Melatonin Nuclear Receptors Mediate Green-and-Blue-Monochromatic-Light-Combinations-Inhibited B Lymphocyte Apoptosis in the Bursa of Chickens via Reducing Oxidative Stress and Nfκb Expression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Sampling
2.3. Lymphocyte Proliferation Assay
2.4. Elisa Assay
2.5. Immunohistochemical Staining
2.6. Real-Time Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
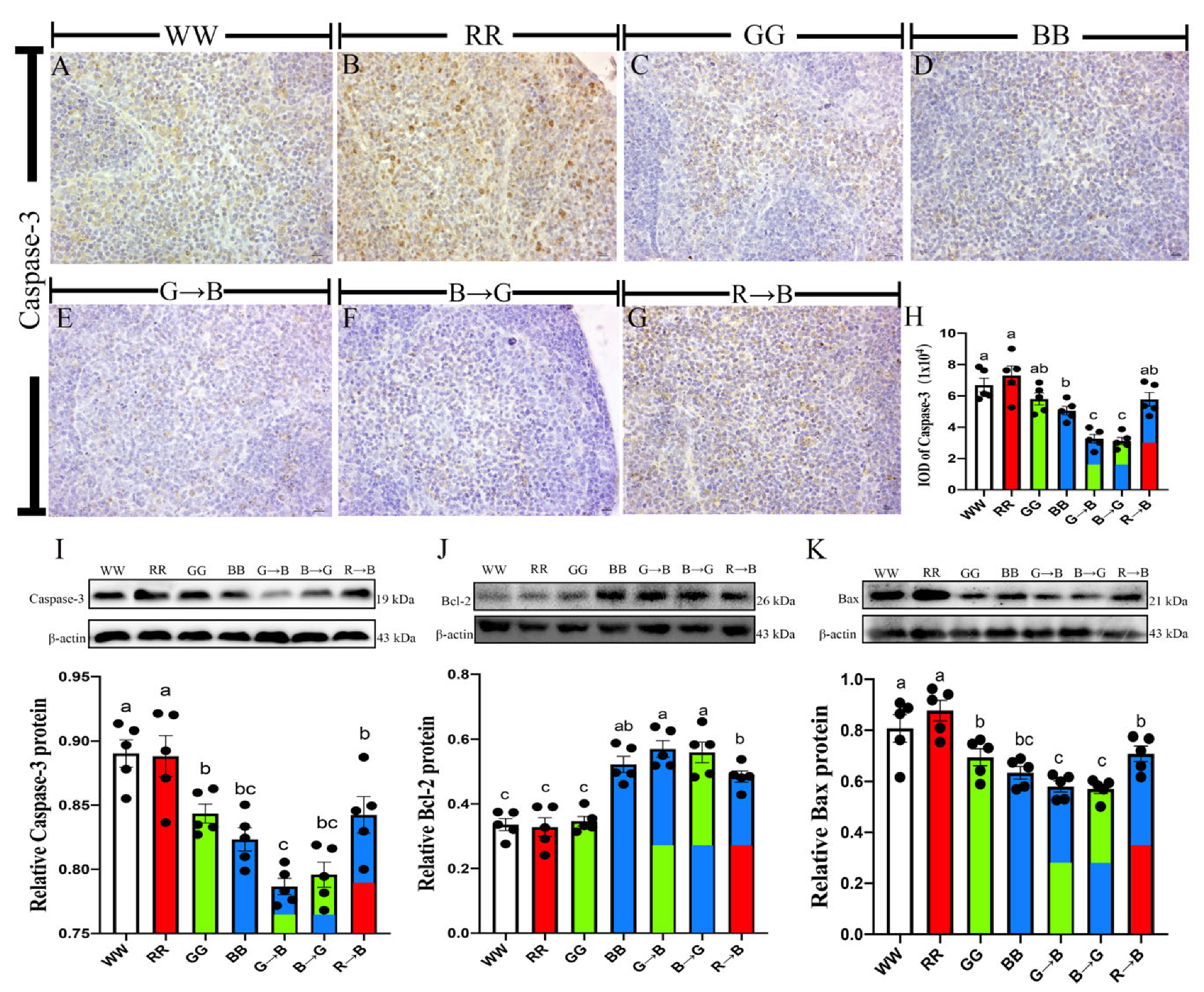
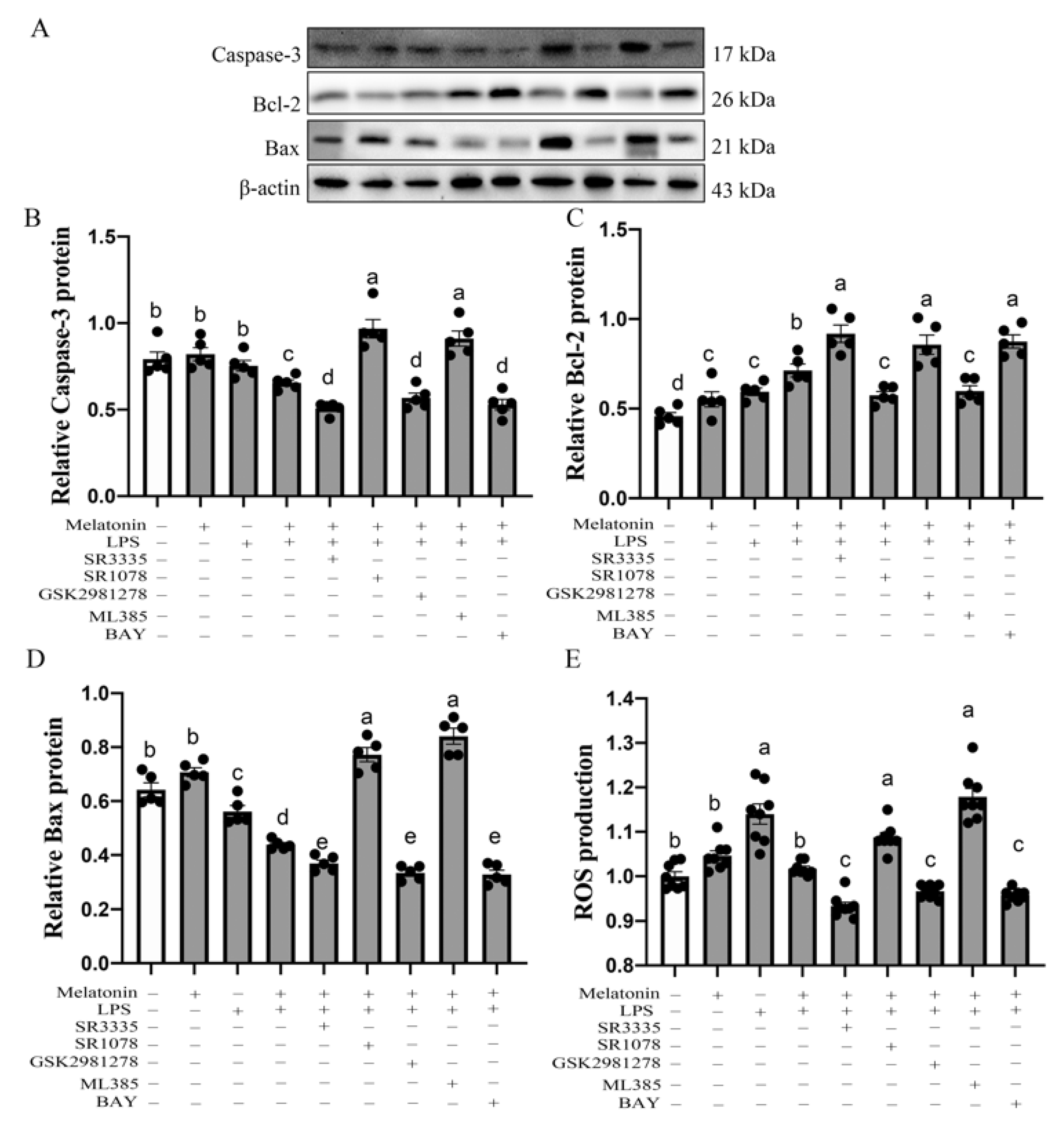
3.1. Caspase-3, Bcl-2, Bax, Protein in the Bursa of Chickens at P42
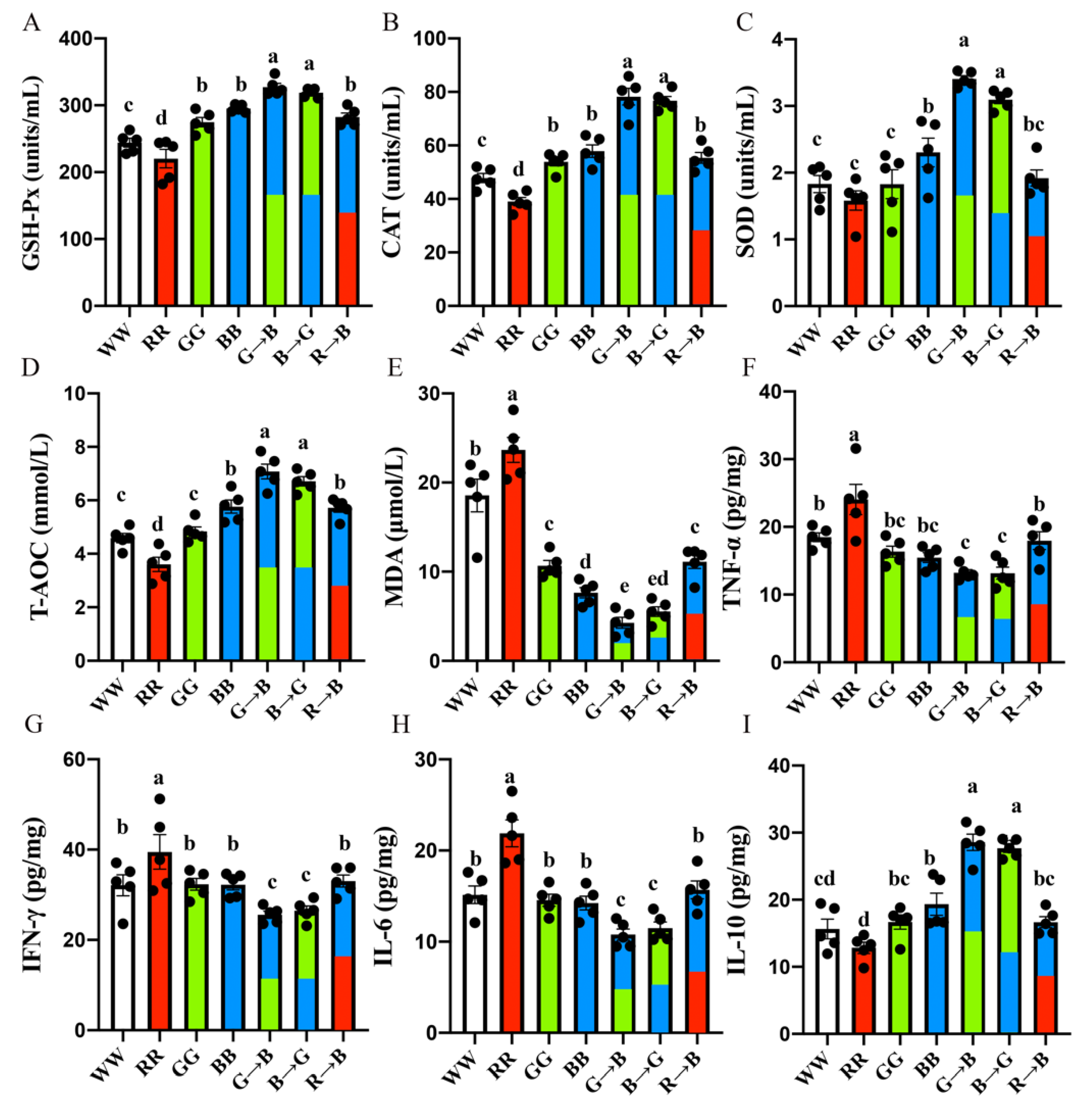
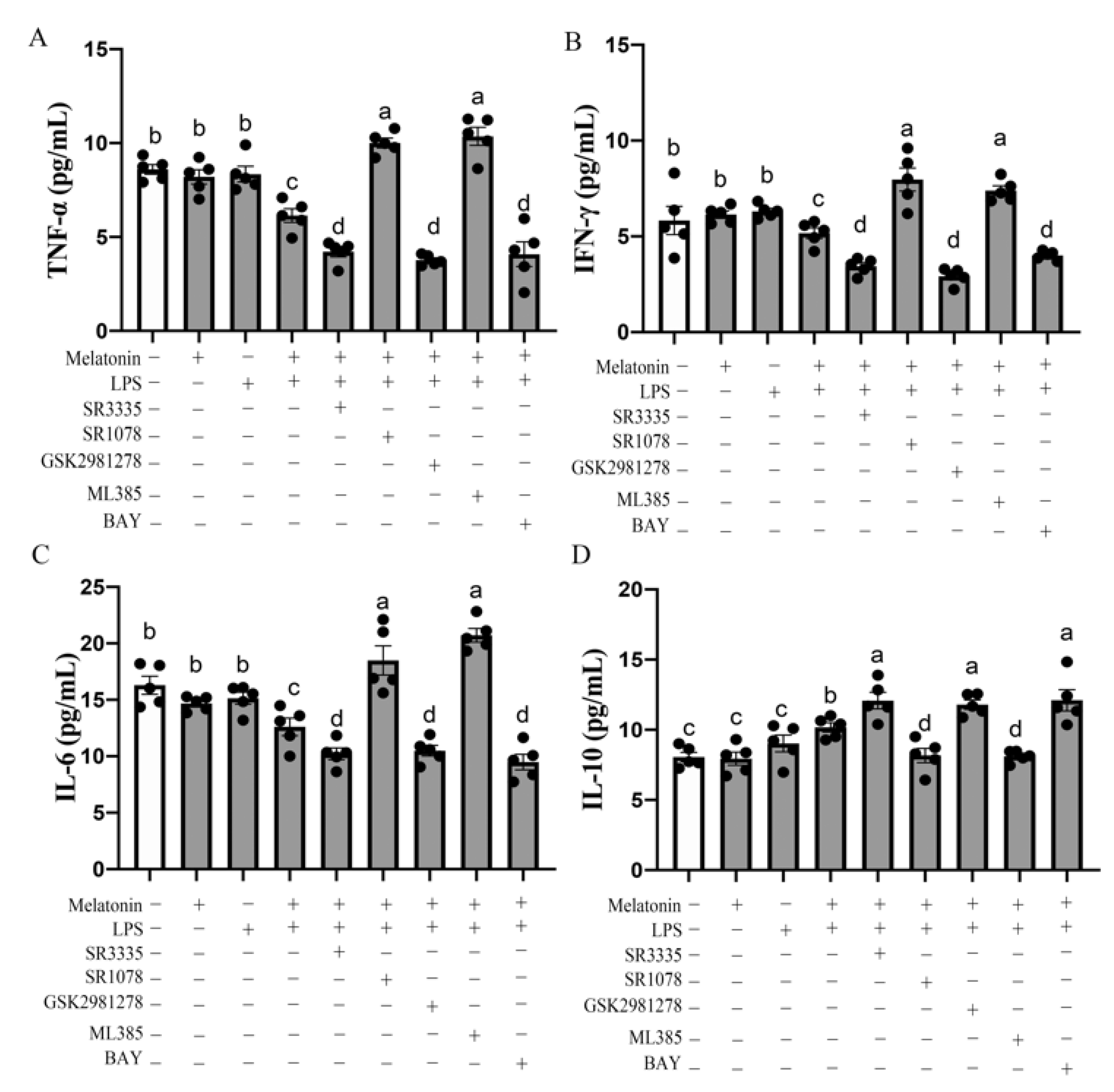
3.2. Plasma GSH-Px, CAT, SOD, T-AOC, MDA and Bursal IL-6, TNF-α, IFN-γ, IL-10 Concentration in Chickens at P42
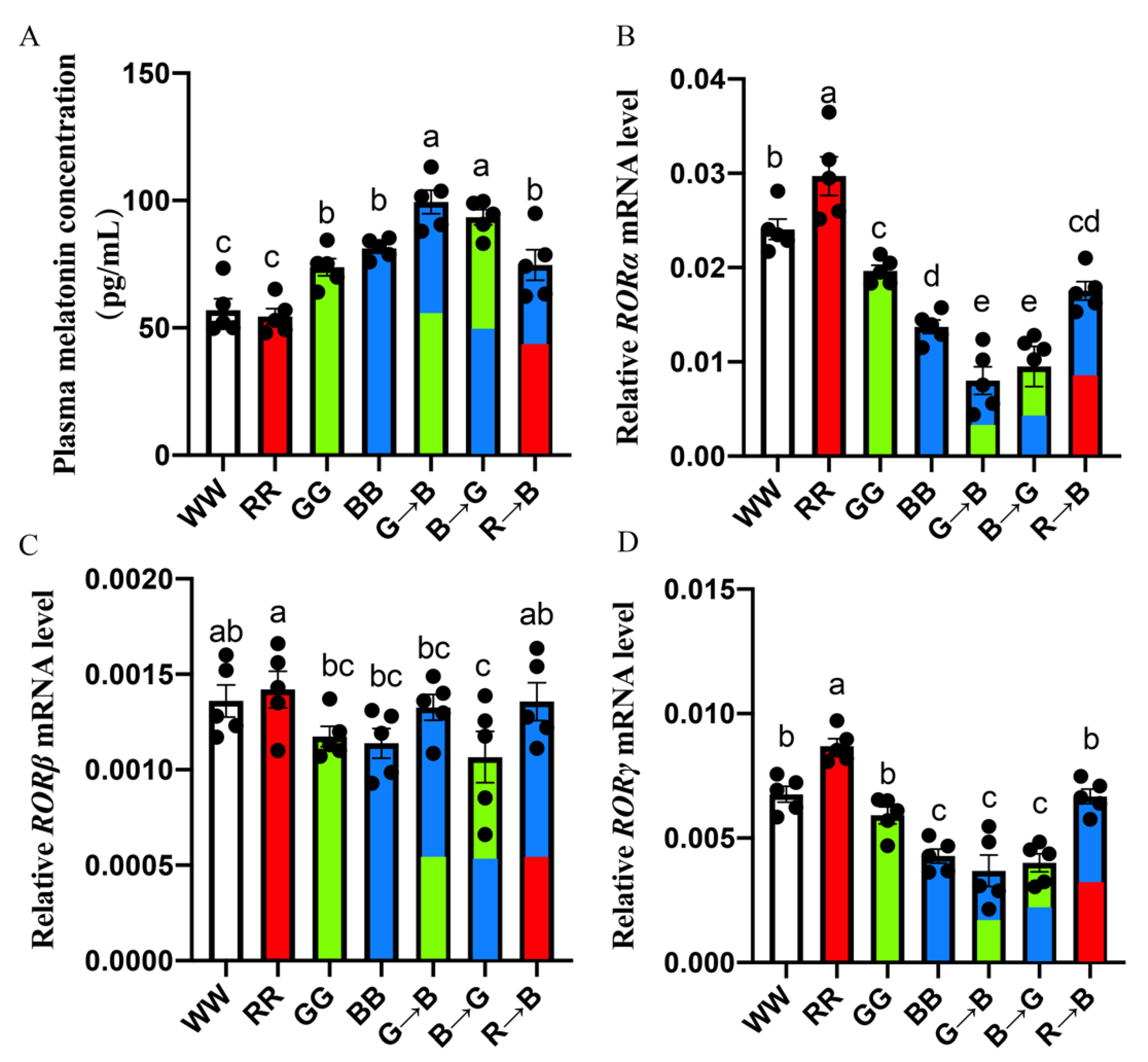
3.3. Plasma Melatonin Concentration and Melatonin Nuclear Expression in the Bursa of Chickens at P42
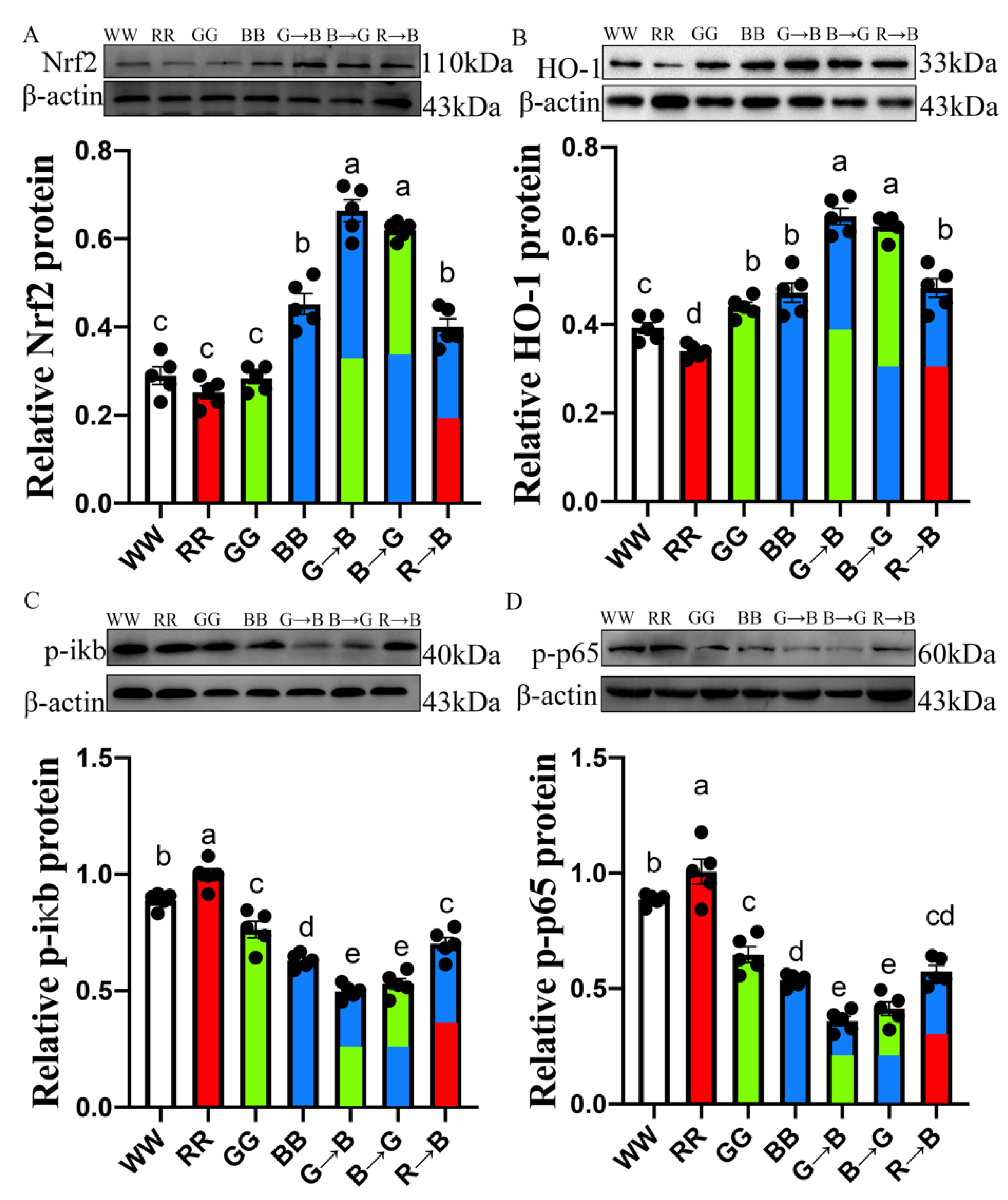
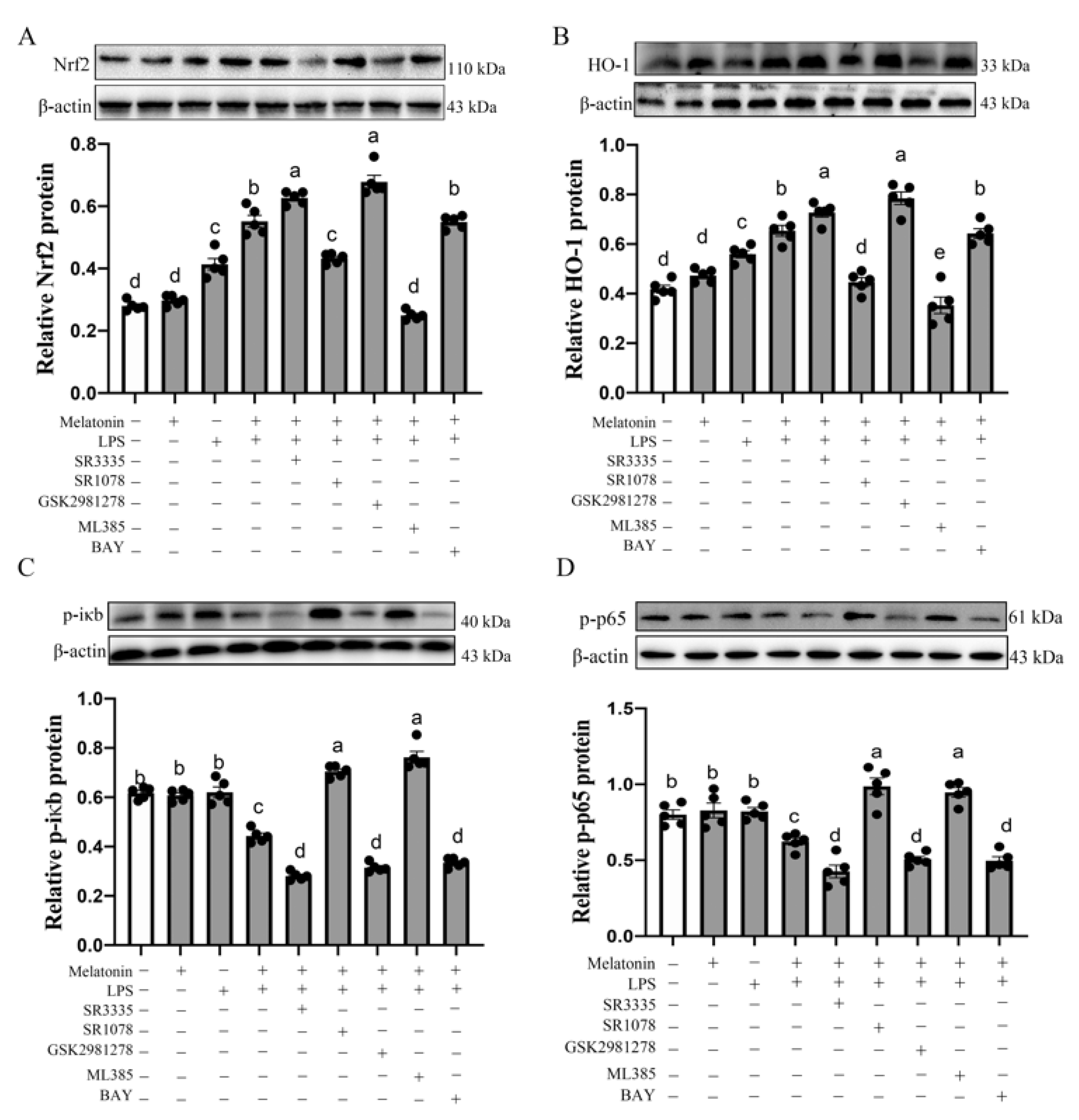
3.4. Nrf2, HO-1, p-iκb, p-p65 Protein Level in the Bursa of Chickens at P42
3.5. Melatonin Nuclear Receptors RORα/RORγ-Mediated G→B-Inhibited B Lymphocyte Apoptosis in the Bursa of Chickens via Nrf2/HO-1 and Nfκb Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Bax | Bcl-2 associated x protein |
| Bcl-2 | B-cell lymphoma-2 |
| BB | Blue light |
| B→G | A combination of blue and green monochromatic light |
| Caspases | Cysteinyl aspartate specific proteinase |
| CAT | Catalase |
| DAB | 3,3′-diaminobenzidine-4HCl |
| GSH-Px | Glutathione peroxidase |
| GG | Green light |
| G→B | A combination of green and blue monochromatic light |
| HRP | Horseradish-peroxidase |
| IOD | Integral optical density |
| INF-γ | Interferon-γ |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| LED | Light Emitting Diode |
| LPS | Lipopolysaccharide |
| Mel | Melatonin |
| Mel1a | Melatonin receptor 1a |
| Mel1b | Melatonin receptor 1b |
| Mel1c | Melatonin receptor 1c |
| MTT | Methyl thiazolyl tetrazolium |
