In Vitro Anticancer Activity of Mucoadhesive Oral Films Loaded with Usnea barbata (L.) F. H. Wigg Dry Acetone Extract, with Potential Applications in Oral Squamous Cell Carcinoma Complementary Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lichen Extract
2.3. Formulation and Manufacturing of Mucoadhesive Oral Films
2.4. Physico-Chemical Characterization of the UBA-Loaded Mucoadhesive Oral Films
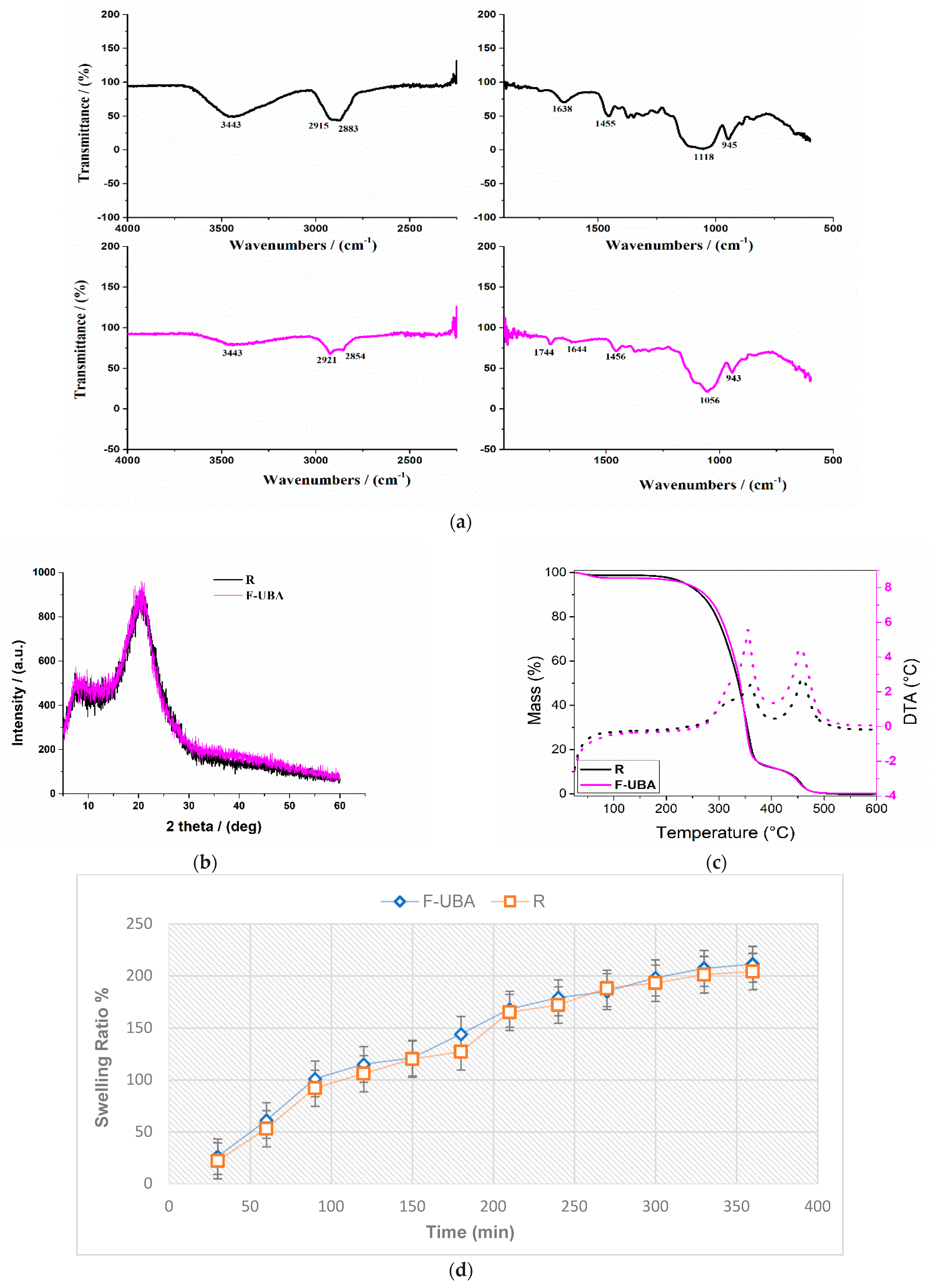
2.4.1. Fourier Transform Infrared Spectroscopy
2.4.2. Powder X-ray Diffractometry
2.4.3. TG/DTA Measurements
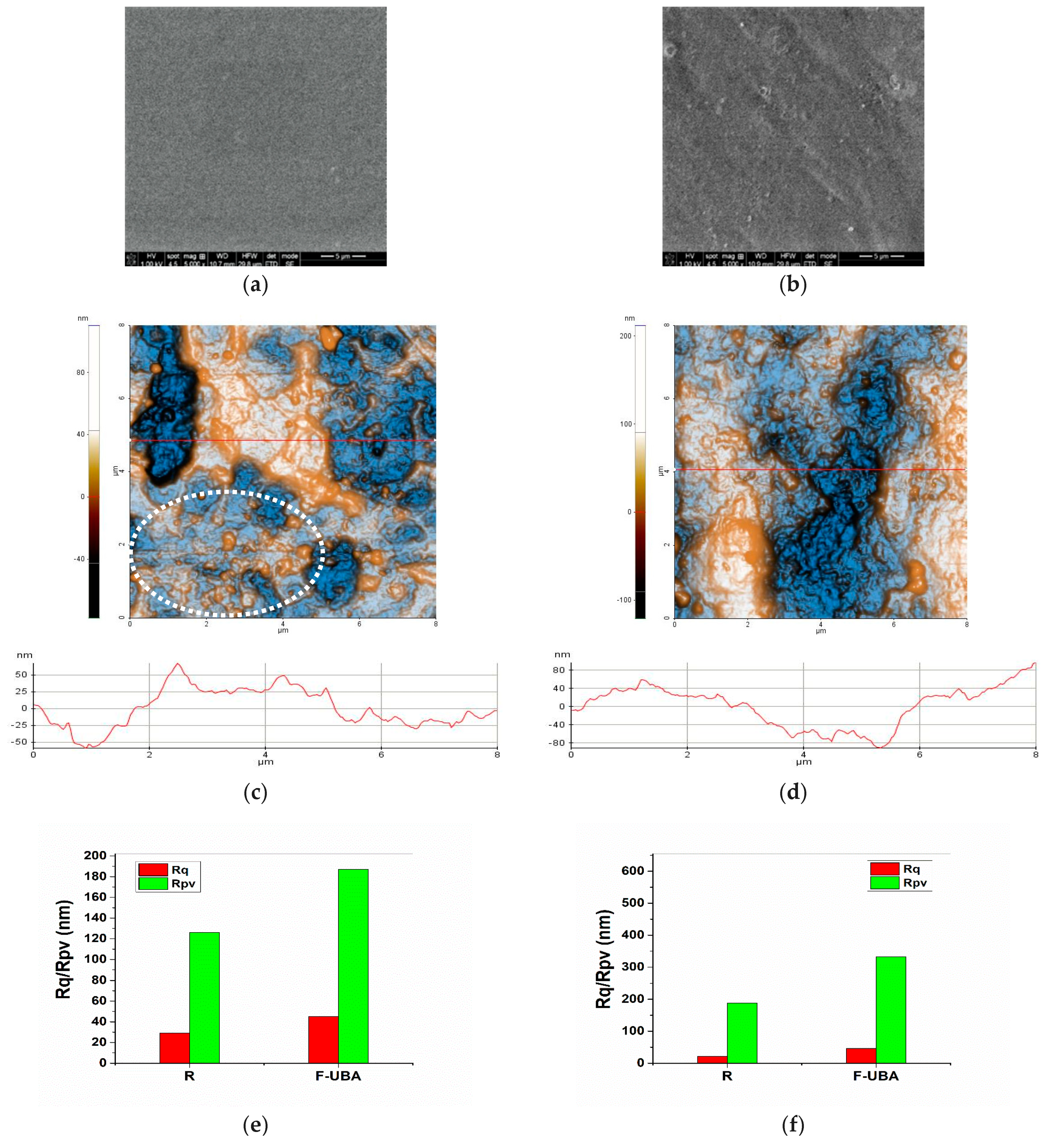
2.4.4. Scanning Electron Microscopy (SEM)
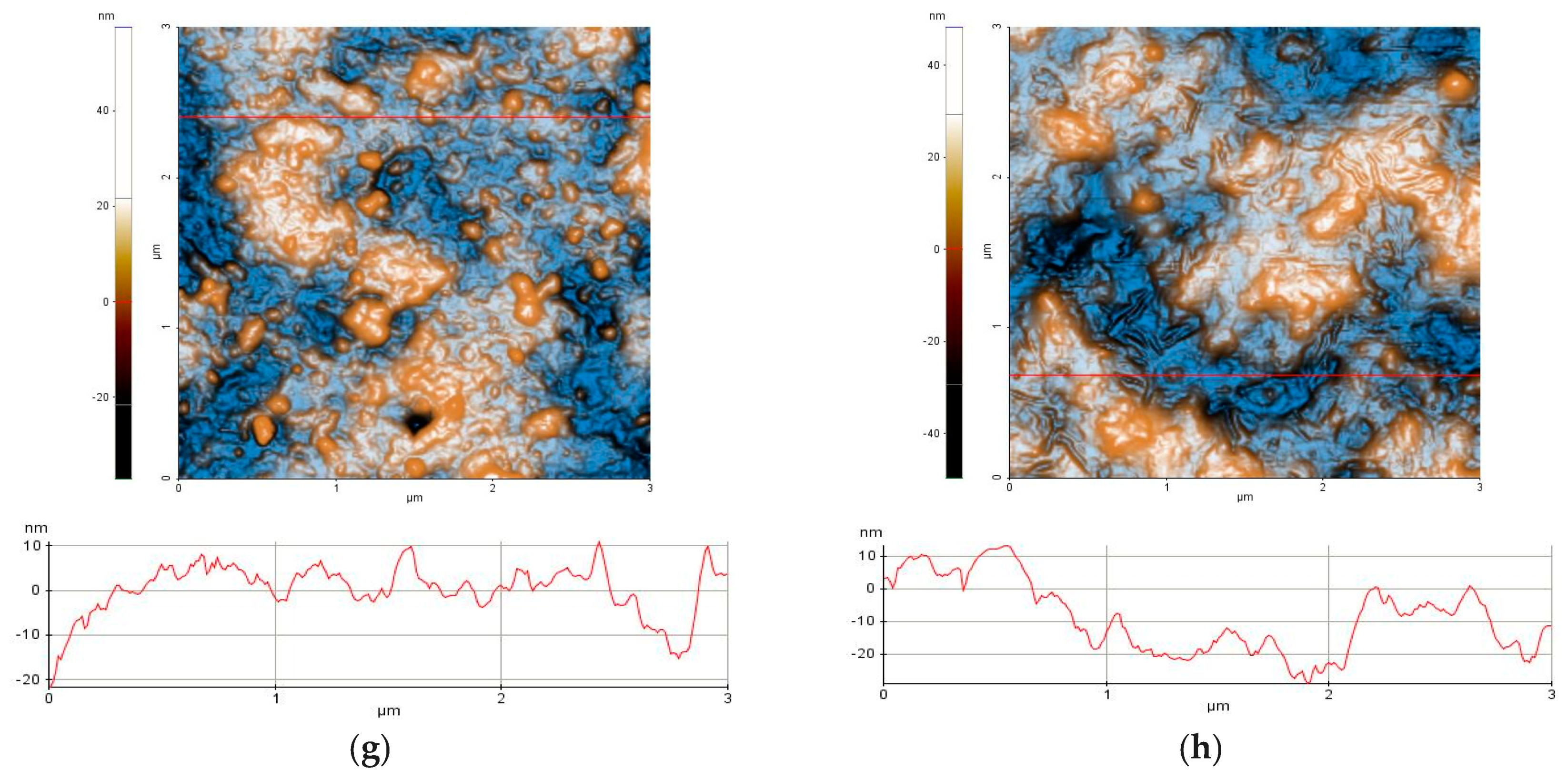
2.4.5. Atomic Force Microscopy (AFM)
2.5. Pharmacotechnical Analysis of the UBA-Loaded Mucoadhesive Oral Films
2.5.1. Weight Uniformity
2.5.2. Thickness
2.5.3. Folding Endurance
2.5.4. Tensile Strength and Elongation Ability
2.5.5. Moisture Content
2.5.6. Surface pH Value
2.5.7. In Vitro Disintegration Time
2.5.8. Swelling Ratio
2.5.9. Ex Vivo Mucoadhesion Time
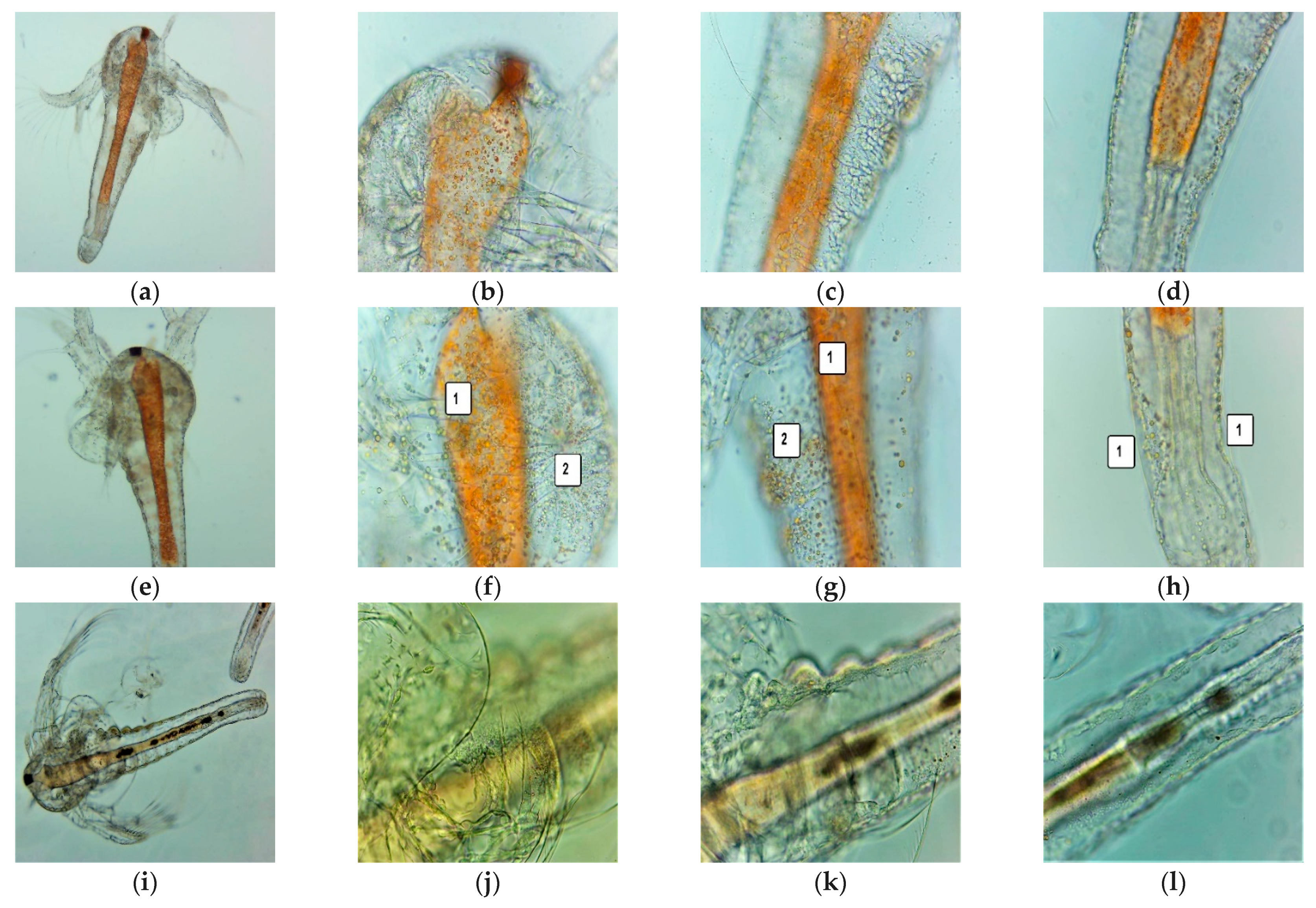
2.6. Evaluation of the Cytotoxic Activity of UBA-Loaded Mucoadhesive Oral Films on A. salina Larvae
2.6.1. Sample Preparation
2.6.2. BSL Assay
2.6.3. Fluorescent Microscopy
2.6.4. Data Processing
2.7. In Vitro Analysis of the Effects of UBA-Loaded Mucoadhesive Oral Films on Human Normal Blood Cells and OSCC CLS-354 Cell Line
2.7.1. Equipment
2.7.2. Data Processing
2.7.3. Human Blood Cells Cultures
2.7.4. CLS-354 Cell Line, Cells Culture
2.7.5. Samples and Control Solutions
2.8. Evaluation of Total ROS Activity
2.9. Evaluation of Caspase 3/7 Activity
2.10. Evaluation of Nuclear Condensation and Lysosomal Activity
2.11. Cell Cycle Analysis
2.12. Annexin V-FITC Apoptosis Assay
