The Influence of Hyaluronic Acid Adjunctive Therapy of Periodontitis on Salivary Markers of Oxidative Stress: Randomized, Controlled Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trial Design
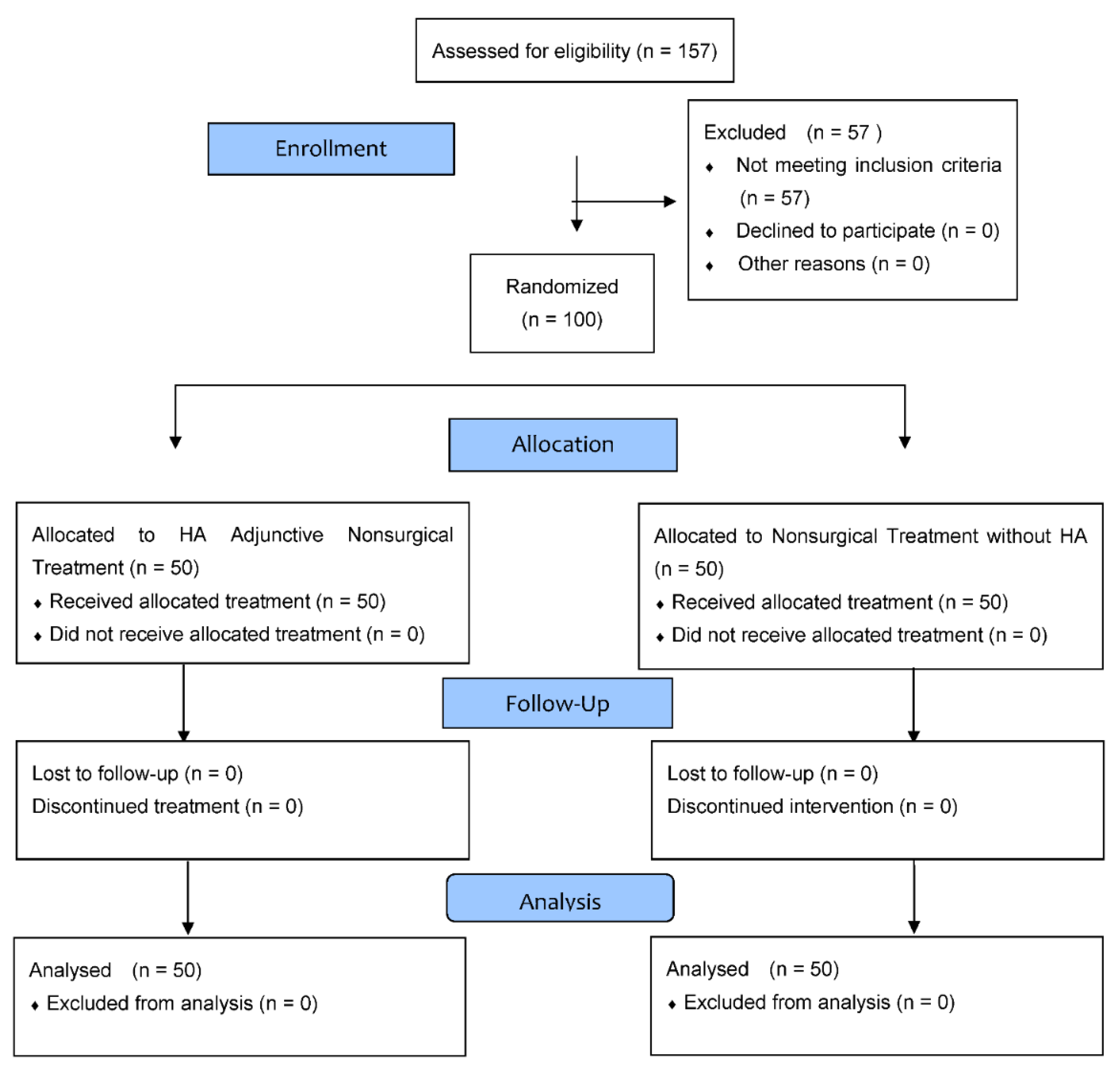
2.2. Randomization and Blinding
2.3. Participants
2.4. Data Collection
2.5. Clinical Parameters
2.6. Intervention
2.7. Saliva Preparation for the Oxidant–Antioxidant Tests
2.8. Measurement of Oxidative Stress Parameters
2.8.1. Total Antioxidant Capacity (TAC)
2.8.2. Total Glutathione (GSH)
2.8.3. Glutathione Reductase Activity (GR)
2.8.4. Uric Acid Concentration (UA)
2.9. Safety Monitoring
2.10. Statistical Analysis
2.11. Adverse Events and Safety Monitoring
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petersen, P.E.; Ogawa, H. The global burden of periodontal disease: Towards integration with chronic disease prevention and control. Periodontol 2000, 60, 15–39. [Google Scholar] [CrossRef]
- Page, R.C.; Offenbacher, S.; Schroeder, H.E.; Seymour, G.J.; Kornman, K.S. Advances in the pathogenesis of periodontitis: Summary of developments, clinical implications and future directions. Periodontol 2000 2000, 1997, 216–248. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Weinberg, A. Role of bacteria in health and disease of periodontal tissues. Periodontol 2000, 40, 50–76. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.M.; Matthews, J.B.; O’ Halloran, D.J.; Griffiths, H.R.; Chapple, I.L. Oxidative and inflammatory status in Type 2 diabetes patients with periodontitis. J. Clin. Periodontol. 2011, 38, 894–901. [Google Scholar] [CrossRef]
- Cheng, Z.; Meade, J.; Mankia, K.; Emery, P.; Devine, D.A. Periodontal disease and periodontal bacteria as triggers for rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2017, 31, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Buczko, P.; Zalewska, A.; Szarmach, I. Saliva and oxidative stress in oral cavity and in some systemic disorders. J. Physiol. Pharmacol. 2015, 66, 3–9. [Google Scholar]
