Ozone Treatment Improves the Texture of Strawberry Fruit during Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ozonation Process and Storage Experiment
2.2. Texture Analysis
2.3. Determination of the Activity of Enzymes Related to Fruit Softening
2.4. Mitochondria Energy Metabolism Analysis
2.4.1. Energy Charge Analysis
2.4.2. Oxidative Phosphorylation Enzymes Activity Assay
2.4.3. Reactive Oxygen Species Generation Analysis
2.4.4. Antioxidant Enzymes Activity
2.5. Determination of Malondialdehyde Level in Fruit and Cell Membrane Electrolyte Leakage
2.6. Statistical Analysis
3. Results
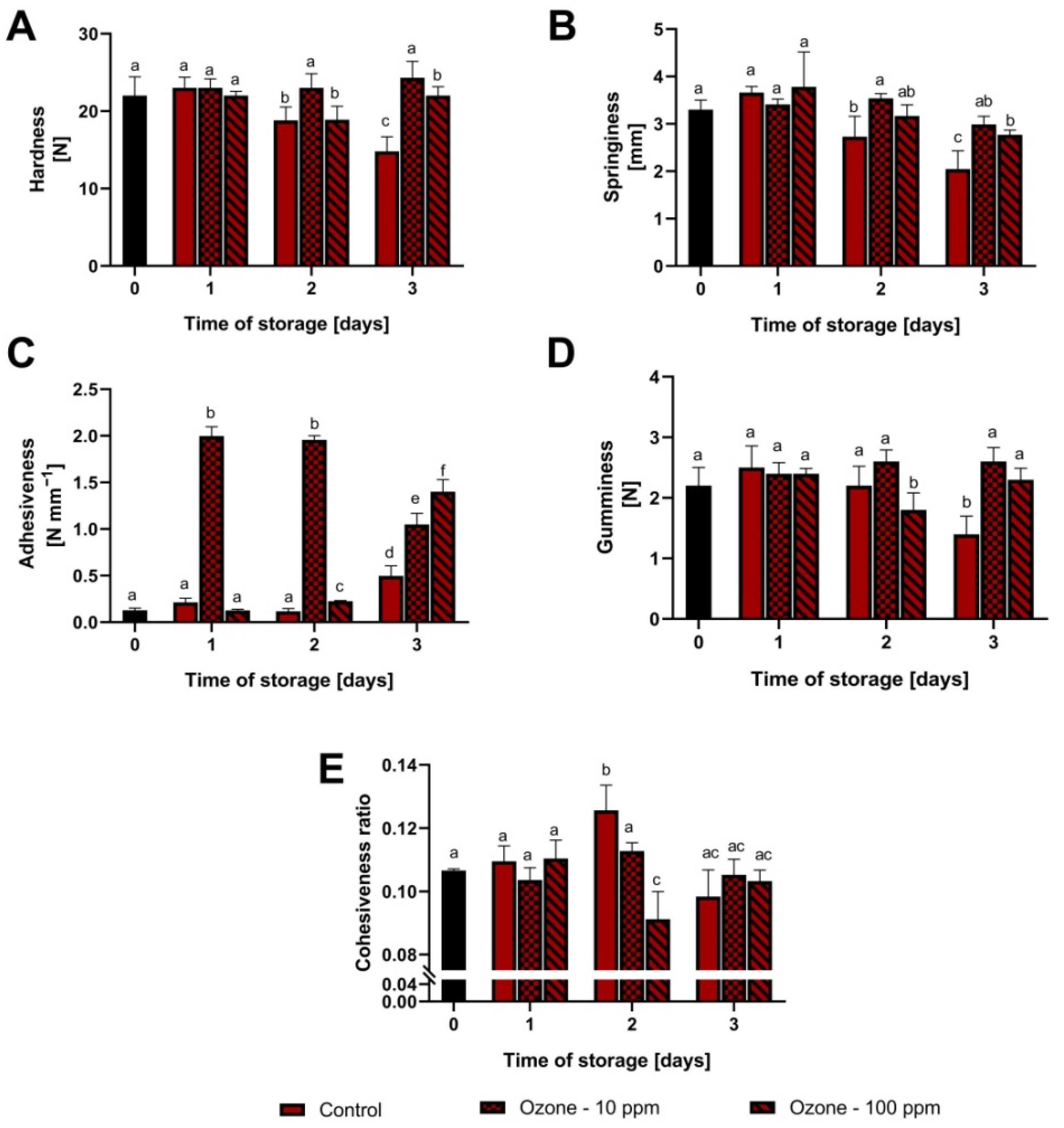
3.1. Changes in Fruit Texture during Storage at Room Temperature
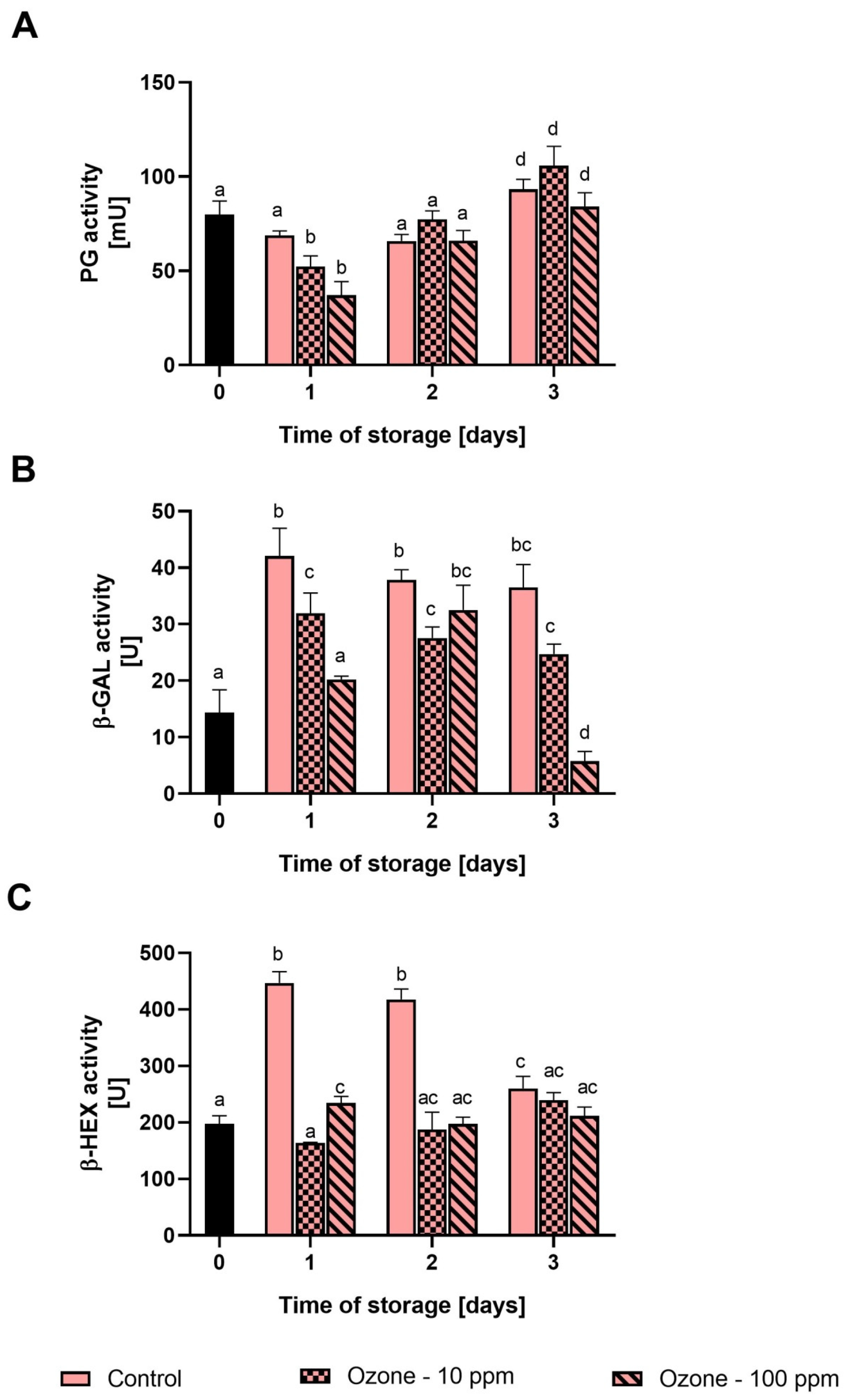
3.2. Changes in the Activity of β-Gal, β-Hex and PG in Strawberries during Storage
3.3. The Effect of Ozone Treatment on the Mitochondria Energy Metabolism in Strawberry Fruit
3.4. Changes in Mitochondrial Oxidative Stress Markers in Strawberry Fruit during Storage
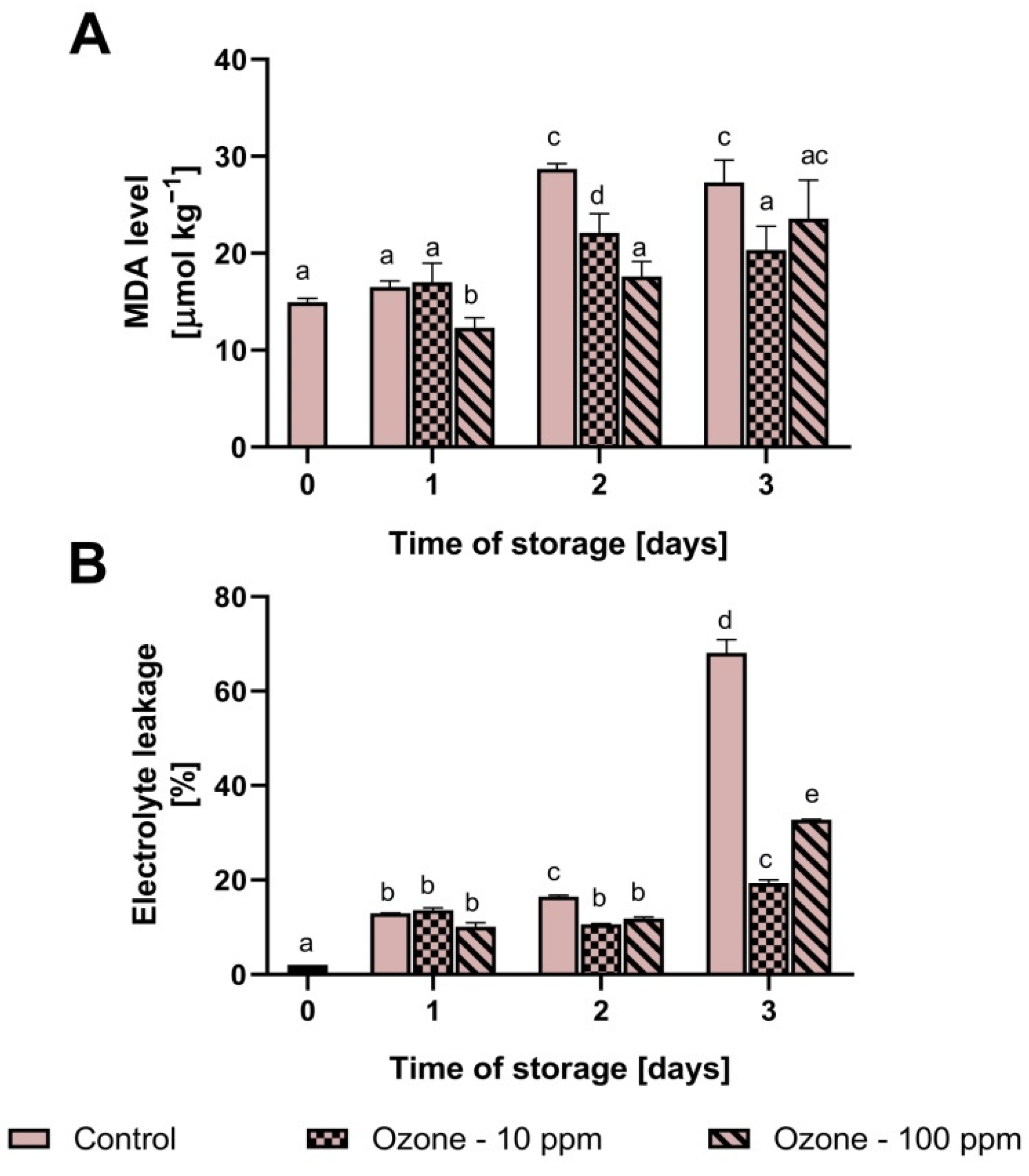
3.5. The Level of MDA and Electrolyte Leakage in Ozonated Strawberry Fruit
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robles-Flores, G.D.C.; Abud-Archila, M.; Ventura-Canseco, L.M.C.; Meza-Gordillo, R.; Grajales-Lagunes, A.; Ruiz-Cabrera, M.A.; Gutiérrez-Miceli, F.A. Development and Evaluation of a Film and Edible Coating Obtained from the Cajanus Cajan Seed Applied to Fresh Strawberry Fruit. Food Bioprocess Technol. 2018, 11, 2172–2181. [Google Scholar] [CrossRef]
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The Strawberry: Composition, Nutritional Quality, and Impact on Human Health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Molina, F.; Gómez, P.L.; Castro, M.A.; Alzamora, S.M. Storage Quality of Strawberry Fruit Treated by Pulsed Light: Fungal Decay, Water Loss and Mechanical Properties. Innov. Food Sci. Emerg. Technol. 2016, 34, 267–274. [Google Scholar] [CrossRef]