2.13. Evaluation of Cell Proliferation
2.14. Antimicrobial Activity Evaluation by Resazurin-Based 96-Well Plate Microdilution Method
2.14.1. Inoculum Preparation
2.14.2. Samples and Standards
2.14.3. Resazurin-Based 96-Well Plate Microdilution Method
2.14.4. Reading and Interpreting
2.15. Data Analysis
3. Results
3.1. Physico-Chemical Characterization of the UBA-Loaded Mucoadhesive Oral Films
3.1.1. FTIR Spectra
3.1.2. XRD Analysis
3.1.3. TG/DTA Measurements
3.1.4. SEM Analysis
3.1.5. AFM Measurements
3.2. Pharmacotechnical Characterization of UBA-Loaded Mucoadhesive Oral Films
3.3. BSL Assay
3.4. In Vitro Analysis of the Effects of UBA-Loaded Mucoadhesive Oral Films on Human Normal Blood Cells and OSCC CLS-354 Cell Line
3.4.1. ROS Levels
3.4.2. Caspase 3/7 Activity
3.4.3. Nuclear Shrinkage and Autophagy
3.4.4. Cell Cycle Analysis
3.4.5. Apoptosis
3.4.6. Cell Proliferation
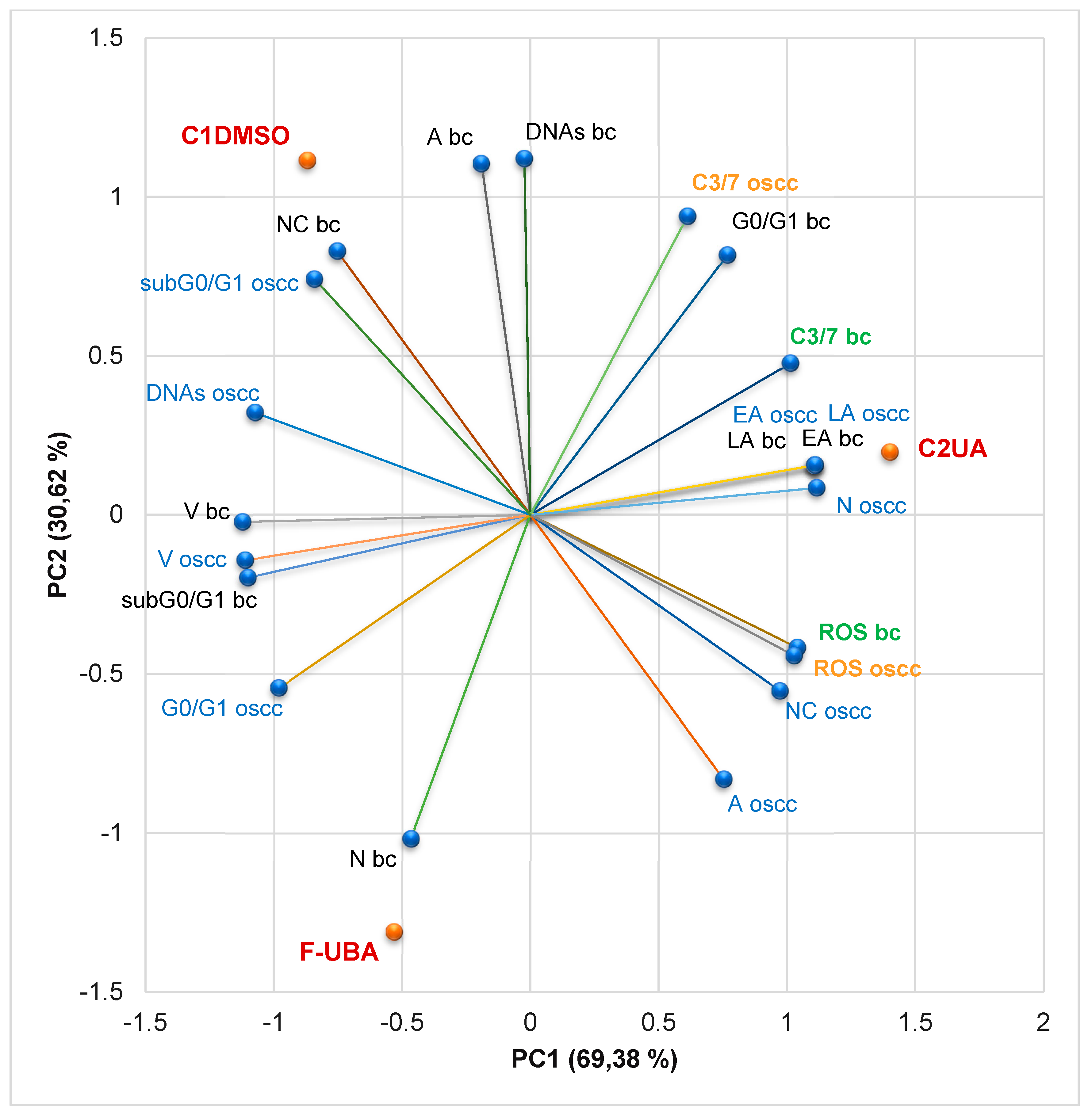
3.4.7. Principal Component Analysis
3.5. Antimicrobial Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, M.; Nanavati, R.; Modi, T.; Dobariya, C. Oral cancer: Etiology and risk factors: A review. J. Cancer Res. Ther. 2016, 12, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Proia, N.K.; Paszkiewicz, G.M.; Nasca, M.A.S.; Franke, G.E.; Pauly, J.L. Smoking and smokeless tobacco-associated human buccal cell mutations and their association with oral cancer-A review. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1061–1077. [Google Scholar] [CrossRef] [PubMed]
- Madathil, S.A.; Rousseau, M.C.; Wynant, W.; Schlecht, N.F.; Netuveli, G.; Franco, E.L.; Nicolau, B. Nonlinear association between betel quid chewing and oral cancer: Implications for prevention. Oral Oncol. 2016, 60, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S. Living with oral cancer: Epidemiology with particular reference to prevalence and life-style changes that influence survival. Oral Oncol. 2010, 46, 407–410. [Google Scholar] [CrossRef]
