Antioxidant and Neuroprotective Effect of a Grape Pomace Extract on Oxaliplatin-Induced Peripheral Neuropathy in Rats: Biochemical, Behavioral and Histopathological Evaluation
Abstract
1. Introduction
2. Materials and Methods
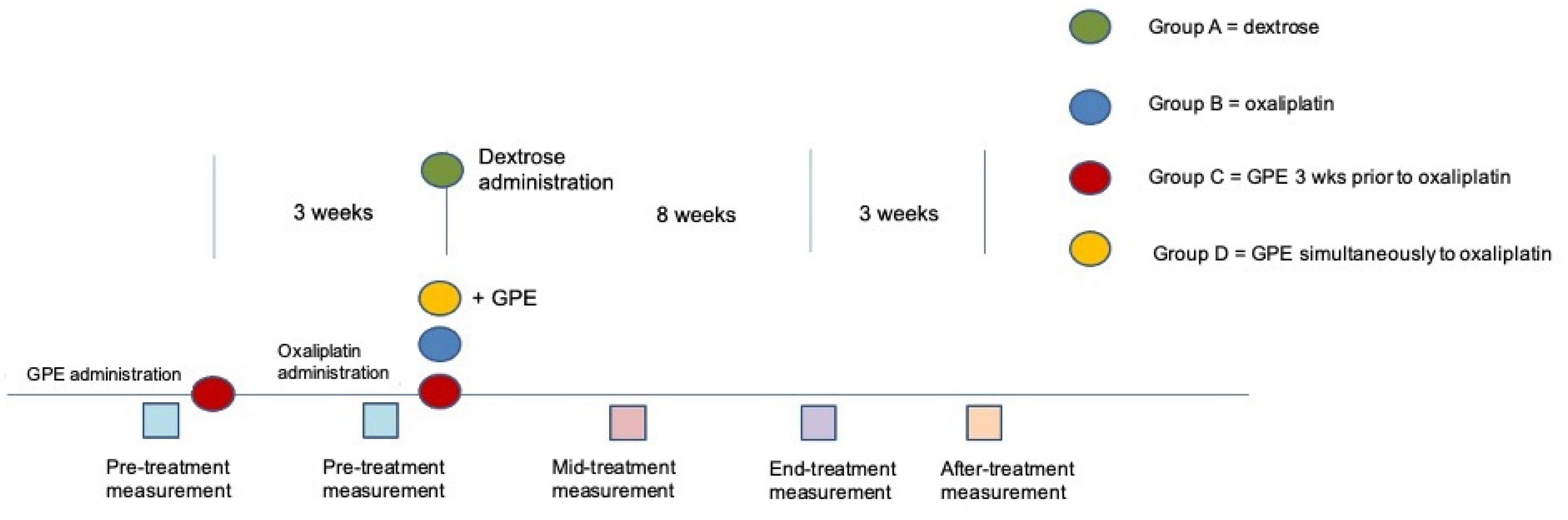
2.1. Animals and Experimental Design
2.2. Oxaliplatin Preparation
2.3. Grape Pomace Extract Preparation
2.4. UHPLC-HRMS Analysis of the Grape Extract
2.5. Grape Pomace Extract Administration
2.6. Behavioral Evaluation
2.7. Blood Sample Collection and Oxidative Stress Biomarkers Evaluation
2.8. Tissue Preparation and Immunohistochemistry
2.9. Morphometrical Analysis
2.10. Statistical Analysis
3. Results
3.1. Oxaliplatin and GPE Administration Effect on General Appearance, Locomotion and Exploratory Activity of Experimental Animals
3.2. Oxaliplatin Administration and GPE Consumption Have No Effect on Body Weight
3.3. Animals Receiving GPE Show NORMAL Sensorimotor Reflexes Whereas in Animals Receiving Only Oxaliplatin Certain Reflexes Were Severely Harmed
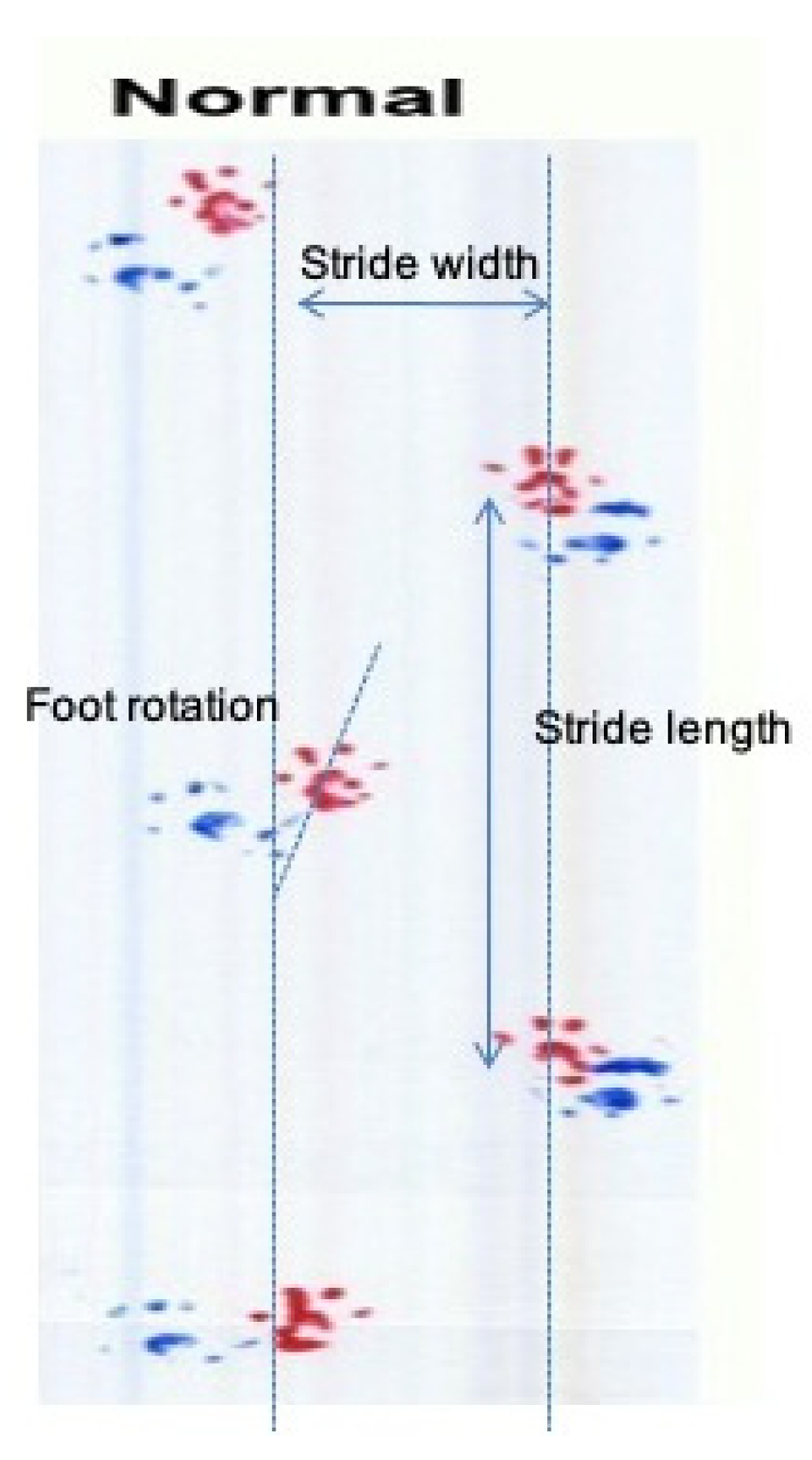
3.4. GPE Administration Has Not Altered Gait Analysis Measurements of Oxaliplatin Treated Animals
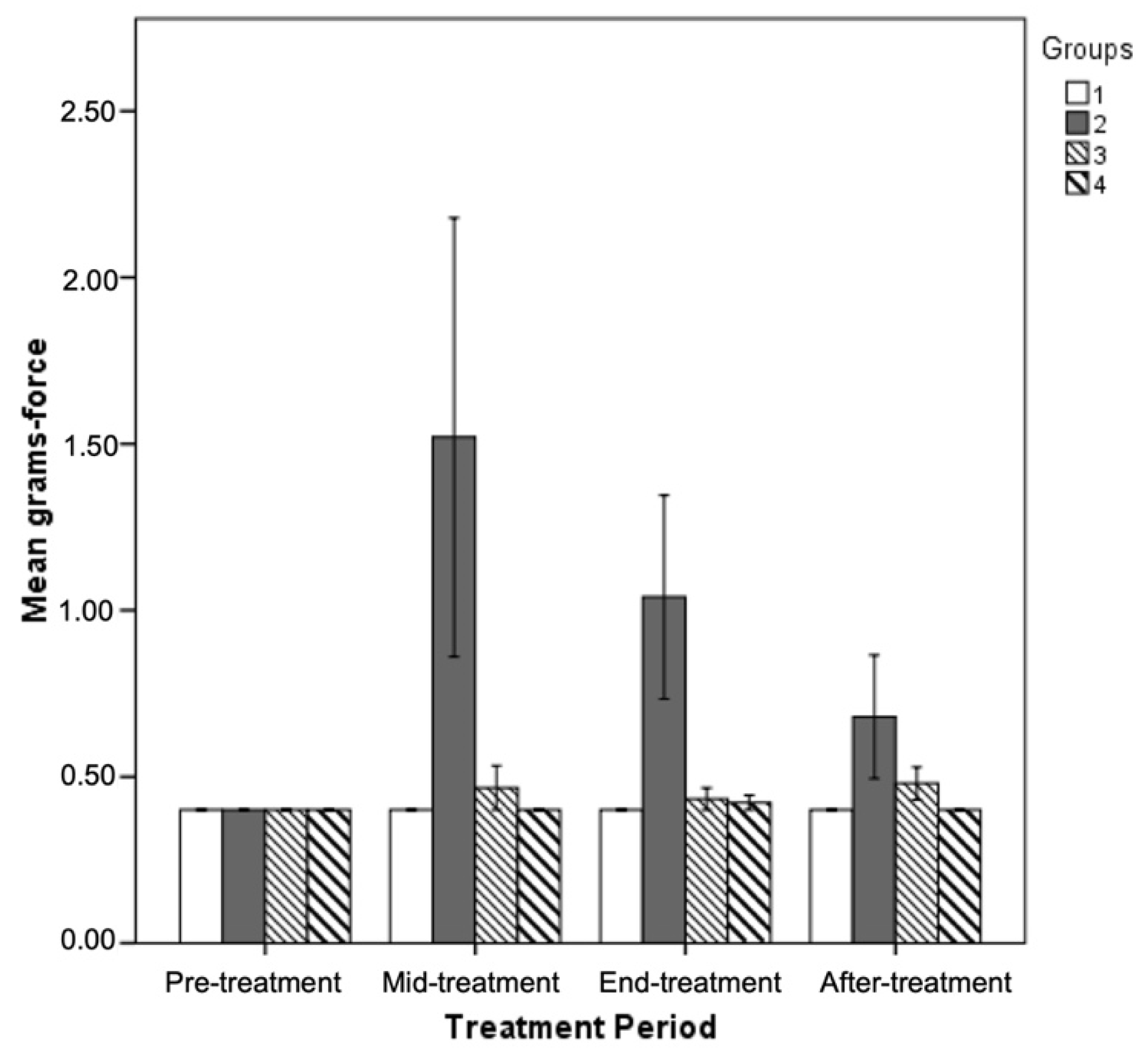
3.5. Effect of GPE Administration on Sensorimotor Tasks
3.6. Oxidative Stress Biomarkers Evaluation
3.7. Oxaliplatin or GPE Administration Have No Effect on Total Number of Lumbar DRG Sensory Neurons
3.8. GPE Protects Large Lumbar DRG Sensory Neurons from Oxaliplatin Induced Atrophy
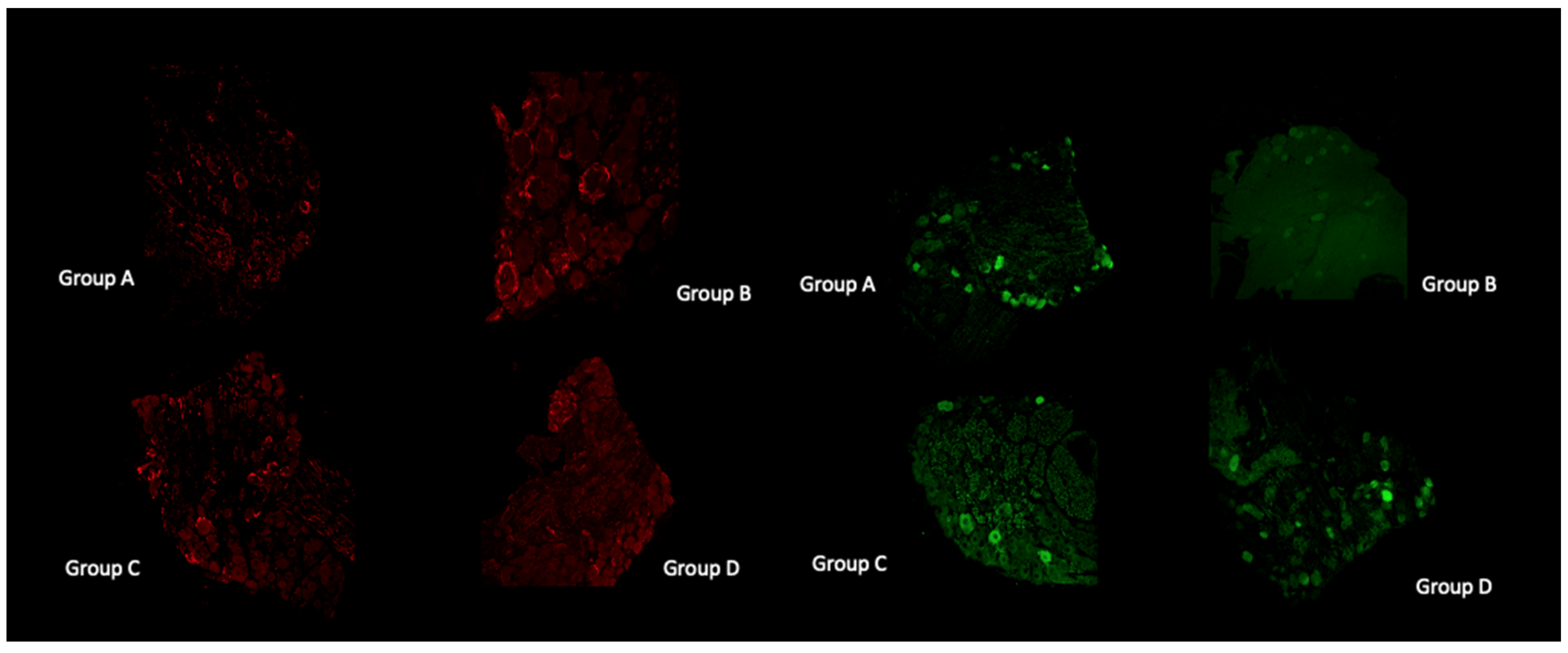
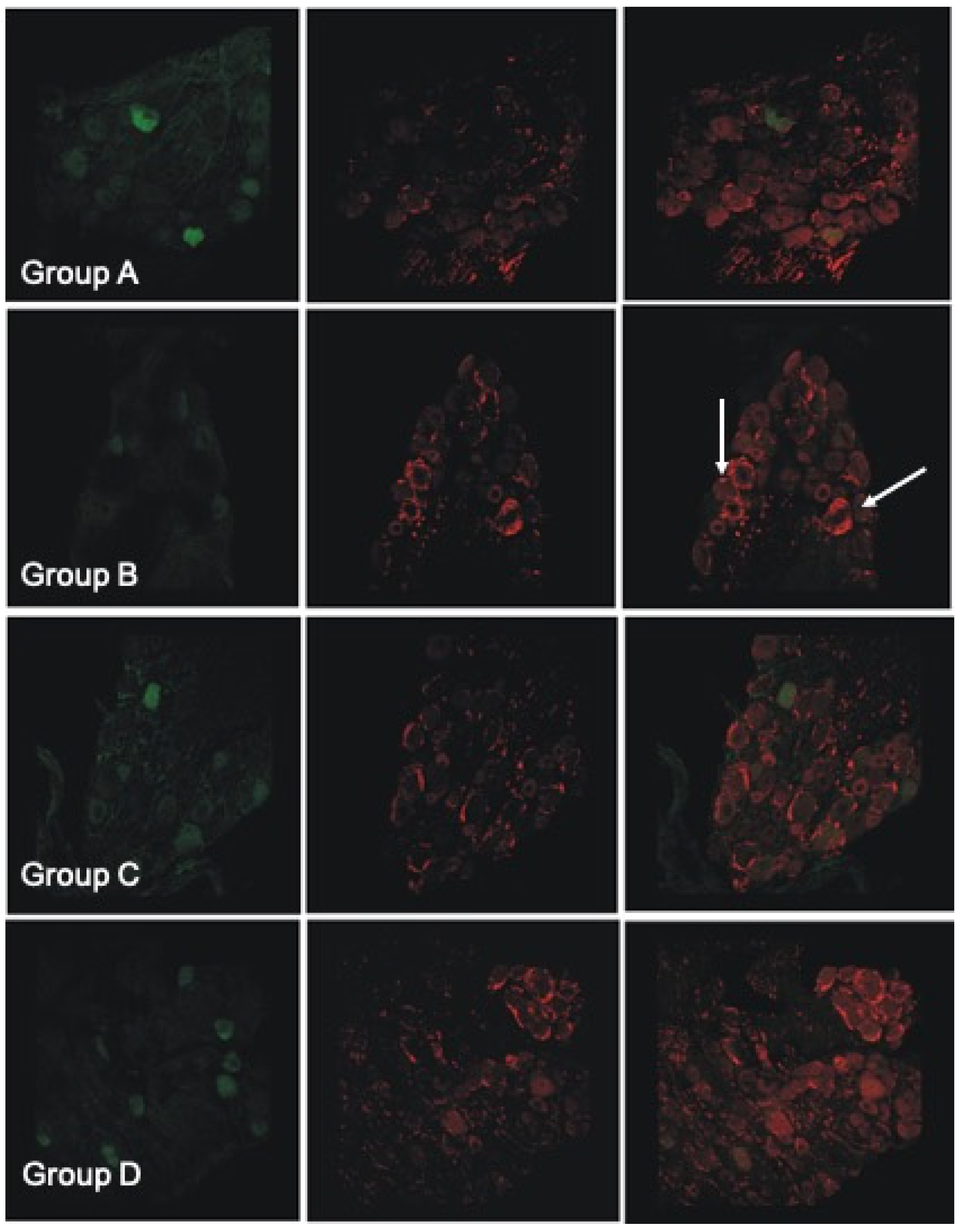
3.9. Histological and Immunofluorescent Staining Revealed Increase in the Satellite GFAP-Stained Cells in the Oxaliplatin Alone Treated Animals
4. Discussion
4.1. Oxaliplatin and GPE Administration Effect on General Appearance, Locomotion and Exploratory Activity of Experimental Animals
4.2. Oxaliplatin Administration and GPE Consumption Have No Effect on Body Weight
4.3. GPE Rescues the Oxaliplatin-Induced Damage on Corticospinal Funtion
4.4. Oxaliplatin and GPE Administration Has Not Altered Gait Analysis Measurements of Oxaliplatin Treated Animals
4.5. Beneficial Effect of GPE Administration on Sensorimotor Tasks
4.6. GPE Effect on the Oxidative STRESS Induced by Oxaliplatin
4.7. Oxaliplatin or GPE Administration Have No Effect on Total Number of Lumbar DRG Sensory Neurons
4.8. GPE Protects Large Lumbar DRG Sensory Neurons from Oxaliplatin Induced Atrophy
4.9. Histological and Immunofluorescent Staining Revealed Increase in the Satellite GFAP-Stained Cells in the Oxaliplatin Alone Treated Animals
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dieras, V.; Bougnoux, P.; Petit, T.; Chollet, P.; Beuzeboc, P.; Borel, C.; Husseini, F.; Goupil, A.; Kebrat, P.; Misset, J.L.; et al. Multicentre Phase II Study of Oxaliplatin as a Single-Agent in Cisplatin/Carboplatin ± Taxane-Pretreated Ovarian Cancer Patients. Ann. Oncol. 2002, 13, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Babaier, A.; Ghatage, P. Mucinous Cancer of the Ovary: Overview and Current Status. Diagnostics 2020, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Passardi, A.; Rapposelli, I.G.; Scarpi, E.; Sullo, F.G.; Bartolini, G.; Neri, E.; Ghigi, G.; Tontini, L.; Ercolani, G.; Monti, M.; et al. Multimodal Treatment with Gemox plus Helical Tomotherapy in Unresectable Locally Advanced Pancreatic Cancer: A Pooled Analysis of Two Phase 2 Studies. Biomolecules 2021, 11, 1200. [Google Scholar] [CrossRef] [PubMed]
