Hemoglobin Oxidation Reactions in Stored Blood
Abstract
:1. Hemoglobin Oxidative Reactions and Toxicity
2. Current Blood Banking Practices and Blood Safety
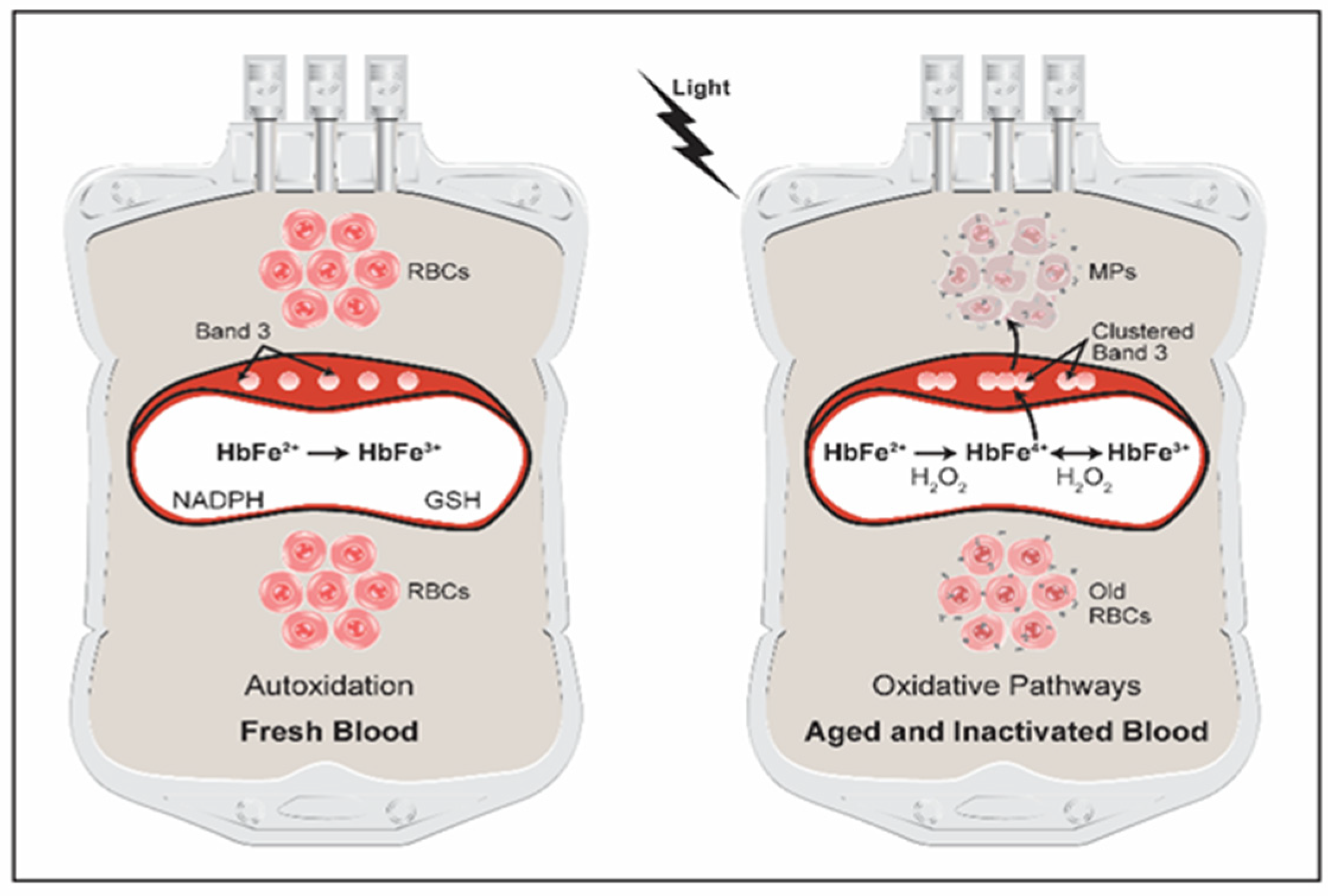
3. Hemoglobin Oxidative Pathways in Stored Blood
4. Pathogen Inactivation of Blood
5. Hemoglobin Oxidation Reactions Drive Changes within RBCs Resulting in Microparticles Formation
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Sadrzadeh, S.M.; Graf, E.; Panter, S.S.; Hallaway, P.E.; Eaton, J.W. Hemoglobin. A biologic fenton reagent. J. Biol. Chem. 1984, 259, 14354–14356. [Google Scholar] [CrossRef]
- Wilson, M.T.; Reeder, B.J. The peroxidatic activities of Myoglobin and Hemoglobin, their pathological consequences and possible medical interventions. Mol. Asp. Med. 2021, 84, 101045. [Google Scholar] [CrossRef] [PubMed]
- Alayash, A.I. Mechanisms of Toxicity and Modulation of Hemoglobin-based Oxygen Carriers. Shock 2019, 52, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Alayash, A.I. βCysteine 93 in human hemoglobin: A gateway to oxidative stability in health and disease. Lab. Investig. 2021, 101, 4–11. [Google Scholar] [CrossRef]
- Posta, N.; Csősz, É.; Oros, M.; Pethő, D.; Potor, L.; Kalló, G.; Hendrik, Z.; Sikura, K.É.; Méhes, G.; Tóth, C.; et al. Hemoglobin oxidation generates globin-derived peptides in atherosclerotic lesions and intraventricular hemorrhage of the brain, provoking endothelial dysfunction. Lab. Investig. 2020, 100, 986–1002. [Google Scholar] [CrossRef]
- Jana, S.; Strader, M.B.; Meng, F.; Hicks, W.; Kassa, T.; Tarandovskiy, I.; De Paoli, S.; Simak, J.; Heaven, M.R.; Belcher, J.D.; et al. Hemoglobin oxidation–dependent reactions promote interactions with band 3 and oxidative changes in sickle cell–derived microparticles. JCI Insight 2018, 3, 120451. [Google Scholar] [CrossRef] [Green Version]
- Strader, M.B.; Jana, S.; Meng, F.; Heaven, M.R.; Shet, A.S.; Thein, S.L.; Alayash, A.I. Post-translational modification as a response to cellular stress induced by hemoglobin oxidation in sickle cell disease. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Cyrklaff, M.; Sanchez, C.P.; Kilian, N.; Bisseye, C.; Simpore, J.; Frischknecht, F.; Lanzer, M. Hemoglobins S and C Interfere with Actin Remodeling in Plasmodium falciparum—Infected Erythrocytes. Science 2011, 334, 1283–1286. [Google Scholar] [CrossRef]
- Vona, R.; Sposi, N.; Mattia, L.; Gambardella, L.; Straface, E.; Pietraforte, D. Sickle Cell Disease: Role of Oxidative Stress and Antioxidant Therapy. Antioxidants 2021, 10, 296. [Google Scholar] [CrossRef]