- Lee, Y.C.A.; Li, S.; Chen, Y.; Li, Q.; Chen, C.J.; Hsu, W.L.; Lou, P.J.; Zhu, C.; Pan, J.; Shen, H.; et al. Tobacco smoking, alcohol drinking, betel quid chewing, and the risk of head and neck cancer in an East Asian population. Head Neck 2019, 41, 92–102. [Google Scholar] [CrossRef]
- Irani, S. Soussan Irani New Insights into Oral Cancer—Risk Factors and Prevention: A Review of Literature. Int. J. Prev. Med. 2020, 11, 202. [Google Scholar] [CrossRef]
- Migueláñez-Medrán, B.D.C.; Pozo-Kreilinger, J.J.; Cebrián-Carretero, J.L.; Martínez-García, M.Á.; López-Sánchez, A.F. Oral squamous cell carcinoma of tongue: Histological risk assessment. A pilot study. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e603–e609. [Google Scholar] [CrossRef]
- Lin, N.C.; Hsien, S.I.; Hsu, J.T.; Chen, M.Y.C. Impact on patients with oral squamous cell carcinoma in different anatomical subsites: A single-center study in Taiwan. Sci. Rep. 2021, 11, 15446. [Google Scholar] [CrossRef]
- Russo, D.; Mariani, P.; Caponio, V.C.A.; Lo Russo, L.; Fiorillo, L.; Zhurakivska, K.; Lo Muzio, L.; Laino, L.; Troiano, G. Development and validation of prognostic models for oral squamous cell carcinoma: A systematic review and appraisal of the literature. Cancers 2021, 13, 5755. [Google Scholar] [CrossRef]
- PDQ® Supportive and Palliative Care Editorial Board. PDQ Oral Complications of Chemotherapy and Head/Neck Radiation. Bethesda, MD: National Cancer Institute. Updated 2022. Available online: https://www.cancer.gov/about-cancer/treatment/side-effects/mouth-throat/oral-complications-hp-pdq (accessed on 6 September 2022).