- Carcuac, O.; Berglundh, T. Composition of human peri-implantitis and periodontitis lesions. J. Dent. Res. 2014, 93, 1083–1088. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.P. Defining functional signatures of dysbiosis in periodontitis progression. Genome Med. 2015, 7, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fine, N.; Hassanpour, S.; Borenstein, A.; Sima, C.; Oveisi, M.; Scholey, J.; Cherney, D.; Glogauer, M. Distinct Oral Neutrophil Subsets Define Health and Periodontal Disease States. J. Dent. Res. 2016, 95, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Baňasová, L.; Kamodyová, N.; Janšáková, K.; Tóthová, Ľ.; Stanko, P.; Turňa, J.; Celec, P. Salivary DNA and markers of oxidative stress in patients with chronic periodontitis. Clin. Oral. Investig. 2015, 19, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Andrukhov, O.; Rausch-Fan, X. Oxidative Stress and Antioxidant System in Periodontitis. Front. Physiol. 2017, 8, 910. [Google Scholar] [CrossRef] [Green Version]
- Tóthová, L.; Kamodyová, N.; Červenka, T.; Celec, P. Salivary markers of oxidative stress in oral diseases. Front. Cell Infect. Microbiol. 2015, 5, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S.; EFP Workshop Participants and Methodological Consultants. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. S22), 4–60. [Google Scholar] [CrossRef] [PubMed]
- Donos, N.; Calciolari, E.; Brusselaers, N.; Goldoni, M.; Bostanci, N.; Belibasakis, G.N. The adjunctive use of host modulators in non-surgical periodontal therapy. A systematic review of randomized, placebo-controlled clinical studies. J. Clin. Periodontol. 2020, 47 (Suppl. S22), 199–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azzi, A. Antioxidants: Wonder drugs or quackery? Biofactors 2017, 43, 785–788. [Google Scholar] [CrossRef] [PubMed]
- Olszewska-Czyz, I.; Kralik, K.; Prpic, J. Biomolecules in Dental Applications: Randomized, Controlled Clinical Trial Evaluating the Influence of Hyaluronic Acid Adjunctive Therapy on Clinical Parameters of Moderate Periodontitis. Biomolecules 2021, 11, 1491. [Google Scholar] [CrossRef]
- Pilloni, A.; Zeza, B.; Kuis, D.; Vrazic, D.; Domic, T.; Olszewska-Czyz, I.; Popova, C.; Kotsilkov, K.; Firkova, E.; Dermendzieva, Y.; et al. Treatment of Residual Periodontal Pockets Using a Hyaluronic Acid-Based Gel: A 12 Month Multicenter Randomized Triple-Blinded Clinical Trial. Antibiotics 2021, 10, 924. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, G.C.; Plous, S. Research Randomizer (Version 4.0) [Computer software]. Available online: http://www.randomizer.org (accessed on 9 October 2020).
