Abstract
Periodontal diseases are caused mainly by inflammation of the gums and bones surrounding the teeth or by dysbiosis of the oral microbiome, and the Global Burden of Disease study (2019) reported that periodontal disease affects 20–50% of the global population. In recent years, more preference has been given to natural therapies compared to synthetic drugs in the treatment of periodontal disease, and several oral care products, such as toothpaste, mouthwash, and dentifrices, have been developed comprising honeybee products, such as propolis, honey, royal jelly, and purified bee venom. In this study, we systematically reviewed the literature on the treatment of periodontitis using honeybee products. A literature search was performed using various databases, including PubMed, Web of Science, ScienceDirect, Scopus, clinicaltrials.gov, and Google Scholar. A total of 31 studies were reviewed using eligibility criteria published between January 2016 and December 2021. In vitro, in vivo, and clinical studies (randomized clinical trials) were included. Based on the results of these studies, honeybee products, such as propolis and purified bee venom, were concluded to be effective and safe for use in the treatment of periodontitis mainly due to their antimicrobial and anti-inflammatory activities. However, to obtain reliable results from randomized clinical trials assessing the effectiveness of honeybee products in periodontal treatment with long-term follow-up, a broader sample size and assessment of various clinical parameters are needed.
1. Introduction
According to the World Health Organization (WHO), a study conducted by the Global Burden of diseases (2019) estimated that 3.5 billion people were affected by oral disease globally which includes different conditions such as oral cancer (causes approximately 180,000 deaths each year), periodontal disease, dental caries of primary teeth (nearly 520 million children suffer each year), dental caries of permanent teeth (2 billion people), birth defects such as cleft palate, and the oral manifestation of the human immunodeficiency virus (HIV) [1,2]. The risk factors include unhealthy diets (high in sugar), tobacco use, and alcohol consumption. However, in recent years, more interest in the adoption of favorable oral health behavior has been observed, which can facilitate better oral health [3,4,5]. In the last two years, 77% of countries have reported complete or partial disruption of oral health services due to the COVID-19 pandemic [2]. Some recent studies reported that risk factors for causing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection might be associated with dental biofilms, especially dental plaque (subgingival), in patients with periodontitis [6]. Studies have also reported indirect and direct mechanisms for the association between poor oral health and the severity of COVID-19. The indirect mechanism relates to bacterial superinfections and inflammatory pathways, and the direct mechanism relates to angiotensin-converting enzyme II receptors (ACE-2) [6,7,8].
According to a Global Burden of Diseases study (2019), among different oral diseases, periodontal disease affects 20–50% of the worldwide population [1]. Periodontitis is caused by an abnormal host response from dental plaque to bacteria and affects teeth supporting structures, such as periodontal ligament, alveolar bone, and root cementum, resulting in tooth loss in some cases [9]. The increase in experimental evidence and clinical studies show a relationship between periodontal disease and systematic diseases, including Alzheimer’s disease, diabetes, cancers, and atherosclerosis [10,11,12,13,14,15]. Immunological research on periodontitis revealed the importance of the local host immune response in the pathogenesis of the periodontal disease [16]. The periodontal disease can be identified by soft periodontal tissue inflammation. The most common type of periodontal disease is gingivitis, which is mostly widespread at all ages. Almost all forms of periodontal disease are reported to be specific chronic bacterial infections caused by the overgrowth of a limited number of species in dental plaque, such as Bacteroides forsythus, Treponema denticola, and Porphyromonas gingivalis [9,17,18]. Meta-transcriptomics and metagenomic studies revealed that in the pathogenesis of periodontitis, a complex microbial community is involved instead of some specific periodontopathic bacteria [19,20,21,22,23]. Microorganisms present in dental plaque are the main cause of periodontitis, and the progression or severity of periodontitis is determined by the local host immune response [24,25]. The characteristics of periodontal disease include an increase in the peripheral polymorphonuclear oxidative response and inflammatory infiltrate presence. In the early phase of periodontitis, studies show that neutrophils arrive at the site of inflammation and kill pathogens using degranulation and phagocytosis [26,27]. In some cases, in the lateral stage of periodontitis, neutrophils become hyperactive, which increases proinflammatory cytokines, superoxides, and destructive enzymes that cause tissue destruction [24,28]. With the increase in reactive oxygen species (ROS) or the number of free radicals, oxidative stress is triggered and causes oxidative damage to the alveolar bone, periodontal ligament, and gingival tissue [29,30,31]. ROS causes the release of proinflammatory cytokines (i.e., tumor necrosis factor-α; interleukin-2, -6, and -8; and interferon-β), which play important roles in the pathogenesis of periodontal diseases [9,32].
In recent years, attention has grown towards the use of natural therapies given the numerous advantages offered by natural products compared to synthetic drugs [33,34,35]. Currently, more preference is given to natural therapies, such as apitherapy, due to the high safety margin, lower cost, and broad bioactivity compared to synthetic medicine [33]. Apitherapy is defined as the science and art of holistic healing and treatment from honeybees and their products, such as royal jelly, propolis, honey, and bee venom [36]. Royal jelly is produced from a combination of pollen and honey and contains essential fatty acids, nutrients, and vitamins, including A, B, C, D, and E [37,38]. Royal jelly is reported to prevent cell damage in HIV and cancer patients, lower blood cholesterol levels, aid in wound healing, and exhibit antimicrobial effects [39,40,41,42]. Purified bee venom is composed of enzymes and several active peptides, including apamin, melittin, mast cell-degranulating peptides, and adolapin, and is a natural toxin produced by Apis mellifera L. (honeybees) [43,44]. Bee venom is reported to treat back pain, rheumatoid arthritis (inflammatory disease), and skin diseases and acts as an anticancer agent in the treatment of breast cancer cells, prostate cancer cells, and lung cancer cells [45,46]. A recent study reported that bee venom was effective in periodontitis treatment, showing anti-periodontitis and anti-inflammatory effects [47].
Propolis is a natural nontoxic resinous compound produced by bees, and its composition includes 10% aromatic and essential oils, 10% pollen and other organic compounds, 30% waxes, and 50% vegetable resins [48,49]. The composition depends on various factors, such as bee species, geographical origin, and botanical origin, and the main component consists of phenolic esters, such as caffeic acid phenethyl ester and flavonoids [50,51]. Propolis is reported to have various bioactivities, such as antioxidant, anticancer, antimicrobial, anti-inflammatory, and anti-fungal activities [9,51,52]. Several in vitro and animal studies have suggested that propolis exhibits potential antioxidant effects. Propolis and its compound pinocembrin upregulate the enzymatic antioxidant pathway and induce Nrf-2 translocation to the nucleus following the expression of ARE-mediated antioxidant genes, including γ-GCS and HO-1. Propolis also regulates the expression of protein and mRNA of other antioxidant markers, including TrxR1, GCLC, LOX-1, GCLM, and γ-GCS [53,54,55]. Some recent studies reported that propolis and its compounds, such as CAPE, rutin, and myricetin inhibit ACE 2 receptors (essential for SARS-CoV-2 virus entry). This activity might be helpful in reducing the risk of COVID-19 complications [6,56,57]. Some studies also reported that propolis is effective in inhibiting periodontal pathogens, including Prevotella intermedia and P. gingivalis, and preventing alveolar bone loss in a periodontitis rat model in vivo [9]. Mouthwash, toothpaste, and dentifrices containing bee products, such as propolis and honey, have shown excellent effects in preventing gingivitis, tooth decay, periodontitis, and biofilm reduction [58,59,60].
In the last five years, more clinical trials and experimental evidence have been published indicating an increase in the trend towards the use of natural therapies with pharmacological activity in the treatment of various “oral bacterial diseases” and to provide better oral health. Given the anti-inflammatory, antimicrobial, and antioxidant activities of bee products, the practical application of bee products in dentistry might be helpful in the treatment of “oral bacterial disease” such as periodontitis, dental caries, and gingivitis. The main objective of this systematic review is to provide insight into the role of apitherapy in the treatment of periodontal disease. However, in vivo and clinical studies assessing the effects of bee products in the treatment of periodontal diseases are limited.
2. Materials and Methods
In the current review, the study selection process was conducted according to the guidelines of ‘Preferred Reporting Items for Systematic Reviews and Meta Analyses’ (PRISMA 2020) for systematic reviews [61].
2.1. Search Strategy
Various in vitro, in vivo, and clinical studies related to the role of apitherapy in the treatment of periodontal diseases were reviewed. An electronic literature search was performed using the PubMed, Web of Science, ScienceDirect, Scopus, clinicaltrials.gov, and Google Scholar databases. The following medical subject heading (MeSH) words were used individually in the search: honey, apitherapy, propolis, periodontitis, gingivitis, royal jelly, bee venom, and periodontal diseases. The following MeSH terms were used in combination: apitherapy and periodontal disease, honey and periodontal disease, propolis and periodontal disease, royal jelly and periodontal disease, bee venom and periodontal disease, propolis and gingivitis treatment, honey and gingivitis treatment, royal jelly and gingivitis treatment, and bee venom and gingivitis treatment. In the current study, an electronic literature search was performed to identify studies published within the period of 2016–2021 and were selected based on eligibility criteria, i.e., inclusion and exclusion criteria.
2.2. Inclusion and Exclusion Criteria
In the current analysis, studies were selected for review based on the following exclusion and inclusion criteria.
Exclusion criteria:
- (i)
- Studies that did not have full text available.
- (ii)
- Clinical trials that do not follow ethical guidelines.
- (iii)
- Published studies in local languages except for English.
- (iv)
- Nonrelevant studies (apitherapy in the treatment of other oral pathologies).
- (v)
- Systematic reviews.
Inclusion criteria:
- (i)
- In vitro, in vivo, and clinical studies evaluating the efficiency of honey, propolis, and royal jelly in the treatment of periodontal diseases.
- (ii)
- Findings published in English.
- (iii)
- Findings published within the period from 2016 to 2021.
- (iv)
- Randomized and nonrandomized clinical trials.
After the selection of in vivo, in vitro, and clinical studies, data related to various bioactivities of honey and its compounds in periodontal disease treatment were collected.
PRISMA flow diagram showing the selection process, including the identified records, inclusion and exclusion criteria, and the number of studies selected for review (Figure 1).