- Mazur, S.P.; Nes, A.; Wold, A.B.; Remberg, S.F.; Martinsen, B.K.; Aaby, K. Effects of Ripeness and Cultivar on Chemical Composition of Strawberry (Fragaria × ananassa Duch.) Fruits and Their Suitability for Jam Production as a Stable Product at Different Storage Temperatures. Food Chem. 2014, 146, 412–422. [Google Scholar] [CrossRef]
- Kelly, K.; Madden, R.; Emond, J.P.; do Nascimento Nunes, M.C. A Novel Approach to Determine the Impact Level of Each Step along the Supply Chain on Strawberry Quality. Postharvest Biol. Technol. 2019, 147, 78–88. [Google Scholar] [CrossRef]
- Marquenie, D.; Michiels, C.W.; Van Impe, J.F.; Schrevens, E.; Nicolaï, B.N. Pulsed White Light in Combination with UV-C and Heat to Reduce Storage Rot of Strawberry. Postharvest Biol. Technol. 2003, 28, 455–461. [Google Scholar] [CrossRef]
- Aday, M.S.; Caner, C. Individual and Combined Effects of Ultrasound, Ozone and Chlorine Dioxide on Strawberry Storage Life. LWT-Food Sci. Technol. 2014, 57, 344–351. [Google Scholar] [CrossRef]
- Chitravathi, K.; Chauhan, O.P.; Raju, P.S.; Madhukar, N. Efficacy of Aqueous Ozone and Chlorine in Combination with Passive Modified Atmosphere Packaging on the Postharvest Shelf-Life Extension of Green Chillies (Capsicum annuum L.). Food Bioprocess Technol. 2015, 8, 1386–1392. [Google Scholar] [CrossRef]
- Sachadyn-Król, M.; Agriopoulou, S. Ozonation as a Method of Abiotic Elicitation Improving the Health-Promoting Properties of Plant Products—A Review. Molecules 2020, 25, 2416. [Google Scholar] [CrossRef]
- Horvitz, S.; Cantalejo, M.J. Application of Ozone for the Postharvest Treatment of Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2014, 54, 312–339. [Google Scholar] [CrossRef]
- Jaramillo-Sánchez, G.; Contigiani, E.V.; Castro, M.A.; Hodara, K.; Alzamora, S.M.; Loredo, A.G.; Nieto, A.B. Freshness Maintenance of Blueberries (Vaccinium corymbosum L.) During Postharvest Using Ozone in Aqueous Phase: Microbiological, Structure, and Mechanical Issues. Food Bioprocess Technol. 2019, 12, 2136–2147. [Google Scholar] [CrossRef]
- Ong, M.K.; Kazi, F.K.; Forney, C.F.; Ali, A. Effect of Gaseous Ozone on Papaya Anthracnose. Food Bioprocess Technol. 2013, 6, 2996–3005. [Google Scholar] [CrossRef]