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S162–S170. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Sanz, M. Implementation of the new classification of periodontal diseases: Decision-making algorithms for clinical practice and education. J. Clin. Periodontol. 2019, 46, 398–405. [Google Scholar] [CrossRef]
- Lange, D.E.; Plagmann, H.C.; Eenboom, A.; Promesberger, A. Klinische Bewertungsverahren zur Objektivierung der Mundhygiene Clinical methods for the objective evaluation of oral hygiene. Dtsch. Zahnarztl. Z. 1977, 32, 44–47. [Google Scholar]
- Ainamo, J.; Bay, I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J. 1975, 25, 229–235. [Google Scholar] [PubMed]
- Haas, A.N.; Furlaneto, F.; Gaio, E.J.; Gomes, S.C.; Palioto, D.B.; Castilho, R.M.; Sanz, M.; Messora, M.R. New tendencies in non-surgical periodontal therapy. Braz, Oral Res. 2021, 35 (Suppl. S2), e095. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Delides, A.; Spooner, R.J.; Goldberg, D.M.; Neal, F.E. An optimized semi-automatic rate method for serum glutathione reductase activity and its application to patients with malignant disease. J. Clin. Pathol. 1976, 29, 73–77. [Google Scholar] [CrossRef] [Green Version]
- Fraisse, L.; Bonnet, M.C.; de Farcy, J.P.; Agut, C.; Dersigny, D.; Bayol, A. A colorimetric 96-well microtiter plate assay for the determination of urate oxidase activity and its kinetic parameters. Anal. Biochem. 2002, 309, 173–179. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Shapira, L.; Borinski, R.; Sela, M.N.; Soskolne, A. Superoxide formation and chemiluminescence of peripheral polymorphonuclear leukocytes in rapidly progressive periodontitis patients. J. Clin. Periodontol. 1991, 18, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L. Reactive oxygen species and antioxidants in inflammatory diseases. J. Clin. Periodontol. 1997, 24, 287–296. [Google Scholar] [CrossRef]
- Tóthová, L.; Celec, P. Oxidative Stress and Antioxidants in the Diagnosis and Therapy of Periodontitis. Front. Physiol. 2017, 8, 1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Liu, Y.; Song, Y.; Zhang, X.; Wang, S.; Wang, Z. Systemic oxidative stress biomarkers in chronic periodontitis: A meta-analysis. Dis. Mark. 2014, 2014, 931083. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, X.; Cueto, R.; Effi, C.; Zhang, Y.; Tan, H.; Qin, X.; Ji, Y.; Yang, X.; Wang, H. Biochemical basis and metabolic interplay of redox regulation. Redox Biol. 2019, 26, 101284. [Google Scholar] [CrossRef] [PubMed]
- Abou Sulaiman, A.E.; Shehadeh, R.M. Assessment of total antioxidant capacity (TAC) and the use of vitamin C in the treatment of non-smokers with chronic periodontitis. J. Periodontol. 2010, 81, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.V.; Srinivas, G.; Reddy, A.A.; Reddy, B.H.; Reddy, C.; Nagarajan, S.; Naveen, A. Locally delivered antioxidant gel as an adjunct to nonsurgical therapy improves measures of oxidative stress and periodontal disease. J. Periodontal Implant Sci. 2013, 43, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Chander Narula, S.; Kumar Sharma, R.; Tewari, S.; Kumar Sehgal, P. Vitamin E supplementation, superoxide dismutase status, and outcome of scaling and root planing in patients with chronic periodontitis: A randomized clinical trial. J. Periodontol. 2014, 85, 242–249. [Google Scholar] [CrossRef]
