Verification of the Relationship between Redox Regulation of Thioredoxin Target Proteins and Their Proximity to Thylakoid Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Protein Extraction from Plant Leaves
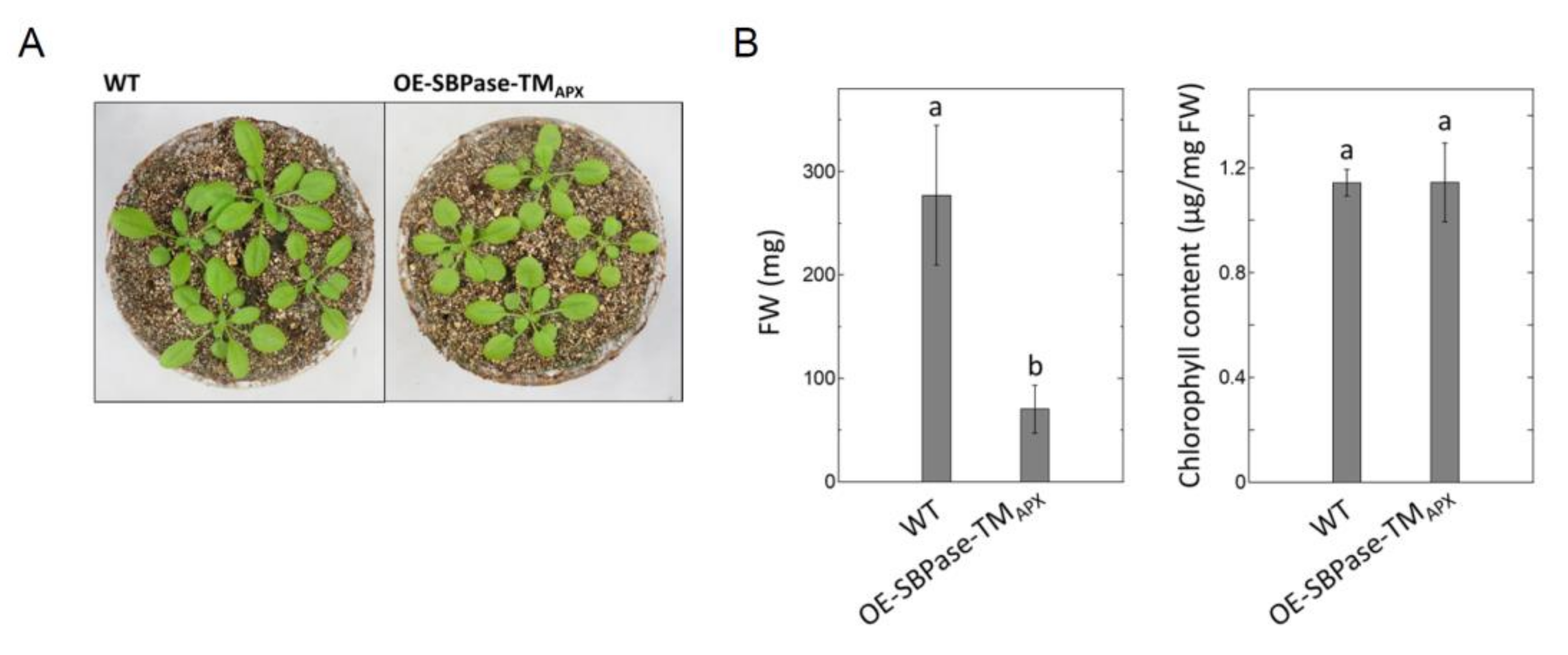
2.3. Measurements of Plant Growth and Chlorophyll Content
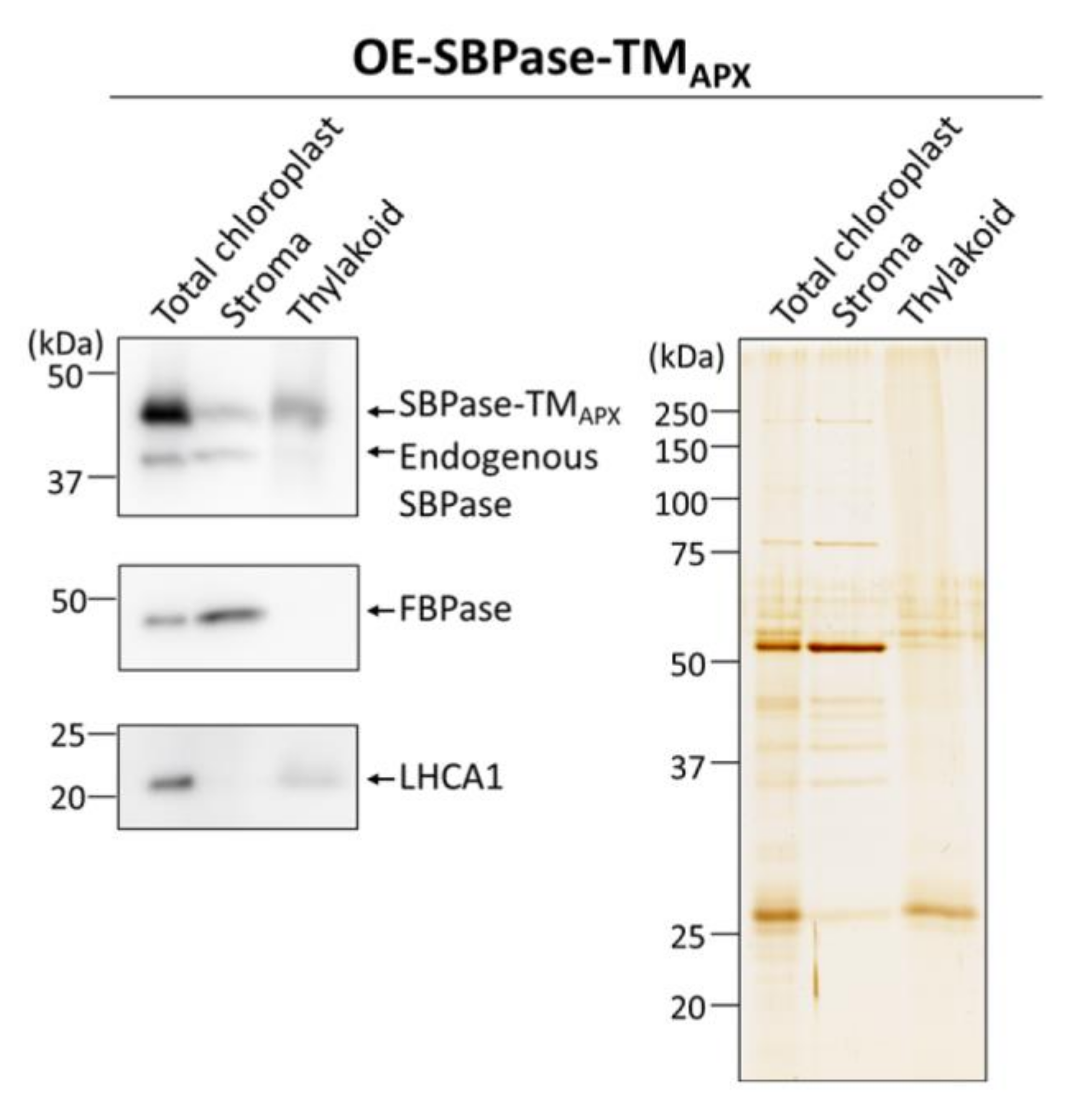
2.4. Isolation and Sub-Fractionation of Chloroplasts
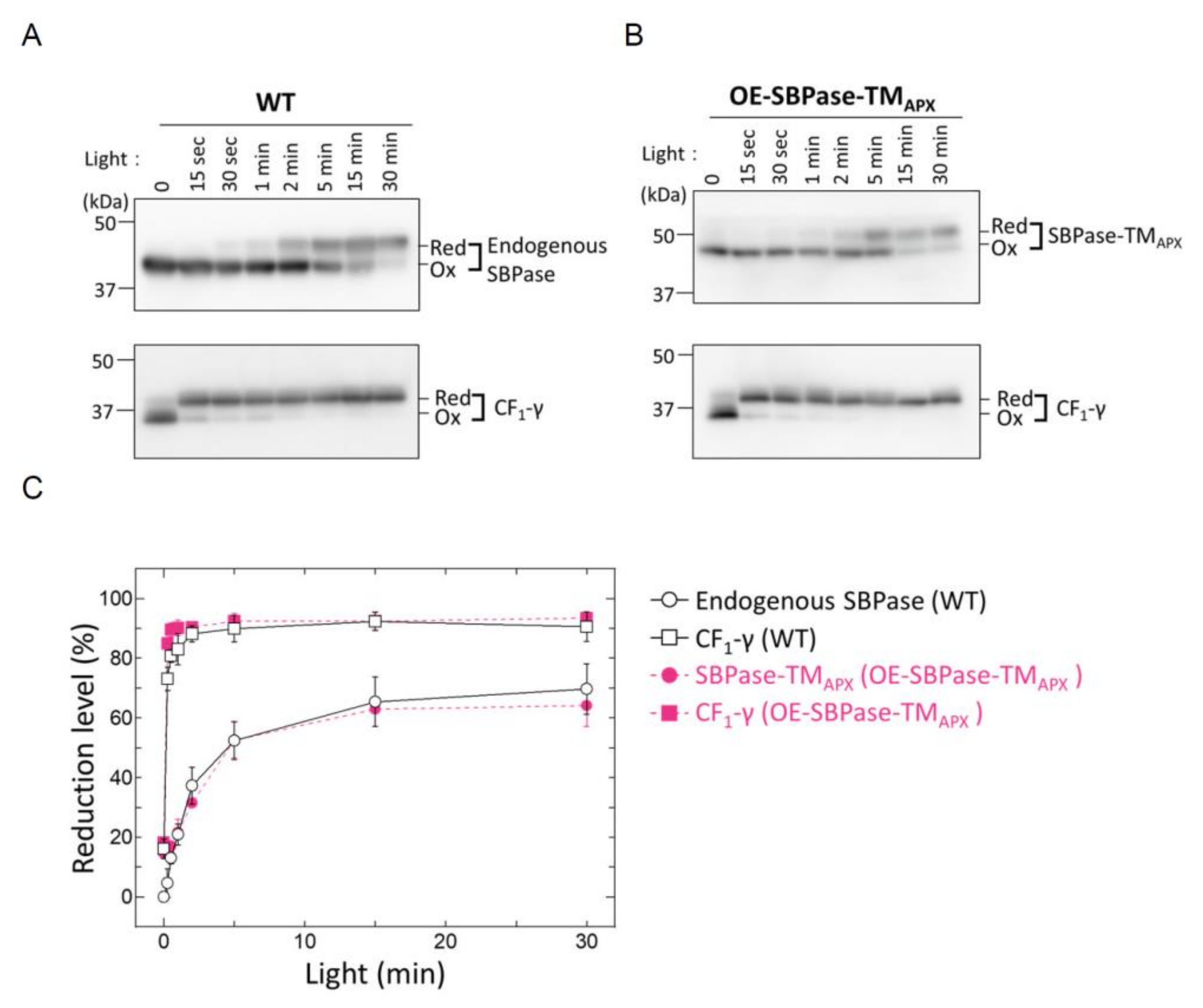
2.5. Determination of Light-Dependent Protein Redox State In Vivo
3. Results and Discussion
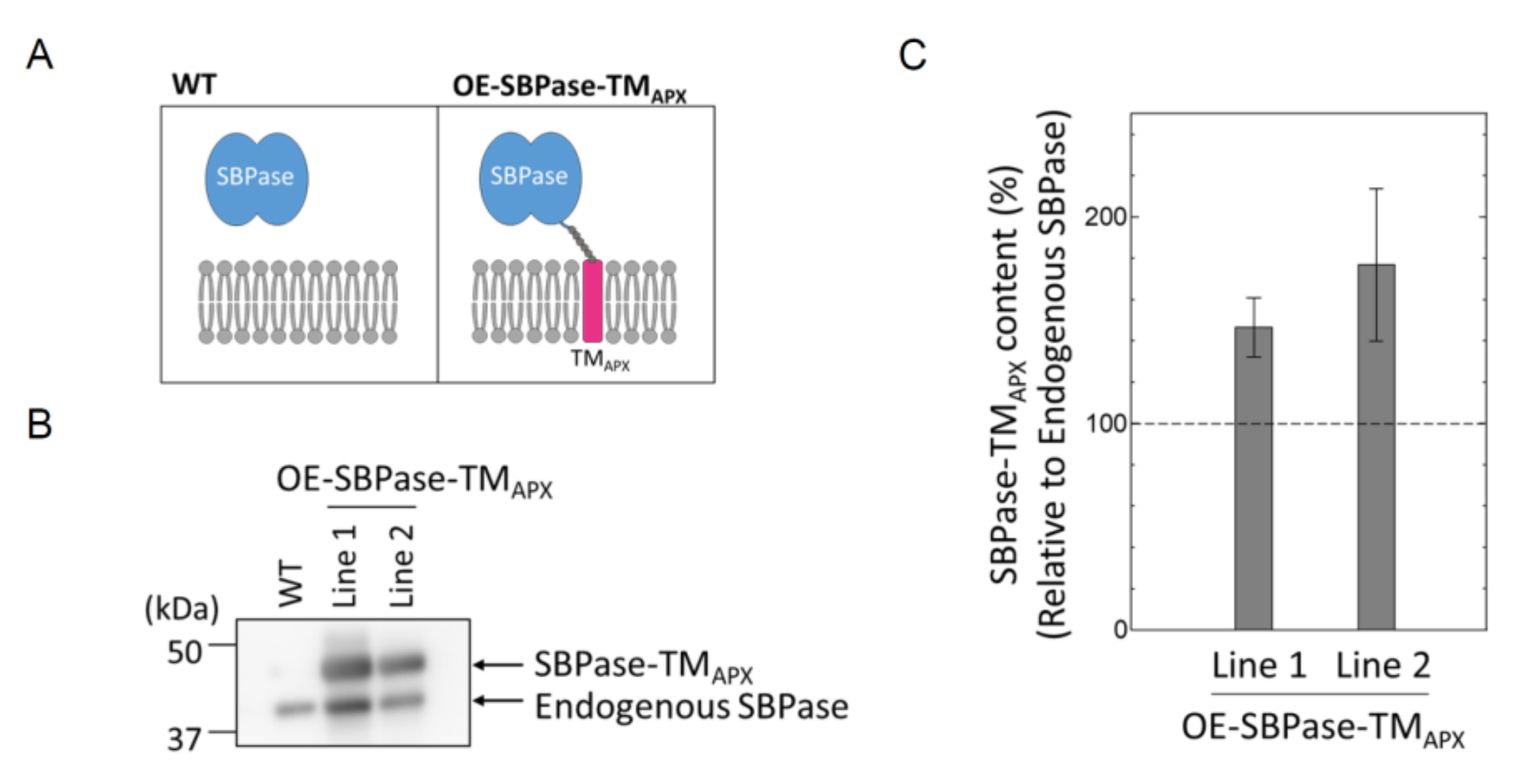
3.1. Generation of Plants Expressing Thylakoid Membrane-Anchored SBPase
3.2. In Vivo Redox Responses of Thylakoid Membrane-Anchored SBPase
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buchanan, B.B. Role of Light in the Regulation of Chloroplast Enzymes. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1980, 31, 341–374. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Schurmann, P.; Wolosiuk, R.A.; Jacquot, J.P. The ferredoxin/thioredoxin system: From discovery to molecular structures and beyond. Photosynth. Res. 2002, 73, 215–222. [Google Scholar] [CrossRef] [PubMed]
- The Arabidopsis Genome Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 2000, 408, 796–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemaire, S.D.; Michelet, L.; Zaffagnini, M.; Massot, V.; Issakidis-Bourguet, E. Thioredoxins in chloroplasts. Curr. Genet. 2007, 51, 343–365. [Google Scholar] [CrossRef] [PubMed]