- PDQ® Adult Treatment Editorial Board. PDQ Lip and Oral Cavity Cancer Treatment (Adult). Bethesda, MD: National Cancer Institute. Updated 2022. Available online: https://www.cancer.gov/types/head-and-neck/hp/adult/lip-mouth-treatment-pdq (accessed on 6 September 2022).
- Morioka, R.; Matsuda, Y.; Kato, A.; Okui, T.; Okuma, S.; Tatsumi, H. Oral functional impairment may cause malnutrition following oral cancer treatment in a single - center cross - sectional study. Sci. Rep. 2022, 12, 14787. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Kumar, M.; Kumari, N.; Thakur, M.; Rathour, S.; Pundir, A.; Sharma, A.K.; Bangar, S.P.; Dhumal, S.; Singh, S.; et al. Plant-based antioxidant extracts and compounds in the management of oral cancer. Antioxidants 2021, 10, 1358. [Google Scholar] [CrossRef] [PubMed]
- Guran, K.; Buzatu, R.; Pinzaru, I.; Boruga, M.; Marcovici, I.; Coricovac, D.; Avram, S.; Poenaru, M.; Susan, M.; Susan, R.; et al. In vitro pharmaco-toxicological characterization of melissa officinalis total extract using oral, pharynx and colorectal carcinoma cell lines. Processes 2021, 9, 850. [Google Scholar] [CrossRef]
- Yeh, C.C.; Yang, J.I.; Lee, J.C.; Tseng, C.N.; Chan, Y.C.; Hseu, Y.C.; Tang, J.Y.; Chuang, L.Y.; Huang, H.W.; Chang, F.R.; et al. Anti-proliferative effect of methanolic extract of Gracilaria tenuistipitata on oral cancer cells involves apoptosis, DNA damage, and oxidative stress. BMC Complement. Altern. Med. 2012, 12, 142. [Google Scholar] [CrossRef]
- Hassabou, N.F.; Farag, A.F. Anticancer effects induced by artichoke extract in oral squamous carcinoma cell lines. J. Egypt. Natl. Canc. Inst. 2020, 32, 17. [Google Scholar] [CrossRef]
- Keshava, R.; Muniyappa, N.; Gope, R.; Ramaswamaiah, A.S. Anti-cancer effects of Imperata cylindrica leaf extract on human oral squamous carcinoma cell line SCC-9 in vitro. Asian Pac. J. Cancer Prev. 2016, 17, 1891–1898. [Google Scholar] [CrossRef]
- KIM, J.H.; Hyun, J.W.; Kim, Y.G. Anticancer Effects of Natural Medicinal Plant Extracts on Oral Carcinoma Cells. Biomol. Ther. 1999, 7, 153–157. [Google Scholar]
- Joo, M.; Heo, J.B.; Kim, S.; Kim, N.; Jeon, H.J.; An, Y.; Song, G.Y.; Kim, J.M.; Lee, H.J. Decursin inhibits tumor progression in head and neck squamous cell carcinoma by downregulating CXCR7 expression in vitro. Oncol. Rep. 2022, 47, 39. [Google Scholar] [CrossRef]
- Di Prima, G.; Conigliaro, A.; De Caro, V. Mucoadhesive Polymeric Films to Enhance Barbaloin Penetration into Buccal Mucosa: A Novel Approach to Chemoprevention. AAPS PharmSciTech 2019, 20, 1–12. [Google Scholar] [CrossRef]
- Zhang, Q.; Alyami, N.M.; Alyami, H.M. Influence of Padina gymnospora on Apoptotic Proteins of Oral Cancer Cells—A Proteome - Wide Analysis. Appl. Biochem. Biotechnol. 2022, 1–18. [Google Scholar] [CrossRef]
- Tang, J.Y.; Wu, K.H.; Wang, Y.Y.; Farooqi, A.A.; Huang, H.W.; Yuan, S.S.F.; Jian, R.I.; Tsao, L.Y.; Chen, P.A.; Chang, F.R.; et al. Methanol extract of usnea barbata induces cell killing, apoptosis, and dna damage against oral cancer cells through oxidative stress. Antioxidants 2020, 9, 694. [Google Scholar] [CrossRef] [PubMed]
- Popovici, V.; Bucur, L.A.; Schröder, V.; Gherghel, D.; Mihai, C.T.; Caraiane, A.; Badea, F.C.; Vochița, G.; Badea, V. Evaluation of the cytotoxic activity of the Usnea barbata (L.) F. H. Wigg dry extract. Molecules 2020, 25, 1865. [Google Scholar] [CrossRef]
- Popovici, V.; Bucur, L.; Vochita, G.; Gherghel, D.; Mihai, C.T.; Rambu, D.; Calcan, S.I.; Costache, T.; Cucolea, I.E.; Matei, E.; et al. In vitro anticancer activity and oxidative stress biomarkers status determined by usnea barbata (L.) f.h. wigg. dry extracts. Antioxidants 2021, 10, 1141. [Google Scholar] [CrossRef] [PubMed]
- Pérez Zamora, C.M.; Michaluk, A.G.; Chiappetta, D.A.; Nuñez, M.B. Herbal buccal films with in vitro antibacterial and anti-inflammatory effects. J. Herb. Med. 2022, 31, 100527. [Google Scholar] [CrossRef]
- Pagano, C.; Luzi, F.; Ricci, M.; Di Michele, A.; Puglia, D.; Ceccarini, M.R.; Beccari, T.; Blasi, F.; Cossignani, L.; Schoubben, A.; et al. Wound Dressing: Combination of Acacia Gum/PVP/Cyclic Dextrin in Bioadhesive Patches Loaded with Grape Seed Extract. Pharmaceutics 2022, 14, 485. [Google Scholar] [CrossRef] [PubMed]
- Utama-ang, N.; Sida, S.; Wanachantararak, P.; Kawee-ai, A. Development of edible Thai rice film fortified with ginger extract using microwave-assisted extraction for oral antimicrobial properties. Sci. Rep. 2021, 11, 1–10. [Google Scholar]
- Manikiran, S.S.; Priya, N.S.; Molly, B.A.; Nori, L.P. Fabrication and characterization of fast dissolving films of eclipta prostrate leaves extract to treat mouth ulcers. Int. J. Appl. Pharm. 2021, 13, 263–271. [Google Scholar]
- Burgess, J.; Van Der Ven, P.D.; Martin, M.; Sherman, J.; Haley, J. Review of over-the-counter treatments for aphthous ulceration and results from use of a dissolving oral patch containing glycyrrhiza complex herbal extract. J. Contemp. Dent. Pract. 2008, 9, 088–098. [Google Scholar] [CrossRef]
- Hashemi, M.; Ramezani, V.; Seyedabadi, M.; Ranjbar, A.M.; Jafari, H.; Honarvar, M.; Fanaei, H. Formulation and optimization of oral mucoadhesive patches of myrtus communis by box behnken design. Adv. Pharm. Bull. 2017, 7, 441–450. [Google Scholar] [CrossRef]
- Nam, S.; Lee, J.J.; Lee, S.Y.; Jeong, J.Y.; Kang, W.S.; Cho, H.J. Angelica gigas Nakai extract-loaded fast-dissolving nanofiber based on poly(vinyl alcohol) and Soluplus for oral cancer therapy. Int. J. Pharm. 2017, 526, 225–234. [Google Scholar] [CrossRef]
- Chiaoprakobkij, N.; Seetabhawang, S.; Okhawilai, M. Multifunctional bacterial cellulose - gelatin containing mangosteen extract films with improved antibacterial and anticancer properties. Cellulose 2022, 29, 6811–6830. [Google Scholar] [CrossRef]
- Popovici, V.; Bucur, L.; Gîrd, C.E.; Popescu, A.; Matei, E.; Caraiane, A.; Botnarciuc, M. Phenolic Secondary Metabolites and Antiradical and Antibacterial Activities of Different Extracts of Usnea barbata (L.) Weber ex F.H. Wigg from C ă limani Mountains, Romania. Pharmaceuticals 2022, 15, 829. [Google Scholar] [CrossRef] [PubMed]
