Sunflower-Assisted Bio-Derived ZnO-NPs as an Efficient Nanocatalyst for the Synthesis of Novel Quinazolines with Highly Antioxidant Activities
Abstract
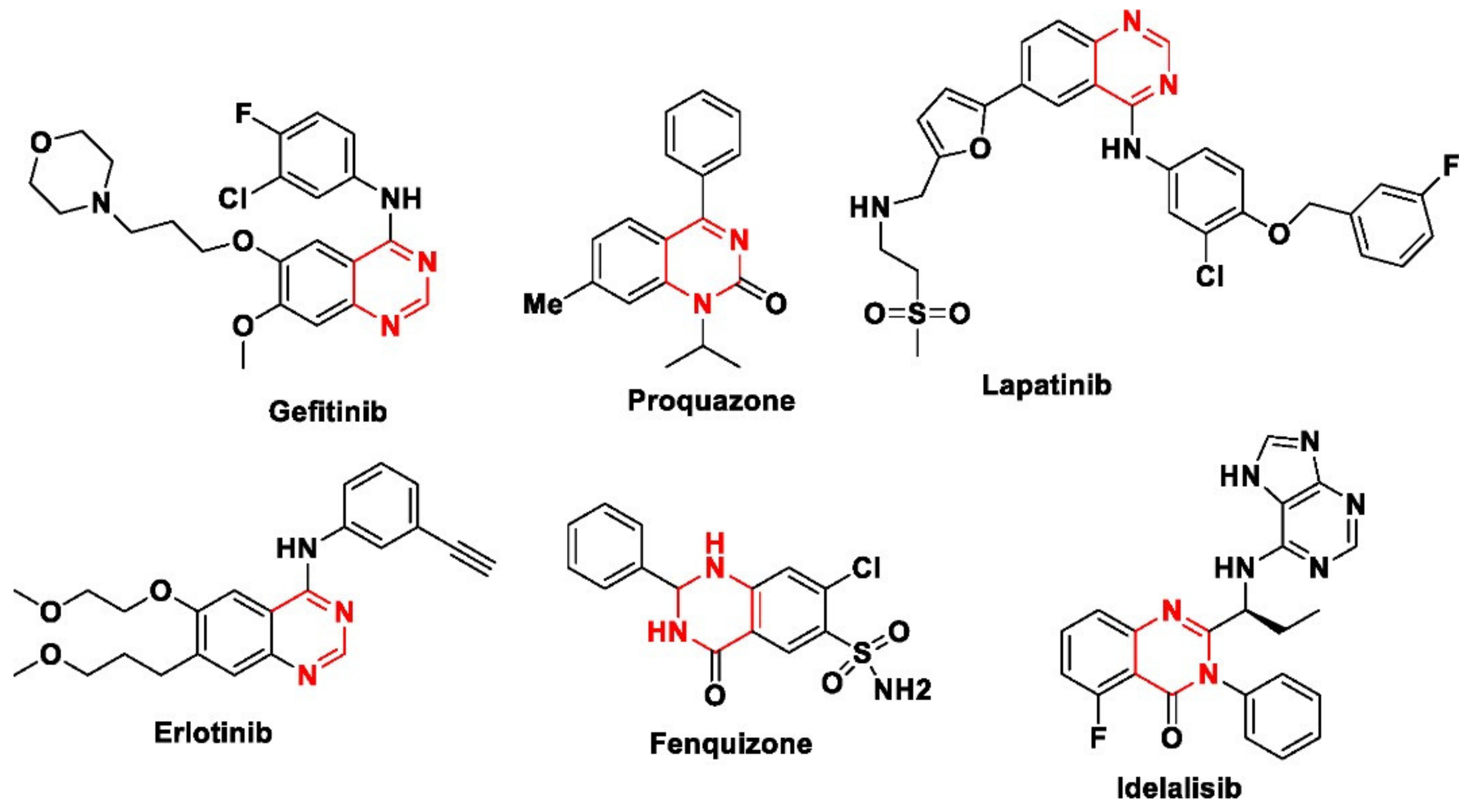
1. Introduction
2. Materials & Methods
2.1. Materials
2.2. Collecting Plant Material and Preparation of the Leaf Extract
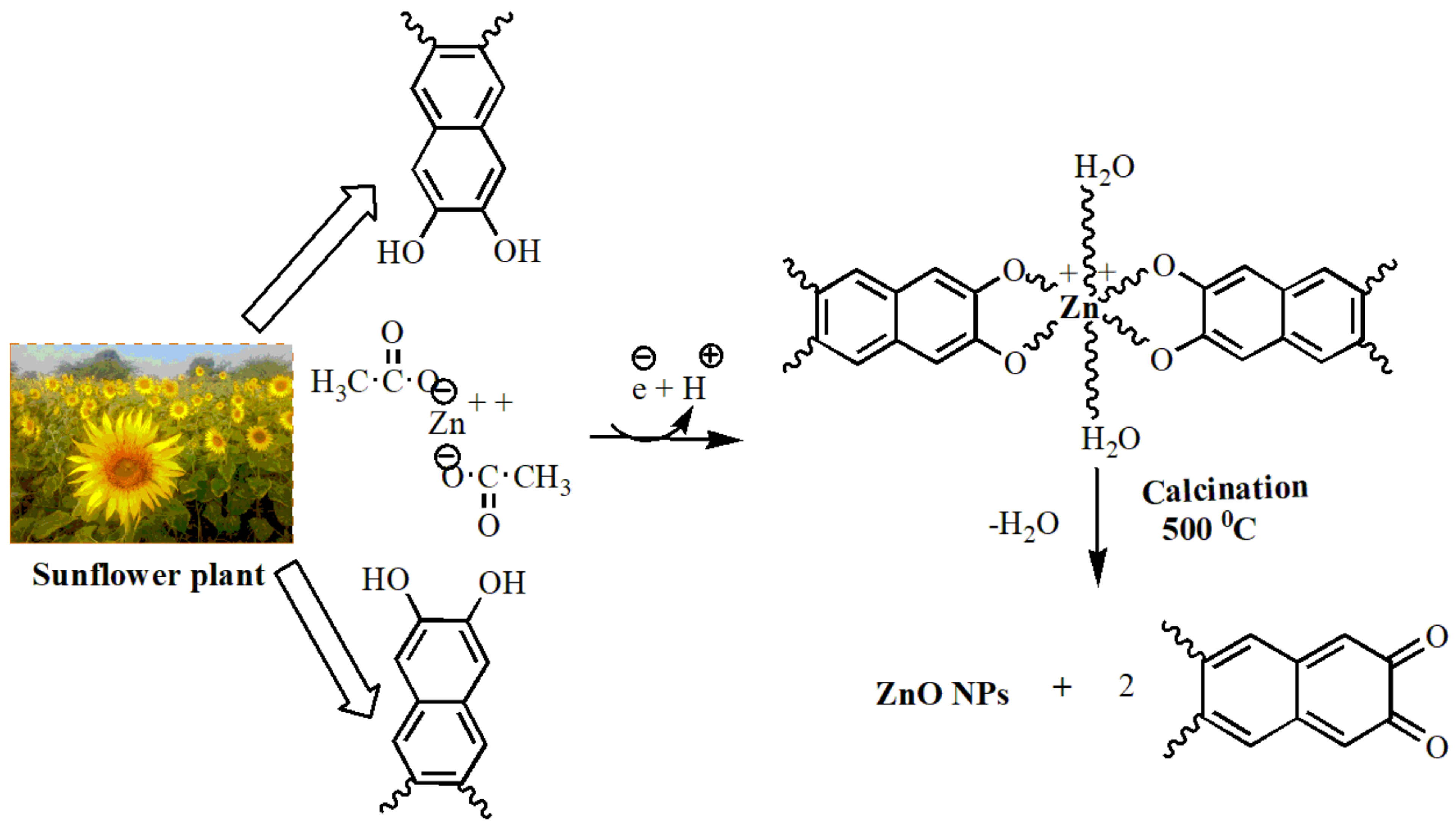
2.3. Preparation of ZnO-NPs Using Sunflower Leaf Extract
2.4. Characterization of Synthesized ZnO NPs
2.5. General Procedure for the Synthesis of 1,2 Dihydroquinazoline Derivatives (4a–o)
2.6. Antioxidant Activity of ZnO-NPs
2.7. General Procedure for Assessing the Antioxidant Activity of 1,2-Dihydroquinazolines with the DPPH Method
3. Results and Discussion
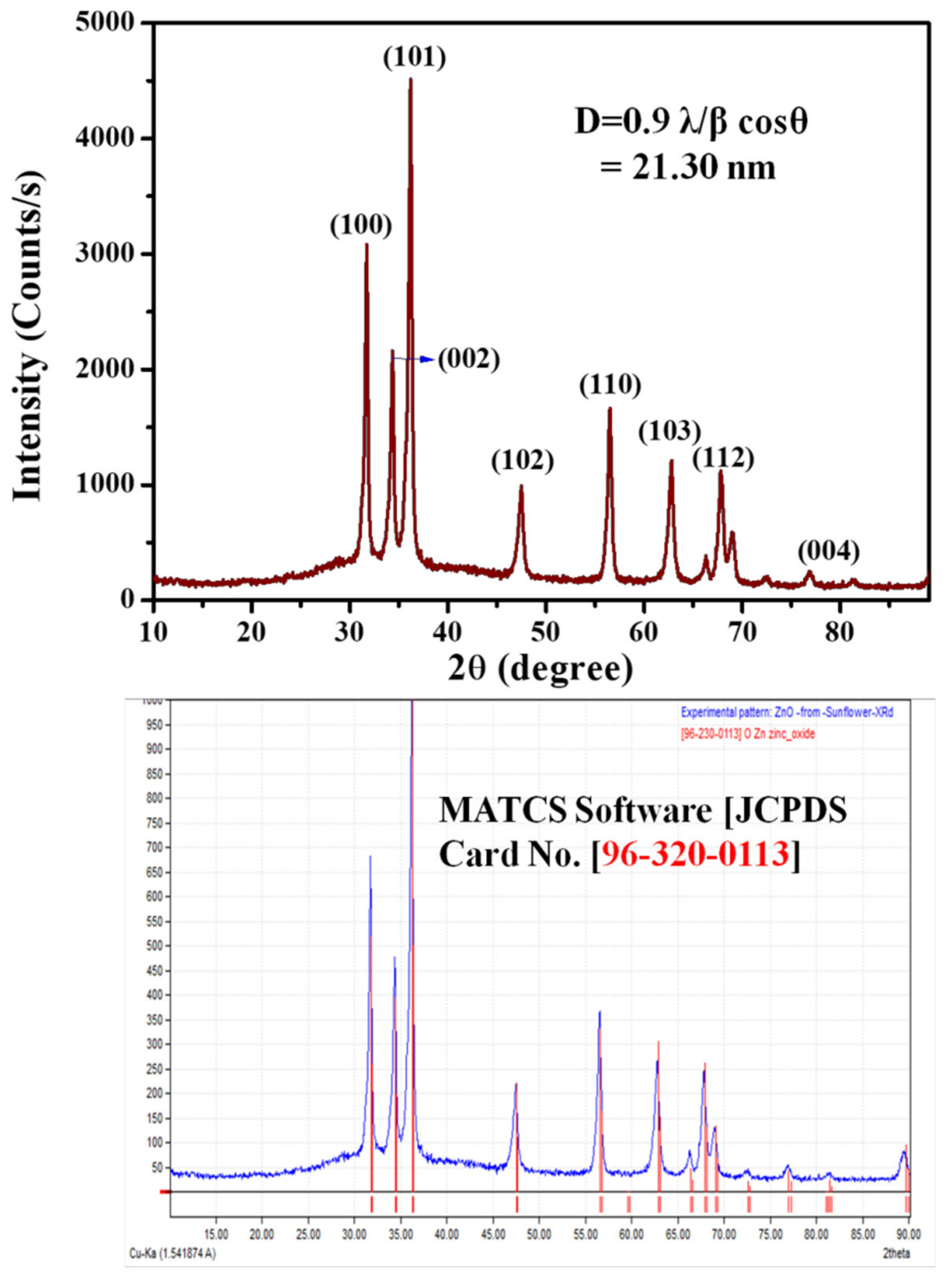
3.1. Catalytic Activity of ZnO Nanoparticles
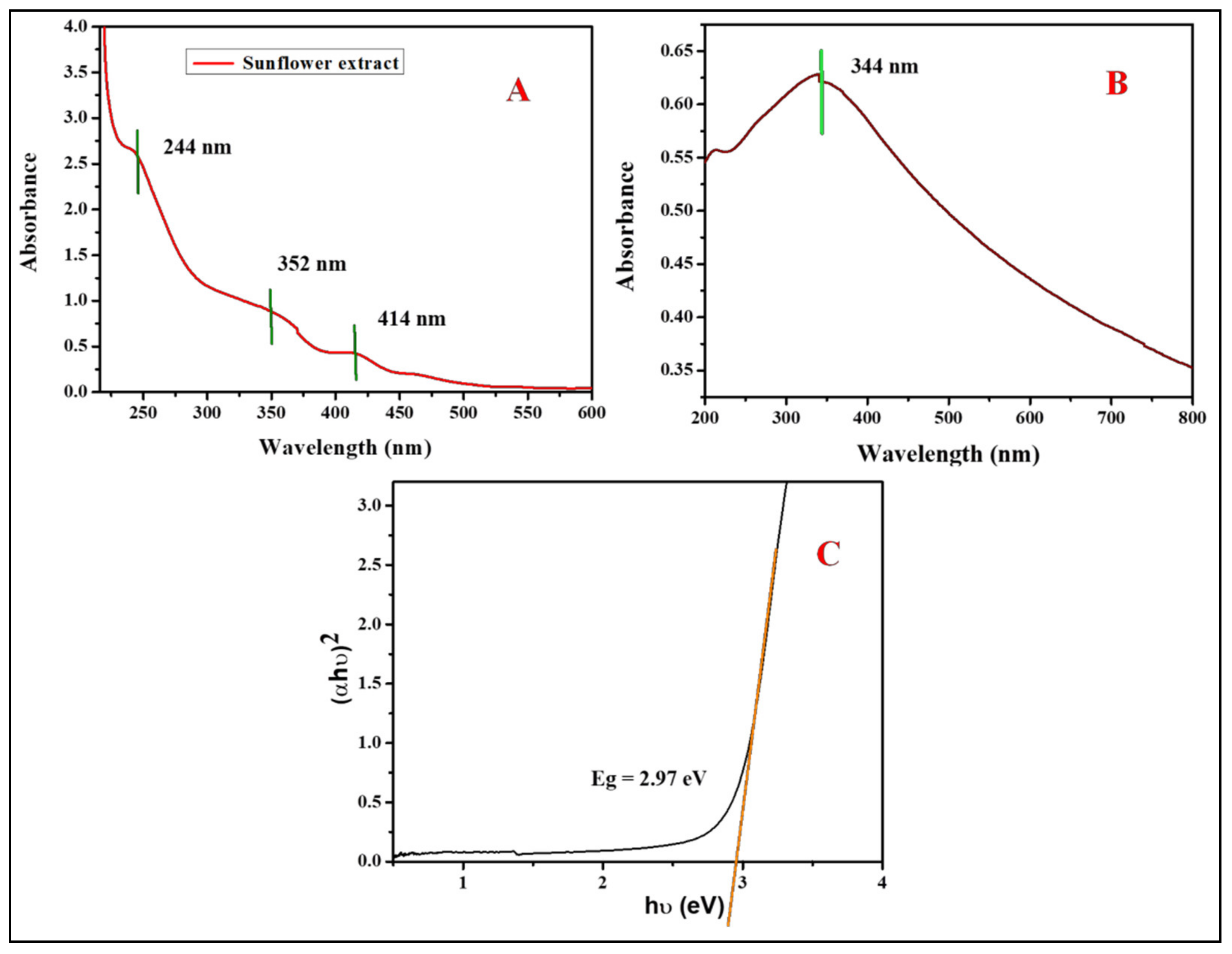
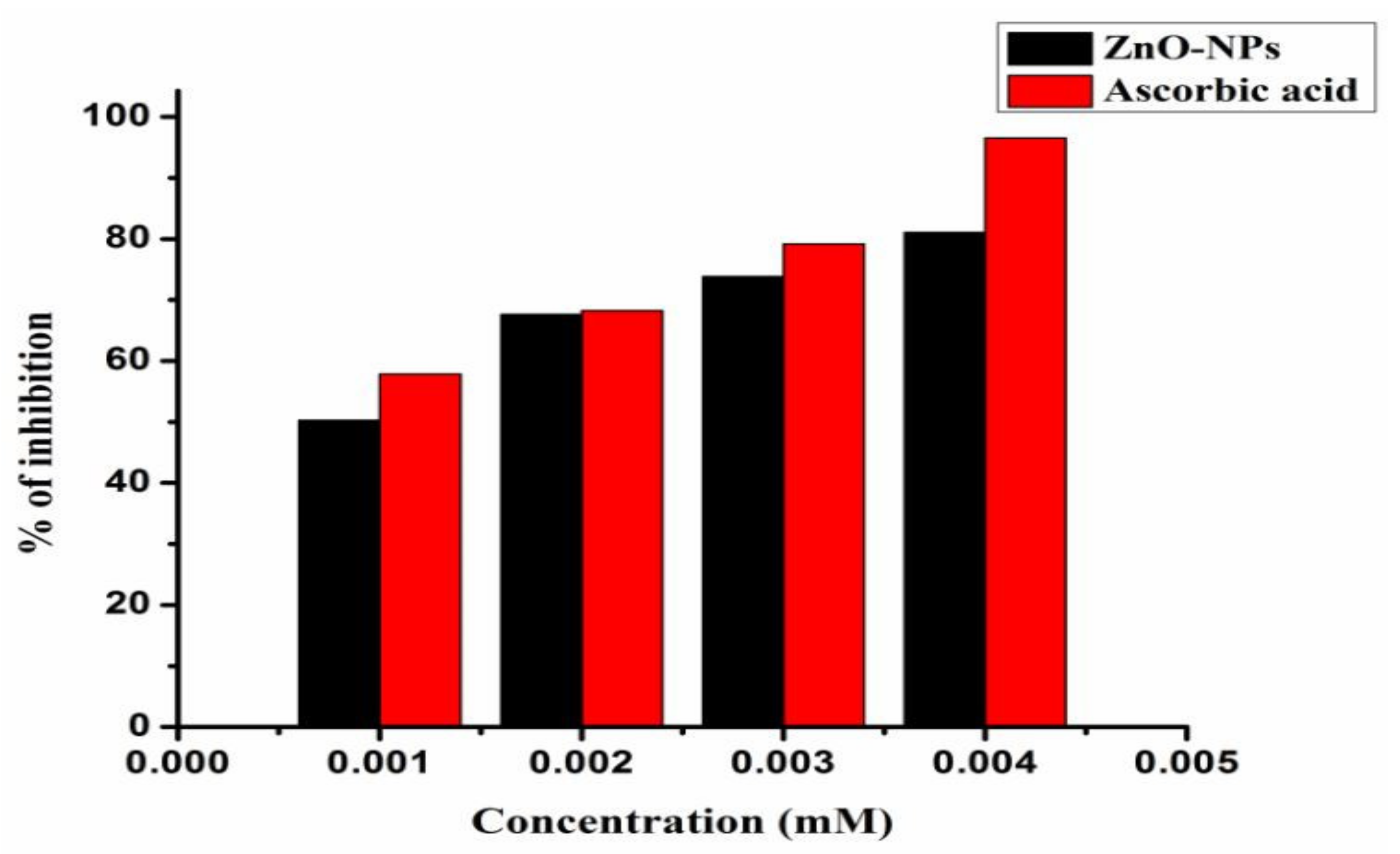
3.2. Antioxidant Activity of ZnO Nanoparticles
3.3. Antioxidant Activity of Synthesized 1,2-Dihydro Quinazolines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verma, R.S. Greener and Sustainable Trends in Synthesis of Organics and Nanomaterials. ACS Sustain. Chem. Eng. 2016, 4, 5866–5878. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, B.; Saha, S.; Chakraborty, M.; Maiti, M.; Das, S.; Basu, R.; Nandy, P. Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: Effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv. 2015, 7, 4993–5003. [Google Scholar]
- Gharpure, S.; Yadwade, R.; Ankamwar, B. Non-antimicrobial and Non-anticancer Properties of ZnO Nanoparticles Biosynthesized Using Different Plant Parts of Bixa orellana. ACS Omega 2022, 7, 1914–1933. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Deng, X.; Haung, J.; Xu, X. Morphology-modulation of SnO2 hierarchical architectures by Zn doping for glycol gas sensing and photo catalytic applications. Sci. Rep. 2015, 5, 7874. [Google Scholar] [CrossRef]
- Narasaiah, B.P.; Mandal, B.K. Remediation of azo-dyes based toxicity by agro-waste cotton boll peels mediated palladium nanoparticles. J. Saudi Chem. Soc. 2020, 24, 267. [Google Scholar] [CrossRef]
