after which the CAT activity was determined as a decrease in absorbance at 240 nm and expressed as units (one unit of CAT was defined as the ng of H2O2 released/mg protein/min).
For the quantification of APX, 1 mL phosphate buffer (50 mM; pH 7.0) containing 1.0 mM ascorbic acid and 1.0 mM EDTA was used for extraction, followed by homogenization at 50 Hz for 30 s and centrifugation at 4830× g at 4 °C for 15 min. Subsequently, the supernatant was added into the phosphate buffer solution (pH 7.0) having 15 mM AsA and 0.3 mM H2O2. At 290 nm, the reaction mixture was examined. A variable quantity of absorbance at 290 nm per minute was defined as one unit of APX. The enzymatic tests were performed three times, with three replications each time.
2.6. Micronutrient Quantification
Silicon quantification was investigated by the method described by Ali et al. [49] via ICP-MS (Optima 7900DV, Perkin-Elmer, Waltham, MA, USA) by using freeze-dried date palm roots and leaves (0.05 g sample). The experiment was repeated three times, and each time comprised of three replications.
2.7. Determination of Organic Acids
Organic acids in seeds were extracted according to the method previously described by Bilal et al. [50]. Briefly, a rotary evaporator was used for evaporating the extracted samples, followed by re-dissolving the dried residues in deionized water and filtering through a 0.45-μm filter. Furthermore, HPLC (Millipore Corp., Waters Chromatography, Milford, MA, USA) was used for quantifying organic acids in samples. Different organic acids such as citric acid, malic acid, succinic acid, and acetic acids were measured by comparing their peak values to the peak values of their respective standards.
2.8. Abscisic Acid and Salicylic Acid Extraction and Quantification
Endogenous abscisic acid (ABA) was extracted and quantified according to the modified protocol as described in Shahzad et al. [51] and Khan et al. [46]. Salicylic acid was extracted and quantified from freeze-dried samples according to Seskar et al. [52] as described by Bilal et al. [53]. For detail, see Supplementary File S1.
2.9. Gene Expression Analysis
RNA was extracted from date palm leaves using an extraction buffer (0.25 M, NaCl; 0.05M, Tris-HCl (pH = 7.5); 20 mM, EDTA; 1% (w/v) SDS; 4% polyvinylpyrrolidone (w/v)) as reported by [41]. Briefly, 750 µL of the extraction buffer and chloroform: isoamyl alcohol (CI; 24:1 v/v) was mixed with a 2-mL RNase-free microcentrifuge tube followed by the addition of 40 µL β-mercaptoethanol. After that, a fine powder (100 mg) of the sample was carefully transferred to a 2 mL tube containing the extraction buffer and CI. Subsequently, the mixture was vortexed, followed by incubation for 15 min at 20 °C and 10 min of centrifugation (12,000× g) at 4.0 °C. Then, supernatant (600 µL) was transferred to a new tube, followed by the addition of the same volume of CI. The gently mixed solution was centrifuged (12,000× g) for 10 min at 4.0 °C. The upper layer was transferred to a new tube and 3 M sodium acetate (pH = 5.2) of 1/10 volume was added. Absolute ethanol was added for precipitation, followed by gentle mixing and incubation for 45 min at 4.0 °C. After that, centrifugation of the sample was carried out at 4.0°C for 10 min. The pellet was collected, and diethyl pyrocarbonate-treated water (200 µL) was added, followed by the addition of 500 µL of 10 M LiCl, and kept on ice for 60 min. Sample was centrifuged at 4.0 °C for 10 min at 12,000× g, pellet was collected and washed with 70% ethanol.
The synthesized cDNA was used to amplify genes (Table S1). A total of 11 genes’ expression was assessed in each sample related to Si and Al3+ transport, the ABA biosynthesis pathway, and oxidative stress regulation. Actin gene was used as a reference for all the primers. Power up “SYBR” green Master Mix was used for the thermo-cycler (Quantstudio 5 by applied biosystems life technologies) PCR reaction. The reaction was performed at a specific condition such as 94 °C for 10 min in stage 1; 35 cycles of PCR reaction at 94 °C for 45 s; 65 °C for 45 s; and 72 °C for 1 min; finally, the extension temperature was set at 72 °C for 10 min. The threshold level of 0.1 was set for gene amplifications. The experiment was repeated three times and each time was comprised of three replications.
2.10. Statistical Analysis
GraphPad Prism was used to create all graphs and data analysis (v8.01; San Diego, CA, USA). All values are expressed as mean ± standard deviation. Mean values were analyzed using Duncan’s multiple range tests with a significant difference among treatments by ANOVA using SAS software (V9.1, Cary, NC, USA) to reveal significant to non-significant treatments by maintaining p < 0.05 and was represented by different lower-case alphabets.
3. Results
3.1. Effects of Si, B, and Their Combination on Date Palm Growth Parameters under Al3+ Phytotoxicity
As shown in Table 1 and Figure 1, in the absence of Al3+ stress, sole Si and B, as well as their combination (Si + B), substantially improved the shoot length by approximately 40%, 33%, and 55%, respectively, and shoot dry weight by 52%, 41%, and 78%, respectively, as compared to the non-treated plants. Similarly, significant enhancement was detected in the root length under sole Si, B, and their combination (Si + B) by approximately 27%, 31%, and 45% and root dry weight by 21%, 14%, and 25%, respectively, relative to non-treated plants in the absence of Al3+ stress. However, exposure to Al3+ toxicity significantly reduced the shoot length and root length of the control, Si, B, and Si + B treatments by 23%, 13%, 15%, and 16%, respectively, and root length by 25%, 8%, 7%, and 4%, respectively, compared to their treatment of control conditions, while in comparison with the respective treatment of no-stress conditions, control (Ct)-, Si-, B-, and Si + B-treated plants under Al3+ stress illustrated decreased shoot dry weight by nearly 28%, 23%, and 15% and root dry weight by 31%, 21%, 26%, and 24%, respectively. However, the application of Si and B and their combination (Si + B) considerably ameliorated Al3+-induced damage to growth attributes compared to non-treated plants, e.g., sole Si, B, and their combination demonstrated significant enhancement in shoot length compared with only Al3+-treated plants by approximately 56%, 46%, and 72% and root length by 57%, 65%, and 87%, respectively (Table 1). On the other hand, the shoot and dry weight of sole and combined Si- and B-treated plants under Al3+ toxicity also presented a statistically significant improvement compared to only Al3+-treated date palm seedlings.

Table 1.
The exogenous application of Si and B alone and in combination with date palm.

Figure 1.
Application of sole silicon (Si) and Boron (B) and their integrative effects on date palm growth under Al stress.
Similarly, date palm seedlings treated with either Si, B, or their combination maintain chlorophyll pigments such as Chl a, Chl b, and carotenoids. Under control conditions, the application of Si, B, and their combination (Si + B) did not present a statistically significant alteration in Chl a level in leaves compared to non-treated plants. On the contrary, an increase was observed in Chl b level by the interaction of Si + B by 91%, which was statistically equally followed by sole Si- and B-treated plants compared to control plants under no-stress conditions, whereas under Al3+ treatment, combined Si + B and sole Si application revealed a significantly higher Chl b, while only Al3+ was detected with the lowest level of Chl b. In the case of carotenoids under no-stress conditions, the combination of Si and B presented a significantly higher level, followed by sole B, Si, and non-treated control plants. Not surprisingly, Al3+ treatment altered the carotenoids level in date palm seedlings, but the supplementation of exogenous Si, B, and their combination considerably improved the level by approximately 66%, 85%, and 144%, respectively, compared to only Al3+-treated date palm seedlings (Table 1). To conclude, Si, B, and their combination (Si + B) showed a synergistic effect against Al3+-induced chlorosis in date palm seedlings.
3.2. Accumulation and Translocation of Si, B, and Al3+ in Date Palm
The accumulation of B, Al3+, and Si and their translocation from the roots to the shoots of date palm were investigated via ICP-MS (Table 2). The accumulation of Al3+ was considerably higher in the roots than the shoots. In the absence of Al3+ stress, combined Si + B-treated plants exhibited a higher uptake of Al3+ in shoots, followed by the control plants and the sole B- and Si-treated plants, respectively. However, combined Si and B application significantly reduced Al3+ accumulation in the roots compared to only Al3+-treated plants by approximately 59%, followed by sole B- and Si-treated plants by 49% and 40%, respectively. Similarly, Al3+ uptake was significantly reduced in shoots of combined Si + B plants by 2.3, 1.3, and 1.6 fold compared to non-treated, sole Si-, and B-treated plants, respectively, under Al3+ stress.

Table 2.
The accumulation and uptake of B, Si, and Al+ by the roots and shoots of date palm under aluminum stress.
Similarly, a significantly higher amount of Si was determined in both the leaves and roots of the date palm seedling with the combined and sole application of exogenous Si and B compared to control plants. Interestingly, the application of exogenous Al3+ inhibited the uptake of Si in roots and shoots compared to non-treated plants of control conditions by 34% and 19%, respectively (Table 2), while under Al3+ toxicity, the combined and sole application of Si and B significantly enhanced the uptake of Si in roots and shoots compared to only Al3+ plants by (65%, 86%, and 44%) and (68%, 84%, and 30%), respectively. Interestingly, Al3+ toxicity did not alter B uptake significantly in the roots and shoots of only Al3+-treated plants compared to control plants. However, exogenous B and combined Si + B under Al3+ stress exhibited the equally maximum accumulation of B in roots and the sole application of B treatment presented higher accumulation in shoots.