- Zare Javid, A.; Seal, C.J.; Heasman, P.; Moynihan, P.J. Impact of a customised dietary intervention on antioxidant status, dietary intakes and periodontal indices in patients with adult periodontitis. J. Hum. Nutr. Diet 2014, 27, 523–532. [Google Scholar] [CrossRef]
- Domínguez, A.; Gómez, C.; García-Kass, A.I.; García-Nuñez, J.A. IL-1beta, TNF-alpha, total antioxidant status and microbiological findings in chronic periodontitis treated with fluorescence-controlled Er: YAG laser radiation. Lasers Surg. Med. 2010, 42, 24–31. [Google Scholar] [CrossRef]
- Muniz, F.W.; Nogueira, S.B.; Mendes, F.L.; Rösing, C.K.; Moreira, M.M.; de Andrade, G.M.; Carvalho Rde, S. The impact of antioxidant agents complimentary to periodontal therapy on oxidative stress and periodontal outcomes: A systematic review. Arch. Oral Biol. 2015, 60, 1203–1214. [Google Scholar] [CrossRef]
- Vernazza, C.; Heasman, P.; Gaunt, F.; Pennington, M. How to measure the cost-effectiveness of periodontal treatments. Periodontol 2000 2012, 60, 138–146. [Google Scholar] [CrossRef]
- Deas, D.E.; Moritz, A.J.; Sagun, R.S.; Gruwell, S.F.; Powell, C.A. Scaling and root planing vs. conservative surgery in the treatment of chronic periodontitis. Periodontol 2000, 71, 128–139. [Google Scholar] [CrossRef]
- Eliezer, M.; Imber, J.C.; Sculean, A.; Pandis, N.; Teich, S. Hyaluronic acid as adjunctive to non-surgical and surgical periodontal therapy: A systematic review and meta-analysis. Clin. Oral Investig. 2019, 23, 3423–3435. [Google Scholar] [CrossRef]
- Ke, C.; Sun, L.; Qiao, D.; Wang, D.; Zeng, X. Antioxidant acitivity of low molecular weight hyaluronic acid. Food Chem. Toxicol. 2011, 49, 2670–2675. [Google Scholar] [CrossRef] [PubMed]
- Bergandi, L.; Skorokhod, O.A.; La Grotta, R.; Schwarzer, E.; Nuzzi, R. Oxidative Stress, Lipid Peroxidation, and Loss of Hyaluronic Acid in the Human Vitreous Affected by Synchysis Scintillans. J. Ophthalmol. 2019, 2019, 7231015. [Google Scholar] [CrossRef] [PubMed]
- Guentsch, A.; Preshaw, P.M.; Bremer-Streck, S.; Klinger, G.; Glockmann, E.; Sigusch, B.W. Lipid peroxidation and antioxidant activity in saliva of periodontitis patients: Effect of smoking and periodontal treatment. Clin. Oral Investig. 2008, 12, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Koromantzos, P.A.; Makrilakis, K.; Dereka, X.; Offenbacher, S.; Katsilambros, N.; Vrotsos, I.A.; Madianos, P.N. Effect of non-surgical periodontal therapy on C-reactive protein, oxidative stress, and matrix metalloproteinase (MMP)-9 and MMP-2 levels in patients with type 2 diabetes: A randomized controlled study. J. Periodontol. 2012, 83, 3–10. [Google Scholar] [CrossRef]
