Potential Antioxidative Components in Azadirachta indica Revealed by Bio-Affinity Ultrafiltration with SOD and XOD
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals and Reagents
2.3. Instruments
2.4. Determinations of Antioxidant Activity of A. indica
2.4.1. ABTS•+ Radical Cation Scavenging Activity Assay
2.4.2. Ferric-Ion-Reducing Antioxidant Power Assay (FRAP)
2.5. Determination of Phenolic Constituents
2.5.1. Total Phenolic Content (TPC)
2.5.2. Determination of Total Flavonoid Contents (TFC)
2.6. Sample Preparation and Screening of the Potential Ligands of SOD and XOD with UF-LC-MS
2.7. UPLC-Q-TOF-MS/MS Analysis
2.8. Molecular Docking Study
2.9. Validation of Potential Ligands Activity by UF-LC-MS
2.10. Statistical Analysis
3. Results and Discussion
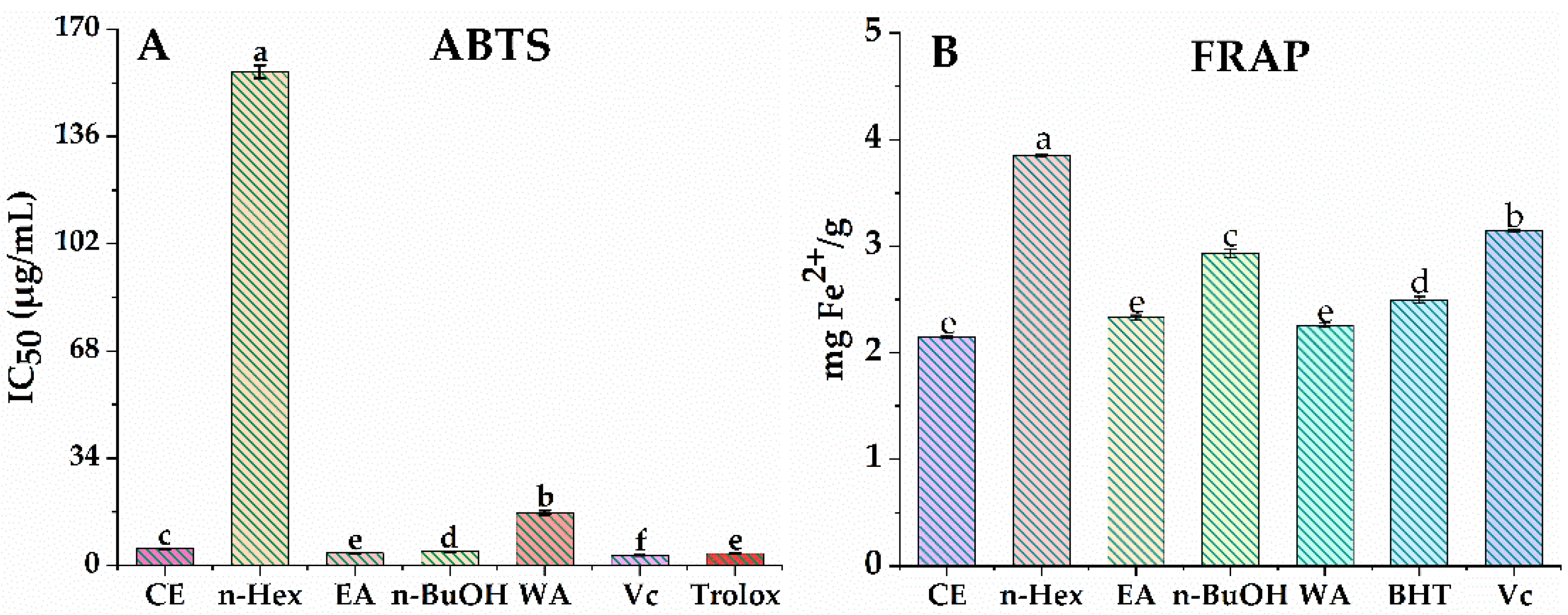
3.1. Evaluation of Antioxidant Activities
3.2. Total Phenolic and Flavonoid Content
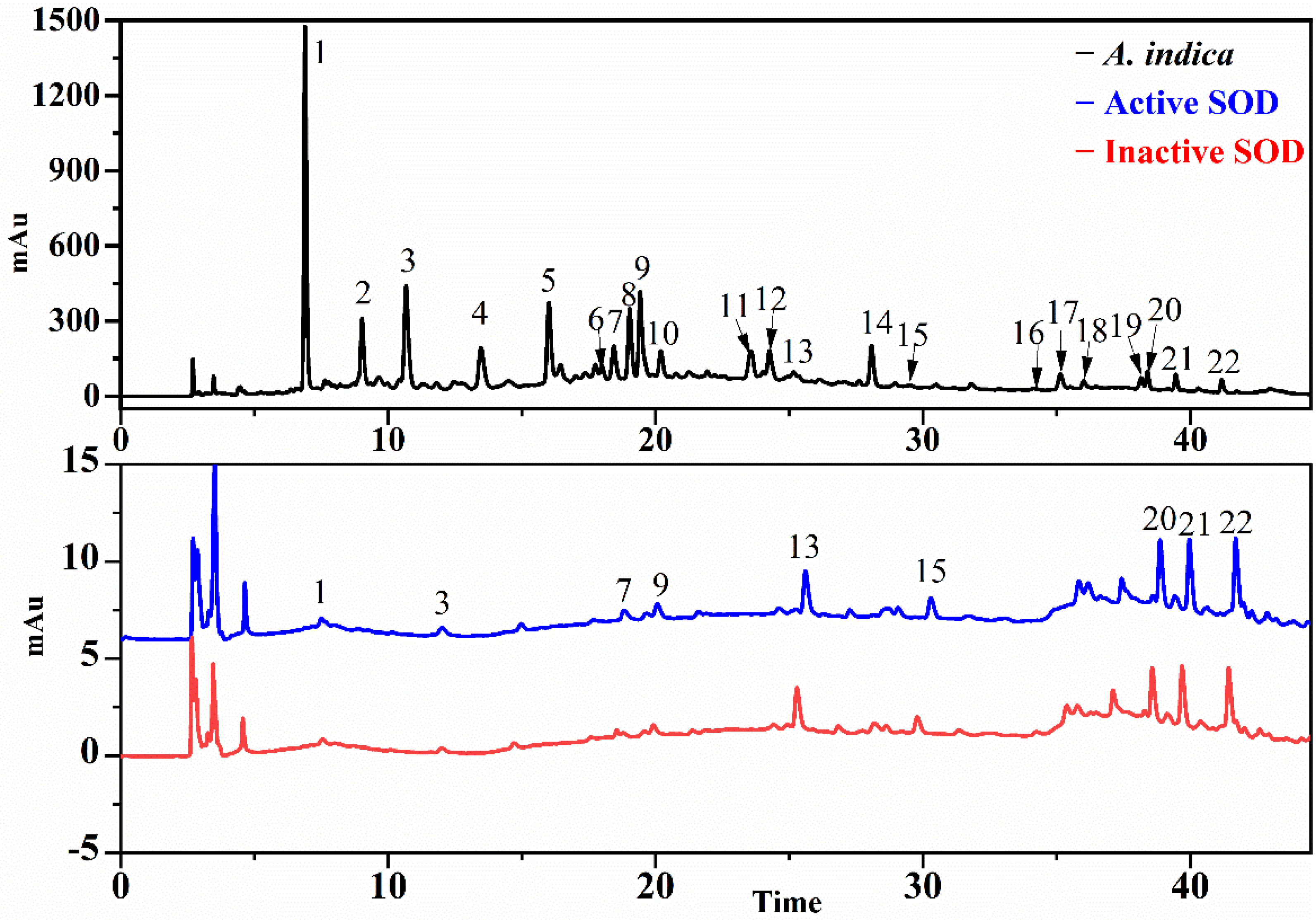
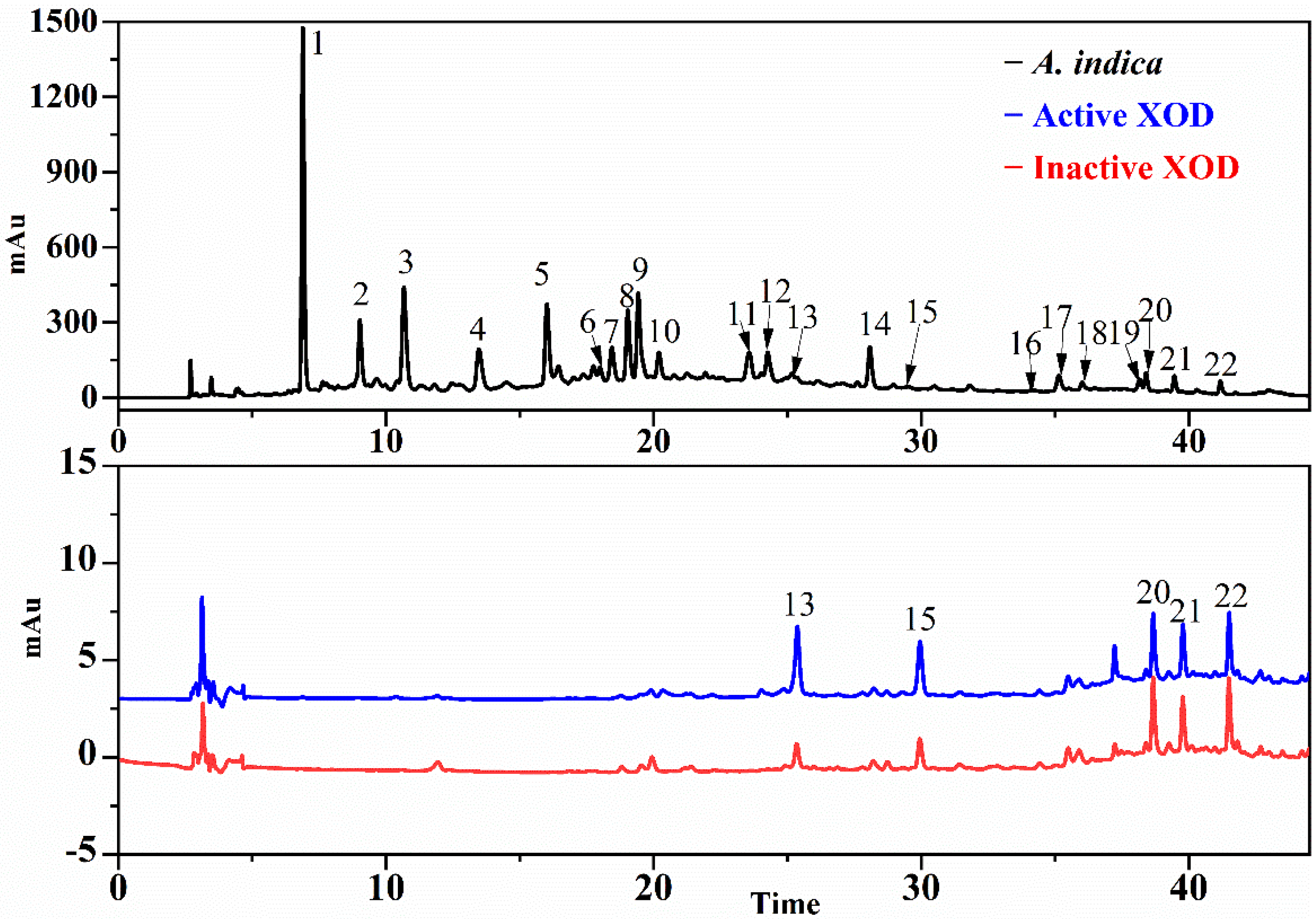
3.3. Screening for SOD and XOD Ligands in A. indica with UF-LC-MS
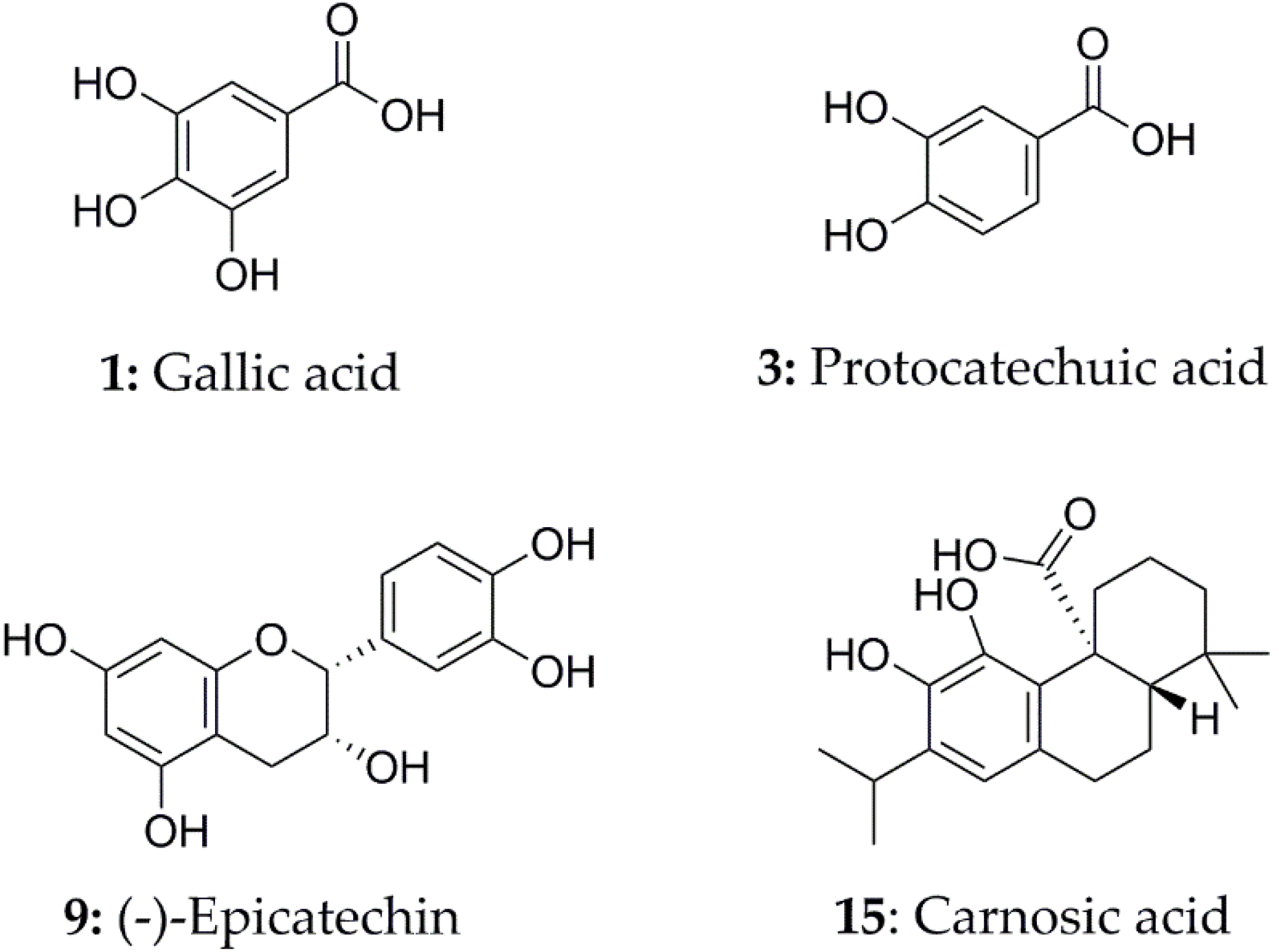
3.4. Identification of SOD and XOD Ligands in A. indica with UPLC-Q-TOF-MS/MS
| No. | Rt/min | [M-H]− (m/z) | MS/MS Spectrum | Identification | RBA | |
|---|---|---|---|---|---|---|
| SOD | XOD | |||||
| 1 | 7.29 | 169.0135 | 125.0253 | Gallic acid [33] | 2.15 | ND |
| 3 | 11.93 | 153.0189 | 109.0286 | Protocatechuic acid [34] | 1.78 | ND |
| 7 | 18.90 | 593.1293 | 467.1010, 441.0726, 423.0718, 407.0664, 305.0650, 289.0655, 245.0418, 125.0238 | (epi)catechin-(epi)gallocatechin isomer [38] | 1.06 | ND |
| 9 | 19.62 | 289.0716 | 245.0879, 221.0811, 205.0494, 203.0702, 125.0197, 109.0290 | (−)-Epicatechin [39] | 1.61 | ND |
| 13 | 24.31 | 459.2732 | 297.2421, 179.0339, 161.0228, 133.0281 | Monohexoside derivatives [41] | 1.12 | 3.14 |
| 15 | 28.11 | 331.1913 | 313.1792, 301.1781, 287.1436, 244.0889 | Carnosic acid [43] | 1.26 | 2.05 |