3.3. Effect of Exogenous Si, B, and Their Interaction on Lipid Peroxidation and Superoxide Anion Accumulation under Al3+ Stress
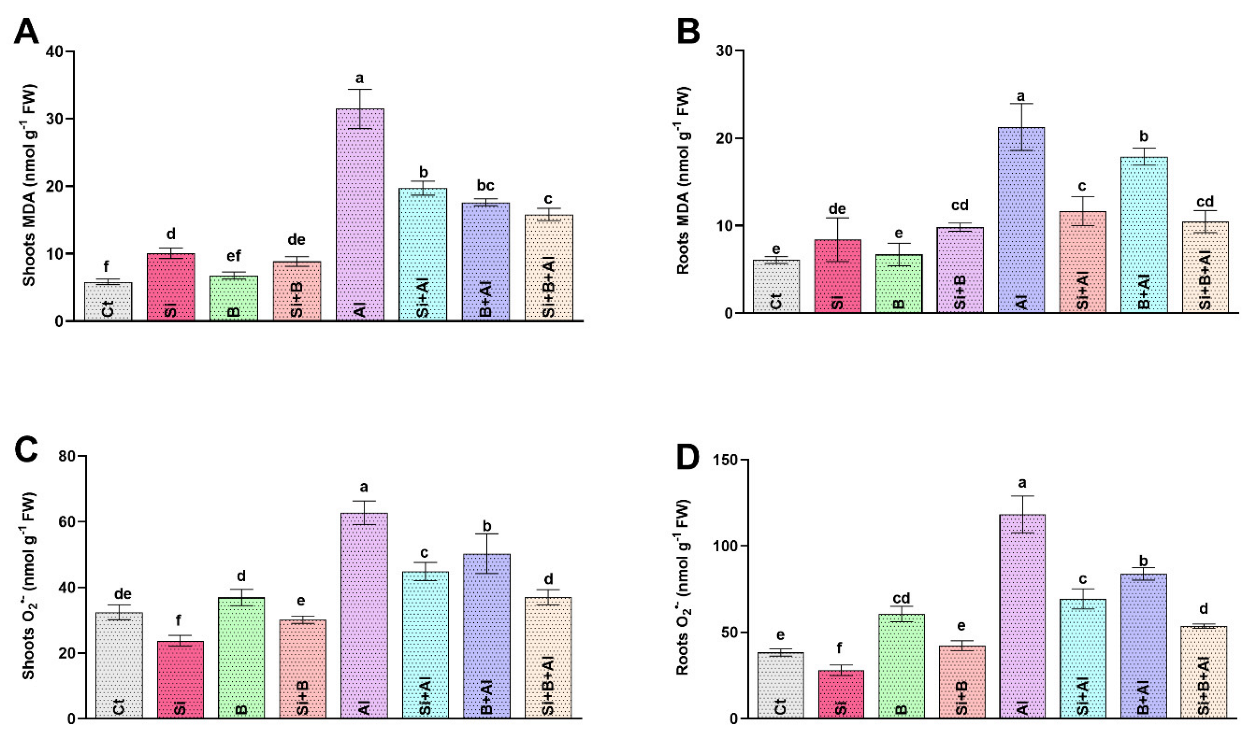
The ROS-induced peroxidation of lipid membranes and superoxide anion (O2•−) produce stress-induced damage at the cellular level. We found that Al3+ stress significantly increased the production of MDA and O2•−, which damage the cell structure due to its highly reactive and toxic nature. A significant augmentation in MDA level was noticed only in the roots of Al3+-treated plants compared to the plants treated with Si, B, and their combination (Figure 2). The interaction of Si and B significantly mitigated the toxic effects of Al3+ toxicity. It exhibited the lowest level of MDA in roots by approximately 50%, followed by sole Si- and B-treated plants with 45% and 16% reduced levels, respectively, compared to only Al3+-treated plants. Likewise, the MDA level in the shoots was either not significantly or less altered by Si, B, and Si + B in the absence of Al3+ stress. However, exposure to Al3+ significantly upregulated MDA levels in only Al3+-treated plants by 37%, 43%, and 50% compared with Si, B, and their combination, respectively.
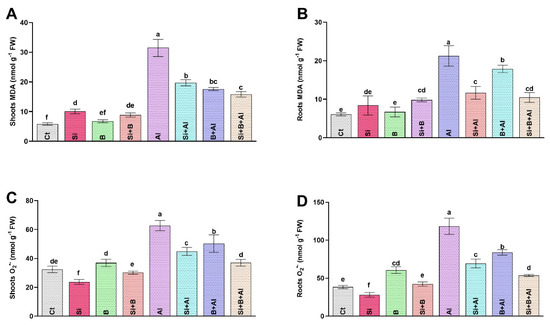
Figure 2.
The effect of sole silicon (Si), boron (B), and their combination on (A,B) lipid peroxidation (malondialdehyde, MDA) and (C,D) superoxide anion (O2•−) modulation under Al-induced toxicity in date palm roots and shoots. Bars with different letters describe statistically significant differences (p < 0.05) between means by using one-way analysis of variance (ANOVA), followed by Duncan’s multiple range test (DMRT). Bars describe mean (of four replicates) ± standard error.
Moreover, in the absence of Al3+ stress, the application of sole Si revealed a significantly reduced level of O2•− in both shoots and roots compared to other treatments. Unlike the absence of Al3+ stress, the O2•− level was significantly elevated under Al3+ stress in both roots and shoots. The alleviated level of O2•− was significantly inhibited under the interaction of Si + B by demonstrating approximately 55%, 23%, and 36% lower accumulation in roots and 40%, 17%, and 26% reduced level in shoots, in comparison with only Al3+-treated, Si-treated, and B-treated plants, respectively.
To conclude, Si, B, or Si + B reduced the MDA and O2•− level more significantly under Al3+ stress than the absence of Al3+ stress.
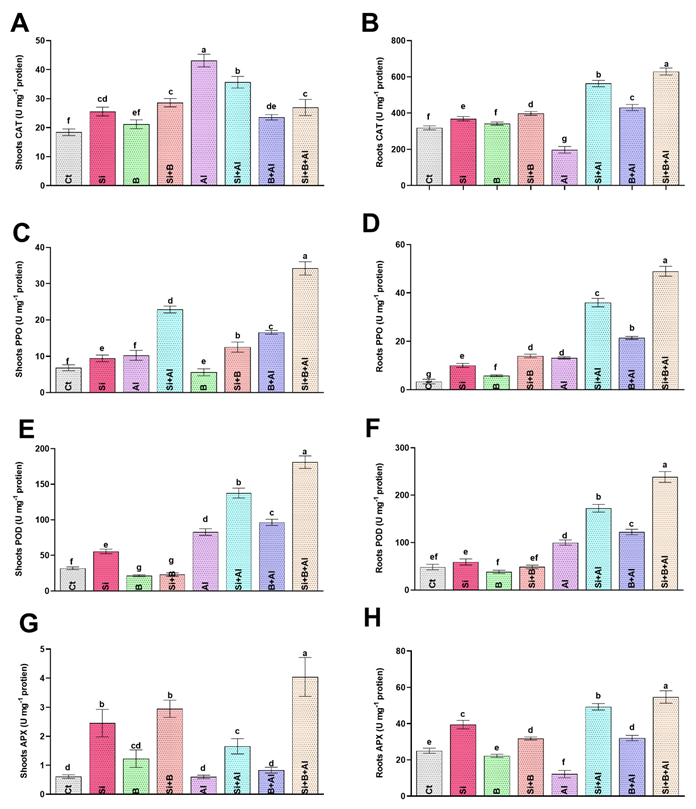
3.4. Modulation of Antioxidant Activities by Si, B, and Their Interaction
Metals toxicity is known for hampering the plant antioxidative system due to excess oxidative stress. Therefore, Si, B, and Si + B interaction were evaluated by analyzing antioxidant enzyme activities (CAT, PPO, POD, and APX) under Al3+ stress in date palm seedlings (Figure 3). In the absence of Al3+ stress, the control treatment exhibited the lowest CAT level in shoots, while Si + B treatment illustrated a significantly enhanced level compared to other treatments. Under the exposure to Al3+ toxicity, sole Al3+-treated plants demonstrated approximately 134%, 21%, 83%, and 60% increase in the CAT level of shoots compared to control, sole Si, B, and their combination, respectively. On the contrary, the CAT level of roots in the absence and presence of Al3+ stress was significantly elevated compared to other treatments. Al3+ toxicity significantly reduced CAT in the roots of only Al3+-treated plants by approximately 2.8-, 2.1-, and 3.1-fold compared to the sole Si, B, and their combination, respectively. Likewise, Si + B slightly increased the activity of PPO in shoots and roots compared to the rest of the treatments under normal conditions. In the case of Al3+ stress, the PPO level was significantly enhanced in all treatments compared to normal conditions. A significantly enhanced PPO level was detected under Al3+ stress in combined Si + B treatment by approximately 3.3-, 1.5-, 2.0-fold in shoots and 3.7-, 1.3- and 2.2-fold in roots compared with Al3+-treated, sole Si-, and B-treated plants, respectively.
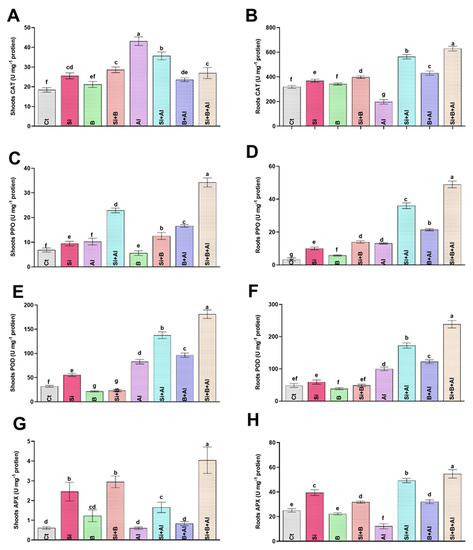
Figure 3.
The effect of sole silicon (Si), boron (B), and their combination on antioxidant regulation (catalase, CAT; polyphenol oxidase; PPO peroxidase, POD; and ascorbate peroxidase, APX;) of date palm roots and shoots under Al-induced stress. (A,B) CAT, (C,D) PPO, (E,F) POD, (G,H) APX. Bars with different letters are describing statistically significant differences (p < 0.05) between means by using one-way analysis of variance (ANOVA), followed by Duncan’s multiple range test (DMRT). Values describe the mean (of four replicates) ± the standard error.