| MDA | Malondialdehyde |
| NF-κB | Nuclear factor kappa B |
| OD | Optical density |
| PBS | Phosphate-buffered saline |
| PCNA | Proliferating cell nuclear antigen |
| RR | Red light |
| R→B | A combination of red and blue monochromatic light |
| ROR | Retinoic acid receptor related Orphan Receptor |
| RORα | Retinoic acid receptor related Orphan Receptor α |
| RORβ | Retinoic acid receptor related Orphan Receptor β |
| RORγ | Retinoic acid receptor related Orphan Receptor γ |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| SDS | Sodium dodecyl sulfate |
| T-AOC | Total antioxidant capacity |
| TNFα | Tumor necrosis factor α |
| WW | White light |
References
- Cao, J.; Liu, W.; Wang, Z.; Xie, D.; Jia, L.; Chen, Y. Green and blue monochromatic lights promote growth and development of broilers via stimulating testosterone secretion and myofiber growth. J. Appl. Poult. Res. 2008, 17, 211–218. [Google Scholar] [CrossRef]
- Rozenboim, I.; Biran, I.; Uni, Z.; Robinzon, B.; Halevy, O. The effect of monochromatic light on broiler growth and development. Poult. Sci. 1999, 78, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cao, J.; Wang, Z.; Dong, Y.; Chen, Y. Effect of a combination of green and blue monochromatic light on broiler immune response. J. Photochem. Photobiol. B Biol. 2014, 138, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.F.; Jiang, J.S.; Pan, J.M.; Ying, Y.B.; Wang, X.S.; Zhang, M.L.; Lu, M.S.; Chen, X.H. The relationship of spectral sensitivity with growth and reproductive response in avian breeders (Gallus gallus). Sci. Rep. 2016, 6, 19291. [Google Scholar] [CrossRef]
- Er, D.; Wang, Z.; Cao, J.; Chen, Y. Effect of monochromatic light on the egg quality of laying hens. J. Appl. Poult. Res. 2007, 16, 605–612. [Google Scholar] [CrossRef]