| 20 | 37.26 | 287.1658 | 255.1365, 199.0797, 186.0686 | Unidentified | 1.30 | 0.86 |
| 21 | 38.40 | 329.1757 | 285.1050, 269.1584, 245.0558, 213.0907 | Carnosol [44,45] | 1.19 | 0.93 |
| 22 | 40.24 | 285.1501 | 269.1133, 255.1002, 199.0743, 187.0725, 173.0576, 157.0664 | Unidentified | 1.29 | 0.83 |
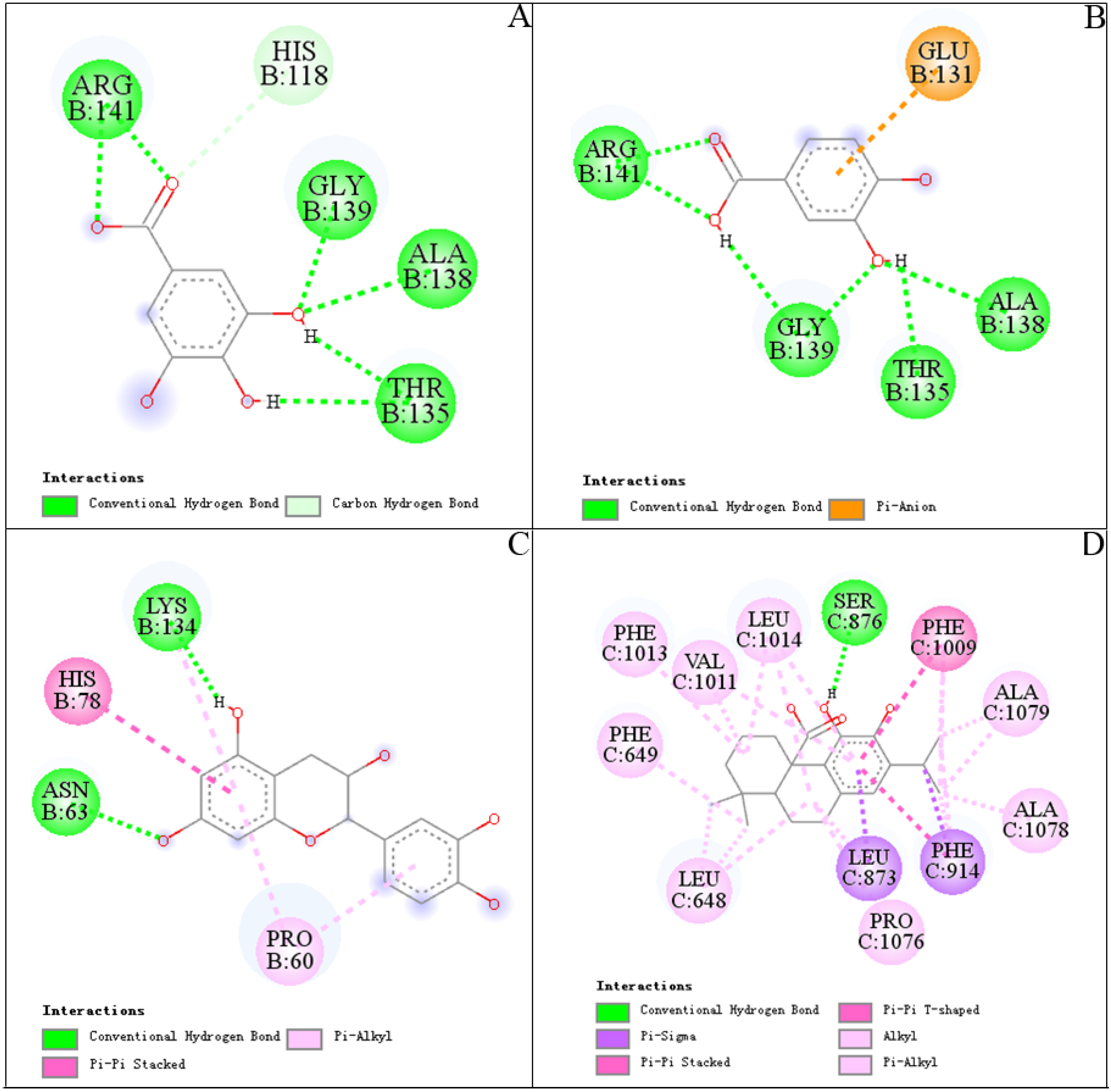
3.5. Molecular Docking
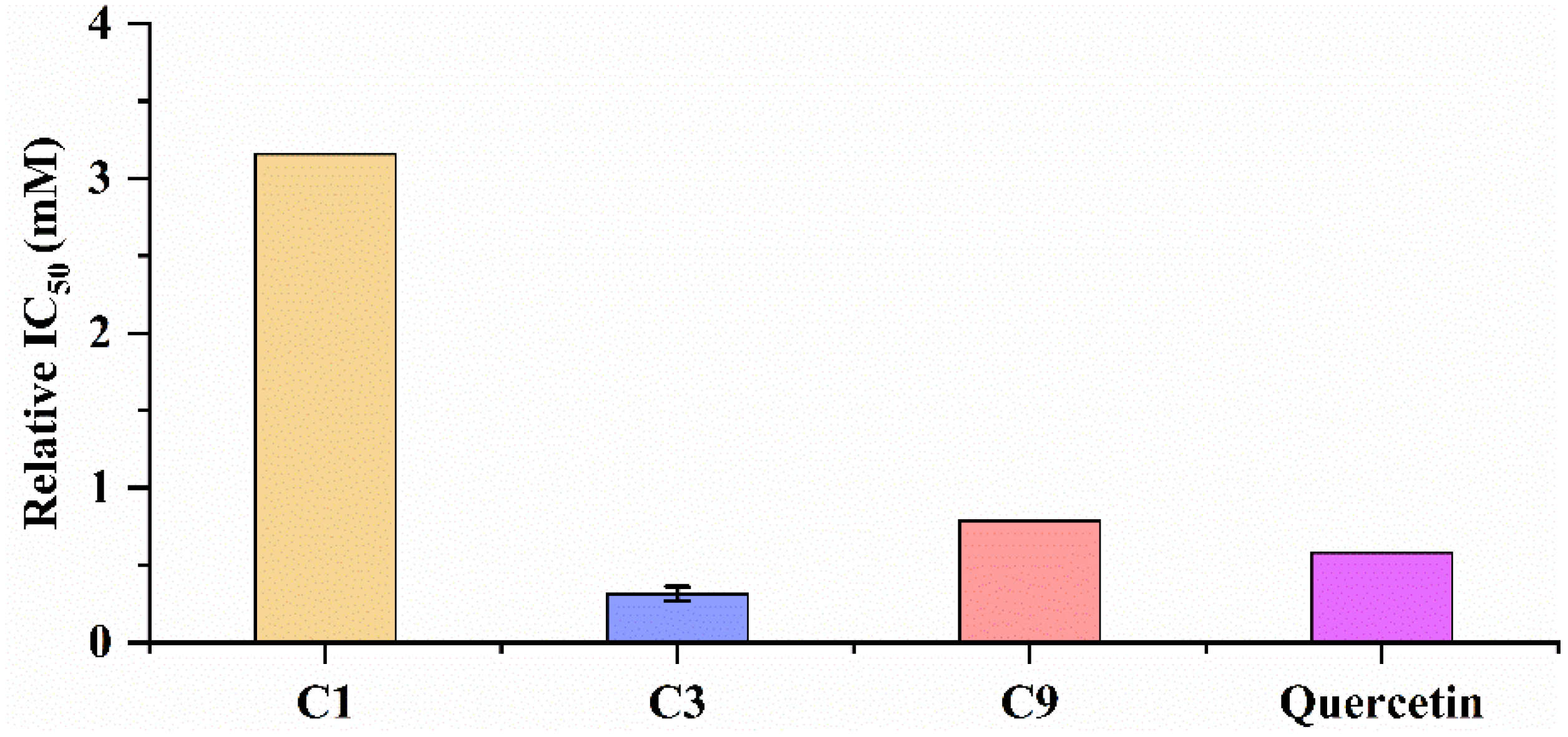
3.6. Antioxidant Capacity of Potential Ligands by UF-LC-MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biney, E.E.; Nkoom, M.; Darkwah, W.K.; Puplampu, J.B. High-performance liquid chromatography analysis and antioxidant activities of extract of Azadirachta indica (Neem) leaves. Pharmacogn. Res. 2020, 12, 29–34. [Google Scholar]
- Gupta, S.C.; Prasad, S.; Tyagi, A.K.; Kunnumakkara, A.B.; Aggarwal, B.B. Neem (Azadirachta indica): An indian traditional panacea with modern molecular basis. Phytomedicine 2017, 34, 14–20. [Google Scholar] [CrossRef]
- Tiwari, R.; Verma, A.K.; Chakraborty, S.; Dhama, K.; Singh, S.V. Neem (Azadirachta indica) and its potential for safeguarding health of animals and humans: A review. J. Biol. Sci. 2014, 14, 110. [Google Scholar] [CrossRef] [Green Version]
- Brahmachari, G. Neem-an omnipotent plant: A retrospection. ChemBioChem 2004, 5, 408–421. [Google Scholar] [CrossRef]
- Rahmani, A.; Almatroudi, A.; Alrumaihi, F.; Khan, A. Pharmacological and therapeutic potential of neem (Azadirachta indica). Pharmacogn. Rev. 2018, 12, 250–255. [Google Scholar] [CrossRef]
- Lim, T. Edible Medicinal and Non-Edible Medicinal Plants. Springer Fruit 2012, 2, 83–108. [Google Scholar]
- Subapriya, R.; Nagini, S. Medicinal properties of neem leaves: A review. Curr. Med. Chem. Anti-Cancer Agents 2005, 5, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Hashmat, I.; Azad, H.; Ahmed, A. Neem (Azadirachta indica A. Juss)—A nature’s drugstore: An overview. Int. Res. J. Biol. Sci. 2012, 1, 76–79. [Google Scholar]