In terms of POD regulation, the sole B and Si + B combination presented significantly decreased POD levels in shoots equally, while significantly reduced POD levels in roots were detected in sole B-treated plants compared to other treatments under normal conditions (Figure 2). However, in response to Al3+-induced ROS, a significant elevation in POD level was observed in roots and shoots compared to normal conditions. The combination of Si + B markedly enhanced the POD level in shoots and roots under Al3+ stress by 2.1- and 2.3-fold, followed by sole Si-treatment with 1.6- and 1.7- fold and sole B-treatments with 1.1- and 1.2-fold, as compared to sole Al3+ treatment, respectively. Likewise, the level of APX was markedly enhanced in the roots compared to the shoots of date palm seedlings. Under normal conditions, sole Si-treated plants illustrated a significantly enhanced level of APX in roots compared to other treatments. However, the APX activity of the roots was significantly inhibited in sole Al3+-treated plants compared to control plants. However, the Al3+ toxicity in roots was substantially mitigated by the combined application of Si + B-treated plants via demonstrating approximately 4.6-, 1.1-, and 1.7-fold higher levels compared to sole Al3+, Si-, and B-treated plants, respectively. A slight variation was noted in the APX level of shoots between normal and Al3+ stress conditions. Nevertheless, the combined treatment of Si + B presented a significantly higher content of APX in shoots in normal conditions, as well as Al3+ stress, in comparison with other treatments.
To conclude, Si, B, and their combination boost Al3+ stress tolerance in date palm seedlings by upregulating the antioxidant enzymes, which help date palm seedlings experience less oxidative stress under normal and Al3+ stress conditions.
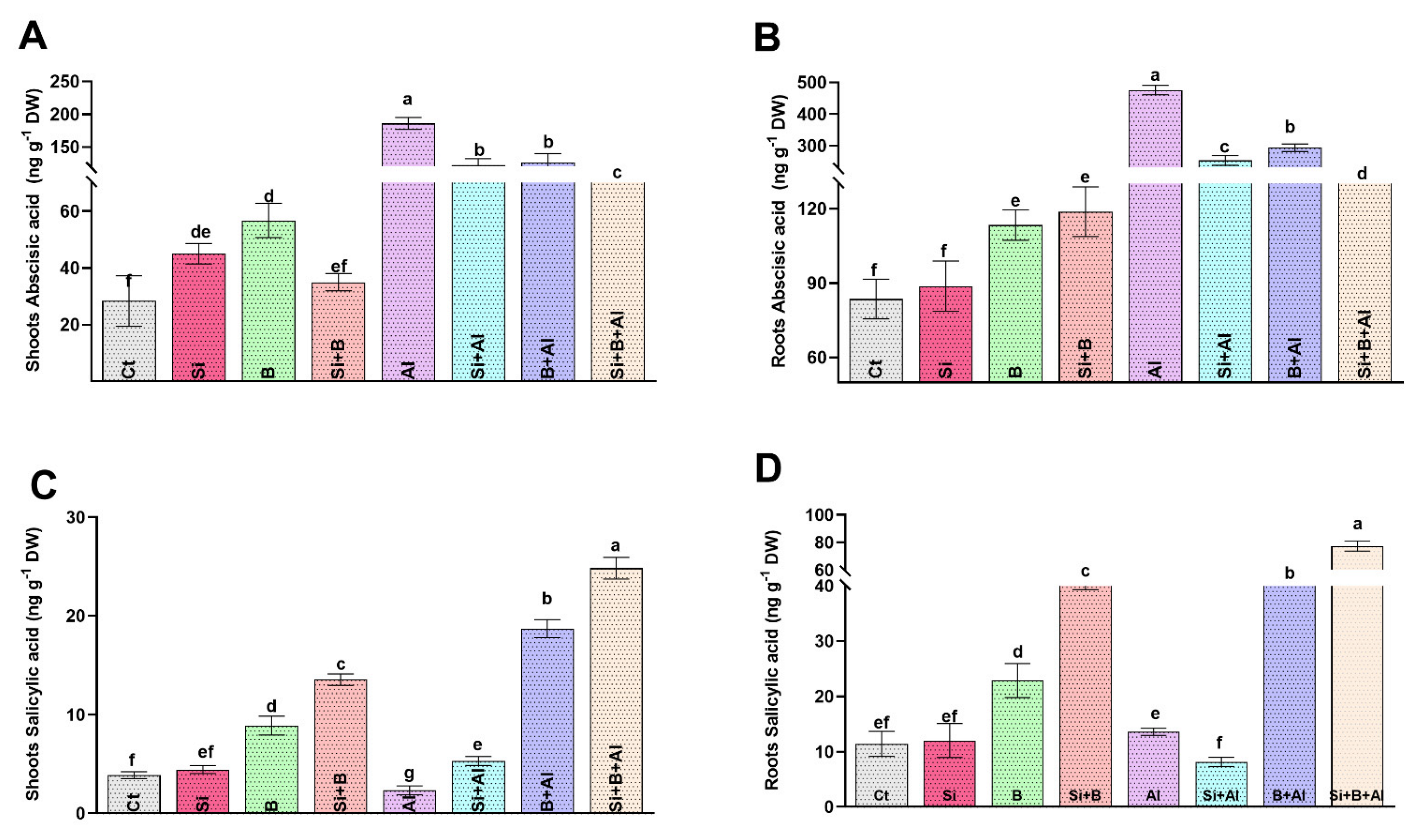
3.5. Effects of Si, B, and Si + B on the Endogenous ABA and SA Levels in Date Palm Seedlings under Normal and Al3+ Stress Conditions
The phytohormones act as signaling molecules under abiotic stresses by initiating different signal transduction pathways to cope with the adverse effect. In the current study, we determine the endogenous ABA and SA levels in all the treatments to understand the crosstalk of exogenous Si and B and their combination with endogenous ABA and SA under normal and Al3+ stress conditions.
In the current study, the ABA level was increased significantly (around 6.5-fold) in the shoot of Al3+-treated seedlings compared to control plants. The escalated level of ABA in date palm leaves under Al3+ stress was significantly reduced by applying sole Si, B, and their combination (Figure 4). For instance, the implementation of Si, B, and Si + B decreased the level of ABA under Al3+ stress conditions by approximately 34%, 32%, and 44%, respectively, relative to sole Al3+-treated seedlings. Likewise, exposure to Al3+ toxicity enhanced the ABA level significantly by approximately 5.8-fold in the root of sole Al3+-treated seedlings compared to control plants. Interestingly, the escalated level of ABA under Al3+ stress in roots decreased significantly upon supplementation of Si, B, and their combination by approximately 1.8-, 1.6-, and 3.0-fold, respectively, compared to sole Al3+-treated plants (Figure 4B).
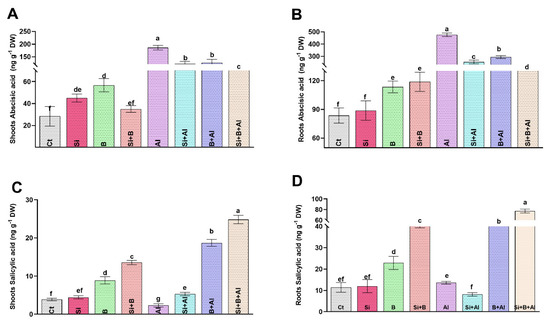
Figure 4.
Endogenous hormonal (abscisic acid and salicylic acid) regulation by the sole and interactive effects of Si and B administration under Al-induced stress in date palm shoots and roots. (A,B) Abscisic acid, (C,D) Salicylic acid. The bars illustrate means (of four replications) ± standard error. The means with different letters are significantly different (p < 0.05) as evaluated by the Duncan Multiple Range Test (DMRT).
Similarly, the SA levels in the shoots of sole Al3+-treated seedlings decreased by approximately 39% compared to the control seedlings (Figure 4C). On the contrary, a significant enhancement in the level of shoots SA was observed upon the supplementation of Si, B, and their combination under Al3+ stress by approximately 56%, 87%, and 90%, respectively, compared with the sole Al3+ plants, while in the roots SA level, no significant changes were noticed between the control and sole Al3+ treated plants. However, the combination of Si + B followed by sole B and Si significantly exhibited an increased level of SA, respectively, compared to the sole Al3+-treated plants.