- Alayash, A.I. Oxidative pathways in the sickle cell and beyond. Blood Cells Mol. Dis. 2018, 70, 78–86. [Google Scholar] [CrossRef]
- Sparrow, R.L. Red blood cell storage and transfusion-related immunomodulation. Blood Transfus. 2010, 8, s26–s30. [Google Scholar] [PubMed]
- Storch, E.K.; Custer, B.S.; Jacobs, M.R.; Menitove, J.E.; Mintz, P.D. Review of current transfusion therapy and blood banking practices. Blood Rev. 2019, 38, 100593. [Google Scholar] [CrossRef] [PubMed]
- Stapley, R.; Owusu, B.Y.; Brandon, A.; Cusick, M.; Rodriguez, C.; Marques, M.B.; Kerby, J.D.; Barnum, S.R.; Weinberg, J.A.; Lancaster, J.R.; et al. Erythrocyte storage increases rates of NO and nitrite scavenging: Implications for transfusion-related toxicity. Biochem. J. 2012, 446, 499–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, J. Seeing red. Transfusion 2010, 50, 116. [Google Scholar] [CrossRef]
- Roback, J.D. Vascular Effects of the Red Blood Cell Storage Lesion. Hematology 2011, 2011, 475–479. [Google Scholar] [CrossRef] [Green Version]
- Campbell-Lee, S.A.; Ness, P.M. Packed Red Blood cells and Related Products. In Blood Banking and Transfusion Medicine; Rudmann, S.V., Ed.; Churchill Livingstone: London, UK, 2007; pp. 250–258. [Google Scholar]
- Chaudhary, R.; Katharia, R. Oxidative injury as contributory factor for red cells storage lesion during twenty eight days of storage. High Speed Blood Transfus. Equip. 2012, 10, 59–62. [Google Scholar] [CrossRef]
- Kanias, T.; Acker, J.P. Biopreservation of red blood cells-the struggle with hemoglobin oxidation. FEBS J. 2010, 277, 343–356. [Google Scholar] [CrossRef]
- Mustafa, I.; Hadwan, T.A.Q. Hemoglobin Oxidation in Stored Blood Accelerates Hemolysis and Oxidative Injury to Red Blood Cells. J. Lab. Physicians 2020, 12, 244–249. [Google Scholar] [CrossRef]
- Buehler, P.W.; D’Agnillo, F.; Hoffman, V.; Alayash, A.I. Effects of endogenous ascorbate on oxidation, oxygenation, and tox-icokinetics of cell-free modified hemoglobin after exchange transfusion in rat and guinea pig. J. Pharmacol. Exp. Ther. 2007, 323, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Baek, J.H.; Yalamanoglu, A.; Gao, Y.; Guenster, R.; Spahn, D.R.; Schaer, D.J.; Buehler, P.W.J. Iron accelerates hemoglobin oxidation increasing mortality in vascular diseased guinea pigs following transfusion of stored blood. J. Clin Investig. Insight 2017, 2, e93577. [Google Scholar] [CrossRef]