- Serrato, A.J.; Fernandez-Trijueque, J.; Barajas-Lopez, J.D.; Chueca, A.; Sahrawy, M. Plastid thioredoxins: A “one-for-all” redox-signaling system in plants. Front. Plant Sci. 2013, 4, 463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, K.; Hisabori, T. Distinct electron transfer from ferredoxin-thioredoxin reductase to multiple thioredoxin isoforms in chloroplasts. Biochem. J. 2017, 474, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Yokochi, Y.; Sugiura, K.; Takemura, K.; Yoshida, K.; Hara, S.; Wakabayashi, K.I.; Kitao, A.; Hisabori, T. Impact of key residues within chloroplast thioredoxin-f on recognition for reduction and oxidation of target proteins. J. Biol. Chem. 2019, 294, 17437–17450. [Google Scholar] [CrossRef]
- Hisabori, T.; Motohashi, K.; Hosoya-Matsuda, N.; Ueoka-Nakanishi, H.; Romano, P.G.N. Towards a Functional Dissection of Thioredoxin Networks in Plant Cells. Photochem. Photobiol. 2007, 83, 145–151. [Google Scholar] [CrossRef]
- Michelet, L.; Zaffagnini, M.; Morisse, S.; Sparla, F.; Perez-Perez, M.E.; Francia, F.; Danon, A.; Marchand, C.H.; Fermani, S.; Trost, P.; et al. Redox regulation of the Calvin-Benson cycle: Something old, something new. Front. Plant Sci. 2013, 4, 470. [Google Scholar] [CrossRef] [Green Version]
- Mills, J.D.; Mitchell, P.; Schurmann, P. Modulation of coupling Factor ATPase Activity in intact chloroplasts, the role of the thioredoxin system. FEBS Lett. 1980, 112, 173–177. [Google Scholar] [CrossRef] [Green Version]