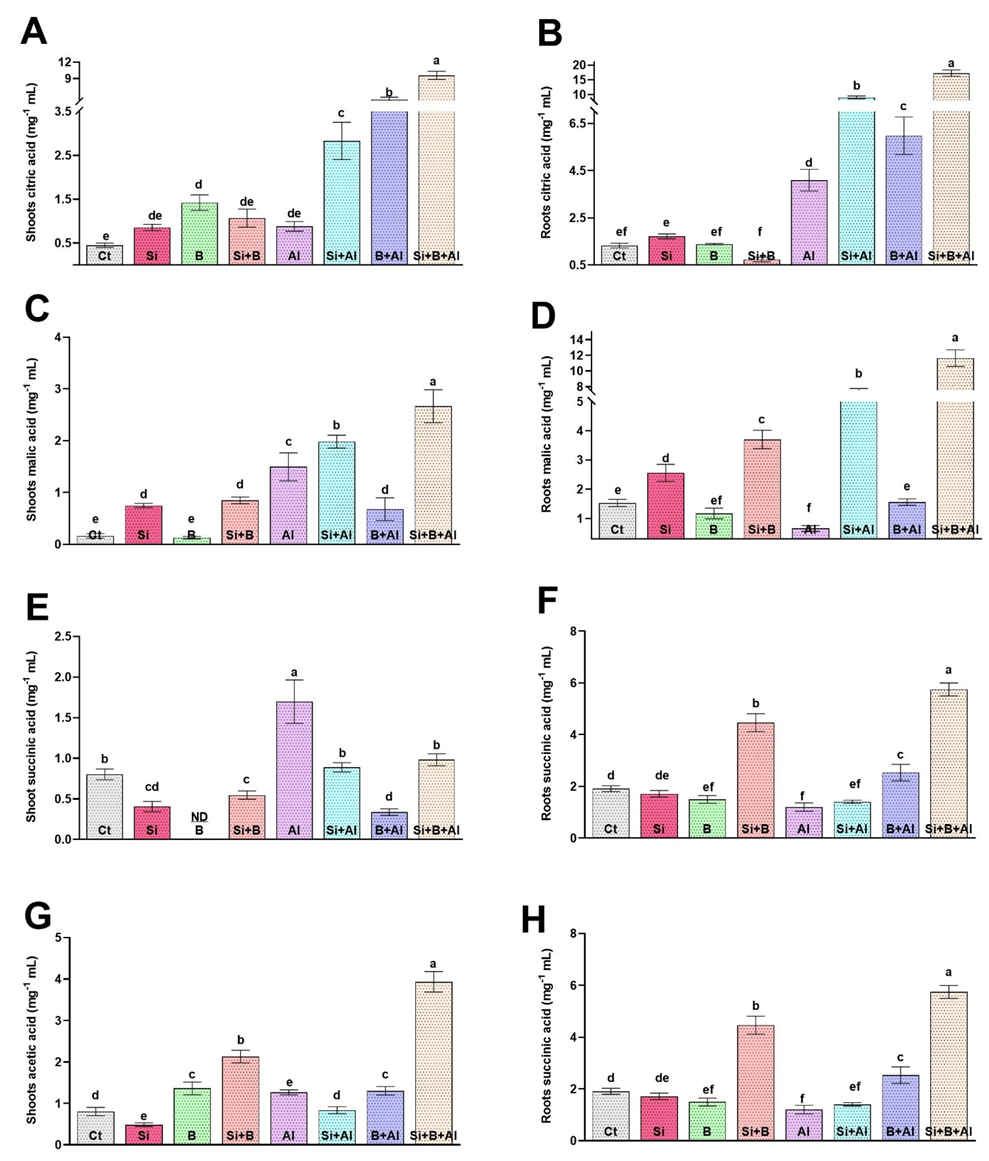
3.6. Effect of Exogenous Si and B on the Regulation of Citric Acid, Malic Acid, Succinic Acid, and Acetic Acid under Al3+ Stress Conditions
Under Al3+ stress, plants adopt various mechanisms to overcome Al3+ toxicity, either preventing Al3+ from root due to the chelating of Al3+ by organic acid (such as citrate, oxalate, and malate) by neutralizing the absorbed Al3+ inside the root and shoot symplasm. The production of organic acids such as citric acid, malic acid, succinic acid, and acetic acid under Al3+ stress conditions is known for its vital role in detoxifying Al3+ toxicity. We found that under Al3+ Stress, citric acid increased significantly as compared to non-Al3+-treated seedlings (Figure 5). Interestingly, a low level of citric acid was detected in shoots under the absence of Al3+ stress in all treatments. However, applying a combination of Si + B significantly enhanced the citric acid level in the shoots under Al3+ stress, followed by sole B-treated and Si-treated plants, respectively, compared with only Al3+-treated plants. In comparison with shoot, the level of citric acid was significantly enhanced in roots by combining Si + B under Al3+ stress. Sole Al3+ treated plants exhibited approximately 4.2-, 2.2-, 1.4-fold lower levels of citric acid in roots compared to the plants treated with combined Si + B and sole Si- and B-treated plants, respectively. A similar trend was detected in the case of malic acid production in shoots and roots under Al3+ stress, and the production of malic acid in roots was detected to be higher than in the shoots. For example, the combined application of Si + B illustrated approximately 19.3- and 1.8-fold higher levels of citric acid in roots and shoots under Al3+ stress compared with sole Al3+ plants, respectively. Likewise, sole and combination Si and B exhibited a very low or insignificant effect compared to control plants on the succinic acid level of shoots and roots in the absence of Al3+ stress, while sole Al3+ treated plants presented significantly higher succinic acid production in both shoots and roots, followed by sole Si-treated and combined Si + B-treated plants, respectively.
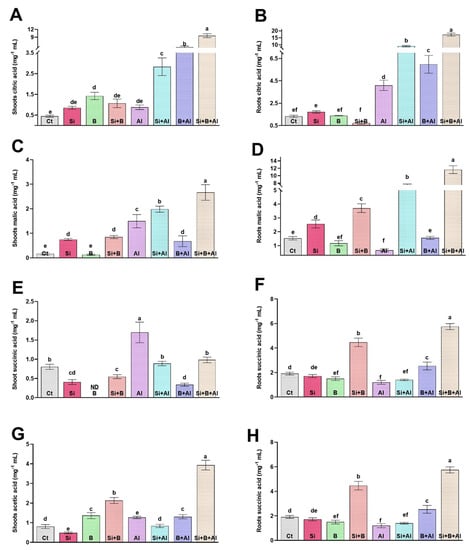
Figure 5.
The sole and in-combination effect of Si and B on the organic acid regulation of date palm shoots and roots under Al-induced stress. (A,B) Citric acid, (C,D) Malic acid, (E,F) Succinic acid, (G,H) APX Bars with different letters are describing statistically significant differences (p < 0.05) between means by using one-way analysis of variance (ANOVA), followed by Duncan’s multiple range test (DMRT). The bars describe the mean (of four replicates) ± standard error.
On the contrary, the acetic acid level was significantly enhanced by the combined treatment of Si + B in both roots and shoots under control and stress conditions, compared to other treatments. The exposure of Al3+ toxicity significantly reduced the acetic acid level in the roots of sole Al3+-treated plants compared to their respective control plants by 1.7-fold, whereas Si + B-treated plants demonstrated approximately 5.1- and 3.2-fold greater levels of succinic acid in comparison with the roots and shoots of sole Al3+ treated plants, respectively.
3.7. Expression of Genes Related to Si and Al3+ Transport, ATPase H+ Pump, and Biosynthesis of ABA
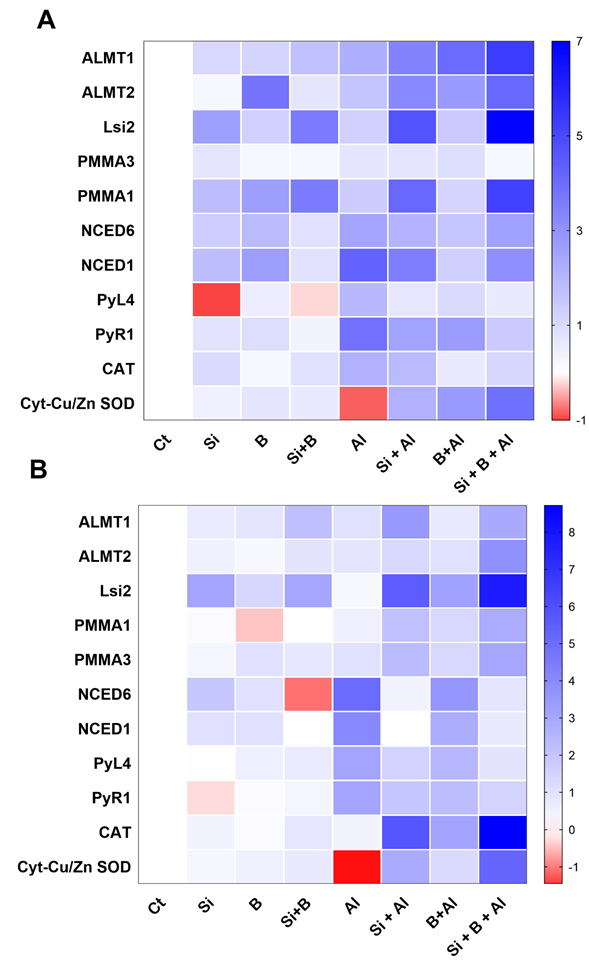
To further understand the protective role of Si and B, we measured the expression pattern of genes in leaves and roots related to Si (silicon efflux transporter; Lsi2) and Al3+ transport (Phoenix dactylifera aluminum-activated malate transporter 1-like; ALMT1, and ALMT2), ATPase H+ pump (Phoenix dactylifera plasma membrane ATPase; PMMA1, and PMMA3), and the biosynthesis of ABA (abscisic acid receptor PYL4-like; PYL4, abscisic acid receptor PYR1; PYR1, CAT, Cyt-Cu/Zn SOD, 9-cis-epoxycarotenoid dioxygenase; NCED1, and 9-cis-epoxycarotenoid dioxygenase; NCED6) at the molecular level by qRT-PCR.
As shown in Figure 6A, the application of Si, B, and their combination induced some vital and significant variations in the expression levels of most of the evaluated genes of date palm shoots compared to control plants under Al3+ stress. The treatment of sole Si, B, and their combination significantly induced the expression of ALMT1 and ALMT2 as compared to sole Al3+-treated plants under aluminum stress. Likewise, the Lsi2 gene was significantly upregulated in all the seedlings treated with Si under conditions and Al3+ stress conditions compared to non-Si treated plant shoots. However, the expression of Lsi2 gene was significantly enhanced under the aluminum stress of Si-treated plants compared to Si-treated plants under control conditions. Additionally, the transcript accumulation of PPMA3 was significantly downregulated by the combination of Si + B under control and Al3+ stress conditions compared to other treatments. However, the combination of Si + B and sole Si significantly induced the transcript accumulation of PPMA1 as compared to sole Al3+ treated and B treated plants under aluminum stress.
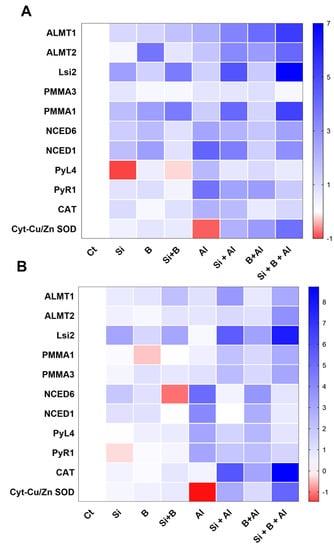
Figure 6.