- Pop, A.L.; Musuc, A.M.; Nicoară, A.C.; Ozon, E.A.; Crisan, S.; Penes, O.N.; Nasui, B.A.; Lupuliasa, D.; Secăreanu, A.A. Optimization of the Preformulation and Formulation Parameters in the Development of New Extended-Release Tablets Containing Felodipine. Appl. Sci. 2022, 12, 5333. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, M.; Li, G. Preparation and characterization of collagen/hydroxypropyl methylcellulose (HPMC) blend film. Carbohydr. Polym. 2015, 119, 194–201. [Google Scholar] [CrossRef]
- Gavriloaia, M.R.; Budura, E.A.; Toma, C.C.; Mitu, M.A.; Karampelas, O.; Arama, C.; Lupuleasa, D. In vitro evaluation of diffusion and rheological profiles for dexamethasone inclusion complexes with β-cyclodextrin or hydroxypropyl β-cyclodextrin. Farmacia 2012, 60, 895–904. [Google Scholar]
- Oana, M.; Simona, M.D.; Dumitru, L.; Adriana, M.M.; Svetlana, G.K.; Ştefan, R.F. The influence of structural characteristics on the in vitro drug release rate of terbinafine from topical gels. Farmacia 2012, 60, 325–333. [Google Scholar]
- Domján, A.; Bajdik, J.; Pintye-Hódi, K. Understanding of the plasticizing effects of glycerol and PEG 400 on chitosan films using solid-state NMR spectroscopy. Macromolecules 2009, 42, 4667–4673. [Google Scholar] [CrossRef]
- Guo, R.; Du, X.; Zhang, R.; Deng, L.; Dong, A.; Zhang, J. Bioadhesive film formed from a novel organic-inorganic hybrid gel for transdermal drug delivery system. Eur. J. Pharm. Biopharm. 2011, 79, 574–583. [Google Scholar] [CrossRef]
- Nafee, N.A.; Ismail, F.A.; Boraie, N.A.; Mortada, L.M. Mucoadhesive buccal patches of miconazole nitrate: In vitro/in vivo performance and effect of ageing. Int. J. Pharm. 2003, 264, 1–14. [Google Scholar] [CrossRef]
- Balaci, T.; Velescu, B.; Karampelas, O.; Musuc, A.M.; Nițulescu, G.M.; Ozon, E.A.; Nițulescu, G.; Gîrd, C.E.; Fița, C.; Lupuliasa, D. Physico-chemical and pharmaco-technical characterization of inclusion complexes formed by rutoside with β-cyclodextrin and hydroxypropyl-β-cyclodextrin used to develop solid dosage forms. Processes 2021, 9, 26. [Google Scholar] [CrossRef]
- Musuc, A.M.; Anuta, V.; Atkinson, I.; Sarbu, I.; Popa, V.T.; Munteanu, C.; Mircioiu, C.; Ozon, E.A.; Nitulescu, G.M.; Mitu, M.A. Formulation of chewable tablets containing carbamazepine-β-cyclodextrin inclusion complex and f-melt disintegration excipient. The mathematical modeling of the release kinetics of carbamazepine. Pharmaceutics 2021, 13, 915. [Google Scholar] [CrossRef] [PubMed]
- Perioli, L.; Ambrogi, V.; Angelici, F.; Ricci, M.; Giovagnoli, S.; Capuccella, M.; Rossi, C. Development of mucoadhesive patches for buccal administration of ibuprofen. J. Control. Release 2004, 99, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Derle, D.; Joshi, O.; Pawar, A.; Patel, J.; Perdeshi, V. Effect of tablet excipients on mucoadhesive properties of polyoxyethylene and Carbopol 971P. Int. J. Pharm. Pharm. Sci. 2009, 1, 198–205. [Google Scholar]
- Don, T.M.; Huang, M.L.; Chiu, A.C.; Kuo, K.H.; Chiu, W.Y.; Chiu, L.H. Preparation of thermo-responsive acrylic hydrogels useful for the application in transdermal drug delivery systems. Mater. Chem. Phys. 2008, 107, 266–273. [Google Scholar] [CrossRef]
- Gupta, A.; Garg, S.; Khar, R.K. Measurement of bioadhesive strength of mucoadhesive buccal tablets: Design of an in-vitro assembly. Indian Drugs 1993, 30, 1–6. [Google Scholar]
- Nazir, S.; Ansari, F.L.; Hussain, T.; Mazhar, K.; Muazzam, A.G.; Qasmi, Z.U.H.; Makhmoor, T.; Noureen, H.; Mirza, B. Brine shrimp lethality assay “an effective prescreen”: Microwave-assisted synthesis, BSL toxicity and 3DQSAR studies-based designing, docking and antitumor evaluation of potent chalcones. Pharm. Biol. 2013, 51, 1091–1103. [Google Scholar] [CrossRef]
- Popovici, V.; Bucur, L.; Gîrd, C.E.; Rambu, D.; Calcan, S.I.; Cucolea, E.I.; Costache, T.; Ungureanu-Iuga, M.; Oroian, M.; Mironeasa, S.; et al. Antioxidant, Cytotoxic, and Rheological Properties of Canola Oil Extract of Usnea barbata (L.) Weber ex F.H. Wigg from Călimani Mountains, Romania. Plants 2022, 11, 854. [Google Scholar] [CrossRef]
- Schröder, V.; Arcus, M.; Anghel, A.H.; Busuricu, F.; Lepadatu, A.C. Cell differentiation process of Artemia sp. larvae tools for natural products testing. 2019, LXII, 149–153. [Google Scholar]
- Iancu, I.M.; Bucur, L.A.; Schroder, V.; Mireșan, H.; Sebastian, M.; Iancu, V.; Badea, V. Phytochemical evaluation and cytotoxicity assay of lythri herba extracts. Farmacia 2021, 69, 51–58. [Google Scholar] [CrossRef]
- Popovici, V.; Bucur, L.; Gîrd, C.E.; Calcan, S.I.; Cucolea, E.I.; Costache, T.; Rambu, D.; Oroian, M.; Mironeasa, S.; Schröder, V.; et al. Advances in the Characterization of Usnea barbata (L.) Weber ex F.H. Wigg from Călimani Mountains, Romania. Appl. Sci. 2022, 12, 4234. [Google Scholar] [CrossRef]
- Popovici, V.; Matei, E.; Cozaru, G.C.; Aschie, M.; Bucur, L.; Rambu, D.; Costache, T.; Cucolea, I.E.; Vochita, G.; Gherghel, D.; et al. Usnic acid and usnea barbata (L.) F.H. wigg. dry extracts promote apoptosis and DNA damage in human blood cells through enhancing ROS levels. Antioxidants 2021, 10, 1171. [Google Scholar] [CrossRef]
- Matei, E.; Aschie, M.; Mitroi, A.F.; Ghinea, M.M.; Gheorghe, E.; Petcu, L.; Dobrin, N.; Chisoi, A.; Mihaela, M. Biomarkers involved in evaluation of platelets function in South-Eastern Romanian patients with hematological malignancies subtypes. Medicine 2021, 100, e25944. [Google Scholar] [CrossRef] [PubMed]
- Chunglok, W.; Utaipan, T.; Somchit, N.; Lertcanawanichakul, M.; Sudjaroen, Y. Antioxidant and antiproliferative activities of non-edible parts of selected tropical fruits. Sains Malays. 2014, 43, 689–696. [Google Scholar]
- Varghese, J.; Radhika, G.; Sarin, A. The role of calpain in caspase activation during etoposide induced apoptosis in T cells. Eur. J. Immunol. 2001, 31, 2035–2041. [Google Scholar] [CrossRef]
- Indurkar, A.; Bangde, P.; Gore, M.; Reddy, P.; Jain, R.; Dandekar, P. Optimization of guar gum-gelatin bioink for 3D printing of mammalian cells. Bioprinting 2020, 20, e00101. [Google Scholar] [CrossRef]
- Thomé, M.P.; Filippi-Chiela, E.C.; Villodre, E.S.; Migliavaca, C.B.; Onzi, G.R.; Felipe, K.B.; Lenz, G. Ratiometric analysis of Acridine Orange staining in the study of acidic organelles and autophagy. J. Cell Sci. 2016, 129, 4622–4632. [Google Scholar] [CrossRef]
- Fathi, F.; Ghobeh, M.; Tabarzad, M. Anti-Microbial Peptides: Strategies of Design and Development and Their Promising Wound-Healing Activities. Mol. Biol. Rep. 2022, 8, 1–12. [Google Scholar] [CrossRef]