- Maryam, A.; Anwar, R.; Malik, A.U.; Raheem, M.I.U.; Khan, A.S.; Hasan, M.U.; Hussain, Z.; Siddique, Z. Combined Aqueous Ozone and Ultrasound Application Inhibits Microbial Spoilage, Reduces Pesticide Residues and Maintains Storage Quality of Strawberry Fruits. J. Food Meas. Charact. 2021, 15, 1437–1451. [Google Scholar] [CrossRef]
- Pérez, A.G.; Sanz, C.; Ríos, J.J.; Olías, R.; Olías, J.M. Effects of Ozone Treatment on Postharvest Strawberry Quality. J. Agric. Food Chem. 1999, 47, 1652–1656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, K.; Zhang, X.; Dong, C.; Ji, H.; Ke, R.; Ban, Z.; Hu, Y.; Lin, S.; Chen, C. Effects of Ozone Treatment on the Antioxidant Capacity of Postharvest Strawberry. RSC Adv. 2020, 10, 38142–38157. [Google Scholar] [CrossRef]
- Carbone, K.; Mencarelli, F. Influence of Short-Term Postharvest Ozone Treatments in Nitrogen or Air Atmosphere on the Metabolic Response of White Wine Grapes. Food Bioprocess Technol. 2015, 8, 1739–1749. [Google Scholar] [CrossRef]
- Piechowiak, T.; Grzelak-Błaszczyk, K.; Sójka, M.; Balawejder, M. Changes in Phenolic Compounds Profile and Glutathione Status in Raspberry Fruit during Storage in Ozone-Enriched Atmosphere. Postharvest Biol. Technol. 2020, 168, 111277. [Google Scholar] [CrossRef]
- Liang, Z.; Xu, L.; De Baerdemaeker, J.; Li, Y.; Saeys, W. Optimisation of a Multi-Duct Cleaning Device for Rice Combine Harvesters Utilising CFD and Experiments. Biosyst. Eng. 2020, 190, 25–40. [Google Scholar] [CrossRef]
- Chaves, V.C.; Calvete, E.; Reginatto, F.H. Quality Properties and Antioxidant Activity of Seven Strawberry (Fragaria × ananassa Duch) Cultivars. Sci. Hortic. 2017, 225, 293–298. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Mousavi Khaneghah, A. Characterization of Novel Basil-Seed Gum Active Edible Films and Coatings Containing Oregano Essential Oil. Prog. Org. Coat. 2017, 110, 35–41. [Google Scholar] [CrossRef]