- Moureau-Zabotto, L.; Phélip, J.M.; Afchain, P.; Mineur, L.; André, T.; Vendrely, V.; Lledo, G.; Dupuis, O.; Huguet, F.; Touboul, E.; et al. Concomitant Administration of Weekly Oxaliplatin, Fluorouracil Continuous Infusion, and Radiotherapy after 2 Months of Gemcitabine and Oxaliplatin Induction in Patients with Locally Advanced Pancreatic Cancer: A Groupe Coordinateur Multidisciplinaire En Oncologie Phase II Study. J. Clin. Oncol. 2008, 26, 1080–1085. [Google Scholar] [CrossRef]
- Delozier, T.; Guastalla, J.P.; Yovine, A.; Levy, C.; Chollet, P.; Mousseau, M.; Delva, R.; Coeffic, D.; Vannetzel, J.M.; Zazzi, E.S.; et al. A Phase II Study of an Oxaliplatin/Vinorelbine/5-Fluorouracil Combination in Patients with Anthracycline-Pretreated and Taxane-Pretreated Metastatic Breast Cancer. Anti-Cancer Drugs 2006, 17, 1067–1073. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Wang, Z.; Hu, X.; Wang, B.; Cao, J.; Lv, F.; Zhen, C.; Zhang, S.; Shao, Z. A Phase II Trial of Biweekly Vinorelbine and Oxaliplatin in Second- or Third-Line Metastatic Triple-Negative Breast Cancer. Cancer Biol. Ther. 2015, 16, 225–232. [Google Scholar] [CrossRef]
- Raez, L.E.; Kobina, S.; Santos, E.S. Oxaliplatin in First-Line Therapy for Advanced Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2010, 11, 18–24. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, T.; Liu, L.; Lu, B. Chemotherapy Oxaliplatin Sensitizes Prostate Cancer to Immune Checkpoint Blockade Therapies via Stimulating Tumor Immunogenicity. Mol. Med. Rep. 2017, 16, 2868–2874. [Google Scholar] [CrossRef][Green Version]
- Cassidy, J.; Misset, J.-L. Oxaliplatin-Related Side Effects: Characteristics and Management. Semin. Oncol. 2002, 29 (Suppl. S15), 11–20. [Google Scholar] [CrossRef]
- Yu, Z.; Huang, R.; Zhao, L.; Wang, X.; Shangguan, X.; Li, W.; Li, M.; Yin, X.; Zhang, C.; Liu, D. Safety Profile of Oxaliplatin in 3687 Patients With Cancer in China: A Post-Marketing Surveillance Study. Front. Oncol. 2021, 11, 4418. [Google Scholar] [CrossRef]
- Sałat, K. Chemotherapy-Induced Peripheral Neuropathy—Part 2: Focus on the Prevention of Oxaliplatin-Induced Neurotoxicity. Pharmacol. Rep. 2020, 72, 508–527. [Google Scholar] [CrossRef] [PubMed]
- Pasetto, L.M.; D’Andrea, M.R.; Rossi, E.; Monfardini, S. Oxaliplatin-Related Neurotoxicity: How and Why? Crit. Rev. Oncol./Hematol. 2006, 59, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Kawashiri, T.; Mine, K.; Kobayashi, D.; Inoue, M.; Ushio, S.; Uchida, M.; Egashira, N.; Shimazoe, T. Therapeutic Agents for Oxaliplatin-Induced Peripheral Neuropathy; Experimental and Clinical Evidence. Int. J. Mol. Sci. 2021, 22, 1393. [Google Scholar] [CrossRef] [PubMed]
- Argyriou, A.A.; Bruna, J.; Marmiroli, P.; Cavaletti, G. Chemotherapy-Induced Peripheral Neurotoxicity (CIPN): An Update. Crit. Rev. Oncol. Hematol. 2012, 82, 51–77. [Google Scholar] [CrossRef]
- Saif, W.; Reardon, J. Management of Oxaliplatin-Induced Peripheral Neuropathy. Ther. Clin. Risk Manag. 2005, 1, 249–258. [Google Scholar]
- Miaskowski, C.; Mastick, J.; Paul, S.M.; Topp, K.; Smoot, B.; Abrams, G.; Chen, L.M.; Kober, K.M.; Conley, Y.P.; Chesney, M.; et al. Chemotherapy-Induced Neuropathy in Cancer Survivors. J. Pain Symptom Manag. 2017, 54, 204–218. [Google Scholar] [CrossRef]