- Elshikh, M.; Ahmed, S.; Funston, S.; Dunlop, P.; McGaw, M.; Marchant, R.; Banat, I.M. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 2016, 38, 1015–1019. [Google Scholar] [CrossRef]
- Madushan, R.; Vidanarachchi, J.K.; Prasanna, P.H.P.; Werellagama, S.; Priyashantha, H. Use of natural plant extracts as a novel microbiological quality indicator in raw milk: An alternative for resazurin dye reduction method. LWT 2021, 144, 111221. [Google Scholar] [CrossRef]
- Cox, K.D.; Quello, K.; Deford, R.J.; Beckerman, J.L. A rapid method to quantify fungicide sensitivity in the brown rot pathogen Monilinia fructicola. Plant Dis. 2009, 93, 328–331. [Google Scholar] [CrossRef]
- Bitacura, J.G. The Use of Baker’s Yeast in the Resazurin Reduction Test: A Simple, Low-Cost Method for Determining Cell Viability in Proliferation and Cytotoxicity Assays. J. Microbiol. Biol. Educ. 2018, 19, jmbe-19-87. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, Z.; Wu, W.; Jing, Y.; Dai, H.; Fang, G. Nanocellulose/Poly(2-(dimethylamino)ethyl methacrylate)Interpenetrating polymer network hydrogels for removal of Pb(II) and Cu(II) ions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 474–480. [Google Scholar] [CrossRef]
- Wang, D.; Yu, H.; Fan, X.; Gu, J.; Ye, S.; Yao, J.; Ni, Q. High Aspect Ratio Carboxylated Cellulose Nanofibers Cross-linked to Robust Aerogels for Superabsorption-Flocculants: Paving Way from Nanoscale to Macroscale. ACS Appl. Mater. Interfaces 2018, 10, 20755–20766. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Liu, Y.; Lai, W.; Huang, F.; Ou, A.; Qin, R.; Liu, X.; Wang, X. Ester Crosslinking Enhanced Hydrophilic Cellulose Nanofibrils Aerogel. ACS Sustain. Chem. Eng. 2018, 6, 11979–11988. [Google Scholar] [CrossRef]
- Chelu, M.; Calderon Moreno, J.; Atkinson, I.; Pandele Cusu, J.; Rusu, A.; Bratan, V.; Aricov, L.; Anastasescu, M.; Seciu-Grama, A.M.; Musuc, A.M. Green synthesis of bioinspired chitosan-ZnO-based polysaccharide gums hydrogels with propolis extract as novel functional natural biomaterials. Int. J. Biol. Macromol. 2022, 211, 410–424. [Google Scholar] [CrossRef]
- George, J.; Kumar, R.; Sajeevkumar, V.A.; Ramana, K.V.; Rajamanickam, R.; Abhishek, V.; Nadanasabapathy, S. Siddaramaiah Hybrid HPMC nanocomposites containing bacterial cellulose nanocrystals and silver nanoparticles. Carbohydr. Polym. 2014, 105, 285–292. [Google Scholar] [CrossRef]
- Wang, T.; Chen, L.; Shen, T.; Wu, D. Preparation and properties of a novel thermo-sensitive hydrogel based on chitosan/hydroxypropyl methylcellulose/glycerol. Int. J. Biol. Macromol. 2016, 93, 775–782. [Google Scholar] [CrossRef]
- Marques, N.d.N.; Balaban, R.d.C.; Halila, S.; Borsali, R. Synthesis and characterization of carboxymethylcellulose grafted with thermoresponsive side chains of high LCST: The high temperature and high salinity self-assembly dependence. Carbohydr. Polym. 2018, 184, 108–117. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef]
- Phe, K.; Dao, D.; Palmer, H.R.; Tama, V.H. In vitro ceftriaxone susceptibility in methicillin-susceptible staphylococcus aureus. Antimicrob. Agents Chemother. 2015, 59, 1370. [Google Scholar] [CrossRef]
- Ochoa, S.A.; Cruz-Córdova, A.; Rodea, G.E.; Cázares-Domínguez, V.; Escalona, G.; Arellano-Galindo, J.; Hernández-Castro, R.; Reyes-López, A.; Xicohtencatl-Cortes, J. Phenotypic characterization of multidrug-resistant Pseudomonas aeruginosa strains isolated from pediatric patients associated to biofilm formation. Microbiol. Res. 2015, 172, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, G.; St. Clair, L.L. Lichens: A promising source of antibiotic and anticancer drugs. Phytochem. Rev. 2013, 12, 229–244. [Google Scholar] [CrossRef]
- Paliya, B.S.; Bajpai, R.; Jadaun, V.; Kumar, J.; Kumar, S.; Upreti, D.K.; Singh, B.R.; Nayaka, S.; Joshi, Y.; Singh, B.N. The genus Usnea: A potent phytomedicine with multifarious ethnobotany, phytochemistry and pharmacology. RSC Adv. 2016, 6, 21672–21696. [Google Scholar]
- Salgado, F.; Albornoz, L.; Cortéz, C.; Stashenko, E.; Urrea-Vallejo, K.; Nagles, E.; Galicia-Virviescas, C.; Cornejo, A.; Ardiles, A.; Simirgiotis, M.; et al. Secondary metabolite profiling of species of the genus usnea by UHPLC-ESI-OT-MS-MS. Molecules 2018, 23, 54. [Google Scholar] [CrossRef]
- Popovici, V.; Bucur, L.; Popescu, A.; Schröder, V.; Costache, T.; Rambu, D.; Cucolea, I.E.; Gîrd, C.E.; Caraiane, A.; Gherghel, D.; et al. Antioxidant and cytotoxic activities of usnea barbata (L.) f.h. wigg. dry extracts in different solvents. Plants 2021, 10, 909. [Google Scholar] [CrossRef]
- Jardón-Romero, E.A.; Lara-Carrillo, E.; González-Pedroza, M.G.; Sánchez-Mendieta, V.; Salmerón-Valdés, E.N.; Toral-Rizo, V.H.; Olea-Mejía, O.F.; López-González, S.; Morales-Luckie, R.A. Antimicrobial Activity of Biogenic Silver Nanoparticles from Syzygium aromaticum against the Five Most Common Microorganisms in the Oral Cavity. Antibiotics 2022, 11, 834. [Google Scholar] [CrossRef]
- Rafey, A.; Amin, A.; Kamran, M.; Haroon, U.; Farooq, K.; Foubert, K.; Pieters, L. Analysis of plant origin antibiotics against oral bacterial infections using in vitro and in silico techniques and characterization of active constituents. Antibiotics 2021, 10, 1504. [Google Scholar] [CrossRef]
- Thiyahuddin, N.M.; Lamping, E.; Rich, A.M.; Cannon, R.D. Yeast species in the oral cavities of older people: A comparison between people living in their own homes and those in rest homes. J. Fungi 2019, 5, 30. [Google Scholar] [CrossRef]
- Popovici, V.; Bucur, L.; Popescu, A.; Caraiane, A.; Badea, V. Evaluation of the Antibacterial Action of the Usnea barbata L. Extracts on Streptococcus Species from the Oro-Dental Cavity. In Proceedings of the Romanian National Congress of Pharmacy, Bucharest, Romania, 26–29 September 2018. 17th ed.. [Google Scholar]
- Popovici, V.; Bucur, L.; Popescu, A.; Caraiane, A.; Badea, V. Comparative study regarding antibacterial action if the Usnea barbata L. In extracts on Gram-positive bacteria from the oro-dental cavity. In Proceedings of the 5th SGEM International Multidisciplinary Scientific Conferences on Social Sciences and Arts, Albena, Bulgaria, 24 August–2 September 2018. [Google Scholar]