- Li, J.; Cao, J.; Wang, Z.; Dong, Y.; Chen, Y. Melatonin plays a critical role in inducing B lymphocyte proliferation of the bursa of fabricius in broilers via monochromatic lights. J. Photochem. Photobiol. B Biol. 2015, 142, 29–34. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Effect of the melatonin nuclear receptor RORα on monochromatic light-induced t-lymphocyte proliferation in chicken thymus. Immunol. Lett. 2019, 213, 21–29. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Effect of melatonin on monochromatic light-induced changes in clock gene circadian expression in the chick liver. J. Photochem. Photobiol. B Biol. 2019, 197, 111537. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Z.; Dong, Y.; Zhang, Z.; Li, J.; Li, F.; Chen, Y. Effect of combinations of monochromatic lights on growth and productive performance of broilers. Poult. Sci. 2012, 91, 3013–3018. [Google Scholar] [CrossRef]
- Ke, Y.; Liu, W.; Wang, Z.; Chen, Y. Effects of monochromatic light on quality properties and antioxidation of meat in broilers. Poult. Sci. 2011, 90, 2632–2637. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Z.; Dong, Y.; Cao, J.; Chen, Y. Physiological crosstalk between the ac/pka and plc/pkc pathways modulates melatonin-mediated, monochromatic-light-induced proliferation of t-lymphocytes in chickens. Cell Tissue Res. 2017, 369, 555–565. [Google Scholar] [CrossRef]
- Xie, D.; Li, J.; Wang, Z.; Cao, J.; Li, T.; Chen, J.; Chen, Y. Effects of monochromatic light on mucosal mechanical and immunological barriers in the small intestine of broilers. Poult. Sci. 2011, 90, 2697–2704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Z.; Dong, Y.; Cao, J.; Chen, Y. Blue light alters the composition of the jejunal microbiota and promotes the development of the small intestine by reducing oxidative stress. Antioxidants 2022, 11, 274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. A green and blue monochromatic light combination therapy reduces oxidative stress and enhances b-lymphocyte proliferation through promoting melatonin secretion. Oxid. Med. Cell Longev. 2021, 2021, 5595376. [Google Scholar] [CrossRef] [PubMed]