- Sharifi, S.; Dzik, W.H.; Sadrzadeh, S.M. Human plasma and Tirilazad mesylate protect stored human erythrocytes against the oxidative damage of gamma-irradiation. Transfus. Med. 2000, 10, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.R. An update on solutions for red cell storage. Vox Sang. 2006, 91, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.; Rugg, N.; Knapp, A.; Gormas, J.; Silberstein, E.; Greenwalt, T. Successful storage of RBCs for 10 weeks in a new additive solution. Transfusion 2000, 40, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.D.; Ahearn, G.S.; Angelo, M.; Zhang, J.; Cobb, F.; Stamler, J.S. S-nitrosohemoglobin deficiency: A mechanism for loss of physiological activity in banked blood. Proc. Natl. Acad. Sci. USA 2007, 104, 17058–17062. [Google Scholar] [CrossRef] [Green Version]
- Kim-Shapiro, D.B.; Lee, J.; Gladwin, M.T. Storage lesion: Role of red blood cell breakdown. Transfusion 2011, 51, 844–851. [Google Scholar] [CrossRef] [Green Version]
- Alayash, A.I.; Fratantoni, J.C.; Bonaventura, C.; Bonaventura, J.; Bucci, E. Consequences of chemical modifications on the free radical reactions of human hemoglobin. Arch. Biochem. Biophys. 1992, 298, 114–120. [Google Scholar] [CrossRef]
- Winslow, R.M.; Intaglietta, M. Red cell age and loss of function: Advance or SNO-job? Transfusion 2008, 48, 411–414. [Google Scholar] [CrossRef]
- Gell, D.A. Structure and function of haemoglobins. Blood Cells Mol. Dis. 2018, 70, 13–42. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Snyder, E.L. Safety of the blood supply: Role of pathogen reduction. Blood Rev. 2003, 17, 111–122. [Google Scholar] [CrossRef]
- Seltsam, A. Pathogen Inactivation of Cellular Blood Products—An Additional Safety Layer in Transfusion Medicine. Front. Med. 2017, 4, 219. [Google Scholar] [CrossRef] [Green Version]
- Schlenke, P. Pathogen Inactivation Technologies for Cellular Blood Components: An Update. Transfus. Med. Hemother. 2014, 41, 309–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seghatchian, J.; Putter, J.S. Pathogen inactivation of whole blood and red cell components: An overview of concept, design, developments, criteria of acceptability and storage lesion. Transfus. Apher. Sci. 2013, 49, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Garel, M.C.; Domenget, C.; Caburi-Martin, J.; Prehu, C.; Galacteros, F.; Beuzard, Y. Covalent binding of glutathione to hemoglobin. I. Inhibition of hemoglobin S polymerization. J. Biol. Chem. 1986, 261, 14704–14709. [Google Scholar] [CrossRef]