- Popovici, V.; Bucur, L.; Calcan, S.I.; Cucolea, E.I.; Costache, T.; Rambu, D.; Schröder, V.; Gîrd, C.E.; Gherghel, D.; Vochita, G.; et al. Elemental Analysis and In Vitro Evaluation of Antibacterial and Antifungal Activities of Usnea barbata (L.) Weber ex F.H. Wigg from C ă limani Mountains, Romania. Plants 2022, 11, 32. [Google Scholar] [CrossRef]
- Rajaram, D.M.; Laxman, S.D. Buccal mucoadhesive films: A review. Syst. Rev. Pharm. 2016, 8, 31–38. [Google Scholar] [CrossRef]
- Gayathri, D.; Jayakumari, L.S. Evaluation of commercial arrowroot starch/CMC film for buccal drug delivery of glipizide. Polimeros 2019, 29, e2019047. [Google Scholar] [CrossRef]
- Saini, S.; Nanda, A.; Dhari, J. Formulation, development & evaluation of oral fast dissolving anti-allergic film of levocetrizine dihydrochloride. J. Pharm. Sci. Res. 2011, 3, 1322–1325. [Google Scholar]
- Pandey, P.; Chauhan, S. Fast dissolving sublingual films of Zolmitriptan: A novel treatment approach for migraine attacks. Indian J. Pharm. Educ. Res. 2014, 48, 67–72. [Google Scholar] [CrossRef]
- Winarti, L.; Laksono, B.T.; Oktora, L.; Sari, R.K. Optimization of hydroxy propyl methyl cellulose and carbomer in diltiazem hydrochloride mucoadhesive buccal film. Indones. J. Pharm. 2021, 32, 43–51. [Google Scholar] [CrossRef]
- Elshafeey, A.H.; El-Dahmy, R.M. Formulation and development of oral fast-dissolving films loaded with nanosuspension to augment paroxetine bioavailability: In vitro characterization, ex vivo permeation, and pharmacokinetic evaluation in healthy human volunteers. Pharmaceutics 2021, 13, 1869. [Google Scholar] [CrossRef]
- Salehi, S.; Boddohi, S. New formulation and approach for mucoadhesive buccal film of rizatriptan benzoate. Prog. Biomater. 2017, 6, 175–187. [Google Scholar] [CrossRef]
- Bergo, P.; Sobral, P.J.A. Effects of plasticizer on physical properties of pigskin gelatin films. Food Hydrocoll. 2007, 21, 1285–1289. [Google Scholar] [CrossRef]
- Meier-Haack, J.; Müller, M.; Lunkwitz, K. Polymers-Opportunities and Risks II: Sustainability, Product Design and Processing, 12th ed.; Eyerer, P., Weller, M., Hübner, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 281–297. [Google Scholar]
- Kraisit, P.; Limmatvapirat, S.; Luangtana-Anan, M.; Sriamornsak, P. Buccal administration of mucoadhesive blend films saturated with propranolol loaded nanoparticles. Asian J. Pharm. Sci. 2018, 13, 34–43. [Google Scholar] [CrossRef]
- Pilicheva, B.; Uzunova, Y.; Marudova, M. Polyelectrolyte Multilayer Films as a Potential Buccal Platform for Drug Delivery. Polymers 2022, 14, 734. [Google Scholar] [CrossRef]
- Aframian, D.J.; Davidowitz, T.; Benoliel, R. The distribution of oral mucosal pH values in healthy saliva secretors. Oral Dis. 2006, 12, 420–423. [Google Scholar] [CrossRef]
- Muzib, Y.i.; Kumari, K.s. Mucoadhesive buccal films of glibenclamide: Development and evaluation. Int. J. Pharm. Investig. 2011, 1, 42. [Google Scholar] [CrossRef] [PubMed]
- Lindert, S.; Breitkreutz, J. Oromucosal multilayer films for tailor-made, controlled drug delivery. Expert Opin. Drug Deliv. 2017, 14, 1265–1279. [Google Scholar] [CrossRef] [PubMed]
- Garipova, V.R.; Gennari, C.G.M.; Selmin, F.; Cilurzo, F.; Moustafine, R.I. Mucoadhesive interpolyelectrolyte complexes for the buccal delivery of clobetasol. Polymers 2018, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Dobaria, N.B.; Badhan, A.C.; Mashru, R.C. A novel itraconazole bioadhesive film for vaginal delivery: Design, optimization, and physicodynamic characterization. AAPS PharmSciTech 2009, 10, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Peh, K.K.; Wong, C.F. Polymeric films as vehicle for buccal delivery: Swelling, mechanical, and bioadhesive properties. J. Pharm. Pharm. Sci. 1999, 2, 53–61. [Google Scholar]
- Vasantha, P.V.; Puratchikody, A.; Mathew, S.T.; Balaraman, A.K. Development and characterization of Eudragit based mucoadhesive buccal patches of salbutamol sulfate. Saudi Pharm. J. 2011, 19, 207–214. [Google Scholar] [CrossRef]
- Caro, V.D.; Murgia, D.; Seidita, F.; Bologna, E.; Alotta, G.; Zingales, M.; Campisi, G. Enhanced in situ availability of Aphanizomenon Flos-Aquae constituents entrapped in buccal films for the treatment of oxidative stress-related oral diseases: Biomechanical characterization and in vitro/ex vivo evaluation. Pharmaceutics 2019, 11, 35. [Google Scholar] [CrossRef]
- Theda, C.; Hwang, S.H.; Czajko, A.; Loke, Y.J.; Leong, P.; Craig, J.M. Quantitation of the cellular content of saliva and buccal swab samples. Sci. Rep. 2018, 8, 4–11. [Google Scholar] [CrossRef]
- Park, J.Y.; Chung, H.; Choi, Y.; Park, J.H. Phenotype and tissue residency of lymphocytes in the murine oral mucosa. Front. Immunol. 2017, 8, 250. [Google Scholar] [CrossRef]
- Düzlü, M.; Karamert, R.; Tutar, H.; Şahin, M.; Türkcan, A.; Yilmaz, M. Diagnostic role of neutrophil-lymphocyte ratio in oral cavity cancers. Niger. J. Clin. Pract. 2018, 21, 49–53. [Google Scholar]
- Al-Ahmadi, A.A.; Ayuob, N.N.; Ali, S.S.; Al-Robai, A.A.; Abo-Khatwa, N.A. Effect of (+)-usnic acid as a fat burner on the rat hepatocyte; Correlated histological and biochemical in vivo study. J. Anim. Vet. Adv. 2012, 11, 1368–1377. [Google Scholar]
- Rabelo, T.K.; Zeidán-Chuliá, F.; Vasques, L.M.; dos Santos, J.P.A.; da Rocha, R.F.; Pasquali, M.A.d.B.; Rybarczyk-Filho, J.L.; Araújo, A.A.S.; Moreira, J.C.F.; Gelain, D.P. Redox characterization of usnic acid and its cytotoxic effect on human neuron-like cells (SH-SY5Y). Toxicol. Vitr. 2012, 26, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Antonenko, Y.N.; Khailova, L.S.; Rokitskaya, T.I.; Nosikova, E.S.; Nazarov, P.A.; Luzina, O.A.; Salakhutdinov, N.F.; Kotova, E.A. Mechanism of action of an old antibiotic revisited: Role of calcium ions in protonophoric activity of usnic acid. Biochim. Biophys. Acta-Bioenerg. 2019, 1860, 310–316. [Google Scholar] [CrossRef]
- Chelombitko, M.A.; Firsov, A.M.; Kotova, E.A.; Rokitskaya, T.I.; Khailova, L.S.; Popova, L.B.; Chernyak, B.V.; Antonenko, Y.N. Usnic acid as calcium ionophore and mast cells stimulator. Biochim. Biophys. Acta-Biomembr. 2020, 1862, 183303. [Google Scholar] [CrossRef] [PubMed]
- Alahmadi, A.A. Usnic acid biological activity: History, evaluation and usage. Int. J. Basic Clin. Pharmacol. 2017, 6, 2752. [Google Scholar] [CrossRef]