- Saraswathi, V.S.; Tatsugi, J.; Shin, P.K.; Santhakumar, K. Facile biosynthesis, characterization, and solar assisted photocatalytic effect of ZnO nanoparticles mediated by leaves of L. speciosa. J. Photochem. Photobiol. B Biol. 2017, 167, 89–98. [Google Scholar] [CrossRef]
- Kumar, K.M.; Mandal, B.K.; Tammina, S.K. Green synthesis of nano platinum using naturally occurring polyphenols. RSC Adv. 2013, 12, 4033–4039. [Google Scholar] [CrossRef]
- Surendra, T.V.; Roopan, S.M.; Arasu, M.V.; Al-dabhi, N.A.; Sridharan, M. Phenolic compounds in drumstick peel for the evaluation of antibacterial, hemolytic and photocatalytic activities. J. Photochem. Photobiol. B Biol. 2016, 161, 463–471. [Google Scholar] [CrossRef]
- Tammina, S.K.; Mandal, B.K.; Rajan, S. Cytotoxicity study of Piper nigrum seed mediated synthesized SnO2 nanoparticles towards colorectal (HCT116) and lung cancer (A549) cell lines. J. Photochem. Photobiol. B Biol. 2016, 166, 158–168. [Google Scholar] [CrossRef]
- Rostamnia, S.; Doustkhah, E. Nanoporous silica-supported organocatalyst: A heterogeneous and green hybrid catalyst for organic transformations. RSC Adv. 2014, 54, 28238–28348. [Google Scholar] [CrossRef]
- Surendra, T.V.; Roopan, S.M.; Arasu, M.V.; Al-dhabhi, N.A.; MokeshRayulu, G. RSM optimized Moringa oleifera peel extract for green synthesis of M. oleifera capped palladium nanoparticles with antibacterial and hemolytic property. J. Photochem. Photobiol. B Biol. 2016, 162, 550–557. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Haung, D.; Chen, X.; Zhu, J.; Sun, D.; Haung, J.; Li, Q. Plant-mediated synthesis of size-controllable Ni nanoparticles with alfalfa extract. Mater. Lett. 2014, 122, 166–169. [Google Scholar] [CrossRef]
- Narasaiah, B.P.; Mandal, B.K.; Sarada, N.C. Synthesis of gold nanoparticles by cottonpeels aqueous extract and their catalyticefficiency for the degradation of dyes andantioxidant activity. IET Nanobiotechnol. 2018, 12, 156–165. [Google Scholar] [CrossRef]
- Yuxin, C.; Anatoly, Z.; Shuzaki, M. Control of a catalytic activity of gold nanoparticles embedded in DNA hydrogel by swelling/shrinking the hydrogel’s matrix. J. Colloid Interface Sci. 2015, 445, 364–370. [Google Scholar]