- Cavaletti, G.; Tredici, G.; Petruccioli, M.G.; Dondè, E.; Tredici, P.; Marmiroli, P.; Minoia, C.; Ronchi, A.; Bayssas, M.; Griffon Etienne, G. Effects of Different Schedules of Oxaliplatin Treatment on the Peripheral Nervous System of the Rat. Eur. J. Cancer 2001, 37, 2457–2463. [Google Scholar] [CrossRef]
- Jamieson, S.M.F.; Subramaniam, J.; Liu, J.J.; Jong, N.N.; Ip, V.; Connor, B.; McKeage, M.J. Oxaliplatin-Induced Loss of Phosphorylated Heavy Neurofilament Subunit Neuronal Immunoreactivity in Rat DRG Tissue. Mol. Pain 2009, 5, 66. [Google Scholar] [CrossRef]
- Liu, J.J.; Jamieson, S.M.F.; Subramaniam, J.; Ip, V.; Jong, N.N.; Mercer, J.F.B.; McKeage, M.J. Neuronal Expression of Copper Transporter 1 in Rat Dorsal Root Ganglia: Association with Platinum Neurotoxicity. Cancer Chemother. Pharmacol. 2009, 64, 847–856. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, B.; Gao, X.; Sun, J.; Ye, J.; Li, J.; Cao, P. Targeting Strategies for Oxaliplatin-Induced Peripheral Neuropathy: Clinical Syndrome, Molecular Basis, and Drug Development. J. Exp. Clin. Cancer Res. 2021, 40, 331. [Google Scholar] [CrossRef]
- Jamieson, S.M.F.; Liu, J.; Connor, B.; McKeage, M.J. Oxaliplatin Causes Selective Atrophy of a Subpopulation of Dorsal Root Ganglion Neurons without Inducing Cell Loss. Cancer Chemother. Pharmacol. 2005, 56, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Ta, L.E.; Espeset, L.; Podratz, J.; Windebank, A.J. Neurotoxicity of Oxaliplatin and Cisplatin for Dorsal Root Ganglion Neurons Correlates with Platinum-DNA Binding. Neurotoxicology 2006, 27, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Adelsberger, H.; Quasthoff, S.; Grosskreutz, J.; Lepier, A.; Eckel, F.; Lersch, C. The Chemotherapeutic Oxaliplatin Alters Voltage-Gated Na+ Channel Kinetics on Rat Sensory Neurons. Eur. J. Pharmacol. 2000, 406, 25–32. [Google Scholar] [CrossRef]
- Sittl, R.; Lampert, A.; Huth, T.; Schuy, E.T.; Link, A.S.; Fleckenstein, J.; Alzheimer, C.; Grafe, P.; Carr, R.W. Anticancer Drug Oxaliplatin Induces Acute Cooling-Aggravated Neuropathy via Sodium Channel Subtype Na V1.6-Resurgent and Persistent Current. Proc. Natl. Acad. Sci. USA 2012, 109, 6704–6709. [Google Scholar] [CrossRef] [PubMed]
- Lersch, C.; Schmelz, R.; Eckel, F.; Erdmann, J.; Mayr, M.; Schulte-Frohlinde, E.; Quasthoff, S.; Grosskreutz, J.; Adelsberger, H. Prevention of Oxaliplatin-Induced Peripheral Sensory Neuropathy by Carbamazepine in Patients with Advanced Colorectal Cancer. Clin. Colorectal Cancer 2002, 2, 54–58. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Zanardelli, M.; Failli, P.; Ghelardini, C. Oxaliplatin-Induced Neuropathy: Oxidative Stress as Pathological Mechanism. Protective Effect of Silibinin. J. Pain 2012, 13, 276–284. [Google Scholar] [CrossRef]
- Carozzi, V.A.; Chiorazzi, A.; Canta, A.; Lapidus, R.G.; Slusher, B.S.; Wozniak, K.M.; Cavaletti, G. Glutamate Carboxypeptidase Inhibition Reduces the Severity of Chemotherapy-Induced Peripheral Neurotoxicity in Rat. Neurotox. Res. 2010, 17, 380–391. [Google Scholar] [CrossRef]