- Currier, N.L.; Sun, Z.Y.; Miller, S.C. Exogenous melatonin: Quantitative enhancement in vivo of cells mediating non-specific immunity. J. Neuroimmunol. 2000, 104, 101–108. [Google Scholar] [CrossRef]
- Ren, W.; Liu, G.; Chen, S.; Yin, J.; Wang, J.; Tan, B.; Wu, G.; Bazer, F.W.; Peng, Y.; Li, T.; et al. Melatonin signaling in t cells: Functions and applications. J. Pineal Res. 2017, 62, e12394. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Kang, J.; Fan, W.; He, H.; Huang, F. ROR: Nuclear receptor for melatonin or not? Molecules 2021, 26, 2693. [Google Scholar] [CrossRef]
- Ding, S.; Lin, N.; Sheng, X.; Zhao, Y.; Su, Y.; Xu, L.; Tong, R.; Yan, Y.; Fu, Y.; He, J.; et al. Melatonin stabilizes rupture-prone vulnerable plaques via regulating macrophage polarization in a nuclear circadian receptor rorα-dependent manner. J. Pineal Res. 2019, 67, e12581. [Google Scholar] [CrossRef]
- Farez, M.F.; Calandri, I.L.; Correale, J.; Quintana, F.J. Anti-inflammatory effects of melatonin in multiple sclerosis. BioEssays 2016, 38, 1016–1026. [Google Scholar] [CrossRef]
- Liu, H.; Aramaki, M.; Fu, Y.; Forrest, D. Retinoid-related orphan receptor β and transcriptional control of neuronal differentiation. Curr. Top. Dev. Biol. 2017, 125, 227–255. [Google Scholar] [CrossRef]
- Jetten, A.M.; Cook, D.N. (inverse) agonists of retinoic acid-related orphan receptor γ: Regulation of immune responses, inflammation, and autoimmune disease. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Takaku, M.; Zou, L.; Gu, A.D.; Chou, W.C.; Zhang, G.; Wu, B.; Kong, Q.; Thomas, S.Y.; Serody, J.S.; et al. Reversing ski-smad4-mediated suppression is essential for t(h)17 cell differentiation. Nature 2017, 551, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Kasal, D.N.; Bendelac, A. Multi-transcription factor reporter mice delineate early precursors to the ilc and lti lineages. J. Exp. Med. 2021, 218, e20200487. [Google Scholar] [CrossRef] [PubMed]