- Paladini, F.; Pollini, M. Antimicrobial silver nanoparticles for wound healing application: Progress and future trends. Materials 2019, 16, 2540. [Google Scholar] [CrossRef]
- Kahrilas, G.A.; Wally, L.M.; Fedreich, S.J.; Hiskey, M.; Prieto, A.L.; Owens, J.E. Microwave-Assisted Green Synthesis of Silver Nanoparticles Using Orange Peel Extract. ACS Sustain. Chem. Eng. 2014, 2, 367–376. [Google Scholar] [CrossRef]
- Renganathan, S.; Subramaniyan, S.; Karunanithi, N.; Vasanthakumar, P.; Kutzner, A.; Kim, P.S.; Heese, K. Antibacterial, Antifungal, and Antioxidant Activities of Silver Nanoparticles Biosynthesized from Bauhinia tomentosa Linn. Antioxidants 2021, 10, 1959. [Google Scholar] [CrossRef]
- Kirankumar, H.; Mandal, B.K. Activity study of biogenic spherical silver nanoparticles towards microbes and oxidants. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 639–645. [Google Scholar]
- Tietze, L.F. Domino Reactions in Organic Synthesis. Chem. Rev. 1996, 96, 115. [Google Scholar] [CrossRef]
- Bogert, M.T. A Review of some recent investigations in the quinazoline group. J. Am. Chem. Soc. 1910, 32, 784. [Google Scholar] [CrossRef]
- Bedi, P.M.S.; Kumar, V.; Mahajan, M.P. Synthesis and biological activity of novel antibacterial quinazolines. Bioorg. Med. Chem. Lett. 2004, 14, 5211. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, P.; Kumari, P.; Kalal, B.L. Exploration of antimicrobial and antioxidant potential of newly synthesized 2,3-disubstituted quinazoline-4(3H)-ones. Bioorg. Med. Chem. Lett. 2011, 21, 4353. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Mchug, R.J.; Cordova, B.C.; Klabe, R.M.; Vitanen, S.E.; Trainorand, G.L.; Rodgers, G.D. Synthesis and evaluation of quinoxalinones as HIV-1 reverse transcriptase inhibitors. Bioorg. Med. Chem. Lett. 2000, 10, 1729. [Google Scholar] [CrossRef]
- Nandy, P.; Vishalakshi, M.T.; Bhat, A.R. Synthesis and antitubercular activity of Mannich bases of 2-methyl-3H-quinazolin-4-ones. Indian J. Heterocycl. Chem. 2006, 15, 293–294. [Google Scholar]
- Henderson, E.A.; Bavetsias, V.; Theti, D.S.; Wilson, S.C.; Clauss, R.; Jackman, A.L. Targeting the α-folate receptor with cyclopenta[g]quinazoline-based inhibitors of thymidylate synthase. Bioorgan. Med. Chem. 2006, 14, 5020. [Google Scholar] [CrossRef]