- Konno, H.; Nakane, T.; Yoshida, M.; Ueoka-Nakanishi, H.; Hara, S.; Hisabori, T. Thiol modulation of the chloroplast ATP synthase is dependent on the energization of thylakoid membranes. Plant Cell Physiol. 2012, 53, 626–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, K.; Matsuoka, Y.; Hara, S.; Konno, H.; Hisabori, T. Distinct redox behaviors of chloroplast thiol enzymes and their relationships with photosynthetic electron transport in Arabidopsis thaliana. Plant Cell Physiol. 2014, 55, 1415–1425. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K.; Hisabori, T. Determining the Rate-Limiting Step for Light-Responsive Redox Regulation in Chloroplasts. Antioxidants 2018, 7, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekiguchi, T.; Yoshida, K.; Okegawa, Y.; Motohashi, K.; Wakabayashi, K.I.; Hisabori, T. Chloroplast ATP synthase is reduced by both f-type and m-type thioredoxins. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148261. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.H.; Hong, J.; Li, X.Q.; Jiang, D.A. Antisense inhibition of Rubisco activase increases Rubisco content and alters the proportion of Rubisco activase in stroma and thylakoids in chloroplasts of rice leaves. Ann. Bot. 2006, 97, 739–744. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Takabayashi, A.; Tanaka, A.; Tanaka, R. Functional analysis of light-harvesting-like protein 3 (LIL3) and its light-harvesting chlorophyll-binding motif in Arabidopsis. J. Biol. Chem. 2014, 289, 987–999. [Google Scholar] [CrossRef] [Green Version]
- Miyake, C.; Asada, K. Thylakoid-Bound Ascorbate Peroxidase in Spinach Chloroplasts and Photoreduction of Its Primary Oxidation Product Monodehydroascorbate Radicals in Thylakoids. Plant Cell Physiol. 1992, 33, 541–553. [Google Scholar]