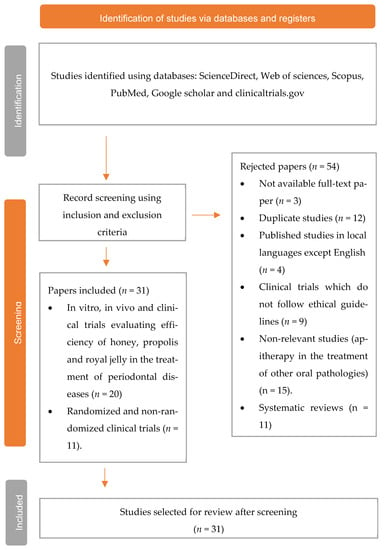
Figure 1.
Flow diagram of the study selection process.
3. Results
3.1. Study Selection
A total of 85 studies were found from the database search; 12 duplicate studies were excluded, and 3 studies with no full text were removed. Thus, a total of 31 studies were selected for review. Bee products, such as honey, propolis, bee venom, and royal jelly, have numerous applications in the treatment of various diseases given their well-known bioactivities, such as antimicrobial, antioxidant, anticancer, and antiseptic activities. In the current study, we discussed 16 in vitro studies evaluating the role of apitherapy in the treatment of periodontal disease.
3.2. Scientific Studies Evaluating Honeybee Products in Periodontal Disease Treatment
3.2.1. Antimicrobial Studies
Six of the studies investigated the antimicrobial effect of propolis against periodontal pathogenic bacteria in vitro. An ethanolic extract of propolis (EEP) shows an inhibitory effect on periodontal pathogenic bacteria, such as Prevotella melaninogenica, Porphyromonas gingivalis, Porphyromonas asaccharolytica, and Prevotella intermedia, based on a 30% w/v concentration. The zone of inhibition was 18.3 ± 0.64 mm for P. melaninogenica, 18.9 ± 0.05 mm for P. gingivalis, 22.8 ± 0.28 mm for P. asaccharolytica, and 22.8 ± 0.18 mm for P. intermedia [62]. Similarly, another study evaluated the antimicrobial activity of EEP and EEP-derived compounds using agar dilution assays and broth microdilution assays against P. gingivalis in vitro, and the results of both assays reported MIC values of 64 μg/mL (broth) and 128 μg/mL (agar). The mechanism of inhibition was also examined. EEP inhibited P. gingivalis activity and induced cell death within 30 min by increasing membrane permeability. EEP on the bacterial surface stimulated aberrant membrane bleb development following bleb fusion. Furthermore, the activity of EEP-derived compounds was examined. The results reported that ursolic acid inhibited bactericidal activity with membrane rupture. Baccharin and artepillin C show bacteriostatic activities with membrane blebbing [63].
The periodontopathic bacteria Fusobacterium nucleatum, Eikenella corrodens, and Actinomyces odontolyticus and oral carcinogenic bacteria (Streptococcus mitis, Lactobacillus acidophilus, Streptococcus mutans, and Streptococcus sanguinis) exhibited inhibitory effects in vitro when treated with propolis with a minimum inhibitory concentration of 12.5 μg/mL. In addition, propolis inhibits all periodontopathic bacteria and oral carcinogenic bacteria except for L. acidophilus with a MIC value of 6.3 μg/mL [64]. Similarly, in another study, propolis showed an inhibitory effect against Streptococcus mutans (bacteria) and Candida albicans (yeast), which are the causative organisms of dental caries. In addition, 50 μL propolis yields a 15.6 mm mean zone of inhibition for Candida albicans compared to 12 mm for probiotics and 14 mm for chlorhexidine. For Streptococcus mutans, the mean zone of inhibition was 9.4 mm for probiotics, 14 mm for chlorhexidine, and 14.6 mm for propolis. Compared to standard chlorhexidine and probiotics, propolis was found to be more effective in inhibiting Streptococcus mutans and Candida albicans [65].
The antibiofilm, cytotoxic and antimicrobial activities of propolis were assessed in an in vitro biofilm of Fusobacterium nucleatum and Streptococcus gordonii. Treatment with the methanolic fraction of propolis (chloroform partition) formed lower-than-average-thickness biofilms of F. nucleatum and S. gordonii at concentrations of 1.563 mg/mL (7.37 ± 1.620 µm and 9.24 ± 0.679 μm) and 0.78 mg/mL (6.84 ± 1.68 μm and 8.02 ± 1.6 μm), respectively. Cytoxicity assay of 0.78 mg/mL propolis (chloroform partition) on a human gingival fibroblast cell line (HGF-1) yielded 92.64% cell viability. An antimicrobial study of the methanolic fraction of propolis (chloroform residue) showed significant inhibition of F. nucleatum and S. gordonii bacteria with zones of inhibition of 12.15 ± 0.19 mm and 12.55 ± 0.19 mm, respectively, in comparison to propolis combined with chlorhexidine (14.33 ± 0.19 mm and 14.55 ± 0.19 mm, respectively) [66].
In another study, the antimicrobial activity of propolis against periodontal pathogens present in multispecies biofilms were examined in vitro. The subgingival biofilm with 32 species (7 days old) was treated with propolis from Day 3 (twice a day for 1 min). Results of microbial composition and metabolic activity determined by DNA–DNA hybridization of biofilms showed that 1600 μg/mL propolis showed no significant difference from the samples treated with chlorohexidine and decreased the metabolic activity by 45%. Based on results, propolis was found to be equally effective in decreasing subgingival biofilm formation compared to chlorhexidine [67]. Similarly, propolis at concentrations of 400, 800, and 1600 μg/mL was found to be effective in reducing the metabolic activity of multispecies biofilms (7 days old) by 57, 56, and 56%, respectively, compared to a 65% reduction with amoxicillin treatment. It was also observed that propolis treatment did not affect the host-compatible Actinomyces species level [68].
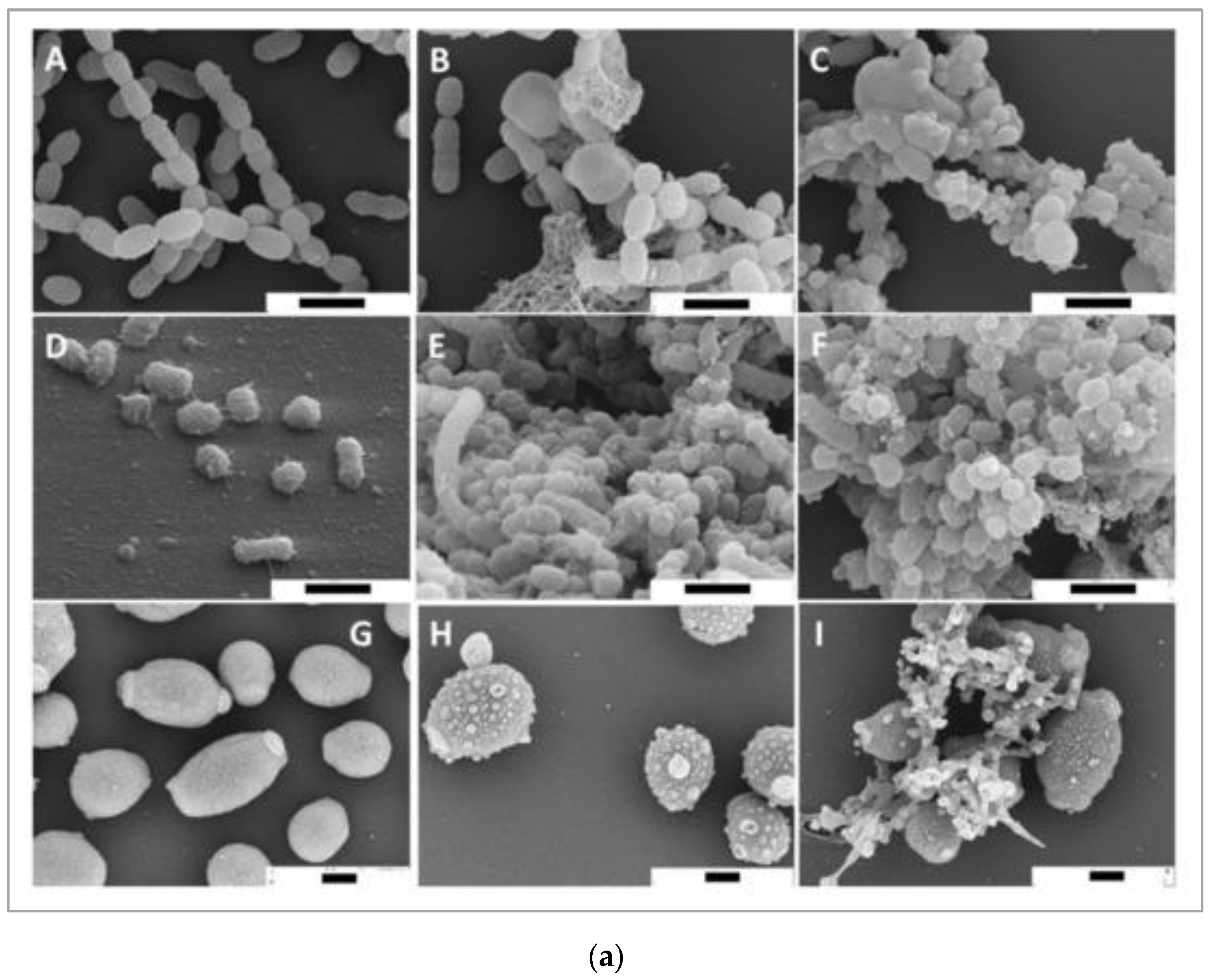
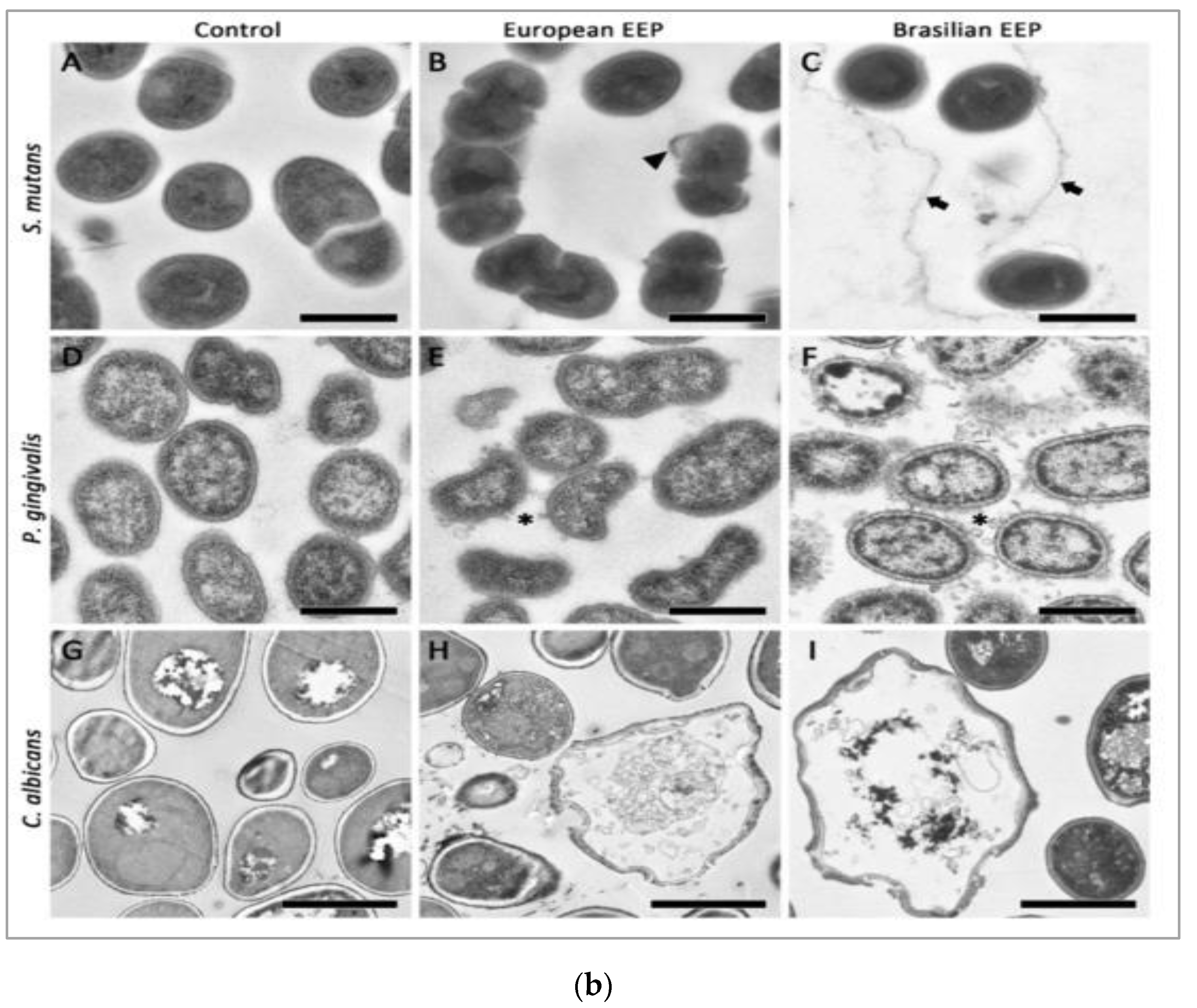