- Abuelizz, H.A.; Marzouk, M.; Ghabbour, H.; Al-Salahi, R. Synthesis and anticancer activity of new quinazoline derivatives. Saudi Pharm. J. 2017, 25, 1047–1054. [Google Scholar] [CrossRef]
- Abuelizz, H.A.; El Dib, R.; Marzouk, M.; Anouar, E.H.; Maklad, A.Y.; Attia, H.N.; Al-Salahi, R. Molecular docking and anticonvulsant activity of newly synthesized quinazoline derivatives. Molecules 2017, 22, 1094. [Google Scholar] [CrossRef]
- Abuelizz, H.A.; Anouar, E.H.; Marzouk, M.; Ezzeldin, E.; Ali, A.A.; Al-Salahi, R. Molecular modeling, enzyme activity, anti-inflammatory and antiarthritic activities of newly synthesized quinazoline derivatives. Future Med. Chem. 2017, 9, 1995–2009. [Google Scholar] [CrossRef]
- Balakumar, C.; Lamba, P.; Prankishore, D.; Narayana, B.L.; Venkat Rao, K.; Rajwinder, A.; Raghuram Rao, B.; Sireesha, B.; Narasaiah, B. Synthesis, anti-inflammatory evaluation and docking studies of some new fluorinated fused quinazolines. Eur. J. Med. Chem. 2010, 45, 4904–4913. [Google Scholar] [CrossRef]
- Alafeefy, A.M.; Kadi, A.A.; Deeb, O.A.; Tahir, K.; Jaber, N.A. Synthesis, analgesic and anti-inflammatory evaluation of some novel quinazoline derivatives. Eur. J. Med. Chem. 2010, 45, 4947–4952. [Google Scholar] [CrossRef]
- Ali, M.M.; Ismail, M.M.F.; Gaby, M.S.A.; Zahran, M.A.; Ammar, Y.A. Synthesis and Antimicrobial Activities of Some Novel Quinoxalinone Derivatives. Molecules 2000, 5, 864–873. [Google Scholar] [CrossRef]
- Guillon, J.; Dallemagne, P.; Pfeiffer, B.; Renard, P.; Manechez, D.; Kervran, A.; Rault, S. Synthesis of new pyrrolo[1,2-a]quinoxalines: Potential non-peptide glucagon receptor antagonists. Eur. J. Med. Chem. 1998, 4, 293–308. [Google Scholar] [CrossRef]
- Feng, L.; Yiqing, F.; Qingqing, M.; Wenhua, L.; Zhiming, L.; Quanrui, W.; Fenggang, T. An efficient construction of quinazolin-4(3H)-ones under microwave irradiation. Arkivoc 2007, 1, 40–50. [Google Scholar]
- Yanlong, L.; Yunyun, W.; Huaming, S.; Zunyuan, X.; Weiqiang, Z.; Ziwei, G. Ethanol promoted titanoceneLewis acid catalyzed synthesis of quinazoline derivatives. RSC Adv. 2016, 70, 66074–66077. [Google Scholar]