- Farez, M.F.; Mascanfroni, I.D.; Méndez-Huergo, S.P.; Yeste, A.; Murugaiyan, G.; Garo, L.P.; Balbuena Aguirre, M.E.; Patel, B.; Ysrraelit, M.C.; Zhu, C.; et al. Melatonin contributes to the seasonality of multiple sclerosis relapses. Cell 2015, 162, 1338–1352. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Leon, J.; Acua-Castroviejo, D.; Sainz, R.M.; Mayo, J.C.; Tan, D.X.; Reiter, R.J. Melatonin and mitochondrial function. Life Sci. 2004, 75, 765–790. [Google Scholar] [CrossRef]
- Shin, Y.H.; Lee, S.H.; Mun, K.C. Effect of melatonin on the antioxidant enzymes in the kidneys of cyclosporine-treated rats. Transplant. Proc. 2002, 34, 2650–2651. [Google Scholar] [CrossRef]
- Bian, J.; Wang, Z.; Dong, Y.; Cao, J.; Chen, Y. Effect of pinealectomy on the circadian clock of the chick retina under different monochromatic lights. Chronobiol. Int. 2019, 36, 548–563. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin mediates monochromatic light—Induced proliferation of T/B lymphocytes in the spleen via the membrane receptor or nuclear receptor. Poult. Sci. 2020, 99, 4294–4302. [Google Scholar] [CrossRef]
- Xie, D.; Wang, Z.; Dong, Y.; Cao, J.; Wang, J.; Chen, J.; Chen, Y. Effects of monochromatic light on immune response of broilers. Poult. Sci. 2008, 87, 1535–1539. [Google Scholar] [CrossRef]
- Xie, D.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Effects of monochromatic light on proliferation response of splencyte in broilers. Anat. Histol. Embryol. 2008, 37, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Abdo, S.E.; El-Kassas, S.; El-Nahas, A.F.; Mahmoud, S. Modulatory effect of monochromatic blue light on heat stress response in commercial broilers. Oxid. Med. Cell Longev. 2017, 2017, 1351945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, S.M.; Belancio, V.P.; Dauchy, R.T.; Xiang, S.; Brimer, S.; Mao, L.; Hauch, A.; Lundberg, P.W.; Summers, W.; Yuan, L.; et al. Melatonin: An inhibitor of breast cancer. Endocr. Relat. Cancer 2015, 22, R183–R204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojtulewicz, K.; Tomaszewska-Zaremba, D.; Herman, A.P. Endotoxin-induced inflammation suppresses the effect of melatonin on the release of lh from the ovine pars tuberalis explants—Ex vivo study. Molecules 2017, 22, 1933. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Miller, S.C.; Osmond, D.G. Melatonin inhibits apoptosis during early b-cell development in mouse bone marrow. J. Pineal Res. 2000, 29, 86–93. [Google Scholar] [CrossRef]
- Silva, S.O.; Rodrigues, M.R.; Ximenes, V.F.; Bueno-da-Silva, A.E.; Amarante-Mendes, G.P.; Campa, A. Neutrophils as a specific target for melatonin and kynuramines: Effects on cytokine release. J. Neuroimmunol. 2004, 156, 146–152. [Google Scholar] [CrossRef]
- Sainz, R.M.; Mayo, J.C.; Uría, H.; Kotler, M.; Antolfn, I.; Rodriguez, C.; Menendez-Pelaez, A. The pineal neurohormone melatonin prevents in vivo and in vitro apoptosis in thymocytes. J. Pineal Res. 1995, 19, 178–188. [Google Scholar] [CrossRef]
- Espino, J.; Bejarano, I.; Paredes, S.D.; Barriga, C.; Reiter, R.J.; Pariente, J.A.; Rodríguez, A.B. Melatonin is able to delay endoplasmic reticulum stress-induced apoptosis in leukocytes from elderly humans. Age 2011, 33, 497–507. [Google Scholar] [CrossRef] [Green Version]
- Acunacastroviejo, D.; Reiter, R.J.; Menendezpelaez, A.; Pablos, M.I.; Burgos, A. Characterization of high-affinity melatonin binding-sites in purified cell nuclei of rat liver. J. Pineal Res. 1994, 16, 100–112. [Google Scholar] [CrossRef]
- Lardone, P.J.; Guerrero, J.M.; Fernandez-Santos, J.M.; Rubio, A.; Martin-Lacave, I.; Carrillo-Vico, A. Melatonin synthesized by t lymphocytes as a ligand of the retinoic acid-related orphan receptor. J. Pineal Res. 2011, 51, 454–462. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Liu, Z.H.; Wang, L.; Xiao, H.M.; Du, C.G.; Zhang, Y.J.; Su, R.; Li, J.Q. Expression of the rorα gene in inner mongolian cashmere goat hair follicles. Genet. Mol. Res. 2015, 14, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.X.; Liu, H.; Xu, L.; Zhang, H.; Zhou, R.X. Melatonin downregulates nuclear receptor rzr/rorγ expression causing growth-inhibitory and anti-angiogenesis activity in human gastric cancer cells in vitro and in vivo. Oncol. Lett. 2016, 12, 897–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Y.; Xia, J.; Wu, S.; Lv, Z.; Huang, S.; Huang, H.; Su, X.; Cheng, J.; Ke, Y. Curcumin inhibits acute vascular inflammation through the activation of heme oxygenase-1. Oxid. Med. Cell Longev. 2018, 2018, 3295807. [Google Scholar] [CrossRef] [Green Version]
- Wakabayashi, N.; Slocum, S.L.; Skoko, J.J.; Shin, S.; Kensler, T.W. When nrf2 talks, who’s listening? Antioxid. Redox Signal. 2010, 13, 1649–1663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lousse, J.C.; Van Langendonckt, A.; Defrere, S.; Ramos, R.G.; Colette, S.; Donnez, J. Peritoneal endometriosis is an inflammatory disease. Front. Biosci. 2012, 4, 23–40. [Google Scholar] [CrossRef]
- Al-Sawaf, O.; Fragoulis, A.; Rosen, C.; Kan, Y.W.; Sönmez, T.T.; Pufe, T.; Wruck, C.J. Nrf2 protects against tweak-mediated skeletal muscle wasting. Sci. Rep. 2014, 4, 3625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.C.; Hsieh, C.W.; Wu, C.C.; Wung, B.S. Chalcone inhibits the activation of nf-kappab and stat3 in endothelial cells via endogenous electrophile. Life Sci. 2007, 80, 1420–1430. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Lin-Shiau, S.Y.; Lin, J.K. Comparative studies on the suppression of nitric oxide synthase by curcumin and its hydrogenated metabolites through down-regulation of ikappab kinase and nfkappab activation in macrophages. Biochem. Pharmacol. 2000, 60, 1665–1676. [Google Scholar] [CrossRef]
| Items | Light Treatments | ||||||
|---|---|---|---|---|---|---|---|
| WW | RR | GG | BB | G→B | B→G | R→B | |
| Light wavelength [nm] (1–26 days) | 400–700 | 660 | 560 | 480 | 560 | 480 | 660 |
| Light wavelength [nm] (27–42 days) | 400–700 | 660 | 560 | 480 | 480 | 560 | 480 |