Bacteria causing periodontal diseases (Porphyromonas gingivalis), yeast causing candida infection (Candida albicans), and bacteria causing dental caries (Streptococcus mutans) treated with propolis showed an inhibitory effect with MIC values of 0.2, 6.25, and 0.2 mg/mL, respectively. The results of propolis treatment on three different biofilms of P. gingivalis, C. albicans, and S. mutans were also reported. Periodontal biofilm containing bacterial counts showed that 8.99 log10 colony forming units (CFU) of biofilm formation after 4 h was reduced to 3.21 log10 CFU by 100 mg/mL propolis after 4 h of treatment. The carcinogenic control biofilm containing 7.99 log10 CFU biofilm formation after 4 h was reduced to a bacterial count of 2.21 log10 CFU by 100 mg/mL propolis after 4 h of treatment. Candida biofilm containing bacterial counts 7.74 log10 CFU biofilm formation after 4 h was reduced to 3.65 log10 CFU by 100 mg/mL propolis after 4 h of treatment [69]. Scanning electron microscopy images suggest microbial cell wall interaction with propolis. After treatment with European propolis, large and small vesicles attached to the cell wall surface were observed. After Brazilian propolis treatment, damaged cells were found to stick together. Transmission electron microscopy images of C. albicans showed loss of cell wall integrity and cell enlargement after propolis treatment. Propolis treatment of S. mutans yielded minor modifications, and vesicles appeared outside of P. gingivalis cells. The results of scanning and transmission electron microscopy (SEM and TEM) showing the effect of propolis on bacteria are shown in Figure 2a,b.
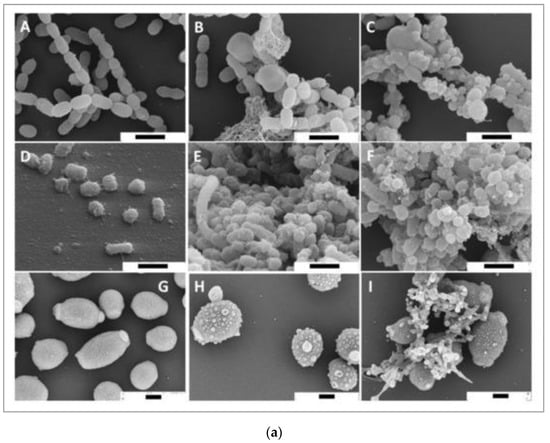
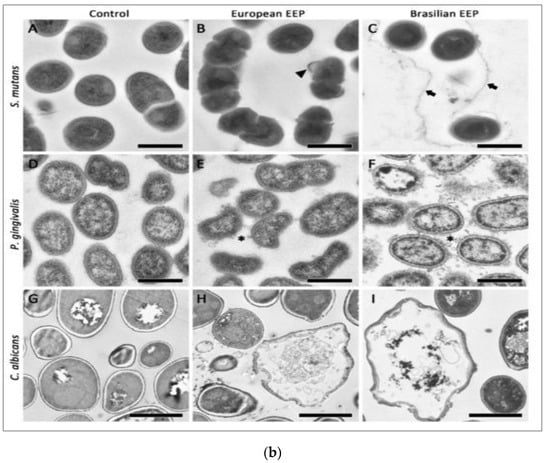
Figure 2.
(a) SEM images of S. mutans (A–C), P. gingivalis (D–F), and C. albicans (G–I). Bar (A–I) 1 μm. European propolis treatment (5 min exposure, 25 mg/mL concentration) (B,E,H). Brazilian propolis treatment (5 min exposure, 25 mg/mL concentration) (C,F,I) [69]. (b) TEM images of S. mutans (A–C), P. gingivalis (D–F), and C. albicans (G–I). TEM images: (A,D,G) are without treatment. European propolis treatment (5 min exposure, 25 mg/mL concentration) (B,E,H) and Brazilian propolis treatment (5 min exposure, 25 mg/mL concentration) (C,F,I). Bar: 2 μm for C. albicans, (A–I) 500 nm for bacteria [69].
The antifungal activity of propolis was evaluated against various Candida species extracted from chronic periodontitis in vitro. The results showed that the MIC values of propolis showed fungicidal and fungistatic activity against various Candida species: 64-152 and 32–64 µg/mL for C. albicans, 64 and 32–64 µg/mL for C. tropicalis, and 64–256 and 64–64 μg/mL for C. glabrata. Based on the results, it was observed that propolis shows antifungal activity against all three Candida species [70].
Comparing the in vitro studies discussed above, it was observed that 12.5–400 μg/mL propolis showed a significant antimicrobial effect against periodontal pathogenic bacteria. It has also been observed that EEP is more effective in the treatment of periodontopathic bacteria than raw propolis [63,64,68].
Four of the selected studies evaluated the effect of propolis on periodontitis treatment in vivo (rat model). The effect of propolis treatment on P. gingivalis-induced impaired glucose and lipid metabolism in C57BL/6 mice was studied. Powdered EEP with 2% carboxymethyl cellulose was administered to mice daily at a concentration of 200 mg/kg and effectively suppressed metabolic changes induced by P. gingivalis. Findings show that propolis treatment inhibited the upregulation of serum endotoxin levels and downregulated P. gingivalis-induced hepatic steatosis [71]. One of the studies reported that 5% and 10% propolis showed no significant therapeutic effect on periodontal disease in a Mus musculus model with ligature silk thread [72].
In another study, the administration of propolis (544 μg) and Garcinia mangostana L. (16 μg) complex (MEC) is effective in the prevention of alveolar bone loss and inhibition of inflammation in ligature-induced periodontal disease in a Wister rat model. The findings showed that MEC administration (ligation + lipopolysaccharide extracted from P. gingivalis + MEC 1:34 group) significantly reduced alveolar bone loss and downregulated the expression levels of COX-2, COX-1, MMP-8, iNOS, PGE2, and IL-8 [73]. In a similar study, propolis (10%) was effective in the treatment of ligature-induced periodontal disease in a Wister rat model. Propolis irrigation after scaling root planning caused downregulation in TNF-α, IL-1β, and malondialdehyde (MDA) serum levels compared to the control group with a statistically significant difference of p < 0.05 [74].
The antimicrobial effects of royal jelly have been examined in vitro against periodontopathic bacterial strains, including Fusobacterium nucleatum, Prevotella intermedia, Porphyromonas gingivalis, and Aggregatibacter actinomycetemcomitans. Specifically, 12.5–100 μg/mL royal jelly shows inhibitory effects on periodontopathic bacteria, and MIC values of royal jelly were higher for F. nucleatum and A. actinomycetemcomitans and lower for P. intermedia and P. gingivalis [75]. In another in vitro study, the antimicrobial activity of royal jelly was examined against periodontopathic bacteria in subgingival plaque. Royal jelly at concentrations of 12.5 and 25 μg/mL show inhibitory effects for anaerobic and aerobic bacteria compared to chlorohexidine, which showed inhibitory effects at concentrations of 6.25 and 3.25 μg/mL for anaerobic and aerobic bacteria, respectively [76]. Comparing the results of both studies, 12.5–100 μg/mL royal jelly showed an inhibitory effect on periodontopathic bacteria.