- Kottschade, L.A.; Sloan, J.A.; Mazurczak, M.A.; Johnson, D.B.; Murphy, B.P.; Rowland, K.M.; Smith, D.A.A.; Berg, A.R.; Stella, P.J.; Loprinzi, C.L. The Use of Vitamin E for the Prevention of Chemotherapy-Induced Peripheral Neuropathy: Results of a Randomized Phase III Clinical Trial. Support. Care Cancer 2011, 19, 1769–1777. [Google Scholar] [CrossRef]
- de Afonseca, S.O.; Cruz, F.M.; Cubero, D.d.I.G.; Lera, A.T.; Schindler, F.; Okawara, M.; de Souza, L.F.; Rodrigues, N.P.; del Giglio, A. Vitamina E Na Prevenção de Neuropatia Periférica Induzida Pela Oxaliplatina: Estudo Clínico Piloto Randomizado. Sao Paulo Med. J. 2013, 131, 35–38. [Google Scholar] [CrossRef]
- Cascinu, S.; Catalano, V.; Cordella, L.; Labianca, R.; Giordani, P.; Baldelli, A.M.; Beretta, G.D.; Ubiali, E.; Catalano, G. Neuroprotective Effect of Reduced Glutathione on Oxaliplatin-Based Chemotherapy in Advanced Colorectal Cancer: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Clin. Oncol. 2002, 20, 3478–3483. [Google Scholar] [CrossRef]
- Lin, P.C.; Lee, M.Y.; Wang, W.S.; Yen, C.C.; Chao, T.C.; Hsiao, L.T.; Yang, M.H.; Chen, P.M.; Lin, K.P.; Chiou, T.J. N-Acetylcysteine Has Neuroprotective Effects against Oxaliplatin-Based Adjuvant Chemotherapy in Colon Cancer Patients: Preliminary Data. Support. Care Cancer 2006, 14, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Agnes, J.P.; dos Santos, V.W.; das Neves, R.N.; Gonçalves, R.M.; Delgobo, M.; Girardi, C.S.; Lückemeyer, D.D.; Ferreira, M.d.A.; Macedo-Júnior, S.J.; Lopes, S.C.; et al. Antioxidants Improve Oxaliplatin-Induced Peripheral Neuropathy in Tumor-Bearing Mice Model: Role of Spinal Cord Oxidative Stress and Inflammation. J. Pain 2021, 22, 996–1013. [Google Scholar] [CrossRef] [PubMed]
- McQuade, R.M.; Stojanovska, V.; Stavely, R.; Timpani, C.; Petersen, A.C.; Abalo, R.; Bornstein, J.C.; Rybalka, E.; Nurgali, K. Oxaliplatin-Induced Enteric Neuronal Loss and Intestinal Dysfunction Is Prevented by Co-Treatment with BGP-15. Br. J. Pharmacol. 2018, 175, 656–677. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, Y.T.; Jeon, E.K.; Won, H.S.; Cho, Y.S.; Ko, Y.H. Effect of Green Tea Extracts on Oxaliplatin-Induced Peripheral Neuropathy in Rats. BMC Complement. Altern. Med. 2012, 12, 124. [Google Scholar] [CrossRef]
- Al Moundhri, M.S.; Al-Salam, S.; Al Mahrouqee, A.; Beegam, S.; Ali, B.H. The Effect of Curcumin on Oxaliplatin and Cisplatin Neurotoxicity in Rats: Some Behavioral, Biochemical, and Histopathological Studies. J. Med. Toxicol. 2013, 9, 25–33. [Google Scholar] [CrossRef]
- Basu, P.; Maier, C.; Basu, A. Effects of Curcumin and Its Different Formulations in Preclinical and Clinical Studies of Peripheral Neuropathic and Postoperative Pain: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 4666. [Google Scholar] [CrossRef]
- Stuart, J.A.; Robb, E.L. Bioactive Polyphenols from Wine Grapes; Springer: New York, NY, USA, 2013; pp. 1–66. [Google Scholar] [CrossRef]
- Stagos, D.; Amoutzias, G.D.; Matakos, A.; Spyrou, A.; Tsatsakis, A.M.; Kouretas, D. Chemoprevention of Liver Cancer by Plant Polyphenols. Food Chem. Toxicol. 2012, 50, 2155–2170. [Google Scholar] [CrossRef]
- He, S.; Yan, X. From Resveratrol to Its Derivatives: New Sources of Natural Antioxidant. Curr. Med. Chem. 2013, 20, 1005–1017. [Google Scholar] [CrossRef]
- Micheli, L.; Mattoli, L.; Maidecchi, A.; Pacini, A.; Ghelardini, C.; Di Cesare Mannelli, L. Effect of Vitis Vinifera Hydroalcoholic Extract against Oxaliplatin Neurotoxicity: In Vitro and in Vivo Evidence. Sci. Rep. 2018, 8, 14364. [Google Scholar] [CrossRef]