- Singh, H.; Kaur, M.; Dhillon, J.S.; Batra, M.; Khurana, J. Neem: A magical herb in endodontics. Stomatol. Dis. Sci. 2017, 1, 50–54. [Google Scholar] [CrossRef] [Green Version]
- Rao, P.S.; Subramanayam, G.; Sridhar, P.R. Flavonol glycosides from Azadirachta indica L. Drug Invent. Today 2018, 10, 1421–1426. [Google Scholar]
- Saleem, S.; Muhammad, G.; Hussain, M.A.; Bukhari, S.N.A. A comprehensive review of phytochemical profile, bioactives for pharmaceuticals, and pharmacological attributes of Azadirachta indica. Phytother. Res. 2018, 32, 1241–1272. [Google Scholar] [CrossRef]
- Biswas, K.; Chattopadhyay, I.; Banerjee, R.K.; Bandyopadhyay, U. Biological activities and medicinal properties of neem (Azadirachta indica). Curr. Sci. 2002, 82, 1336–1345. [Google Scholar]
- Fan, M.; Chen, G.; Sun, B.; Wu, J.; Li, N.; Sarker, S.D.; Nahar, L.; Guo, M. Screening for natural inhibitors of human topoisomerases from medicinal plants with bio-affinity ultrafiltration and LC-MS. Phytochem. Rev. 2020, 19, 1231–1261. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G.L.; Zhang, Y.L.; Yang, M.; Chen, J.M.; Guo, M.Q. Potential hypoglycemic, hypolipidemic, and anti-inflammatory bioactive components in Nelumbo nucifera leaves explored by bioaffinity ultrafiltration with multiple targets. Food Chem. 2022, 375, 131856. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R.H. Guidelines for antioxidant assays for food component. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Borges, F.; Fernandes, E.; Roleira, F. Progress towards the discovery of xanthine oxidase inhibitors. Curr. Med. Chem. 2002, 9, 195–217. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Chen, G.; Zhang, Y.; Nahar, L.; Sarker, S.D.; Hu, G.; Guo, M. Antioxidant and anti-proliferative properties of Hagenia abyssinica roots and their potentially active components. Antioxidants 2020, 9, 143. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eltamany, E.E.; Elhady, S.S.; Ahmed, H.A.; Badr, J.M.; Noor, A.O.; Ahmed, S.A.; Nafie, M.S. Chemical profiling, antioxidant, cytotoxic activities and molecular docking simulation of Carrichtera annua DC. (Cruciferae). Antioxidants 2020, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.C.; Chen, G.L.; Liu, Y.; Zhang, Y.L.; Guo, M.Q. New lignanamides with antioxidant and anti-inflammatory activities screened out and identified from Warburgia ugandensis combining affinity ultrafiltration LC-MS with SOD and XOD Enzymes. Antioxidants 2021, 10, 370. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.L.; Xu, Y.B.; Wu, J.L.; Li, N.; Guo, M.Q. Hypoglycemic and hypolipidemic effects of Moringa oleifera leaves and their functional chemical constituents. Food Chem. 2020, 333, 127478. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.L.; Fan, M.X.; Wu, J.L.; Li, N.; Guo, M.Q. Antioxidant and anti-inflammatory properties of flavonoids from lotus plumule. Food Chem. 2019, 277, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Nadjafi, S.; Hosseini, N.; Farhadi, M.; Khojasteh, F. Interaction of curcumin with one subunit of monoamine oxidase-B in comparison with safinamide: An in silico study. Thrita 2019, 8, e90380. [Google Scholar] [CrossRef]
- Mi, C.; Teng, Y.; Wang, X.; Yu, H.; Huang, Z.; Zong, W.; Zou, L. Molecular interaction of triclosan with superoxide dismutase (SOD) reveals a potentially toxic mechanism of the antimicrobial agent. Ecotoxicol. Environ. Saf. 2018, 153, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.; Han, G.; Wan, J.; Zhang, S.; Yang, J.; Zong, W.; Niu, Q.; Liu, R. Catalase and superoxide dismutase response and the underlying molecular mechanism for naphthalene. Sci. Total Environ. 2020, 736, 139567. [Google Scholar] [CrossRef] [PubMed]