The sole and in combination effect of Si and B on the regulation of gene expressions in date palm (A) shoots and (B) roots under control and Al-induced stress conditions. The red color represents a lower relative transcript accumulation concerning control, and the blue color represents a higher transcript accumulation.
Similarly, no significant differences in the expression pattern of NCED6 were induced under aluminum stress by sole Si, B, and their combination compared to sole Al3+ treatment, whereas the significantly enhanced expression of NCED1 was detected in sole Al3+ treatment, and the lowest was noticed in sole Bi-treated plants shoots. The over expression of PYL4 and PYR1 was detected in sole Al3+-treated plants, while significant downregulation was observed in Si + B-treated plants. Furthermore, Cyt-Cu/Zn SOD expression was detected significantly lower in sole Al3+ treatment, while the combination of Si + B presented a significantly enhanced expression level under aluminum stress.
Likewise, the expression of the genes mentioned earlier was also determined in the roots of date palm seedlings (Figure 6B). Interestingly, plants treated with Si, B, and their combination presented a different expression pattern of Lsi2, NCED6, and PYR1 compared to control plants under the no-Al3+ stress. We found that ALMT1 and ALMT2 genes were upregulated in Si + B-treated seedlings under Al3+ stress compared to sole Al3+-treated plants and sole B-treated plants. Furthermore, the Lsi2 gene revealed a higher expression level in all Si-treated plants, and sole Al3+-treated plants presented significantly lower expression under aluminum stress. Moreover, PPMA1, PPMA3, CAT, and Cyt-Cu/Zn SOD expression level was detected to be extremely lower in sole Al3+-treated plants, while a significantly enhanced expression level was exhibited in combined Si + B-treated plants under aluminum stress. The transcript accumulation of NCED1, NCED6, PYL4, and PYR1 were significantly upregulated in sole Al3+-treated plants and significantly down-regulated in combined Si + B-treated plant roots.
4. Discussion
Excessive metal contamination has destructive impacts on plant growth, physiology, metabolic processes, and productivity. As a toxic metal, aluminum poses a severe threat to agricultural plants and field soil due to excessive acidification by intensive anthropogenic activities and global environmental changes [54]. From past research, it has become evident that the exogenous application of Si can lead to the amelioration of Al toxicity in plants and soil by modulating the soil pH redox potential, enzymatic activities, its accumulation, transportation, and translocation in plants at a cellular level, and the modulation of root-hair morphology, nutrient uptake, and transportation systems [55]. Owing to its monomeric or monosilicic nature, Si is easily absorbed by roots in soil to augment growth attributes and decrease metal accumulation and translocation from roots to shoots, regulating the antioxidant system, metal chelation, and sequestration and inducing the silicon transporters system for alleviating metals stress [56]. However, information is lacking regarding the mitigation and the underlying mechanism of the toxic effects of Al on date palm via the exogenous treatment of Si, particularly in terms of co-synergism with the B application. The findings of the current study illustrate the detrimental effects of Al stress on date palm growth parameters, including shoot and root length, fresh and dry biomass, chlorophyll, and carotenoid contents.
Conversely, the exogenous application of Si to date palm significantly ameliorated the adverse impacts of Al toxicity by enhancing plant growth attributes. The impact of exogenous Si coupled with B was more significant in lessening Al stress in date palm by exhibiting extremely significant growth attributes compared to individual Si and B treatment. The enhanced growth of date palm under Al stress could be attributed to the improved production of chlorophyll (a and b) content and carotenoid levels. Previously, Chen et al. [43] reported that boron and silicon interaction augmented chlorophyll and carotenoids levels in rice and led to improved growth attributes under heavy metals stress. Similarly, an enhanced level of chlorophyll and carotenoids with a significant growth rate was also observed by applying B to sunflower, wheat, and cotton exposed to drought and salinity, respectively [57,58,59].
Moreover, the inhibition of photosynthetic pigments by metals toxicity, including Al stress, is also reported to reduce root and shoot growth in Azolla and Secale cereal plants due to the excessive generation of ROS and interfering in essential nutrients uptake required for chlorophyll synthesis [60,61]. In the current study, the same phenomenon of the degradation photosynthetic pigments was detected in sole Al3+-treated plants, which significantly reduced growth attributes in date palm. However, compared with sole Si, B, and Al3+ treatments, the combined application of Si + B demonstrated the significant mitigation of Al-induced stress and revealed higher photosynthetic pigments for maintaining a better growth rate.
The uptake of Al and its translocation along with Si and B from roots to shoots was scrutinized to gain a better insight into the ameliorating effect of Si coupled with B under Al toxicity in date palm. A significant reduction in Al uptake by roots and its translocation to shoots was observed in sole Si and B treatment compared to sole Al3+ treatment. However, the effect of combined Si + B on inhibiting Al uptake and its translocation was significantly higher than in sole treatments. In parallel, the enhanced deposition of Si in roots and its translocation to the upper part was detected in Si-treated plants, which is known for blocking the passage of lethal cell-degrading ions, such as Al, through forming complexes and the precipitation of metal ions as a co factor [52,62]. Moreover, inhibiting the uptake and translocation of Al by coupled application Si + B could also be ascribed to the significant regulation of organic acids in roots and shoots. It can be assumed from the regulation of organic acids that date palm under Si + B application has adopted an apoplasmic mechanism for restricting Al entry to the cell wall. Previously, for the reduction and inhibition of Al toxicity, the induction of organic acids into the apoplastic spaces for encountering the binding of Al to pectin is reported as the best mechanism of Al tolerance in plants [63,64]. In the current study, the synergistic effect of Si + B significantly enhanced the regulation of aluminum-activated malate transporters genes (ALMT1 and ALMT2), which plants frequently use to exclude Al from the root [65,66]. The significant activation of ALMTs by combined Si and B was also evident from the enhanced production of malic acids and citric acids in roots could be the possible justification for the exclusion of Al from roots cells via encountering the attachment to pectin and thereby lead to the alleviation of the generated toxicity [64,67].
Additionally, the effect of Si, when coupled with B, was more predominant in ameliorating Al toxicity via the upregulation of ALMTs transporters and the activity of silicon efflux transporter (Lsi2) genes, suggesting the intense requirement of Si by tissues for encountering Al toxicity. In line with the current findings, Pontigo et al. [9] reported the upregulation of Silicon efflux transporter genes and silicon uptake for inhibiting Al uptake in ryegrass. Similarly, the overexpression of the ALMT1 gene in blackgram induced organic acid including malate exudation and led to reduced Al3+ uptake and better growth attributes and photosynthetic efficiency. The current findings indicated the Al-mitigating effects of combined Si and B application to date palm via inducing organic acid regulation and silicon efflux transporters to reduce the uptake and accumulation for better plant growth.
Furthermore, plasma membrane ATPases regulation is vital for modulating the plant physiological processes required for resisting or tolerating metals toxicity, including Al stress in plants. The alteration of plasma membrane H+-ATPase-inducing H+ influx is interlinked with the maintaining cytosolic pH, the electrochemical gradient of the plasma membrane, and Al-induced citrate efflux [66]. The current study depicted a strong effect of combined Si and B application on inducing PMMA1 and PMMA3 expression under Al stress, indicating that the higher citric acid production in date palm had subsequently activated plasma membrane H+-ATPase activity for counteracting Al-toxicity. The current results also coincide with Dawood et al. [68], who found suppressed proton adenosine triphosphatase (H+-ATPase) activity barely under aluminum toxicity, leading to the higher accumulation of Al and the inhibition of growth in plants. Moreover, B is also reported to induce root surface alkalization to reduce Al uptake and accumulation in apical zones through the induced auxin efflux transporter and subsequently trigger the downstream regulation of plasma membrane-H+-ATPase [5,69]. The synergistic effects of Si and B highlight their impacts on coping with Al toxicity in date palm via the regulation of plasma membrane H+-ATPase activity and aluminum-activated malate transporter genes via the exudation of organic acids.
Al toxicity is also considered lethal for triggering lipid membrane disruption and producing ROS [70]. The current findings indicated significant lipid peroxidation activities in higher MDA production and O2•− accumulation in the roots and shoots of sole Al3+-treated seedlings. Conversely, the combined application of Si + B demonstrated the significant mitigation of O2•− and MDA by triggering the upregulation of the antioxidant system to combat Al-induced toxicity. The result suggests that the application of Si + B jointly aids plant antioxidative defense systems and provides Al-tolerance to date palm by upregulating the expression of Cyt-Cu/Zn SOD and CAT, thereby inhibiting the ROS indicators i.e., O2•−, and MDA. The in-tandem activation of cyt-Cu/Zn SOD and APX activities by the combined treatment of Si and B is an indicator of conferring resistance to Al toxicity in date palm. In line with our findings, the combined regulation of Cu/Zn SOD and APX significantly improved the tolerance level of transgenic cassava plants to abiotic stress [71]. The activation of the antioxidant system for the amelioration of ROS could also be ascribed to the modulation of the exudation of organic acids such as citric acids, malic acids, and acetic acids via altering the plasma membrane ATPase and aluminium-activated malate transporter gene regulation by combined Si and B effects. In agreement with this, Tahjib-Ul-Arif et al. [72], Zhang et al. [73], and Bilal et al. [70] also revealed the role of the exogenous application and exudation of organic acids, including citric acids, in providing resistance to plants to combat excessive ROS production under abiotic including metals stress. However, further investigation at the transcriptomic level is required to explore the precise mode of action and insights mechanism of the Si-B regulated antioxidant system of the date palm under Al-toxicity.