| Light intensity [W/m2] | 0.19 | 0.19 | 0.19 | 0.19 | 0.19 | 0.19 | 0.19 |
| Photoperiod [Light: Dark] | 23:1 | 23:1 | 23:1 | 23:1 | 23:1 | 23:1 | 23:1 |
| Gene | Product Size | Primer Sequences (5′–3′) | Accession No. |
|---|---|---|---|
| RORα | 140 | F: TGG GCATACCCCTGAAGGTA<break/>R: CCG ATGCTGGTGTGTAGTCA | XM_413763.2 |
| RORβ | 270 | F: AAA TCG TTG CCA ACA CTG CC<break/>R: AGG TCA ATG ACG TGC CCA TT | NM_205093.1 |
| RORγ | 90 | F: GTG GGGTAATATCGGGAGCG<break/>R: CTT ATCGGGACAACCTGCGT | XM_015280013.1 |
| GAPDH | 124 | F: ATCACAGCCACACAGAAGACG<break/>R: TGACTTTCCCCACAGCCTTA | NM_204305 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, Z.; Dong, Y.; Cao, J.; Chen, Y. Melatonin Nuclear Receptors Mediate Green-and-Blue-Monochromatic-Light-Combinations-Inhibited B Lymphocyte Apoptosis in the Bursa of Chickens via Reducing Oxidative Stress and Nfκb Expression. Antioxidants 2022, 11, 748. https://doi.org/10.3390/antiox11040748
Zhang Y, Wang Z, Dong Y, Cao J, Chen Y. Melatonin Nuclear Receptors Mediate Green-and-Blue-Monochromatic-Light-Combinations-Inhibited B Lymphocyte Apoptosis in the Bursa of Chickens via Reducing Oxidative Stress and Nfκb Expression. Antioxidants. 2022; 11(4):748. https://doi.org/10.3390/antiox11040748
Chicago/Turabian StyleZhang, Yijia, Zixu Wang, Yulan Dong, Jing Cao, and Yaoxing Chen. 2022. "Melatonin Nuclear Receptors Mediate Green-and-Blue-Monochromatic-Light-Combinations-Inhibited B Lymphocyte Apoptosis in the Bursa of Chickens via Reducing Oxidative Stress and Nfκb Expression" Antioxidants 11, no. 4: 748. https://doi.org/10.3390/antiox11040748
APA StyleZhang, Y., Wang, Z., Dong, Y., Cao, J., & Chen, Y. (2022). Melatonin Nuclear Receptors Mediate Green-and-Blue-Monochromatic-Light-Combinations-Inhibited B Lymphocyte Apoptosis in the Bursa of Chickens via Reducing Oxidative Stress and Nfκb Expression. Antioxidants, 11(4), 748. https://doi.org/10.3390/antiox11040748