- Salunkhe, V.; van der Meer, P.F.; de Korte, D.; Seghatchian, J.; Gutiérrez, L. Development of blood transfusion product pathogen reduction treatments: A review of methods, current applications and demands. Transfus. Apher. Sci. 2015, 52, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Remigante, A.; Morabito, R.; Marino, A. Band 3 protein function and oxidative stress in erythrocytes. J. Cell. Physiol. 2021, 236, 6225–6234. [Google Scholar] [CrossRef]
- Rogers, S.C.; Ge, X.; Brummet, M.; Lin, X.; Timm, D.D.; D’Avignon, A.; Garbow, J.R.; Kao, J.; Prakash, J.; Issaian, A.; et al. Quantifying dynamic range in red blood cell energetics: Evidence of progressive energy failure during storage. Transfusion 2021, 61, 1586–1599. [Google Scholar] [CrossRef]
- Filho, I.P.T.; Torres, L.N.; Barraza, D.; Williams, C.E.; Hildreth, K. Cellular and Biochemical Effects of Combined X-ray Radiation and Storage on Whole Blood. Dose Response 2022, 20, 1–9. [Google Scholar] [CrossRef]
- Buehler, P.W.; Karnaukhova, E.; Gelderman, M.P.; Alayash, A.I. Blood aging, safety, and transfusion: Capturing the “radical” menace. Antioxid. Redox Signal. 2011, 14, 1713–1728. [Google Scholar] [CrossRef]
- Rubin, O.; Canellini, G.; Delobel, J.; Lion, N.; Tissot, J.-D. Red Blood Cell Microparticles: Clinical Relevance. Transfus. Med. Hemother. 2012, 39, 342–347. [Google Scholar] [CrossRef] [Green Version]
- Ealaarg, A.; Schiffelers, R.M.; Van Solinge, W.W.; Wijk, R.E. Red blood cell vesiculation in hereditary hemolytic anemia. Front. Physiol. 2013, 4, 365. [Google Scholar] [CrossRef]
- Garnier, Y.; Ferdinand, S.; Garnier, M.; Cita, K.-C.; Hierso, R.; Claes, A.; Connes, P.; Hardy-Dessources, M.-D.; Lapouméroulie, C.; Lemonne, N.; et al. Plasma microparticles of sickle patients during crisis or taking hydroxyurea modify endothelium inflammatory properties. Blood 2020, 136, 247–256. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alayash, A.I. Hemoglobin Oxidation Reactions in Stored Blood. Antioxidants 2022, 11, 747. https://doi.org/10.3390/antiox11040747
Alayash AI. Hemoglobin Oxidation Reactions in Stored Blood. Antioxidants. 2022; 11(4):747. https://doi.org/10.3390/antiox11040747
Chicago/Turabian StyleAlayash, Abdu I. 2022. "Hemoglobin Oxidation Reactions in Stored Blood" Antioxidants 11, no. 4: 747. https://doi.org/10.3390/antiox11040747
APA StyleAlayash, A. I. (2022). Hemoglobin Oxidation Reactions in Stored Blood. Antioxidants, 11(4), 747. https://doi.org/10.3390/antiox11040747