- Yong, T.; Chen, S.; Liang, D.; Zuo, D.; Diao, X.; Deng, C.; Wu, Y.; Hu, H.; Xie, Y.; Chen, D. Actions of inonotus obliquus against hyperuricemia through XOD and bioactives screened by molecular modeling. Int. J. Mol. Sci. 2018, 19, 3222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yong, T.; Chen, S.; Xie, Y.; Shuai, O.; Li, X.; Chen, D.; Su, J.; Jiao, C.; Liang, Y. Hypouricemic effects of extracts from Agrocybe aegerita on hyperuricemia mice and virtual prediction of bioactives by molecular docking. Front. Pharmacol. 2018, 9, 498. [Google Scholar] [CrossRef] [Green Version]
- Vianna, C.P.; de Azevedo, W.F. Identification of new potential Mycobacterium tuberculosis shikimate kinase inhibitors through molecular docking simulations. J. Mol. Model. 2012, 18, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Barve, V.; Padhye, S.; Bhonde, R. Reduction of oxidative stress induced vanadium toxicity by complexing with a flavonoid, quercetin: A pragmatic therapeutic approach for diabetes. Biometals 2006, 19, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Munigunti, R.; Mulabagal, V.; Calderón, A.I. Screening of natural compounds for ligands to PfTrxR by ultrafiltration and LC-MS based binding assay. J. Pharm. Biomed. Anal. 2011, 55, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Diamantis, D.A.; Ramesova, S.; Chatzigiannis, C.M.; Degano, I.; Gerogianni, P.S.; Karadima, K.E.; Perikleous, S.; Rekkas, D.; Gerothanassis, I.P.; Galaris, D.; et al. Exploring the oxidation and iron binding profile of a cyclodextrin encapsulated quercetin complex unveiled a controlled complex dissociation through a chemical stimulus. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1913–1924. [Google Scholar] [CrossRef]
- Muhammad, D.R.A.; Tuenter, E.; Patria, G.D.; Foubert, K.; Pieters, L.; Dewettinck, K. Phytochemical composition and antioxidant activity of Cinnamomum burmannii Blume extracts and their potential application in white chocolate. Food Chem. 2021, 340, 127983. [Google Scholar] [CrossRef] [PubMed]