- Piechowiak, T.; Skóra, B.; Balawejder, M. Ozone Treatment Induces Changes in Antioxidative Defense System in Blueberry Fruit During Storage. Food Bioprocess Technol. 2020, 13, 1240–1245. [Google Scholar] [CrossRef]
- Rosli, H.G.; Civello, P.M.; Martínez, G.A. Changes in Cell Wall Composition of Three Fragaria × ananassa Cultivars with Different Softening Rate during Ripening. Plant Physiol. Biochem. 2004, 42, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Contigiani, E.V.; Kronberg, M.F.; Jaramillo Sánchez, G.; Gómez, P.L.; García-Loredo, A.B.; Munarriz, E.; Alzamora, S.M. Ozone Washing Decreases Strawberry Susceptibility to Botrytis Cinerea While Maintaining Antioxidant, Optical and Sensory Quality. Heliyon 2020, 6, e05416. [Google Scholar] [CrossRef]
- Aday, M.S.; Büyükcan, M.B.; Temizkan, R.; Caner, C. Role of Ozone Concentrations and Exposure Times in Extending Shelf Life of Strawberry. Ozone Sci. Eng. 2014, 36, 43–56. [Google Scholar] [CrossRef]
- Piechowiak, T.; Antos, P.; Kosowski, P.; Skrobacz, K.; Józefczyk, R.; Balawejder, M. Impact of Ozonation Process on the Microbiological and Antioxidant Status of Raspberries (Rubus ideaeus L.) fruit during Storage at Room Temperature. Agric. Food Sci. 2019, 28, 35–44. [Google Scholar] [CrossRef]
- Peleg, M. The Instrumental Texture Profile Analysis Revisited. J. Texture Stud. 2019, 50, 362–368. [Google Scholar] [CrossRef]
- Cao, L.; Zhao, C.; Su, S.; Luo, C.; Han, M. The Role of β-Hexosaminidase in Peach (Prunus persica) Fruit Softening. Sci. Hortic. 2014, 169, 226–233. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, Y.; Li, Y. Changes in Firmness, Cell Wall Composition and Cell Wall Hydrolases of Grapes Stored in High Oxygen Atmospheres. Food Res. Int. 2005, 38, 769–776. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, C.; Cheng, S.; Wei, B.; Liu, X.; Ji, S. Changes in Energy Metabolism Accompanying Pitting in Blueberries Stored at Low Temperature. Food Chem. 2014, 164, 493–501. [Google Scholar] [CrossRef]
- Tornheim, K.; Schultz, V. Adenosine Diphosphate and Adenosine Monophosphate: Enzymatic Spectrophotometric Determination. J. Nutr. Biochem. 1990, 1, 440–444. [Google Scholar] [CrossRef]