- Sapanidou, V.G.; Margaritis, I.; Siahos, N.; Arsenopoulos, K.; Dragatidou, E.; Taitzoglou, I.A.; Zervos, I.A.; Theodoridis, A.; Tsantarliotou, M.P. Antioxidant Effect of a Polyphenol-Rich Grape Pomace Extract on Motility, Viability and Lipid Peroxidation of Thawed Bovine Spermatozoa. J. Biol. Res. 2014, 21, 19. [Google Scholar] [CrossRef]
- Chiavaroli, A.; Balaha, M.; Acquaviva, A.; Ferrante, C.; Cataldi, A.; Menghini, L.; Rapino, M.; Orlando, G.; Brunetti, L.; Leone, S.; et al. Phenolic Characterization and Neuroprotective Properties of Grape Pomace Extracts. Molecules 2021, 26, 6216. [Google Scholar] [CrossRef] [PubMed]
- Moser, V.C. Neurobehavioral Screening in Rodents. Curr. Protoc. Toxicol. 1999, 1, 11.2.1–11.2.16. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.; Pavan, C.; Ohlmeier, L.S.; Nilson, B.; Lundgaard, I.; Linder, A.; Bentzer, P. A Functional Observational Battery for Evaluation of Neurological Outcomes in a Rat Model of Acute Bacterial Meningitis. Intensive Care Med. Exp. 2020, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, R.; White, I.; Lieb, W.R.; Franks, N.P. Stereoselective Loss of Righting Reflex in Rats by Isoflurane. Anesthesiology 2000, 93, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.F. Sensorimotor Disturbances in the Aging Rodent. J. Gerontol. 1982, 37, 548–554. [Google Scholar] [CrossRef]
- Sherrington, C.S. Flexion-Reflex of the Limb, Crossed Extension-Reflex, and Reflex Stepping and Standing. J. Physiol. 1910, 40, 28–121. [Google Scholar] [CrossRef]
- Vincent, J.A.; Wieczerzak, K.B.; Gabriel, H.M.; Nardelli, P.; Rich, M.M.; Cope, T.C. A Novel Path to Chronic Proprioceptive Disability with Oxaliplatin: Distortion of Sensory Encoding. Neurobiol. Dis. 2016, 95, 54–65. [Google Scholar] [CrossRef][Green Version]
- Edwards, P.M.; Parker, V. A Simple, Sensitive, and Objective Method for Early Assessment of Acrylamide Neuropathy in Rats. Toxicol. Appl. Pharmacol. 1977, 40, 589–591. [Google Scholar] [CrossRef]
- Classen, W.; Gunson, D.E.; Iverson, W.O.; Traina, V.M.; Vonau, M.H.; Krinke, G.J. Functional and Morphological Characterization of Neuropathy Induced with 5-Lipoxygenase Inhibitor CGS 21595. Exp. Toxicol. Pathol. 1994, 46, 119–125. [Google Scholar] [CrossRef]
- Ahmed, R.U.; Alam, M.; Zheng, Y.P. Experimental Spinal Cord Injury and Behavioral Tests in Laboratory Rats. Heliyon 2019, 5, e01324. [Google Scholar] [CrossRef]
- Deuis, J.R.; Dvorakova, L.S.; Vetter, I. Methods Used to Evaluate Pain Behaviors in Rodents. Front. Mol. Neurosci. 2017, 10, 284. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.Y.; Robinson, C.R.; Zhang, H.; Dougherty, P.M. Spinal Astrocyte Gap Junctions Contribute to Oxaliplatin-Induced Mechanical Hypersensitivity. J. Pain 2013, 14, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Metz, G.A.; Schwab, M.E. Behavioral Characterization in a Comprehensive Mouse Test Battery Reveals Motor and Sensory Impairments in Growth-Associated Protein-43 Null Mutant Mice. Neuroscience 2004, 129, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Kyoto, N.R. Functional Observational Battery (FOB) Observation Procedures. Available online: http://www.anim.med.kyoto-u.ac.jp/nbr/strainsx/FOB_menue.aspx (accessed on 26 December 2021).