- Hidemasa, I.; Yukari, S.; Hideharu, Y.; Yuusaku, J. Pd-Catalyzed Benzylic C–H Amidation with Benzyl Alcohols in Water: A Strategy To Construct Quinazolinones. J. Org. Chem. 2012, 77, 7046–7051. [Google Scholar]
- Tian, X.; Song, L.; Li, E.; Wang, Q.; Yu, W.; Chang, J. Metal-free one-pot synthesis of 1,3-diazaheterocyclic compounds via I2-mediated oxidative C–N bond formation. RSC Adv. 2015, 5, 62194–62201. [Google Scholar] [CrossRef]
- Jintang, Z.; Chenmin, Y.; Sujing, W.; Changfeng, W.; Zhiyong, W. A novel and efficient methodology for the construction of quinazolines based on supported copper oxidenanoparticles. Chem. Commun. 2010, 46, 5244–5246. [Google Scholar]
- Han, B.; Wang, C.; Han, R.F.; Yu, W.; Duan, X.Y.; Fang, R.; Yang, X.L. Efficient aerobic oxidative synthesis of 2-aryl quinazolinesviabenzyl C–H bond amination catalyzed by 4-hydroxy-TEMPO. Chem. Commun. 2011, 47, 7818–7820. [Google Scholar] [CrossRef]
- Sumit Kumar, P.; Nidhi, D.; Satyen, S. I2-Catalyzed three-component protocol for the synthesis of quinazolines. Tetrahedron Lett. 2012, 53, 6167–6172. [Google Scholar]
- Chamseddine, D.; Raouf, B.; Gilbert, K.; Bertrand, C.; Abdelmadjid, D. A DMAP-catalyzed mild and efficient synthesis of 1,2-dihydroquinazolines via a one-pot three-component protocol. Tetrahedron Lett. 2014, 55, 200–204. [Google Scholar]
- Kamal, A.; Babu, K.S.; Chandrasekhar, C.; Nagaraju, B.; Sastry, K.V.; Kumar, C.G. Catalyst-free, one pot and three-component synthesis of 4′-phenyl-1′H-spiro[indoline-3,2′-quinazolin]-2-ones and 2,4-diphenyl-1,2-dihydroquinazolines. Tetrahedron Lett. 2015, 56, 6373–6376. [Google Scholar] [CrossRef]
- Liu, L.Y.; Yan, Y.Z.; Bao, Y.J.; Wang, Z.Y. Efficient synthesis of 2-arylquinazolines via copper-catalyzed dual oxidative benzylic C–H aminations of methylarenes. Chin. Chem. Lett. 2015, 26, 1216–1220. [Google Scholar] [CrossRef]
- Sarma, R.; Dipak, P. Microwave-promoted efficient synthesis of dihydroquinazolines. Green Chem. 2011, 13, 718–722. [Google Scholar] [CrossRef]
- Chaudar, C.; Hakim Siddiki, S.M.A.; Masazumi, T.; Ken-ichi, S. Acceptorless dehydrogenative synthesis of 2-substituted quinazolines from 2-aminobenzylamine with primary alcohols or aldehydes by heterogeneous Pt catalysts. RSC Adv. 2014, 4, 53374–53379. [Google Scholar] [CrossRef]