- Gajdziok, J.; Holešová, S.; Štembírek, J.; Pazdziora, E.; Landová, H.; Doležel, P.; Vetchý, D.; Pillay, V. Carmellose mucoadhesive oral films containing vermiculite/chlorhexidine nanocomposites as innovative biomaterials for treatment of oral infections. Biomed Res. Int. 2015, 2015, 580146. [Google Scholar] [CrossRef]
- Barbălată-Mândru, M.; Serbezeanu, D.; Butnaru, M.; Rîmbu, C.M.; Enache, A.A.; Aflori, M. Poly(vinyl alcohol)/Plant Extracts Films: Preparation, Surface Characterization and Antibacterial Studies against Gram Positive and Gram Negative Bacteria. Materials 2022, 15, 2493. [Google Scholar] [CrossRef]
- Basilicata, M.; Lauro, M.D.; Campolattano, V.; Marrone, G.; Celotto, R.; Mitterhofer, A.P.; Bollero, P.; Daniele, N.D.; Noce, A. Natural Bioactive Compounds in the Management of Oral Diseases in Nephropathic Patients. Int. J. Environ. Res. Public Health 2022, 19, 1665. [Google Scholar] [CrossRef]
- Su, Z.Q.; Liu, Y.H.; Guo, H.Z.; Sun, C.Y.; Xie, J.H.; Li, Y.C.; Chen, J.N.; Lai, X.P.; Su, Z.R.; Chen, H.M. Effect-enhancing and toxicity-reducing activity of usnic acid in ascitic tumor-bearing mice treated with bleomycin. Int. Immunopharmacol. 2017, 46, 146–155. [Google Scholar] [CrossRef]
- Dar, T.U.H.; Dar, S.A.; Islam, S.U.; Mangral, Z.A.; Dar, R.; Singh, B.P.; Verma, P.; Haque, S. Lichens as a repository of bioactive compounds: An open window for green therapy against diverse cancers. Semin. Cancer Biol. 2021, 44, 259–267. [Google Scholar] [CrossRef]
- Guo, L.; Shi, Q.; Fang, J.L.; Mei, N.; Ali, A.A.; Lewis, S.M.; Leakey, J.E.A.; Frankos, V.H. Review of usnic acid and Usnea barbata toxicity. J. Environ. Sci. Health-Part C Environ. Carcinog. Ecotoxicol. Rev. 2008, 26, 317–338. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, W.; Maple, J.T.; Burgart, L.J.; Kamath, P.S. Severe hepatotoxicity associated with use of a dietary supplement containing usnic acid. Mayo Clin. Proc. 2006, 81, 541–544. [Google Scholar] [CrossRef] [PubMed]










| Variable | F-UBA | R | |
|---|---|---|---|
| Composition | |||
| UBA (g) | 0.25 | - | |
| Ethyl alcohol 96% (v/v) (g) | 5 | 5 | |
| Isopropyl alcohol (g) | 5 | 5 | |
| PEG 400 (g) | 5 | 5 | |
| HPMC 15% water dispersion (w/w) (g) | 84.75 | 85 | |
| Physico-chemical properties-TG/DTA parameters | |||
| 1st Step (%) | TG (%) | 2.50% | 1.20% |
| 2nd Step (%) | TG (%)/Tmax (°C) | 85.5%/355.2 °C | 86.9%/360.5 °C |
| 3rd Step (%) | TG (%)/Tmax (°C) | 12.1%/461.8 °C | 11.9%/454.7 °C |
| Pharmacotechnical properties | |||
| Weight uniformity (mg) | 70 ± 3.54 | 66 ± 4.18 | |
| Thickness (mm) | 0.060 ± 0.002 | 0.058 ± 0.003 | |
| Folding endurance value | >300 | >300 | |
| Tensile strength (kg/mm2) | 3.02 ± 2.39 | 2.88 ± 3.43 | |
| Elongation % | 47.26 ± 2.16 | 49.25 ± 2.24 | |
| Moisture content % (w/w) | 4.11 ± 0.35 | 3.98 ± 1.02 | |
| pH | 7.01 ± 0.01 | 7.04 ± 0.02 | |
| Disintegration time (seconds) | 146 ± 5.09 | 138 ± 4.67 | |
| Swelling ratio (% after 6 h) | 211 ± 4.31 | 204 ± 3.29 | |
| Ex vivo bioadhesion time (minutes) | 85 ± 2.33 | 82 ± 2.61 | |
| Micro-Dilution | CTR (mg/mL) | TRF (mg/mL) | F-UBA (mg/mL) | |||||
|---|---|---|---|---|---|---|---|---|
| 30.230 ± 0.630 | 122.330 ± 0.850 | 10.050 ± 0.180 | 70 ± 3.540 | |||||
| 1 | 1.511 ± 0.043 | 6.117 ± 0.042 | 0.500 ± 0.009 | 3.497 ± 0.172 | ||||
| 2 | 0.755 ± 0.022 | 4.893 ± 0.034 | 0.250 ± 0.004 | 1.749 ± 0.086 | ||||
| 3 | 0.377 ± 0.011 | 3.914 ± 0.027 | 0.125 ± 0.002 | 0.874 ± 0.043 | ||||
| 4 | 0.188 ± 0.005 | 3.131 ± 0.021 | 0.061 ± 0.001 | 0.438 ± 0.022 | ||||
| 5 | 0.094 ± 0.002 | 2.505 ± 0.017 | 0.031 ± 0.001 | 0.219 ± 0.011 | ||||
| 6 | 0.047 ± 0.002 | 2.004 ± 0.014 | 0.015 ± 0.001 | 0.110 ± 0.006 | ||||
| 7 | 0.023 ± 0.001 | 1.603 ± 0.011 | 0.007 ± 0.001 | 0.055 ± 0.003 | ||||
| S. aureus | P. aeruginosa | |||||||
| F-UBA | CTR | F-UBA | CTR | |||||
| A * | B ** | A * | B ** | A * | B ** | A * | B ** | |
 |  |  |  |  |  |  |  | |
 * * | ||||||||
| C. albicans | C. parapsilosis | Color *** | Score *** | Signification *** | ||||
| TRF | F-UBA | TRF | F-UBA | |||||
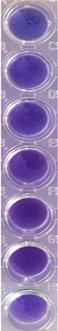 |  |  |  |  | 0 | Blue— cells are dead | ||
| 1 | Violet-blue— cells are partially dead | |||||||
| 2 | Violet— cells are alive; no proliferation | |||||||
| 3 | Light-violet— low proliferation | |||||||
| 4 | Dark pink— moderate proliferation | |||||||
| 5 | Pink— fast proliferation | |||||||
| 6 | Light pink— very fast proliferation | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popovici, V.; Matei, E.; Cozaru, G.C.; Bucur, L.; Gîrd, C.E.; Schröder, V.; Ozon, E.A.; Musuc, A.M.; Mitu, M.A.; Atkinson, I.; et al. In Vitro Anticancer Activity of Mucoadhesive Oral Films Loaded with Usnea barbata (L.) F. H. Wigg Dry Acetone Extract, with Potential Applications in Oral Squamous Cell Carcinoma Complementary Therapy. Antioxidants 2022, 11, 1934. https://doi.org/10.3390/antiox11101934
Popovici V, Matei E, Cozaru GC, Bucur L, Gîrd CE, Schröder V, Ozon EA, Musuc AM, Mitu MA, Atkinson I, et al. In Vitro Anticancer Activity of Mucoadhesive Oral Films Loaded with Usnea barbata (L.) F. H. Wigg Dry Acetone Extract, with Potential Applications in Oral Squamous Cell Carcinoma Complementary Therapy. Antioxidants. 2022; 11(10):1934. https://doi.org/10.3390/antiox11101934
Chicago/Turabian StylePopovici, Violeta, Elena Matei, Georgeta Camelia Cozaru, Laura Bucur, Cerasela Elena Gîrd, Verginica Schröder, Emma Adriana Ozon, Adina Magdalena Musuc, Mirela Adriana Mitu, Irina Atkinson, and et al. 2022. "In Vitro Anticancer Activity of Mucoadhesive Oral Films Loaded with Usnea barbata (L.) F. H. Wigg Dry Acetone Extract, with Potential Applications in Oral Squamous Cell Carcinoma Complementary Therapy" Antioxidants 11, no. 10: 1934. https://doi.org/10.3390/antiox11101934
APA StylePopovici, V., Matei, E., Cozaru, G. C., Bucur, L., Gîrd, C. E., Schröder, V., Ozon, E. A., Musuc, A. M., Mitu, M. A., Atkinson, I., Rusu, A., Petrescu, S., Mitran, R.-A., Anastasescu, M., Caraiane, A., Lupuliasa, D., Aschie, M., & Badea, V. (2022). In Vitro Anticancer Activity of Mucoadhesive Oral Films Loaded with Usnea barbata (L.) F. H. Wigg Dry Acetone Extract, with Potential Applications in Oral Squamous Cell Carcinoma Complementary Therapy. Antioxidants, 11(10), 1934. https://doi.org/10.3390/antiox11101934

