- Janaszewska, A.; Bartosz, G. Assay of Total Antioxidant Capacity: Comparison of Four Methods as Applied to Human Blood Plasma. Scand. J. Clin. Lab. Investig. 2002, 62, 231–236. [Google Scholar] [CrossRef]
- Keles, M.S.; Taysi, S.; Sen, N.; Aksoy, H.; Akçay, F. Effect of Corticosteroid Therapy on Serum and CSF Malondialdehyde and Antioxidant Proteins in Multiple Sclerosis. Can. J. Neurol. Sci. 2001, 28, 141–143. [Google Scholar] [CrossRef]
- Baron, R.; Jänig, W.; Kollmann, W. Sympathetic and Afferent Somata Projecting in Hindlimb Nerves and the Anatomical Organization of the Lumbar Sympathetic Nervous System of the Rat. J. Comp. Neurol. 1988, 275, 460–468. [Google Scholar] [CrossRef]
- Medici, T.; Shortland, P.J. Effects of Peripheral Nerve Injury on Parvalbumin Expression in Adult Rat Dorsal Root Ganglion Neurons. BMC Neurosci. 2015, 16, 93. [Google Scholar] [CrossRef]
- Warwick, R.A.; Hanani, M. The Contribution of Satellite Glial Cells to Chemotherapy-Induced Neuropathic Pain. Eur. J. Pain 2013, 17, 571–580. [Google Scholar] [CrossRef]
- Schicho, R.; Schuligoi, R.; Sirinathsinghji, D.J.S.; Donnerer, J. Increased Expression of GAP-43 in Small Sensory Neurons after Stimulation by NGF Indicative of Neuroregeneration in Capsaicin-Treated Rats. Regul. Pept. 1999, 83, 87–95. [Google Scholar] [CrossRef]
- Tsingotjidou, A.S.; Papadopoulos, G.C. Anatomic Organization of the Ascending Branch of the Milk-Ejection Reflex in Sheep: Primary Afferent Neurons. J. Comp. Neurol. 2003, 460, 66–79. [Google Scholar] [CrossRef]
- Janicke, B.; Coper, H. Tests in Rodents for Assessing Sensorimotor Performance During Aging. Adv. Psychol. 1996, 114, 201–233. [Google Scholar]
- Valvassori, S.S.; Varela, R.B.; Quevedo, J. Animal Models of Mood Disorders: Focus on Bipolar Disorder and Depression. In Animal Models for the Study of Human Disease, 2nd ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 991–1001. [Google Scholar] [CrossRef]
- Sakurai, M.; Egashira, N.; Kawashiri, T.; Yano, T.; Ikesue, H.; Oishi, R. Oxaliplatin-Induced Neuropathy in the Rat: Involvement of Oxalate in Cold Hyperalgesia but Not Mechanical Allodynia. Pain 2009, 147, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Beijers, A.J.M.; Mols, F.; Vreugdenhil, G. A Systematic Review on Chronic Oxaliplatin-Induced Peripheral Neuropathy and the Relation with Oxaliplatin Administration. Supportive Care Cancer 2014, 22, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Ta, L.E.; Schmelzer, J.D.; Bieber, A.J.; Loprinzi, C.L.; Sieck, G.C.; Brederson, J.D.; Low, P.A.; Windebank, A.J. A Novel and Selective Poly (ADP-Ribose) Polymerase Inhibitor Ameliorates Chemotherapy-Induced Painful Neuropathy. PLoS ONE 2013, 8, e54161. [Google Scholar] [CrossRef] [PubMed]
- Romeo, L.; Intrieri, M.; D’Agata, V.; Mangano, N.G.; Oriani, G.; Ontario, M.L.; Scapagnini, G. The Major Green Tea Polyphenol, (-)-Epigallocatechin-3-Gallate, Induces Heme Oxygenase in Rat Neurons and Acts as an Effective Neuroprotective Agent against Oxidative Stress. J. Am. Coll. Nutr. 2009, 28, 492S–499S. [Google Scholar] [CrossRef]
- Han, H.; Wang, H.; Du, Y.; Gao, L. Grape Seed Procyanidins Attenuates Cisplatin-Induced Human Embryonic Renal Cell Cytotoxicity by Modulating Heme Oxygenase-1 in Vitro. Cell Biochem. Biophys. 2019, 77, 367–377. [Google Scholar] [CrossRef]
- Dehghani, M.A.; Shakiba Maram, N.; Moghimipour, E.; Khorsandi, L.; Atefikhah, M.; Mahdavinia, M. Protective Effect of Gallic Acid and Gallic Acid-Loaded Eudragit-RS 100 Nanoparticles on Cisplatin-Induced Mitochondrial Dysfunction and Inflammation in Rat Kidney. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165911. [Google Scholar] [CrossRef]
- Ling, B.; Authier, N.; Balayssac, D.; Eschalier, A.; Coudore, F. Behavioral and Pharmacological Description of Oxaliplatin-Induced Painful Neuropathy in Rat. Pain 2007, 128, 225–234. [Google Scholar] [CrossRef]
- Xiao, W.H.; Zheng, H.; Bennett, G.J. Characterization of Oxaliplatin-Induced Chronic Painful Peripheral Neuropathy in the Rat and Comparison with the Neuropathy Induced by Paclitaxel. Neuroscience 2012, 203, 194–206. [Google Scholar] [CrossRef]
- Wu, B.; Su, X.; Zhang, W.; Zhang, Y.H.; Feng, X.; Ji, Y.H.; Tan, Z.Y. Oxaliplatin Depolarizes the IB4– Dorsal Root Ganglion Neurons to Drive the Development of Neuropathic Pain Through TRPM8 in Mice. Front. Mol. Neurosci. 2021, 14, 690858. [Google Scholar] [CrossRef]
- Martinez, N.W.; Sánchez, A.; Diaz, P.; Broekhuizen, R.; Godoy, J.; Mondaca, S.; Catenaccio, A.; Macanas, P.; Nervi, B.; Calvo, M.; et al. Metformin Protects from Oxaliplatin Induced Peripheral Neuropathy in Rats. Neurobiol. Pain 2020, 8, 100048. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Tian, Y.; Xu, S.; Chen, H. Oxaliplatin-Induced Peripheral Neuropathy: Clinical Features, Mechanisms, Prevention and Treatment. J. Neurol. 2021, 268, 3269–3282. [Google Scholar] [CrossRef] [PubMed]
- Lees, J.G.; Abdulla, M.; Barkl-Luke, M.E.; Livni, L.; Keating, B.A.; Hayes, J.; Fiore, N.T.; Park, S.B.; Moalem-Taylor, G.; Goldstein, D. Effect of Exercise on Neuromuscular Toxicity in Oxaliplatin-Treated Mice. Muscle Nerve 2021, 64, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Olson, W.; Dong, P.; Fleming, M.; Luo, W. The Specification and Wiring of Mammalian Cutaneous Low-Threshold Mechanoreceptors. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Boyette-Davis, J.; Dougherty, P.M. Protection against Oxaliplatin-Induced Mechanical Hyperalgesia and Intraepidermal Nerve Fiber Loss by Minocycline. Exp. Neurol. 2011, 229, 353–357. [Google Scholar] [CrossRef]