Stress-signaling endogenous hormonal modulation and crosstalk are vital for regulating plant metabolism under hostile conditions [74], as is the ability of Si to form complexes with stress-inducing heavy metals and inhibit their uptake and translocation, consequently influencing plant defense-related hormonal regulation [28,56,75,76]. The biosynthesis of ABA in plants for imparting tolerance to stresses, including drought, and metals, is of key importance. The role of ABA signaling in activating a plant defense system to varying levels of Al stress has been reported previously [77]. The current study’s findings demonstrated that the synergistic impacts of Si and B resulted in a significant reduction in the ABA level under Al-stress in date palm. Such a reduction in ABA accumulation under Al toxicity could be ascribed to the generation of less oxidative stress, i.e., ROS elimination and the corresponding downregulation of the abscisic acid receptor PYL4 and PYR1 9-cis-epoxycarotenoid dioxygenase 1 and 6 genes. The current findings highlight the Al protective role of synergistic Si and B application by reducing the hazardous effects of Al toxicity for date palm. Consequently, ABA signaling and accumulation were not activated. However, in the case of sole Al3+ plants, the toxicity of Al led to the significantly higher regulation of NCED genes along with PYL4 and PYR1, leading to the enhanced biosynthesis of ABA to activate the plant defense system to cope with the generated stress. These findings indicate that the combined effect of Si + B induced the ABA-independent pathway in date palm to withstand Al-induced toxicity [78,79].
Moreover, the higher uptake of silicon in combined application compared to the sole application of Si and B could also be attributed to the reduced ABA activities ( Tripathi et al. [80] have also described the effect of silicon uptake on reducing ABA activities under abiotic stress in plants). Moreover, the crosstalk of endogenous SA with ABA is crucial for regulating plant growth and detoxifying excessive ROS under abiotic stresses [81]. The current findings describe the antagonistic trend of SA accumulation compared to reduced ABA accumulation under Al toxicity in combined Si- and B-treated plants. The increase in SA accumulation under combined treatment is reported to contribute to the formation of a protective layer on the leaves; the improvement of the antioxidant system; and the reduction of the lipid peroxidation by the regulation of phenolic compounds, flavonoids, and PAL activities [82]. Contrarily to the current increase of SA content by the synergistic effect of Si and B, the authors of [83] described a significant reduction in SA accumulation under heavy metal toxicity by exogenous Si treatment in rice plants. Such higher regulation of SA accumulation, particularly in roots by the synergistic effect of Si and B, could be the reason for the excessive exudation of organic acids such as citric acid and indicate SA involvement in encountering Al toxicity and thereby enhancing plant tolerance and growth attributes to the stress condition. In concurring with the current findings, the higher regulation of SA is also reported for mediating Al tolerance to plants via modulating citrate efflux from roots and initiating the SA signal transduction pathway in Cassia tora L. plants [84]. The effect of B uptake and accumulation under the combined treatment of Si and B in the control and the Al stress condition could also be ascribed to the higher endogenous SA accumulation in date palm as the uptake and translocation of B are reported to enhance endogenous SA content in plants for modulating plant growth and defense systems under hostile conditions [85]. The current findings demonstrated the ameliorative effects of the synergistic application of Si and B for modulating regulatory hormonal systems to combat Al-toxicity and improve plant physiological processes.
5. Conclusions
The current study revealed the significant growth and biomass enhancement of date palm under toxic effects of Al by the synergistic impacts of Si and B. The synergism of Si and B led to the enhancement of Si accumulation for boosting plant growth and modulating physiological and biochemical alterations in date palm. The synergistic performance of Si and B was characterized by reducing Al3+ uptake and translocation in date palm. Such a response was associated with the differential modulation of ALMTs and PMMAs, thereby inducing the exudation of organic acids such as malic acid and citric acids for counteracting Al3+-induced toxicity in soil. In parallel, Al3+ induced oxidative stress was alleviated by lowering MDA and O2•– via the enhanced regulation of CAT; Cyt-Cu/Zn SOD; and CAT, POD, and APX activities. Likewise, the hazardous effect of Al toxicity was alleviated by combined Si and B application through the downregulation of the abscisic acid receptors PYL4 and PYR1 and the NCED1 and NCED 6 genes, resulting in the lower accumulation of endogenous ABA and the marked regulation of SA. The combined application of Si and B convened significant tolerance in date palm to combat the Al stress-related damages by maintaining plant growth attributes and the organic acids’ exudation and regulation of the oxidative metabolism for triggering hormonal cross talk. Furthermore, the investigation is required at a transcriptomic level to dissect the underlying mechanism of Si and B interaction for ameliorating Al-induced damages in date palm.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox11061063/s1, Supplementary file S1: ABA, SA analysis methodology; Table S1: The gene name, gene description, product size, reference number and oligonucleotide sequences used for qRT PCR.
Author Contributions
Conceptualization, A.L.K.; formal analysis, A.K.; investigation, A.A.-R.; methodology, A.K., M.I. and M.S.A.A.-A. supervision, A.L.K. and I.-J.L.; validation, S.A. and A.L.K.; writing—original draft, S.B.; review and editing, A.L.K., S.B.; and data curation, A.A.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIT) (No. 2022R1A2C1008993).
Data Availability Statement
Data is contained within the article or Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, S.; Ren, X.; Huang, B.; Wang, G.; Zhou, P.; An, Y. Aluminium-induced reduction of plant growth in alfalfa (Medicago sativa) is mediated by interrupting auxin transport and accumulation in roots. Sci. Rep. 2016, 6, 30079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panda, S.K.; Baluška, F.; Matsumoto, H. Aluminum stress signaling in plants. Plant Signal. Behav. 2009, 4, 592–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miotto, A.; Tiecher, T.; Kaminski, J.; Brunetto, G.; De Conti, L.; Tiecher, T.L.; Martins, A.P.; Rheinheimer dos Santos, D. Soil acidity and aluminum speciation affected by liming in the conversion of a natural pasture from the Brazilian Campos Biome into no-tillage system for grain production. Arch. Agron. Soil Sci. 2020, 66, 138–151. [Google Scholar] [CrossRef]
- Alarcón-Poblete, E.; Inostroza-Blancheteau, C.; Alberdi, M.; Rengel, Z.; Reyes-Díaz, M. Molecular regulation of aluminum resistance and sulfur nutrition during root growth. Planta 2018, 247, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Mai, J.; Tao, L.; Qu, M.; Liu, J.; Shen, R.; Xu, G.; Feng, Y.; Xiao, H. Boron alleviates aluminum toxicity by promoting root alkalization in transition zone via polar auxin transport. Plant Physiol. 2018, 177, 1254–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribera-Fonseca, A.; Rumpel, C.; de la Luz Mora, M.; Nikolic, M.; Cartes, P. Sodium silicate and calcium silicate differentially affect silicon and aluminium uptake, antioxidant performance and phenolics metabolism of ryegrass in an acid Andisol. Crop Pasture Sci. 2018, 69, 205–215. [Google Scholar] [CrossRef]
- Gupta, N.; Gaurav, S.S.; Kumar, A. Molecular basis of aluminium toxicity in plants: A review. Am. J. Plant Sci. 2013, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Tripathi, D.K.; Singh, S.; Sharma, S.; Dubey, N.K.; Chauhan, D.K.; Vaculík, M. Toxicity of aluminium on various levels of plant cells and organism: A review. Environ. Exp. Bot. 2017, 137, 177–193. [Google Scholar] [CrossRef]
- Pontigo, S.; Godoy, K.; Jiménez, H.; Gutiérrez-Moraga, A.; Mora, M.D.L.L.; Cartes, P. Silicon-mediated alleviation of aluminum toxicity by modulation of Al/Si uptake and antioxidant performance in ryegrass plants. Front. Plant Sci. 2017, 8, 642. [Google Scholar] [CrossRef] [Green Version]
- Ashenef, A. Essential and toxic metals in tea (Camellia sinensis) imported and produced in Ethiopia. Food Addit. Contam. Part B 2014, 7, 30–36. [Google Scholar] [CrossRef]
- Bojórquez-Quintal, E.; Escalante-Magaña, C.; Echevarría-Machado, I.; Martínez-Estévez, M. Aluminum, a friend or foe of higher plants in acid soils. Front. Plant Sci. 2017, 8, 1767. [Google Scholar] [CrossRef] [PubMed]
- Bilal, S.; Shahzad, R.; Khan, A.L.; Kang, S.-M.; Imran, Q.M.; Al-Harrasi, A.; Yun, B.-W.; Lee, I.-J. Endophytic microbial consortia of phytohormones-producing fungus Paecilomyces formosus LHL10 and bacteria Sphingomonas sp. LK11 to Glycine max L. regulates physio-hormonal changes to attenuate aluminum and zinc stresses. Front. Plant Sci. 2018, 9, 1273. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, N.; Kar, D.; Deepak Mahajan, B.; Nanda, S.; Rahiman, R.; Panchakshari, N.; Bhagavatula, L.; Datta, S. The multitasking abilities of MATE transporters in plants. J. Exp. Bot. 2019, 70, 4643–4656. [Google Scholar] [CrossRef]