- Karnakar, K.; Kumar, A.V.; Murthy, S.N.; Ramesh, K.; Nageswar, Y.V.D. Recyclable graphite oxide promoted efficient synthesis of 2-phenyl quinazoline derivatives in the presence of TBHP as an oxidant. Tetrahedron Lett. 2012, 53, 4613–4617. [Google Scholar] [CrossRef]
- Subashini, R.; Gangadhara, A.; Aggile, K.; Nawaz Khan, F. Microwave-assisted solid acid-catalyzed synthesis of quinolinyl quinolinones and evaluation of their antibacterial, antioxidant activities. Res. Chem. Intermed. 2015, 41, 4899–4912. [Google Scholar] [CrossRef]
- Abid, M.F.; Zablouk, M.A.; Abid-Alameer, A.M. Experimental study of dye removal from industrial wastewater by membrane technologies of reverse osmosis and nano filtration. J. Environ. Health Sci. Eng. 2012, 9, 17. [Google Scholar]
- Sutradhar, P.; Saha, M. Synthesis of zinc oxide nanoparticles using tea leaf extract and its application for solar cell. Bull. Mater. Sci. 2015, 38, 653–657. [Google Scholar] [CrossRef]
- Morales, A.E.; Mora, E.S.; Pal, U. Use of diffuse reflectance spectroscopy for optical characterization of un-supported nanostructures. Revista Mexicana Física 2007, 53, 18–22. [Google Scholar]
- Salim, R.M.; Asik, J.; Sarjadi, M.S. Chemical functional groups of extractives, cellulose and lignin extracted from native Leucaena leucocephala bark. Wood Sci. Technol. 2021, 55, 295–313. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z. Facile Synthesis of Quasi Spherical ZnO Nanoparticles with Excellent Photocatalytic Activity. J. Clust. Sci. 2015, 26, 1187–1201. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Anjum, S.; Hano, C. Differential effects of in vitro cultures of Linum usitatissimum L. (Flax) on biosynthesis, stability, antibacterial and antileishmanial activities of zinc oxide nanoparticles: A mechanistic approach. RSC Adv. 2017, 7, 15931–15943. [Google Scholar] [CrossRef]
- Sangeetha, G.; Rajeswari, S.; Venkatesh, R. Green synthesis of zinc oxide nanoparticles by Aloe barbadensis Miller leaf extract: Structure and optical properties. Mater. Res. Bull. 2011, 46, 2560–2566. [Google Scholar] [CrossRef]