A recent in vitro study in 2021 evaluated the antibacterial efficiency of raw honey against patient-isolated Escherichia coli (reported as a periodontal pathogen due to its more effective lipopolysaccharide compared to Porphyromonas gingivalis). The zone of inhibition for 75% and 100% raw honey against patient-isolated Escherichia coli was 23 ± 0.666 and 27 ± 1.154 mm, respectively, which was equivalent to that of standard tetracycline. It has also been reported that raw honey and commercial honey at 100% concentration show a statistically significant difference (p < 0.01) in the zone of inhibition in treatment against Escherichia coli [77].
The main cause of periodontitis is the formation of bacterial biofilms due to poor oral hygiene [66]. Some periodontal pathogenic bacteria, such as P. melaninogenica, P. gingivalis, P. asaccharolytica, P. intermedia, F. nucleatum, and S. gordonii, were reported to form biofilms that cause periodontitis or gum infection [62,66]. Studies have reported that treatment with bee products, such as EEP, royal jelly, and raw honey, caused significant improvement in the reduction of periodontal biofilm formation by inhibiting different periodontopathic bacteria [67].
3.2.2. Anti-Inflammatory Activity
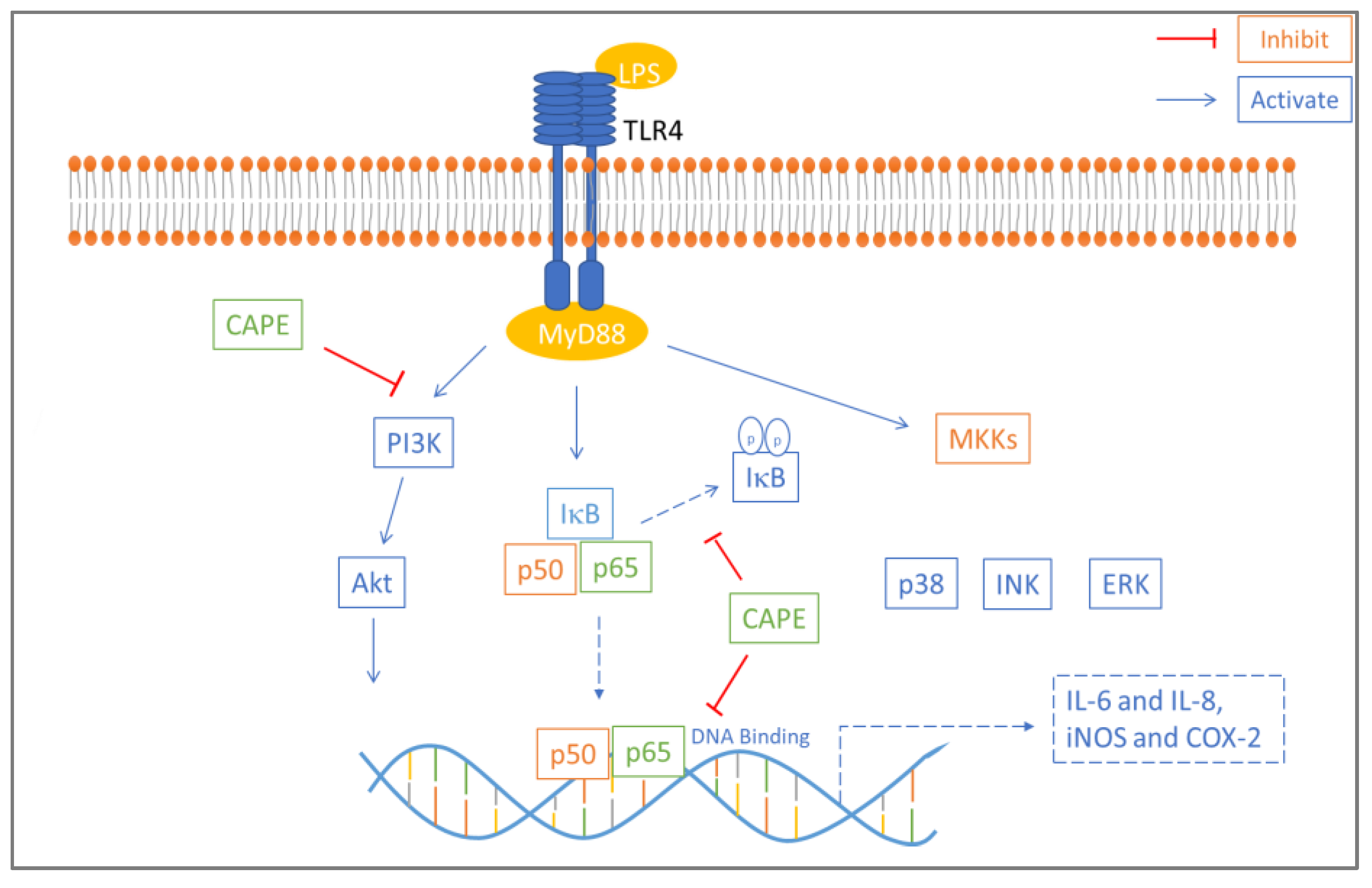
An in vitro study examined the anti-inflammatory effect of caffeic acid phenethyl ester (CAPE) on lipopolysaccharide-induced human gingival fibroblasts (cells present in periodontal soft tissue). CAPE is one of the main active compounds found in propolis and has various well-known bioactivities, such as immune regulation and antioxidant, anti-inflammatory, and antitumor activities. CAPE inhibited LPS-induced inducible nitric oxide synthase (iNOS), cyclooxygenase 2 (COX-2), and interleukin (IL-8 and IL-6) production in a dose-dependent manner and inhibited protein kinase B (AKT) and phosphatidylinositol 3 kinase (PI3K) phosphorylation. Western blot assay results showed that lipopolysaccharide-stimulated nuclear factor kappa B (NF-κB) and TLR4/MyD88 activation were suppressed by CAPE treatment (Figure 3). Based on these results, CAPE reduces the proinflammatory response in lipopolysaccharide-induced human gingival fibroblasts via the NF-κB and PI3K/Akt signaling pathways [78].
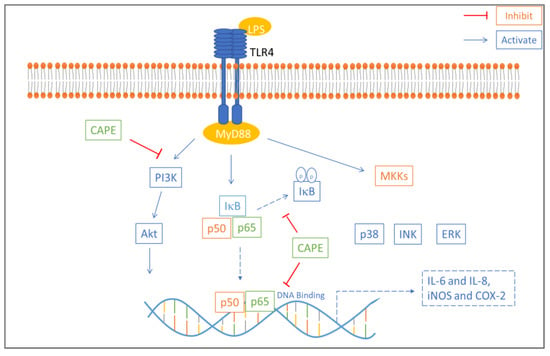
Figure 3.
Mechanism of anti-inflammatory effect of CAPE in LPS-induced HGFs. CAPE inhibited phosphorylation of IκB which reduced NF-κB p50 and p65 nuclear translocation. Akt and PI3K phosphorylation involved in NF-κB activation is also inhibited by CAPE. NF-κB p65 DNA binding is also blocked by CAPE [54].
CAPE inhibits phosphorylation of IκB, which reduces NF-κB p50 and p65 nuclear translocation. Akt and PI3K phosphorylation is important for NF-κB activation and is also inhibited by CAPE. NF-κB p65 DNA binding is also blocked by CAPE [54].
Purified bee venom was examined in vitro to determine whether it can reduce inflammatory periodontitis induced by P. gingivalis and osteoclast differentiation induced by receptor activator of nuclear factor-kappa B ligand signaling (RANKL). The results showed that bee venom (100 μg/kg) treatment reduced inflammatory bone loss-related periodontitis induced by P. gingivalis and reduced the expression of IL-1β and tumor necrosis factor (TNF)-α in vivo. Bee venom treatment also suppressed osteoclast-specific gene expression of tartrate resistant acid phosphate (TRAP), cathepsin K, integrin αVβ3, and nuclear factor of activated T cells 1 (NFATc1) and suppressed multinucleated osteoclast differentiation induced by RANKL [79]. Similarly, another in vitro study examined the anti-inflammatory mechanism of purified bee venom treatment on a P. gingivalis lipopolysaccharide (PGLPS)-induced human keratinocyte cell line (HaCaT). The results showed that PGLPS upregulated the expression of proinflammatory cytokines, including IL-1β, IL-8, IL-6, TNF-α, and toll-like receptor (TLR)-4, and induced signaling pathway activation of the inflammatory cytokine-related transcription factors activator protein 1 (AP-1) and NF-κB. Furthermore, treatment with bee venom (100 ng/mL) inhibited proinflammatory cytokines by downregulating the AP-1 and NF-κB signaling pathways [80].
Melittin, a compound found in bee venom and known for its antibacterial and anti-inflammatory effects, was investigated in vitro for its anti-inflammatory effect on PGLPS-treated HaCaT cells. PGLPS treatment of HaCaT cells upregulated the expression of proinflammatory cytokines, such as interferon (IFN)-γ, IL-6, IL-8, TNF-α, and TLR-4, and induced NF-κB, protein kinase B/Akt, and extracellular signal-regulated kinase (ERK) signaling pathway activation. However, treatment with 1 µg/mL melittin downregulated the expression of proinflammatory cytokines by suppressing the signaling pathway activation of NF-κB, Akt, and ERK. Based on these results, melittin treatment reduced the PGLPS-induced inflammatory response [81].
Cytokines act as the first wave of response to periodontopathic bacteria, are modulators of the inflammatory response and homeostasis and stimulate accessory cell populations and lymphocytes. The disordered regulation of cytokines can induce or accelerate periodontitis, as some studies have shown that single nucleotide polymorphisms in cytokines are related to the severity of periodontal disease [10]. Some studies have reported that bee products, such as propolis and bee venom as well as their compounds CAPE and melittin, can provide a balance to disrupt the regulation of cytokines through the downregulation of their expression and subsequent suppression of signaling pathways. These effects collectively reduce the severity of periodontitis [78,79,80,81].
The outcomes of the various in vitro studies discussed above are summarized in Table 1.

Table 1.
Scientific studies have examined the effects of honeybee products in the treatment of periodontal disease.