- Arabacı, T.; Kermen, E.; Özkanlar, S.; Köse, O.; Kara, A.; Kızıldağ, A.; Duman, Ş.B.; Ibişoğlu, E. Therapeutic Effects of Melatonin on Alveolar Bone Resorption after Experimental Periodontitis in Rats: A Biochemical and Immunohistochemical Study. J. Periodontol. 2015, 86, 874–881. [Google Scholar] [CrossRef]
- Köse, O.; Arabaci, T.; Kizildag, A.; Erdemci, B.; Özkal Eminoğlu, D.; Gedikli, S.; Özkanlar, S.; Zihni, M.; Albayrak, M.; Kara, A.; et al. Melatonin prevents radiation-induced oxidative stress and periodontal tissue breakdown in irradiated rats with experimental periodontitis. J. Periodontal Res. 2017, 52, 438–446. [Google Scholar] [CrossRef] [PubMed]
| Standard Therapy | Standard Therapy + HA | Difference †(95% CI) | p * | |
|---|---|---|---|---|
| Before therapy | ||||
| TAC—total antioxidative capacity | 0.79 (0.6–1) | 0.77 (0.6–1) | −0.003 (−0.09 to 0.05) | 0.94 |
| GR—glutathione reductase | 10.3 (7.3–12.3) | 9.7 (7.42–12.5) | −0.025 (−0.97 to 0.67) | 0.87 |
| UA—uric acid | 237 (201–265) | 226.5 (196–284.75) | 0 (−19 do 16) | 0.99 |
| GSH—total glutathione concentration | 3.79 (1.6–5) | 2.99 (1.55–5.66) | −0.09 (−1.04 to 0.48) | 0.73 |
| After therapy | ||||
| TAC | 0.89 (0.8–1) | 0.98 (0.81–1.08) | 0.08 (0.01 to 0.13) | 0.02 |
| GR | 11.11 (8.3–12.8) | 10.67 (8.76–12.96) | 0.2 (−0.49 to 0.81) | 0.47 |
| UA | 275.5 (256–296) | 296 (286.3–327.8) | 26 (14 to 39) | <0.001 |
| GSH | 3.92 (2–5.4) | 3.86 (2.56–6.01) | 0.3 (−0.45 to 0.96) | 0.35 |
| Median (Interquartile Range) | Difference †(95% CI) | p * | ||
|---|---|---|---|---|
| Before Therapy | After Therapy | |||
| Standard therapy | ||||
| TAC | 0.79 (0.6–1) | 0.89 (0.8–1) | 0.06 (0.03 to 0.09) | <0.001 |
| GR | 10.3 (7.3–12.3) | 11.11 (8.3–12.8) | 0.59 (0.45 to 0.72) | <0.001 |
| UA | 237 (201–265) | 275.5 (256–296) | 36 (27 to 45.5) | <0.001 |
| GSH | 3.79 (1.6–5) | 3.92 (2–5.4) | 0.31 (0.23 to 0.40) | <0.001 |
| Standard therapy + HA | ||||
| TAC | 0.77 (0.6–1) | 0.98 (0.81–1.08) | 0.15 (0.12 to 0.19) | <0.001 |
| GR | 9.7 (7.42–12.5) | 10.67 (8.76–12.96) | 0.9 (0.65 to 1.17) | <0.001 |
| UA | 226.5 (196–284.75) | 296 (286.3–327.8) | 64.5 (53.5 to 77) | <0.001 |
| GSH | 2.99 (1.55–5.66) | 3.86 (2.56–6.01) | 0.74 (0.6 to 0.88) | <0.001 |
| Median (Interquartile Range) of Difference Before–After Therapy | Difference †(95% CI) | p * | ||
|---|---|---|---|---|
| Standard Therapy | Standard Therapy + HA | |||
| TAC | −0.04 (−0.13–0) | −0.18 (−0.23 to −0.04) | −0.09 (−0.14 do −0.04) | <0.001 |
| GR | −0.56 (−0.9 to −0.31) | −0.81 (−1.46 to −0.23) | −0.22 (−0.52 do 0) | 0.05 |
| UA | −34 (−59 to −12.75) | −72 (−92 to −34.5) | −29 (−43 to −15) | <0.001 |
| GSH | −0.27 (−0.55 to −0.12) | −0.77 (−1.06 to −0.36) | −0.42 (−0.60 to −0.25) | <0.001 |
| Standard Therapy | Spearman Correlation Coefficient Rho (p Values)–before Therapy | |||
|---|---|---|---|---|
| TAC | GR | UA | GSH | |
| Before therapy | oxidative stress parameters before therapy | |||