- Ishikawa, T.; Sakai, K.; Yoshimura, K.; Takeda, T.; Shigeoka, S. cDNAs encoding spinach stromal and thylakoid-bound ascorbate peroxidase, differing in the presence or absence of their 3′-coding regions. FEBS Lett. 1996, 384, 289–293. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, K.; Yabuta, Y.; Tamoi, M.; Ishikawa, T.; Shigeoka, S. Alternatively spliced mRNA variants of chloroplast ascorbate peroxidase isoenzymes in spinach leaves. Biochem. J. 1999, 338, 41–48. [Google Scholar] [CrossRef]
- Higuchi, R.; Krummel, B.; Saiki, R.K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: Study of protein and DNA interactions. Nucleic Acids Res. 1988, 16, 7351–7367. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K.; Hara, S.; Hisabori, T. Thioredoxin selectivity for thiol-based redox regulation of target proteins in chloroplasts. J. Biol. Chem. 2015, 290, 14278–14288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [Green Version]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of Accurate Extinction Coefficients and Simultaneous-Equations for Assaying Chlorophyll-a and Chlorophyll-B Extracted with 4 Different Solvents—Verification of the Concentration of Chlorophyll Standards by Atomic-Absorption Spectroscopy. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Yoshida, K.; Hara, A.; Sugiura, K.; Fukaya, Y.; Hisabori, T. Thioredoxin-like2/2-Cys peroxiredoxin redox cascade supports oxidative thiol modulation in chloroplasts. Proc. Natl. Acad. Sci. USA 2018, 