3.3. Safety of Honeybee Products in Periodontal Disease Treatment
In the current study, we discussed 11 clinical studies evaluating the role of apitherapy in the treatment of periodontal disease. A randomized double-blind controlled clinical trial investigated the effect of propolis (topical administration) into >5 mm periodontal pockets of periodontitis patients. A total of 24 patients diagnosed with chronic periodontitis were selected and divided into four groups (6 patients in each group). Each group underwent treatment with a different ointment: Group I—placebo group (placebo carboxymethyl cellulose sodium salt (CMC) ointment); group II—propolis group (0.01 mg/mL EEP in CMC ointment); group III—curry leaf group (1 mg/mL water-extracted curry leaf in CMC ointment); and group IV—minocycline group (2% minocycline hydrochloride ointment). Propolis ointment was administered thrice at 1-month intervals. The results showed that P. gingivalis was significantly reduced in gingival crevicular fluid after treatment with propolis. An improvement in the score of the clinical attachment level was noted in the propolis group (1.67 ± 1.22 mm) compared to the placebo group (0.33 ± 0.82 mm), but the difference was not significant (mean difference (MD)—1.33 mm, p = 0.160 and confidence interval (CI) 95% of difference—0.42 to 3.08 mm). Propolis also improved the score of probing pocket depth (1.83 ± 1.17 mm) compared to the placebo (0.33 ± 0.82 mm), but the difference was not significant (CI 95% of difference—0.11 to 2.89 mm, p = 0.033 and MD—1.50 mm) [82].
The efficacy of propolis-containing mouthwash in the treatment of gingivitis was evaluated in a double-blinded randomized clinical trial (Registered in Iranian Randomized Clinical Trial site with IRCT ID: IRCT20150210021029N3). A total of 32 patients diagnosed with gingivitis were selected and divided into two groups: Group I received propolis extract containing mouthwash, and Group II received the same mouthwash without propolis extract (each group had 16 patients allocated). The propolis mouthwash (30 drops mixed with 20 mL water) was given to patients twice a day (gargle 1 min) with a 12-hour interval. The results showed no significant difference (p = 0.91) in the plaque index (PI) score of the propolis group (85.19 ± 51.6%) compared to the placebo group (83.93 ± 36.1%). The results showed a significant reduction in the papillary bleeding index (PBI) of the propolis compared with the placebo group with a significant difference of p < 0.001 between the two groups. The tooth color change over time was insignificant in the propolis group (p = 0.14) and significant in the placebo group [83].
In another double-blind randomized clinical trial, propolis extract, nano vitamin E, and nano vitamin C in gel formulation were examined for their efficiency as adjuvants to mechanical debridement in peri-implant mucositis (PM) treatment. In this study, a total of 46 patients with at least one implant with PM were selected and were divided into two groups: Group I was treated with a 2% propolis extract-containing gel, and Group II served as the control group without propolis gel. The test group and control group included 23 participants each and were advised to use gel as toothpaste for 1 month 3 times/day. The results showed that after treatment, 0% of patients in the control group and 26.1% of patients in the test group showed complete PM resolution (p = 0.02). In the test group, a significant reduction was reported in probing depths (p = 0.27), plaque index score (p = 0.03), and bleeding on probing (p = 0.04) compared to the control groups. From baseline to the 1-month follow-up, significant reductions in Porphyromonas gingivalis (p = 0.05) and Tannerella forsythia (p = 0.02) were observed in the test group compared with the control group. Based on the results, the test gel shows antimicrobial activity after the course of 1 month and clinically improved PM [84].
The effectiveness of manuka honey and raw honey mouthwash on GI and PI was evaluated in a double-blind randomized controlled field trial (CTRI no: CTRI/2017/11/010565). A total of 135 school children were selected for the study and were divided into three groups with 45 participants each: Group I used manuka honey, Group II used raw honey, and Group III used chlorhexidine mouthwash (control). Participants were instructed to use 10 mL of honey mouthwash twice/day for the course of 21 days. Examination of participants was performed at baseline, one day after mouthwash discontinuation, and 1week after mouthwash discontinuation. The results of the clinical parameters PI and GI score showed statistically significant reductions in the test groups (manuka and raw honey mouthwash) and control group (chlorhexidine mouthwash). The GI score in the raw honey mouthwash group decreased from baseline (1.465 ± 0.17) to the 22nd day (0.927 ± 0.26). The GI score in the manuka honey mouthwash group decreased from baseline (1.457 ± 0.18) to the 22nd day (0.976 ± 0.15). The score of the chlorhexidine mouthwash group decreased from baseline (1.452 ± 0.19) to the 22nd day (0.498 ± 0.5). The PI score of the raw honey mouthwash group decreased from baseline (1.525 ± 0.2) to the 22nd day (0.723 ± 0.11). The score of the manuka honey mouthwash group decreased from baseline (1.525 ± 0.2) to the 22nd day (0.72 ± 0.12), and the score of the chlorhexidine mouthwash group decreased from baseline (1.505 ± 0.23) to the 22nd day (0.495 ± 0.13). Based on the results, honey-based mouthwash shows similar antimicrobial effects on PI and GI scores compared to chlorhexidine mouthwash [85].
A randomized controlled clinical trial investigated the immunological and clinical efficacy of propolis and mangosteen extract (PME) on gingivitis and early periodontitis. A total of 80 patients diagnosed with incipient periodontitis or gingivitis were selected and randomly allocated to two groups, including Group I—test (capsule with PME) and Group II—control (same capsule without PME) with 41 and 39 participants, respectively. Test group patients were advised to take 194 mg of PME capsules, and control group patients were advised to take the same capsule without PME daily for the course of 8 weeks. The results showed a significant difference of p = 0.0406 in the modified GI between the test and control groups at 4 and 8 weeks. The results of the test group also showed an increase in salivary matrix metalloproteinase-9 and a reduction in IL-6 after 8 weeks. Patient-reported outcomes assessed by oral health impact profile (OHIP)-14 questionnaires also showed improvement after 4 weeks in the test group compared to the placebo group [86].
In another randomized clinical trial, the antimicrobial effect of propolis (mouthwash and pate formulation) was investigated in patients (after tooth extraction) with peri-odontal disease. A total of 60 patients for the study of propolis paste and 40 patients for the propolis mouthwash study were selected. Furthermore, the mouthwash patients were divided into four groups: Group I—placebo (control mouthwash); Group II—used 0.2% chlorhexidine containing mouthwash; Group III—used 2% propolis containing mouthwash; and Group IV—used 0.2% chlorhexidine + 2% propolis-containing mouthwash. Each group had 10 participants, separately. The result of the propolis mouthwash assay shows a reduction in bacterial proliferation. In particular, patients using the mouthwash formulation of 0.2% chlorhexidine + 2% propolis exhibited < 105 CFU. The results of the propolis paste assay reported 90% complete healing in periodontal sockets in comparison with the control paste, which showed 13.4% complete healing after three days of surgery. Based on these results, propolis paste was found to be a viable alternative for periodontal socket healing after dental extraction [87].
The anti-inflammatory effect of polyherbal mouthwash containing Salvia officinalis, Plantago lanceolata leaf extract, 1.75% essential oil, and propolis extract was evaluated in a single-blind randomized controlled trial. A total of 40 patients were selected with moderate or severe periodontitis and were divided into two groups: Group I—phytoherbal mouthwash; and Group II—placebo mouthwash. Twenty participants were allocated to each group. The test group was instructed to rinse with phytoherbal mouthwash, and the control group was instructed to rinse with placebo mouthwash for 2 min twice/day for the course of 3 months. The results of probing depth (PD), clinical attachment level (CAL), full month plaque score (FMBS), and full month bleeding score (FMBS) were recorded at baseline and after the course of 3 months. Both the control group and test group showed a statistically significant reduction from baseline to 3 months in the P.D (CG p = 0.011, TG p = 0.001), FMPS (CG p = 0.003, TG p = 0.001), CAL (CG p = 0.020, TG p < 0.001), and FMBS (CG p = 0.002, TG p = 0.001) [88].
In another randomized controlled clinical trial, the efficiency of propolis and herbs (antioxidant-based formula) as adjunctive therapy to nonstandard periodontal treatment was examined. In this study, a total of 40 patients were selected and randomly allocated to the test group or control group. The results of clinical parameters were recorded at baseline, 1 month, and after 3 months. No significant clinical difference was noted between the two groups (p > 0.05). It has also been reported that the results of the test group show better oxidation stress reduction results than those of the placebo group [89].
In a triple-blind randomized controlled clinical trial, the efficacy of a propolis mouth rinse on oral pathogens was investigated. A total of 120 participants were selected and randomly assigned to four different groups: Group I—hot EEP; Group II—cold EEP; Group III—0.2% chlorhexidine gluconate; and Group IV—placebo (distilled water). In total, 30 participants were included in each group. Participants were advised to rinse twice a day for the course of 3 months. For the microbial assay, saliva was collected at baseline, 5 min, and 1 h, and GI and PI were recorded at baseline, 15 days, 1 month, and 3 months. The results show a decline in the S. mutans concentration after the use of mouth rinse (p < 0.05). The cell counts of S. mutans and L. acidophilus were decreased compared with baseline with the use of chlorhexidine mouthwash (5.8 × 102) and hot ethanolic propolis mouthwash (5.5 × 102). Additionally, a significant reduction in plaque scores was observed after the course of 3 months in the cold ethanolic propolis (0.46), hot ethanolic propolis (0.47), and chlorhexidine (0.45) mouthwash groups. Based on the results, propolis mouthwash was found to be as effective as chlorhexidine mouthwash in reducing dental caries pathogens and dental plaque [90].
In a randomized placebo–control study, the effect of Polish propolis and plant oils (toothpaste) on the oral cavity health of patients with orthodontically treated oral clefts was examined. A total of 50 patients were selected and were randomly assigned into two groups: Group I—test group (used toothpaste with active ingredients, including menthol, rosemary oil, Polish propolis, and tea tree oil); and Group II—control group (used toothpaste without active ingredients as placebo). In total, 25 patients were allocated to each group. Patients were advised to brush their teeth with propolis toothpaste or placebo toothpaste 3 times/day for 3 min over the course of 35 days. The results show that after the use of propolis toothpaste in Group I (toothpaste with propolis and plant oils) for gingival conditions, the gingival bleeding index (GBI) was significantly decreased for molars (p = 0.0017), incisors (p = 0.007), and total GBI (p = 0.002). A significant improvement in the oral hygiene index (OHI) was observed (p = 0.011). Based on the results, propolis and plant oil toothpaste can be effective in preventing and controlling oral infectious diseases that occur during orthodontic treatment of oral clefts [91].