- Ahn, B.S.; Kim, S.K.; Kim, H.N.; Lee, J.H.; Lee, J.H.; Hwang, D.S.; Bae, H.; Min, B.I.; Kim, S.K. Gyejigachulbu-Tang Relieves Oxaliplatin-Induced Neuropathic Cold and Mechanical Hypersensitivity in Rats via the Suppression of Spinal Glial Activation. Evid. Based Complement. Altern. Med. 2014, 2014, 436482. [Google Scholar] [CrossRef]
- Kim, S.T.; Chung, Y.H.; Lee, H.S.; Chung, S.J.; Lee, J.H.; Sohn, U.D.; Shin, Y.K.; Park, E.S.; Kim, H.C.; Bang, J.S.; et al. Protective Effects of Phosphatidylcholine on Oxaliplatin-Induced Neuropathy in Rats. Life Sci. 2015, 130, 81–87. [Google Scholar] [CrossRef]
- Ameyaw, E.O.; Woode, E.; Boakye-Gyasi, E.; Abotsi, W.K.M.; Kyekyeku, J.O.; Adosraku, R.K. Anti-Allodynic and Anti-Hyperalgesic Effects of an Ethanolic Extract and Xylopic Acid from the Fruits of Xylopia Aethiopica in Murine Models of Neuropathic Pain. Pharmacogn. Res. 2014, 6, 172–179. [Google Scholar] [CrossRef]
- Fumagalli, G.; Monza, L.; Cavaletti, G.; Rigolio, R.; Meregalli, C. Neuroinflammatory Process Involved in Different Preclinical Models of Chemotherapy-Induced Peripheral Neuropathy. Front. Immunol. 2021, 11, 626687. [Google Scholar] [CrossRef]
- Nascimento, D.S.M.; Castro-Lopes, J.M.; Neto, F.L.M. Satellite Glial Cells Surrounding Primary Afferent Neurons Are Activated and Proliferate during Monoarthritis in Rats: Is There a Role for ATF3? PLoS ONE 2014, 9, e108152. [Google Scholar] [CrossRef]
- Xu, J.; Wu, S.; Wang, J.; Wang, J.; Yan, Y.; Zhu, M.; Zhang, D.; Jiang, C.; Liu, T. Oxidative Stress Induced by NOX2 Contributes to Neuropathic Pain via Plasma Membrane Translocation of PKCε in Rat Dorsal Root Ganglion Neurons. J. Neuroinflamm. 2021, 18, 106. [Google Scholar] [CrossRef] [PubMed]
- Hanani, M.; Blum, E.; Liu, S.; Peng, L.; Liang, S. Satellite Glial Cells in Dorsal Root Ganglia Are Activated in Streptozotocin-Treated Rodents. J. Cell. Mol. Med. 2014, 18, 2367–2371. [Google Scholar] [CrossRef] [PubMed]
- Woodham, P.; Anderson, P.N.; Nadim, W.; Turmaine, M. Satellite Cells Surrounding Axotomised Rat Dorsal Root Ganglion Cells Increase Expression of a GFAP-like Protein. Neurosci. Lett. 1989, 98, 8–12. [Google Scholar] [CrossRef]

| Rt (min) | Compounds | Theoretical [M-H]− m/z | Experimental [M-H]− m/z | Molecular Formula | RDBeq. | Delta (ppm) | |
|---|---|---|---|---|---|---|---|
| 1 | 0.75 | Gluconic acid | 195.0510 | 195.0511 | C6H12O7 | 1.5 | 0.50 |
| 2 | 1.10 | Gallic acid | 169.0142 | 169.0145 | C7H6O5 | 5.5 | 1.32 |
| 3 | 1.90 | Protocatechuic acid | 153.0193 | 153.0195 | C7H6O4 | 5.5 | 1.29 |
| 4 | 2.17 | Dihydroxybenzoic acid hexoside isomer 1 | 315.0722 | 315.0724 | C13H16O9 | 6.5 | 0.84 |
| 5 | 3.01 | Procyanidin B1 or B2 | 577.1351 | 577.135 | C30H26O12 | 18.5 | −0.20 |
| 6 | 3.03/3.34 | Caffeic acid hexoside | 341.0878 | 341.087 | C15H18O9 | 7.5 | −2.36 |
| 7 | 3.17 | Caffeoyltartaric acid | 311.0409 | 311.041 | C13H12O9 | 8.5 | 0.30 |
| 8 | 3.24 | 2-Isopropylmalic acid | 175.0612 | 175.0612 | C7H12O5 | 2.5 | 0.10 |
| 9 | 3.32 | Catechin or Epicatechin | 289.0718 | 289.0717 | C15H14O6 | 9.5 | −0.03 |
| 10 | 3.67 | Coumaric acid hexoside isomer 1 | 325.0929 | 325.093 | C15H18O8 | 7.5 | 0.49 |
| 11 | 3.69 | Caffeic acid | 179.035 | 179.0352 | C9H8O4 | 6.5 | 1.50 |
| 12 | 3.71 | Procyanidin B1 or B2 | 577.1351 | 577.1352 | C30H26O12 | 18.5 | 0.07 |
| 13 | 3.79 | Coumaroyltartaric acid isomer 1 | 295.0459 | 295.0461 | C13H12O8 | 8.5 | 0.37 |
| 14 | 3.90 | Ferulic acid pentoside | 325.0929 | 325.093 | C15H18O8 | 7.5 | 0.39 |
| 15 | 3.93 | Catechin or Epicatechin | 289.0718 | 289.0718 | C15H14O6 | 9.5 | 0.27 |
| 16 | 4.38 | Myricetin-3-O-galactoside | 479.0831 | 479.0828 | C21H20O13 | 12.5 | −0.67 |
| 17 | 4.49 | Syringic acid | 197.0455 | 197.0456 | C9H10O5 | 5.5 | 0.32 |
| 18 | 4.80 | Isoquercitrin | 463.0882 | 463.0881 | C21H20O12 | 12.5 | −0.20 |
| 19 | 4.85 | Quercetin 3-O-glucuronide | 477.0675 | 477.0675 | C21H18O13 | 13.5 | 0.07 |
| 20 | 5.59 | p-Hydroxybenzoic acid | 137.0244 | 137.0245 | C7H6O3 | 5.5 | 0.31 |
| 21 | 5.20 | Isorhamnetin 3-glucoside | 477.1038 | 477.1038 | C22H22O12 | 12.5 | −0.12 |
| 22 | 6.22 | Quercetin | 301.0354 | 301.0356 | C15H10O7 | 11.5 | 0.57 |
| 23 | 6.94 | Kaempferol | 285.0405 | 285.0408 | C15H10O6 | 11.5 | 1.08 |
| FOB Test | Tested Parameter | Validation | Scale |
|---|---|---|---|
| Home-cage measurements | |||
| Body position | General condition, pain | R | 1 to 3 |
| Respiration | Respiration rate, weakness | R | 1 to 6 |
| Vocalization | Pain | Y/N | |
| Palpebral closure | R | 1 to 3 | |
| Hand-held observations | |||
| Reactivity | R | 1 to 5 | |
| Handling | R | 1 to 4 | |
| Palpebral closure | R | 1 to 3 | |
| Open field activity | |||
| Number of rearings | Exploratory activity | N | |
| Gait | Posture | R | 1 to 6 |
| Arousal | Activity over time | R | 1 to 6 |
| Defecations number | Autonomic system function | N | |
| Diarrhea | Autonomic system function | Y/N | |
| Urinations number | Autonomic system function | N | |