115, E8296–E8304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koochak, H.; Puthiyaveetil, S.; Mullendore, D.L.; Li, M.; Kirchhoff, H. The structural and functional domains of plant thylakoid membranes. Plant J. 2019, 97, 412–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konno, H.; Yodogawa, M.; Stumpp, M.T.; Kroth, P.; Strotmann, H.; Motohashi, K.; Amano, T.; Hisabori, T. Inverse regulation of F1-ATPase activity by a mutation at the regulatory region on the γ subunit of chloroplast ATP synthase. Biochem. J. 2000, 352, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Ueoka-Nakanishi, H.; Nakanishi, Y.; Konno, H.; Motohashi, K.; Bald, D.; Hisabori, T. Inverse regulation of rotation of F1-ATPase by the mutation at the regulatory region on the γ subunit of chloroplast ATP synthase. J. Biol. Chem. 2004, 279, 16272–16277. [Google Scholar] [CrossRef] [Green Version]
- Kohzuma, K.; Dal Bosco, C.; Meurer, J.; Kramer, D.M. Light- and Metabolism-related Regulation of the Chloroplast ATP Synthase Has Distinct Mechanisms and Functions. J. Biol. Chem. 2013, 288, 13156–13163. [Google Scholar] [CrossRef] [Green Version]
- Carrillo, L.R.; Froehlich, J.E.; Cruz, J.A.; Savage, L.J.; Kramer, D.M. Multi-level regulation of the chloroplast ATP synthase: The chloroplast NADPH thioredoxin reductase C (NTRC) is required for redox modulation specifically under low irradiance. Plant J. 2016, 87, 654–663. [Google Scholar] [CrossRef]
- Naranjo, B.; Mignee, C.; Krieger-Liszkay, A.; Hornero-Mendez, D.; Gallardo-Guerrero, L.; Cejudo, F.J.; Lindahl, M. The chloroplast NADPH thioredoxin reductase C, NTRC, controls non-photochemical quenching of light energy and photosynthetic electron transport in Arabidopsis. Plant Cell Environ. 2016, 39, 804–822. [Google Scholar] [CrossRef] [Green Version]