In a triple-blind parallel-group clinical trial, the effect of propolis mouthwash treatment on GI and PI was evaluated in patients undergoing orthodontic treatment. In this study, a total of 40 patients were selected and randomly assigned to two groups: Group I—test group (propolis aqueous extract); and Group II—control group (chlorhexidine mouthwash). Twenty patients were allocated to each group. The test group and control group were advised to use mouthwash for 3 weeks after brushing their teeth twice/day consecutively. The GI, PI, and periodontal index results were evaluated at baseline and after 3 weeks. A statistically significant difference between the scores of periodontal index (p = 0.005), PI (p < 0.001), and GI (p = 0.006) in the test group was observed. In the chlorhexidine group, significant differences were also observed in the periodontal index (p = 0.003), GI (p = 0.001), and PI (p < 0.001). Based on results, propolis mouthwash was found to be as effective as chlorhexidine mouthwash [92].
Out of the 11 selected clinical trials discussed above, 8 were randomized clinical trials, including 4 single randomized clinical trials, 3 double-blinded randomized clinical trials, 1 triple-blinded clinical trial, and 3 non-randomized clinical trials. The majority of clinical trials investigated the effect of propolis, and one of the clinical trials investigated the effect of raw honey in periodontitis treatment [85]. Out of the four double-blind randomized clinical trials, one study showed no significant difference in clinical attachment level, CI or probing pocket depth with treatment with propolis ointment compared to the control group [82]. All the clinical trials suggested that propolis and honey-based products, such as mouthwash [83,85,87,88,90,92], gel [89], ointment [82], capsule [86], and toothpaste [84,91], were significantly effective compared to control groups in the treatment of periodontal disease.
The outcomes of clinical trials investigating the safety of honeybee products in periodontal disease treatment are shown in Table 2.

Table 2.
Clinical trials were conducted to evaluate the potential of bee products in periodontal disease treatment.
The observed limitation of this study is the use of various indices and assessment criteria to determine the effect of bee products on periodontal disease. The plaque index was measured in only three studies [59,64,68] with an 85% reduction compared to the control group, 83%. One study measured the plaque score [66], and the other seven studies did not use the plaque index. The gingival index was measured in only two studies [61,68]. Bleeding was measured using two different criteria: probing bleeding [64] and papillary bleeding index [59]. Probing depth was measured in three studies [58,64,66]. Regarding other assessment criteria, CAL was measured in two studies [58,66] with a confidence interval reported in one study [58]. A reduction in bacterial proliferation and healing of the periodontal socket was noted with 90% recovery compared to the control showing 13.4% recovery [63]. Furthermore, there are limitations in sample size and assessment time. Different numbers of participants were selected in all studies. Most studies had small sample sizes, and heterogeneity was noted in the assessment time of each clinical trial, ranging from 1 week to a few months. Bee products, including bee venom, royal jelly, and honey, have shown good results in the treatment of periodontal disease; however, limited clinical studies and experimental evidence are available to date supporting the effect of bee products. However, in the case of propolis, the antimicrobial and antioxidant activities are well known, but a limited number of randomized clinical trials are available. Therefore, in the future, it is important to perform more randomized clinical trials to assess periodontal parameters, such as bleeding, gingival index, plaque, and oral hygiene index, using unified criteria, performing well-defined research with a broader sample size, and following standard ethical guidelines to compare the use of bee products with the control group.
4. Conclusions
Over a long period of time, apitherapy has maintained its popularity, and various bee products, such as propolis, bee venom, and honey, have been scientifically demonstrated to have numerous applications in dentistry due to their antimicrobial, anti-inflammatory, anticancer, immune-modulating, and antioxidant properties. Based on clinical and experimental evidence, it is suggested that propolis is the most effective bee product in the treatment of periodontal disease with the concentration range of 12–400 μg/mL. These bee products are likely to represent an alternative to synthetic drugs in periodontal disease treatment in the future; however, to date, limited in vivo and clinical evidence validating the application of bee products, especially bee venom, honey, and royal jelly is available. Furthermore, numerous findings supported by in vitro, in vivo, and clinical trials have validated that propolis products, such as mouthwash, gels, ointments, and toothpaste, have potential application in periodontitis treatment, showing antioxidant and antimicrobial activity. Based on these studies, it can be concluded that bee products are safer to use in the treatment of periodontitis; however, there is a need for more clinical and in vivo experimental evidence to explore the underlying mechanism of the bioactivities of bee products in periodontitis treatment.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank the University of Kiel and Schleswig-Holstein for their support through the OA program.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Oral Health. Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 11 February 2022).
- Prakash, S.; Radha; Kumar, M.; Kumari, N.; Thakur, M.; Rathour, S.; Pundir, A.; Sharma, A.K.; Bangar, S.P.; Dhumal, S.; et al. Plant-Based Antioxidant Extracts and Compounds in the Management of Oral Cancer. Antioxidants 2021, 10, 1358. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Prakash, S.; Radha; Kumari, N.; Pundir, A.; Punia, S.; Saurabh, V.; Choudhary, P.; Changan, S.; Dhumal, S.; et al. Beneficial role of antioxidant secondary metabolites from medicinal plants in maintaining oral health. Antioxidants 2021, 10, 1061. [Google Scholar] [CrossRef] [PubMed]
- Sasi, M.; Kumar, S.; Kumar, M.; Thapa, S.; Prajapati, U.; Tak, Y.; Changan, S.; Saurabh, V.; Kumari, S.; Kumar, A.; et al. Garlic (Allium sativum L.) bioactives and its role in alleviating oral pathologies. Antioxidants 2021, 10, 1847. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Walczyńska-Dragon, K.; Felitti, R.; Nitecka-Buchta, A.; Baron, S.; Olczyk, P. The Influence of Propolis on Dental Plaque Reduction and the Correlation between Dental Plaque and Severity of COVID-19 Complications-A Literature Review. Molecules 2021, 26, 5516. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Propolis, Bee Honey, and Their Components Protect against Coronavirus Disease 2019 (COVID-19): A Review of In Silico, In vitro, and Clinical Studies. Molecules 2021, 26, 1232. [Google Scholar] [CrossRef]
- Elmahallawy, E.K.; Mohamed, Y.; Abdo, W.; El-Gohary, F.A.; Ahmed Awad Ali, S.; Yanai, T. New Insights into Potential Benefits of Bioactive Compounds of Bee Products on COVID-19: A Review and Assessment of Recent Research. Front. Mol. Biosci. 2021, 7, 513. [Google Scholar] [CrossRef]
- López-Valverde, N.; Pardal-Peláez, B.; López-Valverde, A.; Flores-Fraile, J.; Herrero-Hernández, S.; Macedo-De-sousa, B.; Herrero-Payo, J.; Ramírez, J.M. Effectiveness of Propolis in the Treatment of Periodontal Disease: Updated Systematic Review with Meta-Analysis. Antioxidants 2021, 10, 269. [Google Scholar] [CrossRef]
- Pan, W.; Wang, Q.; Chen, Q. The cytokine network involved in the host immune response to periodontitis. Int. J. Oral Sci. 2019, 11, 30. [Google Scholar] [CrossRef] [Green Version]
- Madianos, P.N.; Bobetsis, Y.A.; Offenbacher, S. Adverse pregnancy outcomes (APOs) and periodontal disease: Pathogenic mechanisms. J. Clin. Periodontol. 2013, 40 (Suppl. S14), S170–S180. [Google Scholar] [CrossRef]
- Lundberg, K.; Wegner, N.; Yucel-Lindberg, T.; Venables, P.J. Periodontitis in RA-the citrullinated enolase connection. Nat. Rev. Rheumatol. 2010, 6, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Lalla, E.; Papapanou, P.N. Diabetes mellitus and periodontitis: A tale of two common interrelated diseases. Nat. Rev. Endocrinol. 2011, 7, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Kebschull, M.; Demmer, R.T.; Papapanou, P.N. “Gum Bug, Leave My Heart Alone!”—Epidemiologic and Mechanistic Evidence Linking Periodontal Infections and Atherosclerosis. J. Dent. Res. 2010, 89, 879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genco, R.J.; Van Dyke, T.E. Prevention: Reducing the risk of CVD in patients with periodontitis. Nat. Rev. Cardiol. 2010, 7, 479–480. [Google Scholar] [CrossRef] [PubMed]
- Darveau, R.P. Periodontitis: A polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010, 8, 481–490. [Google Scholar] [CrossRef]
- Eley, B.M. Antibacterial agents in the control of supragingival plaque--a review. Br. Dent. J. 1999, 186, 286–296. [Google Scholar] [CrossRef]