| BoP | −0.927 (<0.001) | −0.939 (<0.001) | −0.832 (<0.001) | −0.92 (<0.001) |
| mean CAL | −0.505 (<0.001) | −0.511 (<0.001) | −0.392(<0.001) | −0.445 (<0.001) |
| PPD | −0.143 (0.32) | −0.057 (0.70) | −0.061 (0.68) | −0.038 (0.80) |
| After therapy | oxidative stress parameters before therapy | |||
| BoP | −0.688 (<0.001) | −0.600(<0.001) | −0.627 (<0.001) | −0.609 (<0.001) |
| mean CAL | −0.261 (0.07) | −0.213 (0.14) | −0.056 (0.70) | −0.212 (0.14) |
| PPD | −0.079 (0.58) | 0.001 (0.99) | −0.144 (0.32) | 0.046 (0.75) |
| After therapy | oxidative stress parameters after therapy | |||
| BoP | −0.656 (<0.001) | −0.588 (<0.001) | −0.501 (<0.001) | −0.591 (<0.001) |
| mean CAL | −0.219 (0.13) | −0.204 (0.16) | −0.221 (0.12) | −0.221 (0.12) |
| PPD | −0.084 (0.56) | −0.018 (0.90) | 0.154 (0.29) | 0.031 (0.83) |
| Standard Therapy + HA | Spearman Correlation Coefficient Rho (p Values)–before Therapy | |||
|---|---|---|---|---|
| TAC | GR | UA | GSH | |
| Before therapy | oxidative stress indices before therapy | |||
| BoP | −0.904 (<0.001) | −0.946 (<0.001) | −0.920 (<0.001) | −0.867 (<0.001) |
| Mean CAL | −0.572 (<0.001) | −0.627 (<0.001) | −0.535 (<0.001) | −0.543 (<0.001) |
| PPD | −0.318 (0.02) | −0.292 (0.04) | −0.268 (0.06) | −0.234 (0.10) |
| After therapy | oxidative stress indices before therapy | |||
| BoP | −0.753 (<0.001) | −0.902 (<0.001) | −0.778 (<0.001) | −0.871 (<0.001) |
| Mean CAL | −0.469 (<0.001) | −0.633 (<0.001) | −0.519 (<0.001) | −0.548 (<0.001) |
| PPD | −0.303 (0.03) | −0.287 (0.04) | −0.268 (0.06) | −0.219 (0.13) |
| After therapy | oxidative stress indices after therapy | |||
| BoP | −0.394 (<0.001) | −0.406 (<0.001) | −0.492 (<0.001) | −0.201 (0.16) |
| Mean CAL | −0.075 (0.61) | −0.097 (0.50) | −0.195 (0.17) | −0.248 (0.08) |
| PPD | −0.313 (0.03) | −0.38 (0.01) | −0.303 (0.03) | −0.3 (0.03) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olszewska-Czyz, I.; Kralik, K.; Tota, M.; Prpic, J. The Influence of Hyaluronic Acid Adjunctive Therapy of Periodontitis on Salivary Markers of Oxidative Stress: Randomized, Controlled Clinical Trial. Antioxidants 2022, 11, 135. https://doi.org/10.3390/antiox11010135
Olszewska-Czyz I, Kralik K, Tota M, Prpic J. The Influence of Hyaluronic Acid Adjunctive Therapy of Periodontitis on Salivary Markers of Oxidative Stress: Randomized, Controlled Clinical Trial. Antioxidants. 2022; 11(1):135. https://doi.org/10.3390/antiox11010135
Chicago/Turabian StyleOlszewska-Czyz, Iwona, Kristina Kralik, Marin Tota, and Jelena Prpic. 2022. "The Influence of Hyaluronic Acid Adjunctive Therapy of Periodontitis on Salivary Markers of Oxidative Stress: Randomized, Controlled Clinical Trial" Antioxidants 11, no. 1: 135. https://doi.org/10.3390/antiox11010135
APA StyleOlszewska-Czyz, I., Kralik, K., Tota, M., & Prpic, J. (2022). The Influence of Hyaluronic Acid Adjunctive Therapy of Periodontitis on Salivary Markers of Oxidative Stress: Randomized, Controlled Clinical Trial. Antioxidants, 11(1), 135. https://doi.org/10.3390/antiox11010135