- Zhang, J.; Li, D.; Wei, J.; Ma, W.; Kong, X.; Rengel, Z.; Chen, Q. Melatonin alleviates aluminum-induced root growth inhibition by interfering with nitric oxide production in Arabidopsis. Environ. Exp. Bot. 2019, 161, 157–165. [Google Scholar] [CrossRef]
- Jones, D.; Blancaflor, E.; Kochian, L.; Gilroy, S. Spatial coordination of aluminium uptake, production of reactive oxygen species, callose production and wall rigidification in maize roots. Plant Cell Environ. 2006, 29, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Borsani, O.; Valpuesta, V.; Botella, M.A. Evidence for a Role of Salicylic Acid in the Oxidative Damage Generated by NaCl and Osmotic Stress in Arabidopsis Seedlings. Plant Physiol. 2001, 126, 1024–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cartes, P.; McManus, M.; Wulff-Zottele, C.; Leung, S.; Gutiérrez-Moraga, A.; de la Luz Mora, M. Differential superoxide dismutase expression in ryegrass cultivars in response to short term aluminium stress. Plant Soil 2012, 350, 353–363. [Google Scholar] [CrossRef]
- Riaz, M.; Yan, L.; Wu, X.; Hussain, S.; Aziz, O.; Wang, Y.; Imran, M.; Jiang, C. Boron alleviates the aluminum toxicity in trifoliate orange by regulating antioxidant defense system and reducing root cell injury. J. Environ. Manag. 2018, 208, 149–158. [Google Scholar] [CrossRef]
- Ribeiro, M.A.Q.; Almeida, A.-A.F.d.; Mielke, M.S.; Gomes, F.P.; Pires, M.V.; Baligar, V.C. Aluminum effects on growth, photosynthesis, and mineral nutrition of cacao genotypes. J. Plant Nutr. 2013, 36, 1161–1179. [Google Scholar] [CrossRef]
- Montpetit, J.; Vivancos, J.; Mitani-Ueno, N.; Yamaji, N.; Rémus-Borel, W.; Belzile, F.; Ma, J.F.; Bélanger, R.R. Cloning, functional characterization and heterologous expression of TaLsi1, a wheat silicon transporter gene. Plant Mol. Biol. 2012, 79, 35–46. [Google Scholar] [CrossRef]
- Shahnaz, G.; Shekoofeh, E.; Kourosh, D.; Moohamadbagher, B. Interactive effects of silicon and aluminum on the malondialdehyde (MDA), proline, protein and phenolic compounds in Borago officinalis L. J. Med. Plants Res. 2011, 5, 5818–5827. [Google Scholar]
- Tripathi, D.K.; Singh, V.P.; Ahmad, P.; Chauhan, D.K.; Prasad, S.M. Silicon in Plants: Advances and Future Prospects; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Kopittke, P.M.; Gianoncelli, A.; Kourousias, G.; Green, K.; McKenna, B.A. Alleviation of Al Toxicity by Si Is Associated with the Formation of Al–Si Complexes in Root Tissues of Sorghum. Front. Plant Sci. 2017, 8, 2189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirisuntornlak, N.; Ullah, H.; Sonjaroon, W.; Anusontpornperm, S.; Arirob, W.; Datta, A. Interactive effects of silicon and soil pH on growth, yield and nutrient uptake of maize. Silicon 2021, 13, 289–299. [Google Scholar] [CrossRef]
- Pavlovic, J.; Kostic, L.; Bosnic, P.; Kirkby, E.A.; Nikolic, M. Interactions of silicon with essential and beneficial elements in plants. Front. Plant Sci. 2021, 12, 1224. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Khan, A.L.; Lee, I.-J. Silicon: A duo synergy for regulating crop growth and hormonal signaling under abiotic stress conditions. Crit. Rev. Biotechnol. 2016, 36, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Khan, A.L.; Waqas, M.; Lee, I.-J. Silicon regulates antioxidant activities of crop plants under abiotic-induced oxidative stress: A review. Front. Plant Sci. 2017, 8, 510. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, D.; Bashri, G.; Shweta, S.; Ahmad, P.; Singh, V. Efficacy of silicon against aluminum toxicity in plants: An overview. Silicon Plants: Adv. Future Prospect. 2017, 1, 355–366. [Google Scholar]
- Zhou, X.-X.; Yang, L.-T.; Qi, Y.-P.; Guo, P.; Chen, L.-S. Mechanisms on boron-induced alleviation of aluminum-toxicity in Citrus grandis seedlings at a transcriptional level revealed by cDNA-AFLP analysis. PLoS ONE 2015, 10, e0115485. [Google Scholar] [CrossRef] [Green Version]
- Li, X.W.; Liu, J.Y.; Fang, J.; Tao, L.; Shen, R.F.; Li, Y.L.; Xiao, H.D.; Feng, Y.M.; Wen, H.X.; Guan, J.H. Boron supply enhances aluminum tolerance in root border cells of pea (Pisum sativum) by interacting with cell wall pectins. Front. Plant Sci. 2017, 8, 742. [Google Scholar] [CrossRef] [Green Version]
- Riaz, M.; Yan, L.; Wu, X.; Hussain, S.; Aziz, O.; Imran, M.; Rana, M.S.; Jiang, C. Boron reduces aluminum-induced growth inhibition, oxidative damage and alterations in the cell wall components in the roots of trifoliate orange. Ecotoxicol. Environ. Saf. 2018, 153, 107–115. [Google Scholar] [CrossRef]
- Riaz, M.; Yan, L.; Wu, X.; Hussain, S.; Aziz, O.; Jiang, C. Boron deprivation induced inhibition of root elongation is provoked by oxidative damage, root injuries and changes in cell wall structure. Environ. Exp. Bot. 2018, 156, 74–85. [Google Scholar] [CrossRef]
- Riaz, M.; Yan, L.; Wu, X.; Hussain, S.; Aziz, O.; Jiang, C. Boron increases root elongation by reducing aluminum induced disorganized distribution of HG epitopes and alterations in subcellular cell wall structure of trifoliate orange roots. Ecotoxicol. Environ. Saf. 2018, 165, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Riaz, M.; Liu, Y.; Zeng, Y.; Jiang, C. Aluminum toxicity could be mitigated with boron by altering the metabolic patterns of amino acids and carbohydrates rather than organic acids in trifoliate orange. Tree Physiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.Q.; Cao, X.C.; Zhu, L.F.; Hu, W.J.; Hu, A.Y.; Abliz, B.; Bai, Z.G.; Huang, J.; Liang, Q.D.; Sajid, H. Boron reduces cell wall aluminum content in rice (Oryza sativa) roots by decreasing H2O2 accumulation. Plant Physiol. Biochem. 2019, 138, 80–90. [Google Scholar] [CrossRef]
- Al-Yahyai, R.; Khan, M.M. Date palm status and perspective in Oman. In Date Palm Genetic Resources and Utilization; Springer: Berlin/Heidelberg, Germany, 2015; pp. 207–240. [Google Scholar]
- Yaish, M.W.; Kumar, P.P. Salt tolerance research in date palm tree (Phoenix dactylifera L.), past, present, and future perspectives. Front. Plant Sci. 2015, 6, 348. [Google Scholar] [CrossRef] [Green Version]
- Alansi, S.; Al-Qurainy, F.; Nadeem, M.; Khan, S.; Tarroum, M.; Alshameri, A.; Gaafar, A.-R.Z. Cryopreservation: A tool to conserve date palm in Saudi Arabia. Saudi J. Biol. Sci. 2019, 26, 1896–1902. [Google Scholar] [CrossRef]
- Chaâbene, Z.; Rorat, A.; Hakim, I.R.; Bernard, F.; Douglas, G.C.; Elleuch, A.; Vandenbulcke, F.; Mejdoub, H. Insight into the expression variation of metal-responsive genes in the seedling of date palm (Phoenix dactylifera). Chemosphere 2018, 197, 123–134. [Google Scholar] [CrossRef]
- Awad, K.M.; Salih, A.M.; Khalaf, Y.; Suhim, A.A.; Abass, M.H. Phytotoxic and genotoxic effect of Aluminum to date palm (Phoenix dactylifera L.) in vitro cultures. J. Genet. Eng. Biotechnol. 2019, 17, 1–8. [Google Scholar] [CrossRef]
- Khan, A.; Bilal, S.; Khan, A.L.; Imran, M.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.-J. Silicon-mediated alleviation of combined salinity and cadmium stress in date palm (Phoenix dactylifera L.) by regulating physio-hormonal alteration. Ecotoxicol. Environ. Saf. 2020, 188, 109885. [Google Scholar] [CrossRef]
- Khan, A.; Kamran, M.; Imran, M.; Al-Harrasi, A.; Al-Rawahi, A.; Al-Amri, I.; Lee, I.-J.; Khan, A.L. Silicon and salicylic acid confer high-pH stress tolerance in tomato seedlings. Sci. Rep. 2019, 9, 1–16. [Google Scholar]
- Chen, D.; Chen, D.; Xue, R.; Long, J.; Lin, X.; Lin, Y.; Jia, L.; Zeng, R.; Song, Y. Effects of boron, silicon and their interactions on cadmium accumulation and toxicity in rice plants. J. Hazard. Mater. 2019, 367, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. ISSN 2014, 2231, 606X. [Google Scholar]
- Bilal, S.; Shahzad, R.; Khan, A.L.; Al-Harrasi, A.; Kim, C.K.; Lee, I.-J. Phytohormones enabled endophytic Penicillium funiculosum LHL06 protects Glycine max L. from synergistic toxicity of heavy metals by hormonal and stress-responsive proteins modulation. J. Hazard. Mater. 2019, 379, 120824. [Google Scholar] [CrossRef]
- Khan, A.; Bilal, S.; Khan, A.L.; Imran, M.; Shahzad, R.; Al-Harrasi, A.; Al-Rawahi, A.; Al-Azhri, M.; Mohanta, T.K.; Lee, I.-J. Silicon and gibberellins: Synergistic function in harnessing ABA signaling and heat stress tolerance in date palm (Phoenix dactylifera L.). Plants 2020, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, E.; Skłodowska, M. Differential biochemical responses of wheat shoots and roots to nickel stress: Antioxidative reactions and proline accumulation. Plant Growth Regul. 2008, 54, 179–188. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Ali, A.; Bilal, S.; Khan, A.L.; Mabood, F.; Al-Harrasi, A.; Lee, I.-J. Endophytic Aureobasidium pullulans BSS6 assisted developments in phytoremediation potentials of Cucumis sativus under Cd and Pb stress. J. Plant Interact. 2019, 14, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Bilal, S.; Khan, A.L.; Waqas, M.; Shahzad, R.; Kim, I.-D.; Lee, I.-J.; Shin, D.-H. Biochemical constituents and in vitro antioxidant and anticholinesterase potential of seeds from native Korean persimmon genotypes. Molecules 2016, 21, 893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahzad, R.; Khan, A.L.; Waqas, M.; Ullah, I.; Bilal, S.; Kim, Y.-H.; Asaf, S.; Kang, S.-M.; Lee, I.-J. Metabolic and proteomic alteration in phytohormone-producing endophytic Bacillus amyloliquefaciens RWL-1 during methanol utilization. Metabolomics 2019, 15, 16. [Google Scholar] [CrossRef]