- Kim, E.H.; Kim, S.; Kim, H.J.; Jeong, H.O.; Lee, J.; Jang, J.; Joo, J.Y.; Shin, Y.; Kang, J.; Park, A.K.; et al. Prediction of Chronic Periodontitis Severity Using Machine Learning Models Based on Salivary Bacterial Copy Number. Front. Cell. Infect. Microbiol. 2020, 10, 698. [Google Scholar] [CrossRef]
- Abusleme, L.; Dupuy, A.K.; Dutzan, N.; Silva, N.; Burleson, J.A.; Strausbaugh, L.D.; Gamonal, J.; Diaz, P.I. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013, 7, 1016–1025. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Y.; Darzi, Y.; Tawaratsumida, K.; Marchesan, J.T.; Hasegawa, M.; Moon, H.; Chen, G.Y.; Núñez, G.; Giannobile, W.V.; Raes, J.; et al. Induction of bone loss by pathobiont-mediated Nod1 signaling in the oral cavity. Cell Host Microbe 2013, 13, 595–601. [Google Scholar] [CrossRef] [Green Version]
- Hajishengallis, G.; Liang, S.; Payne, M.A.; Hashim, A.; Jotwani, R.; Eskan, M.A.; McIntosh, M.L.; Alsam, A.; Kirkwood, K.L.; Lambris, J.D.; et al. A Low-Abundance Biofilm Species Orchestrates Inflammatory Periodontal Disease through the Commensal Microbiota and the Complement Pathway. Cell Host Microbe 2011, 10, 497. [Google Scholar] [CrossRef] [Green Version]
- Settem, R.P.; El-Hassan, A.T.; Honma, K.; Stafford, G.P.; Sharma, A. Fusobacterium nucleatum and Tannerella forsythia Induce Synergistic Alveolar Bone Loss in a Mouse Periodontitis Model. Infect. Immun. 2012, 80, 2436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orth, R.K.H.; O’Brien-Simpson, N.M.; Dashper, S.G.; Reynolds, E.C. Synergistic virulence of Porphyromonas gingivalis and Treponema denticola in a murine periodontitis model. Mol. Oral Microbiol. 2011, 26, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Prim. 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Dikilitas, A.; Karaaslan, F.; Aydin, E.Ö.; Yigit, U.; Ertugrul, A.S. Granulocyte-macrophage colony-stimulating factor (GM-CSF) in subjects with different stages of periodontitis according to the new classification. J. Appl. Oral Sci. 2022, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nędzi-Góra, M.; Kowalski, J.; Górska, R. The Immune Response in Periodontal Tissues. Arch. Immunol. Ther. Exp. 2017, 65, 421–429. [Google Scholar] [CrossRef]
- Sochalska, M.; Potempa, J. Manipulation of Neutrophils by Porphyromonas gingivalis in the Development of Periodontitis. Front. Cell. Infect. Microbiol. 2017, 7, 197. [Google Scholar] [CrossRef] [Green Version]
- Tsantikos, E.; Lau, M.; Castelino, C.M.N.; Maxwell, M.J.; Passey, S.L.; Hansen, M.J.; McGregor, N.E.; Sims, N.A.; Steinfort, D.P.; Irving, L.B.; et al. Granulocyte-CSF links destructive inflammation and comorbidities in obstructive lung disease. J. Clin. Investig. 2018, 128, 2406–2418. [Google Scholar] [CrossRef]
- Pendyala, G.; Thomas, B.; Kumari, S. The challenge of antioxidants to free radicals in periodontitis. J. Indian Soc. Periodontol. 2008, 12, 79. [Google Scholar] [CrossRef]
- Borges, I.; Machado Moreira, E.A.; Filho, D.W.; De Oliveira, T.B.; Da Silva, M.B.S.; Fröde, T.S. Proinflammatory and Oxidative Stress Markers in Patients with Periodontal Disease. Mediat. Inflamm. 2007, 2007, 045794. [Google Scholar] [CrossRef]
- Abou Sulaiman, A.E.; Shehadeh, R.M.H. Assessment of Total Antioxidant Capacity and the Use of Vitamin C in the Treatment of Non-Smokers with Chronic Periodontitis. J. Periodontol. 2010, 81, 1547–1554. [Google Scholar] [CrossRef]
- Wang, Y.; Andrukhov, O.; Rausch-Fan, X. Oxidative Stress and Antioxidant System in Periodontitis. Front. Physiol. 2017, 8, 910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ara, S.A.; Ashraf, S.; Arora, V.; Rampure, P. Use of Apitherapy as a Novel Practice in the Management of Oral Diseases: A Review of Literature. J. Contemp. Dent. 2013, 3, 25–31. [Google Scholar] [CrossRef]
- Singh, R.P.; Prakash, S.; Bhatia, R.; Negi, M.; Singh, J.; Bishnoi, M.; Kondepudi, K.K. Generation of structurally diverse pectin oligosaccharides having prebiotic attributes. Food Hydrocoll. 2020, 108, 105988. [Google Scholar] [CrossRef]
- Kumar, M.; Radha; Devi, H.; Prakash, S.; Rathore, S.; Thakur, M.; Puri, S.; Pundir, A.; Bangar, S.P.; Changan, S.; et al. Ethnomedicinal plants used in the health care system: Survey of the mid hills of solan district, Himachal Pradesh, India. Plants 2021, 10, 1842. [Google Scholar] [CrossRef]
- Hellner, M.; Winter, D.; Von Georgi, R.; Münstedt, K. Apitherapy: Usage and Experience in German Beekeepers. Evid. Based Complement. Altern. Med. 2008, 5, 475. [Google Scholar] [CrossRef] [Green Version]
- Nabavi, S.M.; Silva, A.S. (Eds.) Nonvitamin and Nonmineral Nutritional Supplements; Academic Press: Cambridge, MA, USA, 2018; pp. 1–552. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, L.; Zhang, W.; Cui, X.; Wang, H.; Xu, B. Comparison of the nutrient composition of royal jelly and worker jelly of honeybees (Apis mellifera). Apidologie 2016, 47, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Melliou, E.; Chinou, I. Chemistry and Bioactivities of Royal Jelly. Stud. Nat. Prod. Chem. 2014, 43, 261–290. [Google Scholar] [CrossRef]
- Ahmad, S.; Campos, M.G.; Fratini, F.; Altaye, S.Z.; Li, J. New Insights into the Biological and Pharmaceutical Properties of Royal Jelly. Int. J. Mol. Sci. 2020, 21, 382. [Google Scholar] [CrossRef] [Green Version]
- Bălan, A.; Moga, M.A.; Dima, L.; Toma, S.; Neculau, A.E.; Anastasiu, C.V. Royal Jelly—A traditional and natural remedy for postmenopausal symptoms and aging-related pathologies. Molecules 2020, 25, 3291. [Google Scholar] [CrossRef]
- Fratini, F.; Cilia, G.; Mancini, S.; Felicioli, A. Royal Jelly: An ancient remedy with remarkable antibacterial properties. Microbiol. Res. 2016, 192, 130–141. [Google Scholar] [CrossRef]
- Abd El-Wahed, A.A.; Khalifa, S.A.M.; Sheikh, B.Y.; Farag, M.A.; Saeed, A.; Larik, F.A.; Koca-Caliskan, U.; AlAjmi, M.F.; Hassan, M.; Wahabi, H.A.; et al. Bee Venom Composition: From Chemistry to Biological Activity. Stud. Nat. Prod. Chem. 2019, 60, 459–484. [Google Scholar] [CrossRef]
- Wehbe, R.; Frangieh, J.; Rima, M.; El Obeid, D.; Sabatier, J.M.; Fajloun, Z. Bee Venom: Overview of Main Compounds and Bioactivities for Therapeutic Interests. Molecules 2019, 24, 2997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fratellone, P.M.; Tsimis, F.; Fratellone, G. Apitherapy products for medicinal use. J. Altern. Complement. Med. 2016, 22, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Tanuğur-Samanc, A.E.; Kekeçoğlu, M. An evaluation of the chemical content and microbiological contamination of Anatolian bee venom. PLoS ONE 2021, 16, e0255161. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; Eid, N.; Abd El-Wahed, A.A.; Rateb, M.E.; Afifi, H.S.; Algethami, A.F.; Zhao, C.; Al Naggar, Y.; Alsharif, S.M.; Tahir, H.E.; et al. Honeybee Products: Preclinical and Clinical Studies of Their Anti-inflammatory and Immunomodulatory Properties. Front. Nutr. 2022, 8, 1109. [Google Scholar] [CrossRef]
- Wagh, V.D. Propolis: A Wonder Bees Product and Its Pharmacological Potentials. Adv. Pharmacol. Sci. 2013, 2013, 11. [Google Scholar] [CrossRef] [Green Version]
- Toreti, V.C.; Sato, H.H.; Pastore, G.M.; Park, Y.K. Recent progress of propolis for its biological and chemical compositions and its botanical origin. Evid.-Based Complement. Altern. Med. 2013, 2013, 697390. [Google Scholar] [CrossRef]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef]
- da Silva Barboza, A.; Aitken-Saavedra, J.P.; Ferreira, M.L.; Fábio Aranha, A.M.; Lund, R.G. Are propolis extracts potential pharmacological agents in human oral health? -A scoping review and technology prospecting. J. Ethnopharmacol. 2021, 271, 113846. [Google Scholar] [CrossRef]
- Elnakady, Y.A.; Rushdi, A.I.; Franke, R.; Abutaha, N.; Ebaid, H.; Baabbad, M.; Omar, M.O.M.; Al Ghamdi, A.A. Characteristics, chemical compositions and biological activities of propolis from Al-Bahah, Saudi Arabia. Sci. Rep. 2017, 7, 41453. [Google Scholar] [CrossRef] [Green Version]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxid. Med. Cell. Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef] [PubMed]
- El Adham, E.K.; Hassan, A.I.; Dawoud, M.M.A. Evaluating the role of propolis and bee venom on the oxidative stress induced by gamma rays in rats. Sci. Rep. 2022, 12, 2656. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, T.; Starowicz, M.; Kłębukowska, L.; Hanus, P. The Profile of Polyphenolic Compounds, Contents of Total Phenolics and Flavonoids, and Antioxidant and Antimicrobial Properties of Bee Products. Molecules 2022, 27, 1301. [Google Scholar] [CrossRef] [PubMed]
- Berretta, A.A.; Silveira, M.A.D.; Cóndor Capcha, J.M.; De Jong, D. Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease: Running title: Propolis against SARS-CoV-2 infection and COVID-19. Biomed. Pharmacother. 2020, 131, 110622. [Google Scholar] [CrossRef]
- Ripari, N.; Sartori, A.A.; Honorio, M.D.S.; Conte, F.L.; Tasca, K.I.; Santiago, K.B.; Sforcin, J.M. Propolis antiviral and immunomodulatory activity: A review and perspectives for COVID-19 treatment. J. Pharm. Pharmacol. 2021, 73, 281–299. [Google Scholar] [CrossRef]