- Suganthi, K.S.; Rajan, K.S. Temperature induced changes in ZnO–water nanofluid: Zeta potential, size distribution and viscosity profiles. Int. J. Heat Mass Transf. 2012, 55, 7969–7980. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Nada, A.A.; Hassabo, A.G.; Eid, B.M.; Azza, M.; Deen, N.E.; Abou-Zeid, N.Y. Effect of different capping agents on physicochemical and antimicrobial properties of ZnO nanoparticles. Chem. Pap. 2017, 71, 1365. [Google Scholar] [CrossRef]
- Narasaiah, B.P.; Mandal, B.K. Waste to wealth: A solution to textile dyes related pollution. Mater. Res. Express 2020, 7, 024001. [Google Scholar] [CrossRef]

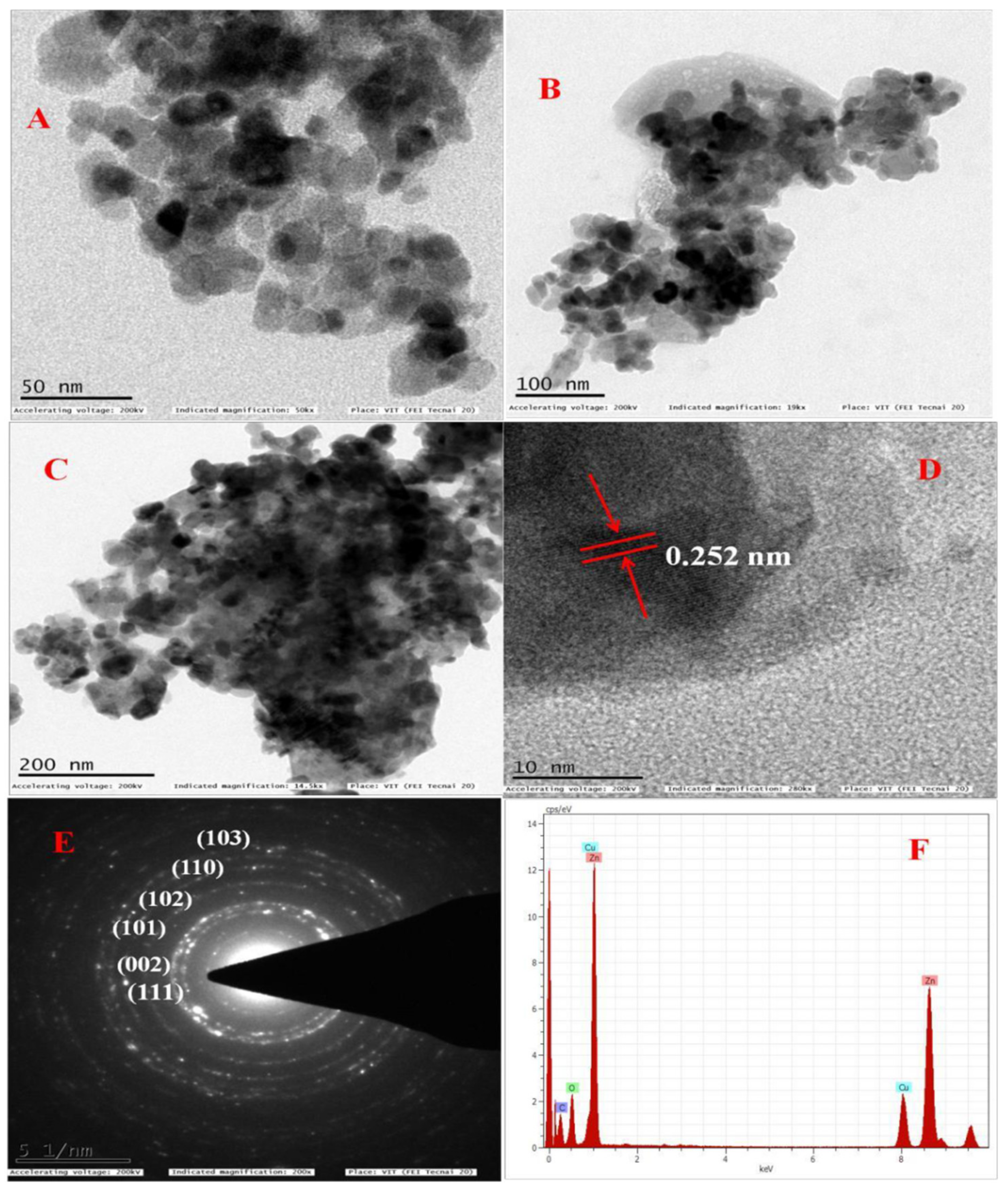

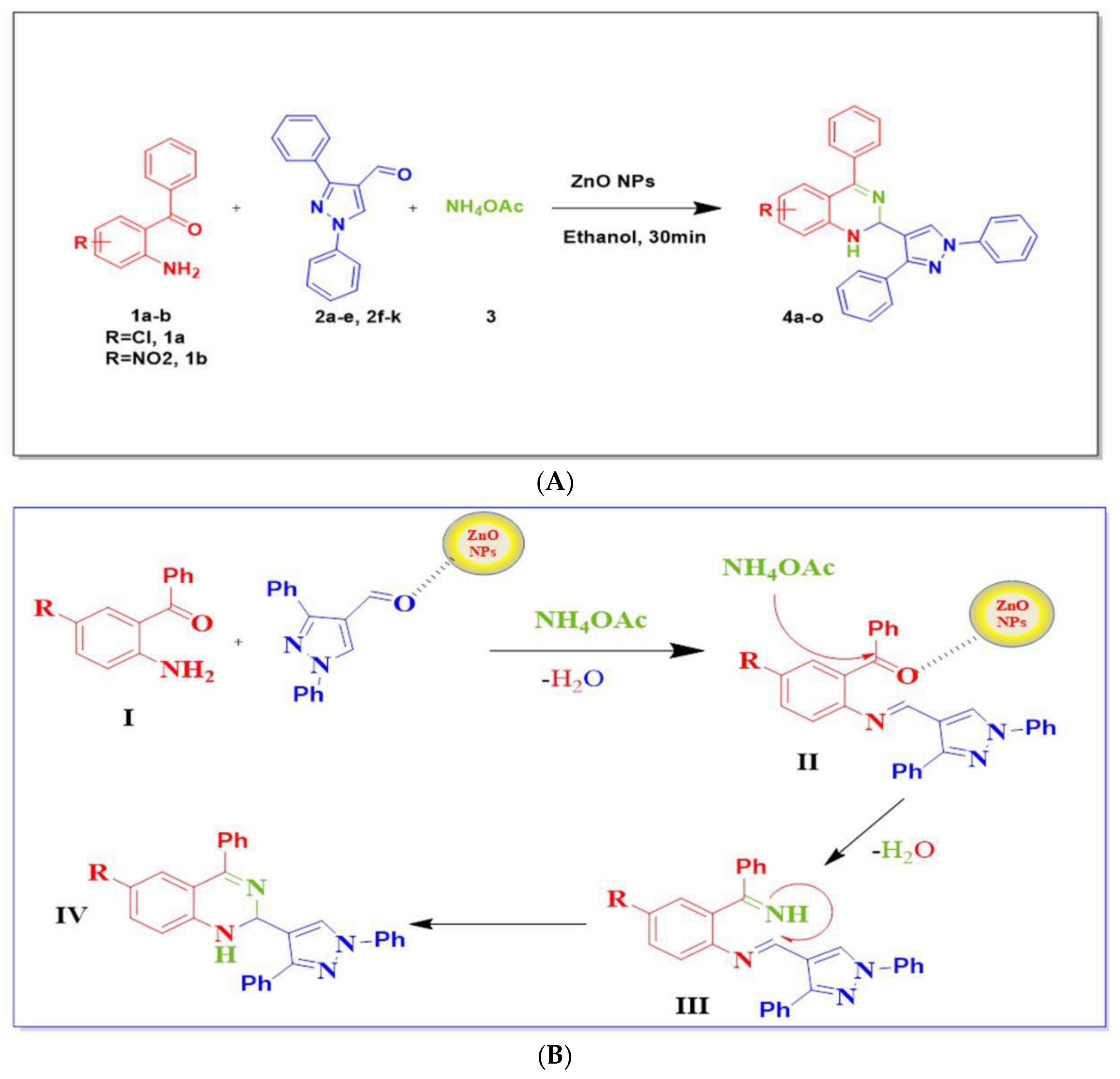
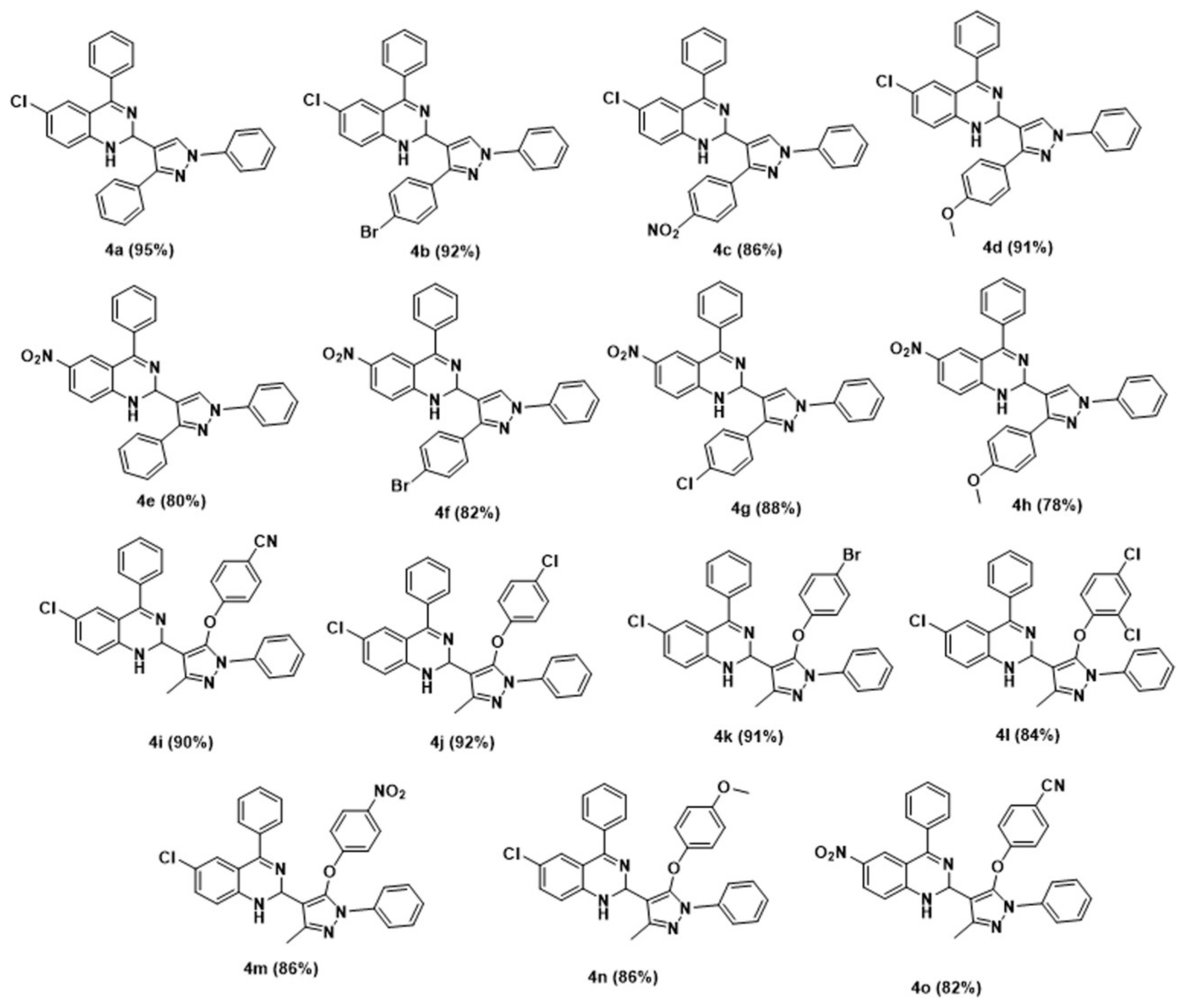

| Entry | Catalyst | Catalyst Loading (Mol%) | Yield (%) 4a |
|---|---|---|---|
| 1 | ZnO-NPs | 05 | 90 |
| 2 | ZnO-NPs | 10 | 95 |
| 3 | ZnO-NPs | 15 | 94 |
| 4 | ZnO-NPs | 20 | 90 |
| 2 Theta | FWHM Value | d-Spacing (Å) | Cos (θ) | Crystalline Size (nm) |
|---|---|---|---|---|
| 31.61 | 0.35 | 2.83 | 0.96221 | 24.65 |
| 34.23 | 0.38 | 2.61 | 0.95574 | 22.86 |
| 36.16 | 0.42 | 2.48 | 0.91262 | 20.79 |
| 47.61 | 0.53 | 1.90 | 0.95062 | 17.12 |
| 56.64 | 0.48 | 1.62 | 0.88031 | 19.65 |
| 62.83 | 0.55 | 1.47 | 0.85345 | 17.69 |
| Average size | 20.46 | |||
| Entry | Catalyst | Catalyst Loading (Mol %) | Solvent | Temperature (°C) | Time (h) | Yield (%) 4a |
|---|---|---|---|---|---|---|
| 1 | --- | --- | Ethanol | RT | 12 | |
| 2 | --- | --- | Ethanol | Reflux | 6 | 20 |
| 3 | Piperidine | 20 | Ethanol | 50 | 5 | 53 |
| 4 | DMAP | 20 | Ethanol | Reflux | 6 | 62 |
| 5 | TEA | 25 | Ethanol | Reflux | 5 | 60 |
| 6 | KOH | 20 | Ethanol | Reflux | 4 | 62 |
| 7 | AIBN | 20 | Ethanol | Reflux | 6 | 24 |
| 8 | PTSA | 20 | Ethanol | Reflux | 4 | 76 |
| 9 | H3PO4 | 20 | Ethanol | 60 | 6 | 72 |
| 10 | MK-10 | 20 | Ethanol | Reflux | 5 | 78 |
| 11 | ZnO-NPs | 10 | Ethanol | Reflux | 0.5 | 95 |
| 12 | ZnO-NPs | 10 | Methanol | Reflux | 1 | 91 |
| 13 | ZnO-NPs | 10 | DMF | Reflux | 2 | 72 |
| 14 | ZnO-NPs | 10 | Ethyl acetate | 60 | 2 | 79 |
| 15 | ZnO-NPs | 10 | Water | Reflux | 3 | 82 |
| Percentage of Inhibition at Different Concentrations (mM) | ||||||
|---|---|---|---|---|---|---|