- Tornheim, K.; Schultz, V. Adenosine Triphosphate: Enzymatic Spectrophotometric Determination. J. Nutr. Biochem. 1990, 1, 172–176. [Google Scholar] [CrossRef]
- Piechowiak, T.; Sowa, P.; Tarapatskyy, M.; Balawejder, M. The Role of Mitochondrial Energy Metabolism in Shaping the Quality of Highbush Blueberry Fruit During Storage in Ozone-Enriched Atmosphere. Food Bioprocess Technol. 2021, 14, 1973–1982. [Google Scholar] [CrossRef]
- Piechowiak, T.; Balawejder, M. Impact of Ozonation Process on the Level of Selected Oxidative Stress Markers in Raspberries Stored at Room Temperature. Food Chem. 2019, 298, 125093. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Ma, C.; Cheng, S.; Wei, B.; Liu, X.; Ji, S. Changes in Antioxidative Metabolism Accompanying Pitting Development in Stored Blueberry Fruit. Postharvest Biol. Technol. 2014, 88, 88–95. [Google Scholar] [CrossRef]
- Li-Qin, Z.; Jie, Z.; Shu-Hua, Z.; Lai-Hui, G. Inhibition of Browning on the Surface of Peach Slices by Short-Term Exposure to Nitric Oxide and Ascorbic Acid. Food Chem. 2009, 114, 174–179. [Google Scholar] [CrossRef]
- Alzamora, S.M.; Viollaz, P.E.; Martínez, V.Y.; Nieto, A.B.; Salvatori, D. Exploring the Linear Viscoelastic Properties Structure Relationship in Processed Fruit Tissues. In Food Engineering: Integrated Approaches; Springer: New York, NY, USA, 2008. [Google Scholar]
- Contigiani, E.V.; Jaramillo-Sánchez, G.; Castro, M.A.; Gómez, P.L.; Alzamora, S.M. Postharvest Quality of Strawberry Fruit (Fragaria × Ananassa Duch Cv. Albion) as Affected by Ozone Washing: Fungal Spoilage, Mechanical Properties, and Structure. Food Bioprocess Technol. 2018, 11, 1639–1650. [Google Scholar] [CrossRef]
- Barrett, D.M.; Beaulieu, J.C.; Shewfelt, R. Color, Flavor, Texture, and Nutritional Quality of Fresh-Cut Fruits and Vegetables: Desirable Levels, Instrumental and Sensory Measurement, and the Effects of Processing. Crit. Rev. Food Sci. Nutr. 2010, 50, 369–389. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, X.; Zhang, H.; Ban, Z.; Li, L.; Dong, C.; Ji, H.; Xue, W. Label-Free Quantitative Proteomics to Investigate the Response of Strawberry Fruit after Controlled Ozone Treatment. RSC Adv. 2019, 9, 676–689. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Ong, M.K.; Forney, C.F. Effect of Ozone Pre-Conditioning on Quality and Antioxidant Capacity of Papaya Fruit during Ambient Storage. Food Chem. 2014, 142, 19–26. [Google Scholar] [CrossRef]
- Panou, A.A.; Akrida-Demertzi, K.; Demertzis, P.; Riganakos, K.A. Effect of Gaseous Ozone and Heat Treatment on Quality and Shelf Life of Fresh Strawberries during Cold Storage. Int. J. Fruit Sci. 2021, 21, 218–231. [Google Scholar] [CrossRef]
- Yang, H.; Liu, J.; Dang, M.; Zhang, B.; Li, H.; Meng, R.; Qu, D.; Yang, Y.; Zhao, Z. Analysis of β-Galactosidase during Fruit Development and Ripening in Two Different Texture Types of Apple Cultivars. Front. Plant Sci. 2018, 9, 539. [Google Scholar] [CrossRef] [PubMed]
- Natalini, A.; Vanesa, M.D.; Ferrante, A.; Pardossi, A. Effect of Temperature and Ripening Stages on Membrane Integrity of Fresh-Cut Tomatoes. Acta Physiol. Plant. 2014, 36, 191–198. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Jannatizadeh, A.; Luo, Z.; Paliyath, G. Ensuring Sufficient Intracellular ATP Supplying and Friendly Extracellular ATP Signaling Attenuates Stresses, Delays Senescence and Maintains Quality in Horticultural Crops during Postharvest Life. Trends Food Sci. Technol. 2018, 76, 67–81. [Google Scholar] [CrossRef]
- Piechowiak, T.; Sowa, P.; Balawejder, M. Effect of Ozonation Process on the Energy Metabolism in Raspberry Fruit During Storage at Room Temperature. Food Bioprocess Technol. 2021, 14, 483–491. [Google Scholar] [CrossRef]
- Jin, P.; Zhu, H.; Wang, J.; Chen, J.; Wang, X.; Zheng, Y. Effect of Methyl Jasmonate on Energy Metabolism in Peach Fruit during Chilling Stress. J. Sci. Food Agric. 2013, 93, 1827–1832. [Google Scholar] [CrossRef]
- Shu, C.; Zhang, W.; Zhao, H.; Cao, J.; Jiang, W. Chlorogenic Acid Treatment Alleviates the Adverse Physiological Responses of Vibration Injury in Apple Fruit through the Regulation of Energy Metabolism. Postharvest Biol. Technol. 2020, 159, 110997. [Google Scholar] [CrossRef]