- Seskar, M.; Shulaev, V.; Raskin, I. Endogenous methyl salicylate in pathogen-inoculated tobacco plants. Plant Physiol. 1998, 116, 387–392. [Google Scholar] [CrossRef] [Green Version]
- Bilal, S.; Khan, A.L.; Shahzad, R.; Kim, Y.-H.; Imran, M.; Khan, M.J.; Al-Harrasi, A.; Kim, T.H.; Lee, I.-J. Mechanisms of Cr (VI) resistance by endophytic Sphingomonas sp. LK11 and its Cr (VI) phytotoxic mitigating effects in soybean (Glycine max L.). Ecotoxicol. Environ. Saf. 2018, 164, 648–658. [Google Scholar] [CrossRef]
- Shetty, R.; Vidya, C.S.-N.; Prakash, N.B.; Lux, A.; Vaculík, M. Aluminum toxicity in plants and its possible mitigation in acid soils by biochar: A review. Sci. Total Environ. 2021, 765, 142744. [Google Scholar] [CrossRef] [PubMed]
- Hodson, M.J.; Evans, D.E. Aluminium–silicon interactions in higher plants: An update. J. Exp. Bot. 2020, 71, 6719–6729. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Awan, S.A.; Rizwan, M.; Ali, S.; Hassan, M.J.; Brestic, M.; Zhang, X.; Huang, L. Effects of silicon on heavy metal uptake at the soil-plant interphase: A review. Ecotoxicol. Environ. Saf. 2021, 222, 112510. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, M.A.; Maqsood, M.; Nawaz, F.; Abbas, T.; Yasin, S. Boron-induced improvement in physiological, biochemical and growth attributes in sunflower (Helianthus annuus L.) exposed to terminal drought stress. J. Plant Nutr. 2018, 41, 943–955. [Google Scholar] [CrossRef]
- Abdel-Motagally, F.; El-Zohri, M. Improvement of wheat yield grown under drought stress by boron foliar application at different growth stages. J. Saudi Soc. Agric. Sci. 2018, 17, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Azeem, M.; Shoujun, Y.; Qasim, M.; Abbasi, M.W.; Ahmed, N.; Hanif, T.; Adnan, M.Y.; Ahmad, R.; Dong, R. Foliar enrichment of potassium and boron overcomes salinity barriers to improve growth and yield potential of cotton (Gossypium hirsutum L.). J. Plant Nutr. 2021, 44, 438–454. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mishra, A.; Verma, E.; Tiwari, B.; Mishra, A.K.; Singh, S.S. Physiological mechanisms of aluminum (Al) toxicity tolerance in nitrogen-fixing aquatic macrophyte Azolla microphylla Kaulf: Phytoremediation, metabolic rearrangements, and antioxidative enzyme responses. Environ. Sci. Pollut. Res. 2019, 26, 9041–9054. [Google Scholar] [CrossRef]
- Silva, S.; Pinto, G.; Dias, M.C.; Correia, C.M.; Moutinho-Pereira, J.; Pinto-Carnide, O.; Santos, C. Aluminium long-term stress differently affects photosynthesis in rye genotypes. Plant Physiol. Biochem. 2012, 54, 105–112. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Xie, Y.; Sangari, S. Silicon mechanisms to ameliorate heavy metal stress in plants. BioMed Res. Int. 2018, 2018, 8492898. [Google Scholar] [CrossRef] [Green Version]
- Ikegawa, H.; Yamamoto, Y.; Matsumoto, H. Responses to aluminium of suspension-cultured tobacco cells in a simple calcium solution. Soil Sci. Plant Nutr. 2000, 46, 503–514. [Google Scholar]
- Chauhan, D.K.; Yadav, V.; Vaculík, M.; Gassmann, W.; Pike, S.; Arif, N.; Singh, V.P.; Deshmukh, R.; Sahi, S.; Tripathi, D.K. Aluminum toxicity and aluminum stress-induced physiological tolerance responses in higher plants. Crit. Rev. Biotechnol. 2021, 41, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-L.; Fan, W.; Zheng, S.-J. Mechanisms and regulation of aluminum-induced secretion of organic acid anions from plant roots. J. Zhejiang Univ.-Sci. B 2019, 20, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Long, Y.; Huang, J.; Xia, J. Molecular mechanisms for coping with Al toxicity in plants. Int. J. Mol. Sci. 2019, 20, 1551. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.F.; Ryan, P.R.; Delhaize, E. Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci. 2001, 6, 273–278. [Google Scholar] [CrossRef]
- Dawood, M.; Cao, F.; Jahangir, M.M.; Zhang, G.; Wu, F. Alleviation of aluminum toxicity by hydrogen sulfide is related to elevated ATPase, and suppressed aluminum uptake and oxidative stress in barley. J. Hazard. Mater. 2012, 209, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.A.; Hippler, F.W.; Prado, L.A.d.S.; Rima, J.A.; Boaretto, R.M.; Quaggio, J.A.; Façanha, A.R.; Mattos-Jr, D. Boron modulates the plasma membrane H+-ATPase activity affecting nutrient uptake of Citrus trees. Ann. Appl. Biol. 2021, 178, 293–303. [Google Scholar] [CrossRef]
- Bilal, S.; Shahzad, R.; Lee, I.-J. Synergistic interaction of fungal endophytes, Paecilomyces formosus LHL10 and Penicillium funiculosum LHL06, in alleviating multi-metal toxicity stress in Glycine max L. Environ. Sci. Pollut. Res. 2021, 28, 67429–67444. [Google Scholar] [CrossRef]
- Xu, J.; Yang, J.; Duan, X.; Jiang, Y.; Zhang, P. Increased expression of native cytosolic Cu/Zn superoxide dismutase and ascorbate peroxidase improves tolerance to oxidative and chilling stresses in cassava (Manihot esculenta Crantz). BMC Plant Biol. 2014, 14, 1–14. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Zahan, M.; Karim, M.; Imran, S.; Hunter, C.T.; Islam, M.; Mia, M.; Hannan, M.; Rhaman, M.S.; Hossain, M. Citric acid-mediated abiotic stress tolerance in plants. Int. J. Mol. Sci. 2021, 22, 7235. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Liu, X.; Chen, X.; Liu, L.; Niu, Y.; Wang, R. The relief effects of organic acids on Scirpus triqueter L. under pyrene–lead stress. Environ. Sci. Pollut. Res. 2019, 26, 15828–15837. [Google Scholar] [CrossRef]
- Shahzad, R.; Bilal, S.; Imran, M.; Khan, A.L.; Alosaimi, A.A.; Al-Shwyeh, H.A.; Almahasheer, H.; Rehman, S.; Lee, I.-J. Amelioration of heavy metal stress by endophytic Bacillus amyloliquefaciens RWL-1 in rice by regulating metabolic changes: Potential for bacterial bioremediation. Biochem. J. 2019, 476, 3385–3400. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, A.L.; Imran, M.; Asaf, S.; Kim, Y.-H.; Bilal, S.; Numan, M.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.-J. Silicon-induced thermotolerance in Solanum lycopersicum L. via activation of antioxidant system, heat shock proteins, and endogenous phytohormones. BMC Plant Biol. 2020, 20, 248. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, A.L.; Muneer, S.; Kim, Y.-H.; Al-Rawahi, A.; Al-Harrasi, A. Silicon and salinity: Crosstalk in crop-mediated stress tolerance mechanisms. Front. Plant Sci. 2019, 10, 1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranjan, A.; Sinha, R.; Sharma, T.R.; Pattanayak, A.; Singh, A.K. Alleviating aluminum toxicity in plants: Implications of reactive oxygen species signaling and crosstalk with other signaling pathways. Physiol. Plant. 2021, 173, 1765–1784. [Google Scholar] [CrossRef]
- Shukla, P.S.; Shotton, K.; Norman, E.; Neily, W.; Critchley, A.T.; Prithiviraj, B. Seaweed extract improve drought tolerance of soybean by regulating stress-response genes. AoB Plants 2018, 10, plx051. [Google Scholar] [CrossRef]
- Bilal, S.; Shahzad, R.; Imran, M.; Jan, R.; Kim, K.M.; Lee, I.-J. Synergistic association of endophytic fungi enhances Glycine max L. resilience to combined abiotic stresses: Heavy metals, high temperature and drought stress. Ind. Crops Prod. 2020, 143, 111931. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Vishwakarma, K.; Singh, V.P.; Prakash, V.; Sharma, S.; Muneer, S.; Nikolic, M.; Deshmukh, R.; Vaculík, M.; Corpas, F.J. Silicon crosstalk with reactive oxygen species, phytohormones and other signaling molecules. J. Hazard. Mater. 2020, 408, 124820. [Google Scholar] [CrossRef]
- Jogawat, A.; Yadav, B.; Lakra, N.; Singh, A.K.; Narayan, O.P. Crosstalk between phytohormones and secondary metabolites in the drought stress tolerance of crop plants: A review. Physiol. Plant. 2021, 172, 1106–1132. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Ashfaque, F.; Chhillar, H.; Irfan, M.; Khan, N.A. The intricacy of silicon, plant growth regulators and other signaling molecules for abiotic stress tolerance: An entrancing crosstalk between stress alleviators. Plant Physiol. Biochem. 2021, 162, 36–47. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Khan, A.L.; Kim, D.-H.; Lee, S.-Y.; Kim, K.-M.; Waqas, M.; Jung, H.-Y.; Shin, J.-H.; Kim, J.-G.; Lee, I.-J. Silicon mitigates heavy metal stress by regulating P-type heavy metal ATPases, Oryza sativa low silicon genes, and endogenous phytohormones. BMC Plant Biol. 2014, 14, 13. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.-M.; Wang, J.; Wang, S.-H.; Xu, L.-L. Salicylic acid-induced aluminum tolerance by modulation of citrate efflux from roots of Cassia tora L. Planta 2003, 217, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, K. Transcriptomic and hormonal control of boron uptake, accumulation and toxicity tolerance in poplar. Environ. Exp. Bot. 2017, 141, 60–73. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).