- Spínola, V.; Castilho, P.C. Phytochemical profile, chemotaxonomic studies, and in vitro antioxidant activities of two endemisms from Madeira Archipelago: Melanoselinum decipiens and Monizia edulis (Apiaceae). Chem. Biodivers. 2016, 13, 1290–1306. [Google Scholar] [CrossRef]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. Int. Sch. Res. Not. 2014, 2014, 952943. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Gabaldón-Hernández, J.A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. RP-HPLC-ESI-QTOF/MS2 based strategy for the comprehensive metabolite profiling of Sclerocarya Birrea (Marula) bark. Ind. Crops Prod. 2015, 71, 214–234. [Google Scholar] [CrossRef]
- Ismail, B.B.; Pu, Y.; Fan, L.; Dandago, M.A.; Guo, M.; Liu, D. Characterizing the phenolic constituents of baobab (Adansonia digitata) fruit shell by LC-MS/QTOF and their in vitro biological activities. Sci. Total Environ. 2019, 694, 133387. [Google Scholar] [CrossRef]
- Jug, U.; Naumoska, K.; Vovk, I. (−)-Epicatechin-an important contributor to the antioxidant activity of Japanese knotweed rhizome bark extract as determined by antioxidant activity-guided fractionation. Antioxidants 2021, 10, 133. [Google Scholar] [CrossRef]
- Shrestha, A. Phytochemical Analysis of Rhododendron Species. Ph.D. Thesis, Jacobs University Bremen, Bremen, Germany, 2016. [Google Scholar]
- Koutsoulas, A.; Arnecká, M.; Slanina, J.; Tóth, J.; Slaninová, I. Characterization of phenolic compounds and antiproliferative effects of Salvia pomifera and Salvia fruticosa extracts. Molecules 2019, 24, 2921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedro, M.; Martina, C.; Michele, T.; Kelli, H.; Chiara, D.; Daniele, D.R. Phytochemical profiling of flavonoids, phenolic acids, terpenoids, and volatile fraction of a Rosemary (Rosmarinus officinalis L.) extract. Molecules 2016, 21, 1576. [Google Scholar]