- Bernal-Bayard, P.; Hervas, M.; Cejudo, F.J.; Navarro, J.A. Electron transfer pathways and dynamics of chloroplast NADPH-dependent thioredoxin reductase C (NTRC). J. Biol. Chem. 2012, 287, 33865–33872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukushi, Y.; Yokochi, Y.; Wakabayashi, K.-i.; Yoshida, K.; Hisabori, T. Verification of the Relationship between Redox Regulation of Thioredoxin Target Proteins and Their Proximity to Thylakoid Membranes. Antioxidants 2022, 11, 773. https://doi.org/10.3390/antiox11040773
Fukushi Y, Yokochi Y, Wakabayashi K-i, Yoshida K, Hisabori T. Verification of the Relationship between Redox Regulation of Thioredoxin Target Proteins and Their Proximity to Thylakoid Membranes. Antioxidants. 2022; 11(4):773. https://doi.org/10.3390/antiox11040773
Chicago/Turabian StyleFukushi, Yuka, Yuichi Yokochi, Ken-ichi Wakabayashi, Keisuke Yoshida, and Toru Hisabori. 2022. "Verification of the Relationship between Redox Regulation of Thioredoxin Target Proteins and Their Proximity to Thylakoid Membranes" Antioxidants 11, no. 4: 773. https://doi.org/10.3390/antiox11040773
APA StyleFukushi, Y., Yokochi, Y., Wakabayashi, K.-i., Yoshida, K., & Hisabori, T. (2022). Verification of the Relationship between Redox Regulation of Thioredoxin Target Proteins and Their Proximity to Thylakoid Membranes. Antioxidants, 11(4), 773. https://doi.org/10.3390/antiox11040773