| Stereotypical behavior | Y/N | ||
| Sensorimotor reflexes | |||
| Approach response | R | 1 to 4 | |
| Touch response | R | 1 to 4 | |
| Eyelid reflex | Y/N | ||
| Sound response | R | 1 to 3 | |
| Tail pinch response | Pain response/locomotion | R | 1 to 4 |
| Righting reflex | Proprioception | R | 1 to 4 |
| Contact placing response | Corticospinal system function/Proprioception | R | 0 to 2 |
| Crossed extensor reflex | Corticospinal system function/Proprioception | R | 1 to 2 |
| Sensorimotor measurements | |||
| Footprint/gait analysis | |||
| Stride length | Ataxia, coordination, locomotion | M | |
| Stride width | Base of support, locomotion | M | |
| Foot rotation (R) | Sciatic nerve function | M | |
| Foot rotation (L) | Sciatic nerve function | M | |
| Interpedal distance | Ataxia, coordination, locomotion | M | |
| Landing foot splay | Vestibular and proprioceptive sensation/motor efferent fibres | M | |
| Grip strength | Touch and proprioceptive sensation/motor efferent fibres | M | |
| Sticky paper | Touch receptors/motor efferent fibres | M | |
| Von Frey hair pinch test | Mechanoreceptors, pain receptors/motor efferent fibres | M | |
| Group | Pre-Treatment | Mid-Treatment | End-Treatment | Post-Treatment |
|---|---|---|---|---|
| Control (group A) | 213 ± 15.62 | 228 ± 12.5 | 225 ± 9.75 | 232 ± 9.3 |
| Group B | 196 ± 10.77 | 201 ± 11.77 | 207 ± 11.79 | 221 ± 4.3 |
| Group C | 226 ± 10.77 | 220 ± 10.25 | 218 ± 10.07 | 227 ± 15.46 |
| Group D | 199 ± 1.05 | 202 ± 10.9 | 192.5 ± 7.77 | 212.5 ± 6 |
| Group A | Group B | Group C | Group D | |
|---|---|---|---|---|
| Foot rotation R | 7.9 ± 1.63 | 11.15 ± 1.63 | 8.3 ± 1.4 | 11.68 ± 1.44 |
| Foot rotation L | 10.7 ± 1.9 | 11.85 ± 1.9 | 11.57 ± 1.6 | 12.51 ± 1.65 |
| Stride length R | 10.65 ± 0.44 | 11.08 ± 0.35 | 11.03 ± 0.3 | 10.9 ± 0.33 |
| Stride length L | 10.81 ± 0.42 | 11.04 ± 0.32 | 10.98 ± 0.34 | 11.33 ± 0.29 |
| Stride width | 4.49 ± 0.2 | 4.35 ± 0.21 | 4.15 ± 0.16 | 4.13 ± 0.17 |
| Interpedal distance | 5.47 ± 0.2 | 5.5 ± 0.19 | 5.5 ± 0.15 | 5.48 ± 0.16 |
| Sensorimotor Tasks | Group A | Group B | Group C | Group D | |
|---|---|---|---|---|---|
| Landing foot splay (cm) | 3.99 ± 0.5 | 4.35 ± 0.34 | 4.43 ± 0.29 | 4.58 ± 0.28 | N.S. |
| Grip strength (N) | 3.11 ± 0.18 * | 2.81 ± 0.18 **/*** | 3.4 ± 0.15 ** | 3.53 ± 0.15 */*** | * p ≤ 0.1 ** p ≤ 0.05 *** p ≤ 0.01 |
| Sticky paper (sec) | 157.7 ± 24 | 124.9 ± 24.3 | 126.8 ± 19 | 161.1 ± 18.5 | N.S. |
| Von Frey hair pinch test (grams) | 0.40 ± 0.0 * | 1.04 ± 0.3 */**/*** | 0.43 ± 0.03 | 0.42 ± 0.02 | * p ≤ 0.05 ** p ≤ 0.05 *** p ≤ 0.05 |
| TAC (mmol DPPH/L Plasma) | SEM | TBARS (μmol/L Plasma) | SEM | |
|---|---|---|---|---|
| group A | 0.77 | 0.048 | 48.4 | 2.11 |
| group B | 0.77 | 0.006 | 40.3 | 1.07 |
| group C | 0.79 | 0.076 | 47.8 | 5.14 |
| group D | 0.67 | 0.012 | 40.4 | 4.68 |
| Morphometric Parameters | Group A | Group B | Group C | Group D | p |
|---|---|---|---|---|---|
| Total DRG Neuron Number c | 14.138 ± 1.590.37 | 17.020 ± 2.278.97 | 15.090 ± 2.0000 | 16.782 ± 1.194.75 | N.S. |
| Large DRG neurons b | 10.4% | 2.8% | 3.3% | 3.8% | - |
| Medium DRG neurons b | 30.8% | 24.1% | 21.2% | 26% | - |
| Small DRG neurons b | 58.8% | 73.2% | 75.6% | 70.2% | - |
| Mean somatic Area (μm2) a | 526.96 ± 4.24 * | 390.94 ± 2.48 */** | 393.92 ± 3.39 | 428.87 ± 2.82 ** | *,** p < 0.01 |
| Area of Large DRG neurons (μm2) a | 1.322.18 ± 10.54 */** | 1.196.91 ± 12.48 */*** | 1.275.81 ± 21.35 *** | 1.237.48 ± 13.27 ** | *,** p < 0.001 *** p < 0.01 |
| Diameter a | 24.13 ± 0.48 */** | 20.43 ± 0.79 * | 20.32 ± 0.65 ** | 21.49 ± 0.38 | *,** p < 0.01 |
| Perimeter a | 86.25 ± 1.61 */**/*** | 71.97 ± 2.44 * | 72.19 ± 3.21 ** | 75.26 ± 1.88 *** | *,** p < 0.01 *** p < 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekiari, C.; Tekos, F.; Skaperda, Z.; Argyropoulou, A.; Skaltsounis, A.-L.; Kouretas, D.; Tsingotjidou, A. Antioxidant and Neuroprotective Effect of a Grape Pomace Extract on Oxaliplatin-Induced Peripheral Neuropathy in Rats: Biochemical, Behavioral and Histopathological Evaluation. Antioxidants 2022, 11, 1062. https://doi.org/10.3390/antiox11061062
Bekiari C, Tekos F, Skaperda Z, Argyropoulou A, Skaltsounis A-L, Kouretas D, Tsingotjidou A. Antioxidant and Neuroprotective Effect of a Grape Pomace Extract on Oxaliplatin-Induced Peripheral Neuropathy in Rats: Biochemical, Behavioral and Histopathological Evaluation. Antioxidants. 2022; 11(6):1062. https://doi.org/10.3390/antiox11061062
Chicago/Turabian StyleBekiari, Chryssa, Fotios Tekos, Zoi Skaperda, Aikaterini Argyropoulou, Alexios-Leandros Skaltsounis, Demetrios Kouretas, and Anastasia Tsingotjidou. 2022. "Antioxidant and Neuroprotective Effect of a Grape Pomace Extract on Oxaliplatin-Induced Peripheral Neuropathy in Rats: Biochemical, Behavioral and Histopathological Evaluation" Antioxidants 11, no. 6: 1062. https://doi.org/10.3390/antiox11061062
APA StyleBekiari, C., Tekos, F., Skaperda, Z., Argyropoulou, A., Skaltsounis, A.-L., Kouretas, D., & Tsingotjidou, A. (2022). Antioxidant and Neuroprotective Effect of a Grape Pomace Extract on Oxaliplatin-Induced Peripheral Neuropathy in Rats: Biochemical, Behavioral and Histopathological Evaluation. Antioxidants, 11(6), 1062. https://doi.org/10.3390/antiox11061062