- Dodwad, V.; Kukreja, B. Propolis mouthwash: A new beginning. J. Indian Soc. Periodontol. 2011, 15, 121. [Google Scholar] [CrossRef]
- Halboub, E.; Al-Maweri, S.A.; Al-Wesabi, M.; Al-Kamel, A.; Shamala, A.; Al-Sharani, A.; Koppolu, P. Efficacy of propolis-based mouthwashes on dental plaque and gingival inflammation: A systematic review. BMC Oral Health 2020, 20, 198. [Google Scholar] [CrossRef]
- Coutinho, A. Honeybee propolis extract in periodontal treatment: A clinical and microbiological study of propolis in periodontal treatment. Indian J. Dent. Res. 2012, 23, 294. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 105906. [Google Scholar] [CrossRef]
- Shabbir, A.; Rashid, M.; Tipu, H.N. Propolis, A Hope for the Future in Treating Resistant Periodontal Pathogens. Cureus 2016, 8, e682. [Google Scholar] [CrossRef] [Green Version]
- Yoshimasu, Y.; Ikeda, T.; Sakai, N.; Yagi, A.; Hirayama, S.; Morinaga, Y.; Furukawa, S.; Nakao, R. Rapid Bactericidal Action of Propolis against Porphyromonas gingivalis. J. Dent. Res. 2018, 97, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Tambur, Z.; Miljkovic-Selimovic, B.; Opacic, D.; Vukovic, B.; Malesevic, A.; Ivancajic, L.; Aleksic, E. Inhibitory effects of propolis and essential oils on oral bacteria. J. Infect. Dev. Ctries. 2021, 15, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Mary George, R.; Kasliwal, A.V. Effectiveness of Propolis, Probiotics and Chlorhexidine on Streptococcus Mutans and Candida Albicans: An In-Vitro Study. IOSR J. Dent. Med. Sci. 2017, 16, 15–18. [Google Scholar] [CrossRef]
- Gómez, P.A.; Jon, L.Y.; Torres, D.J.; Amaranto, R.E.; Díaz, I.E.; Medina, C.A. Antibacterial, antibiofilm, and cytotoxic activities and chemical compositions of Peruvian propolis in an In vitro oral biofilm. F1000Research 2021, 10, 1093. [Google Scholar] [CrossRef] [PubMed]
- Miranda, S.L.F.; Damasceno, J.T.; Faveri, M.; Figueiredo, L.; da Silva, H.D.; de Alencar Alencar, S.M.; Rosalen, P.L.; Feres, M.; Bueno-Silva, B. Brazilian red propolis reduces orange-complex periodontopathogens growing in multispecies biofilms. Biofouling 2019, 35, 308–319. [Google Scholar] [CrossRef] [PubMed]
- De Figueiredo, K.A.; Da Silva, H.D.P.; Miranda, S.L.F.; Gonçalves, F.J.D.S.; de Sousa, A.P.; de Figueiredo, L.C.; Feres, M.; Bueno-Silva, B. Brazilian Red Propolis Is as Effective as Amoxicillin in Controlling Red-Complex of Multispecies Subgingival Mature Biofilm In vitro. Antibiotics 2020, 9, 432. [Google Scholar] [CrossRef] [PubMed]
- Stähli, A.; Schröter, H.; Bullitta, S.; Serralutzu, F.; Dore, A.; Nietzsche, S.; Milia, E.; Sculean, A.; Eick, S. In vitro Activity of Propolis on Oral Microorganisms and Biofilms. Antibiotics 2021, 10, 1045. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, A.B.S.; de Araújo Rodriguez, L.R.N.; Santos, R.K.B.; Marinho, R.R.B.; Abreu, S.; Peixoto, R.F.; de Vasconcelos Gurgel, B.C. Antifungal activity of propolis against Candida species isolated from cases of chronic periodontitis. Braz. Oral Res. 2015, 29, 1–6. [Google Scholar] [CrossRef]
- Nakajima, M.; Arimatsu, K.; Minagawa, T.; Matsuda, Y.; Sato, K.; Takahashi, N.; Nakajima, T.; Yamazaki, K. Brazilian propolis mitigates impaired glucose and lipid metabolism in experimental periodontitis in mice. BMC Complement. Altern. Med. 2016, 16, 329. [Google Scholar] [CrossRef] [Green Version]
- Soekanto, S.A.; Safitri, Y.N.; Zeid, H.; Gultom, F.P.; Djais, A.A.; Darwita, R.R.; Sahlan, M. Effectiveness of propolis gel on Mus musculus (Swiss Webster) periodontitis model with ligature silk thread application. AIP Conf. Proc. 2021, 2344, 040008. [Google Scholar] [CrossRef]
- Sung, S.-J.; Kang, K.-M.; Lee, K.-H.; Yoo, S.-Y.; Kook, J.-K.; Lee, D.S.; Yu, S.-J. Effect of Garcinia mangostana L. and propolis extracts on the inhibition of inflammation and alveolar bone loss in ligature-induced periodontitis in rats. Int. J. Oral Biol. 2019, 44, 55–61. [Google Scholar] [CrossRef]
- Ali, K.; Saleh, Z.; Jalal, J. Effect of local propolis irrigation in experimental periodontitis in rats on inflammatory markers (IL-1β and TNF-α) and oxidative stress. Indian J. Dent. Res. 2020, 31, 893. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, D.; Karibasappa, S.N.; Mehta, D.S. Royal Jelly Antimicrobial Activity against Periodontopathic Bacteria. J. Interdiscip. Dent. 2018, 8, 18. [Google Scholar] [CrossRef]
- Khosla, A.; Gupta, S.J.; Jain, A.; Shetty, D.C.; Sharma, N. Evaluation and comparison of the antimicrobial activity of royal jelly—A holistic healer against periodontopathic bacteria: An In vitro study. J. Indian Soc. Periodontol. 2020, 24, 221. [Google Scholar] [CrossRef] [PubMed]
- Gupta, I.; Das, N.; Ranjan, P.; Sujatha, R.; Gupta, R.; Gupta, N. A Preliminary Study on the Evaluation of In-vitro Inhibition Potential of Antimicrobial Efficacy of Raw and Commercial Honey on Escherichia coli: An Emerging Periodontal Pathogen. Mymensingh Med. J. 2021, 30, 547–554. [Google Scholar] [PubMed]
- Li, L.; Sun, W.; Wu, T.; Lu, R.; Shi, B. Caffeic acid phenethyl ester attenuates lipopolysaccharide-stimulated proinflammatory responses in human gingival fibroblasts via NF-κB and PI3K/Akt signaling pathway. Eur. J. Pharmacol. 2017, 794, 61–68. [Google Scholar] [CrossRef]
- Gu, H.; An, H.J.; Kim, J.Y.; Kim, W.H.; Gwon, M.G.; Kim, H.J.; Han, S.M.; Park, I.S.; Park, S.C.; Leem, J.; et al. Bee venom attenuates Porphyromonas gingivalis and RANKL-induced bone resorption with osteoclastogenic differentiation. Food Chem. Toxicol. 2019, 129, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; An, H.J.; Kim, J.Y.; Gwon, M.G.; Gu, H.; Park, J.B.; Sung, W.J.; Kwon, Y.C.; Park, K.D.; Han, S.M.; et al. Bee Venom Inhibits Porphyromonas gingivalis Lipopolysaccharides-Induced Pro-Inflammatory Cytokines through Suppression of NF-κB and AP-1 Signaling Pathways. Molecules 2016, 21, 1508. [Google Scholar] [CrossRef]
- Kim, W.H.; An, H.J.; Kim, J.Y.; Gwon, M.G.; Gu, H.; Jeon, M.; Kim, M.K.; Han, S.M.; Park, K.K. Anti-inflammatory effect of melittin on Porphyromonas gingivalis LPS-stimulated human keratinocytes. Molecules 2018, 23, 332. [Google Scholar] [CrossRef] [Green Version]
- Nakao, R.; Senpuku, H.; Ohnishi, M.; Takai, H.; Ogata, Y. Effect of topical administration of propolis in chronic periodontitis. Odontology 2020, 108, 704–714. [Google Scholar] [CrossRef]
- Kiani, S.; Birang, R.; Jamshidian, N. Effect of Propolis mouthwash on clinical periodontal parameters in patients with gingivitis: A double-blinded randomized clinical trial. Int. J. Dent. Hyg. 2021, 2021, 1–19. [Google Scholar] [CrossRef] [PubMed]
- González-Serrano, J.; López-Pintor, R.M.; Serrano, J.; Torres, J.; Hernández, G.; Sanz, M. Short-term efficacy of a gel containing propolis extract, nanovitamin C and nanovitamin E on peri-implant mucositis: A double-blind, randomized, clinical trial. J. Periodontal Res. 2021, 56, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Singhal, R.; Siddibhavi, M.; Sankeshwari, R.; Patil, P.; Jalihal, S.; Ankola, A. Effectiveness of three mouthwashes—Manuka honey, Raw honey, and Chlorhexidine on plaque and gingival scores of 12–15-year-old school children: A randomized controlled field trial. J. Indian Soc. Periodontol. 2018, 22, 34. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Ko, K.A.; Lee, J.Y.; Oh, J.W.; Lim, H.C.; Lee, D.W.; Choi, S.H.; Cha, J.K. Clinical and Immunological Efficacy of Mangosteen and Propolis Extracted Complex in Patients with Gingivitis: A Multi-Centered Randomized Controlled Clinical Trial. Nutrients 2021, 13, 2604. [Google Scholar] [CrossRef]
- Lisbona-González, M.J.; Muñoz-Soto, E.; Lisbona-González, C.; Vallecillo-Rivas, M.; Diaz-Castro, J.; Moreno-Fernandez, J. Effect of Propolis Paste and Mouthwash Formulation on Healing after Teeth Extraction in Periodontal Disease. Plants 2021, 10, 1603. [Google Scholar] [CrossRef]
- Sparabombe, S.; Monterubbianesi, R.; Tosco, V.; Orilisi, G.; Hosein, A.; Ferrante, L.; Putignano, A.; Orsini, G. Efficacy of an All-Natural Polyherbal Mouthwash in Patients with Periodontitis: A Single-Blind Randomized Controlled Trial. Front. Physiol. 2019, 10, 632. [Google Scholar] [CrossRef]
- Giammarinaro, E.; Marconcini, S.; Genovesi, A.; Poli, G.; Lorenzi, C.; Covani, U. Propolis as an adjuvant to non-surgical periodontal treatment: A clinical study with salivary antioxidant capacity assessment. Minerva Stomatol. 2018, 67, 183–188. [Google Scholar] [CrossRef]
- Bapat, S.; Nagarajappa, R.; Ramesh, G.; Bapat, K. Effect of propolis mouth rinse on oral microorganisms—A randomized controlled trial. Clin. Oral Investig. 2021, 25, 6139–6146. [Google Scholar] [CrossRef]
- Machorowska-Pieniążek, A.; Morawiec, T.; Olek, M.; Mertas, A.; Aebisher, D.; Bartusik-Aebisher, D.; Cieślar, G.; Kawczyk-Krupka, A. Advantages of using toothpaste containing propolis and plant oils for gingivitis prevention and oral cavity hygiene in cleft lip/palate patients. Biomed. Pharmacother. 2021, 142, 111992. [Google Scholar] [CrossRef]
- Dehghani, M.; Abtahi, M.; Hasanzadeh, N.; Farahzad, Z.; Noori, M.; Noori, M. Effect of Propolis mouthwash on plaque and gingival indices over fixed orthodontic patients. J. Clin. Exp. Dent. 2019, 11, e244. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).