- Celano, R.; Piccinelli, A.L.; Pagano, I.; Roscigno, G.; Campone, L.; De Falco, E.; Russo, M.; Rastrelli, L. Oil distillation wastewaters from aromatic herbs as new natural source of antioxidant compounds. Food Res. Int. 2017, 99, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Samani, M.R.; D’Urso, G.; Montoro, P.; Pirbalouti, A.G.; Piacente, S. Effects of bio-fertilizers on the production of specialized metabolites in Salvia officinalis L. leaves: An analytical approach based on LC-ESI/LTQ-Orbitrap/MS and multivariate data analysis. J. Pharm. Biomed. Anal. 2021, 197, 113951. [Google Scholar]
- Choi, S.H.; Jang, G.W.; Choi, S.I.; Jung, T.D.; Cho, B.Y.; Sim, W.S.; Han, X.; Lee, J.S.; Kim, D.Y.; Kim, D.B.; et al. Development and validation of an analytical method for carnosol, carnosic acid and rosmarinic acid in food matrices and evaluation of the antioxidant activity of rosemary extract as a food additive. Antioxidants 2019, 8, 76. [Google Scholar] [CrossRef] [Green Version]
- Mira-Sánchez, M.D.; Castillo-Sánchez, J.; Morillas-Ruiz, J.M. Comparative study of rosemary extracts and several synthetic and natural food antioxidants relevance of carnosic acid/carnosol ratio. Food Chem. 2020, 309, 125688. [Google Scholar] [CrossRef]
- Paital, B.; Sablok, G.; Kumar, S.; Singh, S.K.; Chainy, G.B.N. Investigating the conformational structure and potential site interactions of SOD inhibitors on Ec-SOD in marine mud crab Scylla serrata: A molecular modeling approach. Interdiscip. Sci. Comput. Life Sci. 2016, 8, 312–318. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.; Ma, B.; Chen, L.; Song, F.; Liu, Z.; Liu, C.M. Rapid screening and detection of XOD inhibitors from S. tamariscina by ultrafiltration LC-PDA-ESI-MS combined with HPCCC. Anal. Bioanal. Chem. 2014, 406, 7379–7387. [Google Scholar] [CrossRef]