| Entry | Compound | 0.001 | 0.002 | 0.003 | 0.004 | IC50 |
| 1 | 4a | 50.51 | 64.81 | 77.57 | 82.98 | 0.837 |
| 2 | 4b | 51.15 | 64.04 | 77.06 | 81.18 | 0.794 |
| 3 | 4c | 51.54 | 60.56 | 68.81 | 75.25 | 0.740 |
| 4 | 4d | 51.80 | 58.37 | 67.91 | 76.28 | 0.870 |
| 5 | 4e | 53.35 | 64.43 | 74.35 | 80.67 | 0.546 |
| 6 | 4f | 49.74 | 67.91 | 75.25 | 84.66 | 0.842 |
| 7 | 4g | 52.44 | 61.21 | 69.58 | 77.70 | 0.690 |
| 8 | 4h | 50.77 | 60.30 | 71.77 | 83.37 | 0.988 |
| 9 | 4i | 51.80 | 58.24 | 70.23 | 76.80 | 0.885 |
| 10 | 4j | 50.25 | 61.08 | 68.29 | 73.71 | 0.823 |
| 11 | 4k | 49.61 | 60.18 | 68.81 | 77.57 | 0.985 |
| 12 | 4l | 52.44 | 65.97 | 67.26 | 86.08 | 0.902 |
| 13 | 4m | 53.09 | 63.40 | 74.48 | 72.16 | 0.596 |
| 14 | 4n | 51.93 | 61.46 | 71.26 | 68.81 | 0.715 |
| 15 | 4o | 50.38 | 59.79 | 68.94 | 78.22 | 0.953 |
| 16 | ZnO NPs | 50.29 | 67.63 | 73.84 | 81.04 | 0.769 |
| 17 | Ascorbic acid | 57.98 | 64.94 | 75.12 | 90.59 | 0.511 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
S., M.; Narasaiah, B.P.; B., H.; G. L., B.; Pradeepkiran, J.A.; Padhy, H. Sunflower-Assisted Bio-Derived ZnO-NPs as an Efficient Nanocatalyst for the Synthesis of Novel Quinazolines with Highly Antioxidant Activities. Antioxidants 2022, 11, 688. https://doi.org/10.3390/antiox11040688
S. M, Narasaiah BP, B. H, G. L. B, Pradeepkiran JA, Padhy H. Sunflower-Assisted Bio-Derived ZnO-NPs as an Efficient Nanocatalyst for the Synthesis of Novel Quinazolines with Highly Antioxidant Activities. Antioxidants. 2022; 11(4):688. https://doi.org/10.3390/antiox11040688
Chicago/Turabian StyleS., Mahesh, Boya Palajonnala Narasaiah, Himabindu B., Balaji G. L., Jangampalli Adi Pradeepkiran, and Harihara Padhy. 2022. "Sunflower-Assisted Bio-Derived ZnO-NPs as an Efficient Nanocatalyst for the Synthesis of Novel Quinazolines with Highly Antioxidant Activities" Antioxidants 11, no. 4: 688. https://doi.org/10.3390/antiox11040688
APA StyleS., M., Narasaiah, B. P., B., H., G. L., B., Pradeepkiran, J. A., & Padhy, H. (2022). Sunflower-Assisted Bio-Derived ZnO-NPs as an Efficient Nanocatalyst for the Synthesis of Novel Quinazolines with Highly Antioxidant Activities. Antioxidants, 11(4), 688. https://doi.org/10.3390/antiox11040688