- Piechowiak, T. Effect of Ozone Treatment on Glutathione (GSH) Status in Selected Berry Fruit. Phytochemistry 2021, 187, 112767. [Google Scholar] [CrossRef]
- Millar, A.H.; Eubel, H.; Jänsch, L.; Kruft, V.; Heazlewood, J.L.; Braun, H.P. Mitochondrial Cytochrome c Oxidase and Succinate Dehydrogenase Complexes Contain Plant Specific Subunits. Plant Mol. Biol. 2004, 56, 77–90. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, Y.; Lin, H.; Ritenour, M.A.; Shi, J.; Zhang, S.; Chen, Y.; Wang, H. Hydrogen Peroxide-Induced Pericarp Browning of Harvested Longan Fruit in Association with Energy Metabolism. Food Chem. 2017, 225, 31–36. [Google Scholar] [CrossRef]
- Mhamdi, A.; Van Breusegem, F. Reactive Oxygen Species in Plant Development. Development 2018, 145, dev164376. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cheng, X.; Wu, C.; Fan, G.; Li, T.; Dong, C. Retardation of Postharvest Softening of Blueberry Fruit by Methyl Jasmonate Is Correlated with Altered Cell Wall Modification and Energy Metabolism. Sci. Hortic. 2021, 276, 109752. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piechowiak, T.; Migut, D.; Józefczyk, R.; Balawejder, M. Ozone Treatment Improves the Texture of Strawberry Fruit during Storage. Antioxidants 2022, 11, 821. https://doi.org/10.3390/antiox11050821
Piechowiak T, Migut D, Józefczyk R, Balawejder M. Ozone Treatment Improves the Texture of Strawberry Fruit during Storage. Antioxidants. 2022; 11(5):821. https://doi.org/10.3390/antiox11050821
Chicago/Turabian StylePiechowiak, Tomasz, Dagmara Migut, Radosław Józefczyk, and Maciej Balawejder. 2022. "Ozone Treatment Improves the Texture of Strawberry Fruit during Storage" Antioxidants 11, no. 5: 821. https://doi.org/10.3390/antiox11050821
APA StylePiechowiak, T., Migut, D., Józefczyk, R., & Balawejder, M. (2022). Ozone Treatment Improves the Texture of Strawberry Fruit during Storage. Antioxidants, 11(5), 821. https://doi.org/10.3390/antiox11050821