| Samples | TPC (mg GAE/g dw) | TFC (mg RE/g dw) |
|---|---|---|
| CE | 541.111 ± 1.432 b | 180.667 ± 0.301 b |
| n-Hex | 96.622 ± 0.779 e | 51.270 ± 0.366 e |
| EA | 590.526 ± 1.468 a | 280.800 ± 0.980 a |
| n-BuOH | 533.485 ± 2.143 c | 89.333 ± 0.562 c |
| WA | 287.583 ± 0.745 d | 69.387 ± 0.261 d |
| Peaks | SOD (PDB 1CBJ) | XOD (PDB 1FIQ) | ||||
|---|---|---|---|---|---|---|
| BE (Kcal/mol) | Ki | Hydrogen Bonds | BE (Kcal/mol) | Ki | Hydrogen Bonds | |
| 1 | −3.84 ± 0.37 | 1.69 ± 0.99 mM | Arg141, Gly139, Ala138, Thr135 | ND | ND | ND |
| 3 | −4.41 ± 0.01 | 588.63 ± 7.84 µM | Arg141, Gly139, Thr135, Ala138 | ND | ND | ND |
| 9 | −5.04 ± 0.01 | 203.14 ± 1.47 µM | Lys134, Asn63 | ND | ND | ND |
| 15 | ND | ND | ND | −8.24 ± 0.71 | 1.27 ± 1.24 µM | Ser 876 |
| DTC # | −2.77 ± 0.13 | 9.52 ± 2.05 mM | His61, Asn63 | ND | ND | ND |
| ALL ## | ND | ND | ND | −6.28 ± 0.00 | 24.82 ± 0.00 µM | Phe1009, Ala1149 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, M.-X.; Chen, G.-L.; Guo, M.-Q. Potential Antioxidative Components in Azadirachta indica Revealed by Bio-Affinity Ultrafiltration with SOD and XOD. Antioxidants 2022, 11, 658. https://doi.org/10.3390/antiox11040658
Fan M-X, Chen G-L, Guo M-Q. Potential Antioxidative Components in Azadirachta indica Revealed by Bio-Affinity Ultrafiltration with SOD and XOD. Antioxidants. 2022; 11(4):658. https://doi.org/10.3390/antiox11040658
Chicago/Turabian StyleFan, Min-Xia, Gui-Lin Chen, and Ming-Quan Guo. 2022. "Potential Antioxidative Components in Azadirachta indica Revealed by Bio-Affinity Ultrafiltration with SOD and XOD" Antioxidants 11, no. 4: 658. https://doi.org/10.3390/antiox11040658
APA StyleFan, M.-X., Chen, G.-L., & Guo, M.-Q. (2022). Potential Antioxidative Components in Azadirachta indica Revealed by Bio-Affinity Ultrafiltration with SOD and XOD. Antioxidants, 11(4), 658. https://doi.org/10.3390/antiox11040658






