Effects of Sodium Pyruvate on Vanadyl Sulphate-Induced Reactive Species Generation and Mitochondrial Destabilisation in CHO-K1 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Dose Selection
2.4. Determination of Cytotoxicity Using the Resazurin Assay
2.5. Determination of Total and Mitochondrial ROS Production Using DCFH-DA and DHR123 Dyes
2.6. Mitochondrial Membrane Potential (MMP) Assessment with MitoTell Orange Dye
2.7. Mitochondrial Membrane Potential (MMP) Assessment with JC-10 Dye
2.8. Statistical Analysis
3. Results
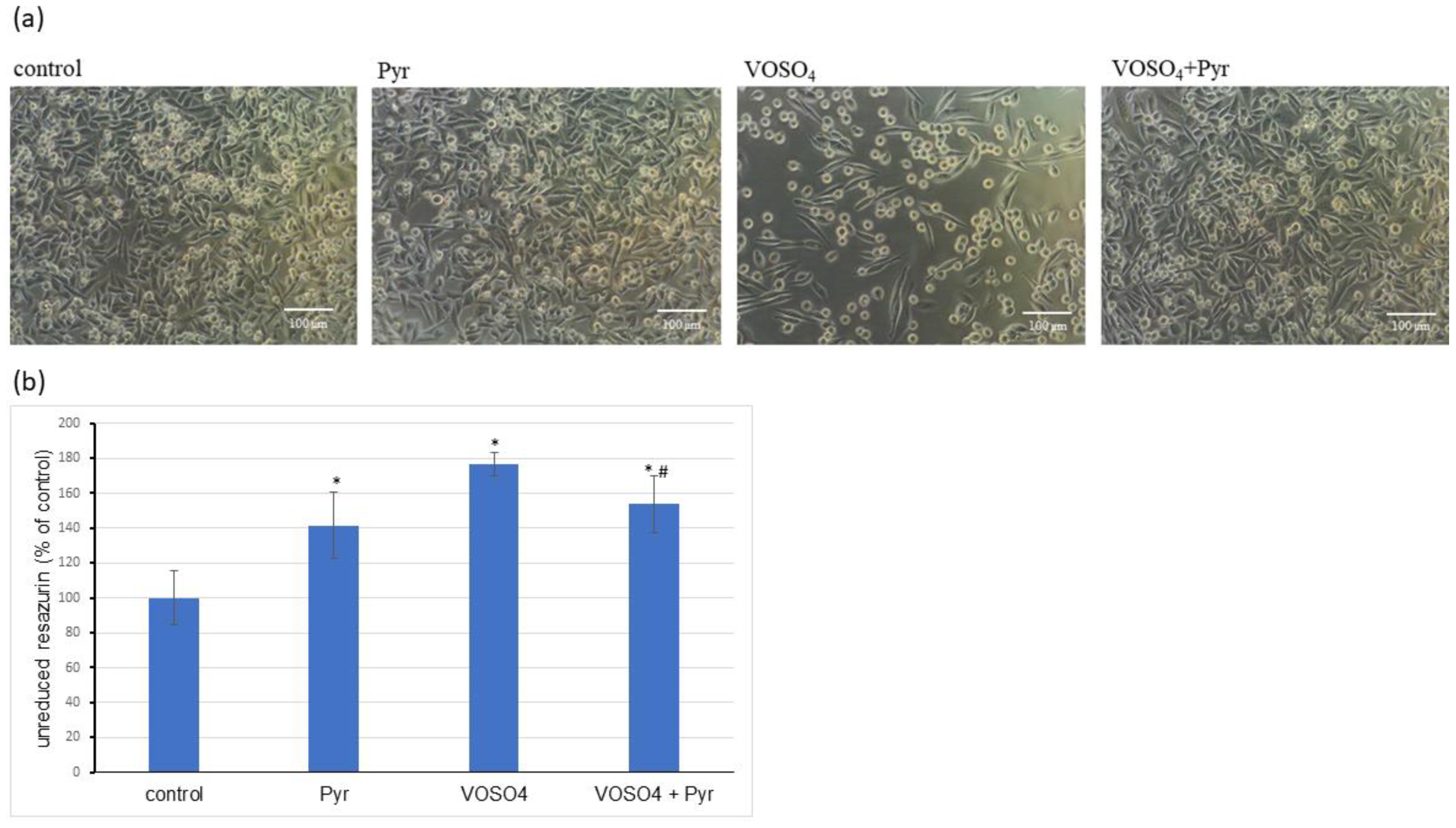
3.1. Cytotoxicity Assessment
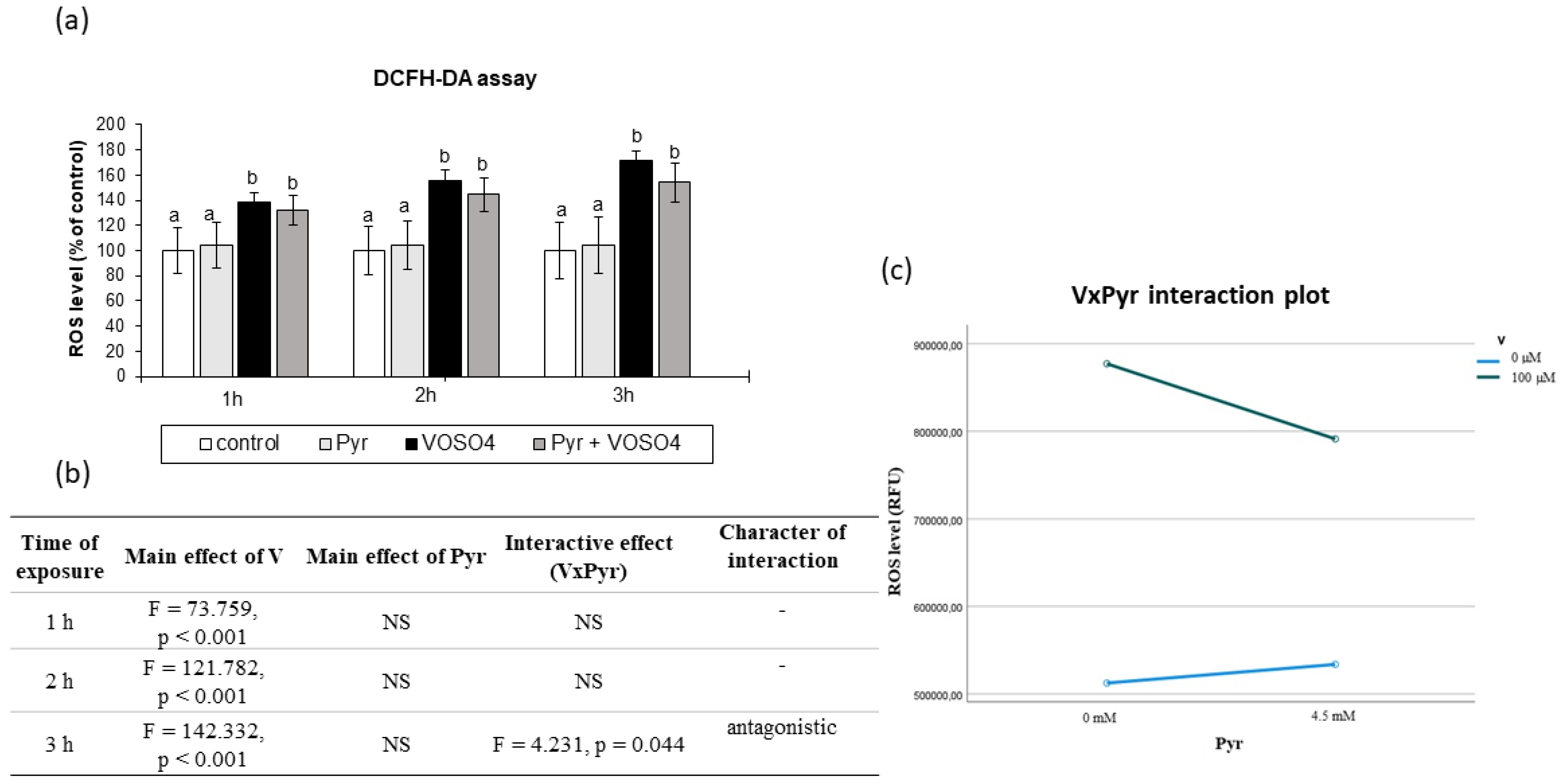
3.2. ROS Generation Assessed by DCFH-DA Staining
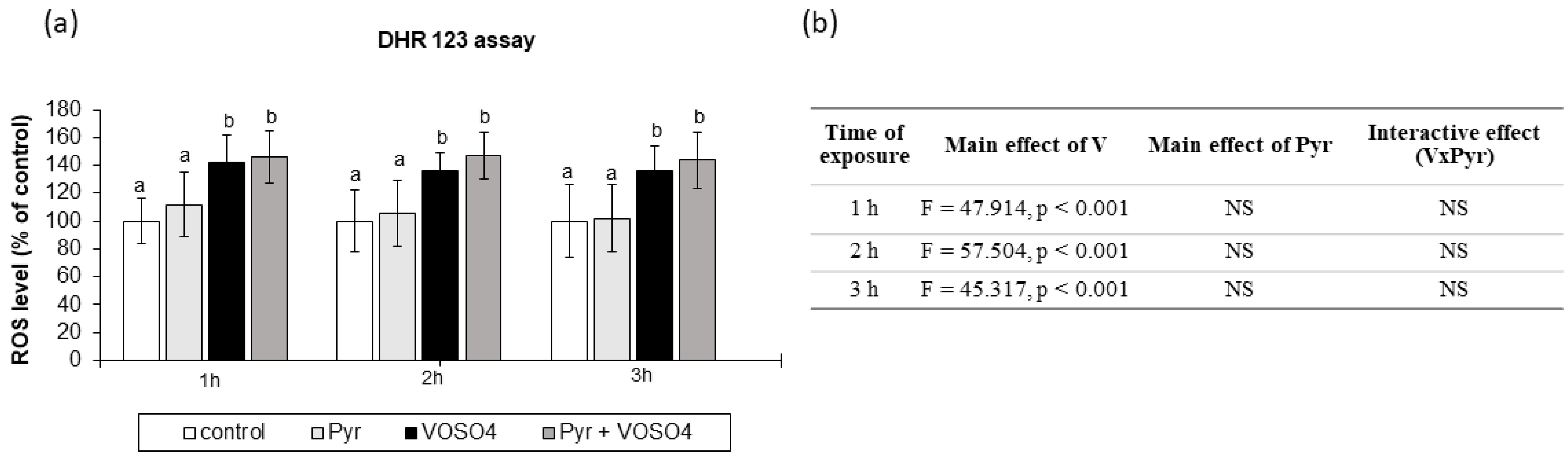
3.3. ROS Generation Measured by DHR123 Staining
3.4. Mitochondrial Membrane Potential Assessed by MitoTell Orange Staining
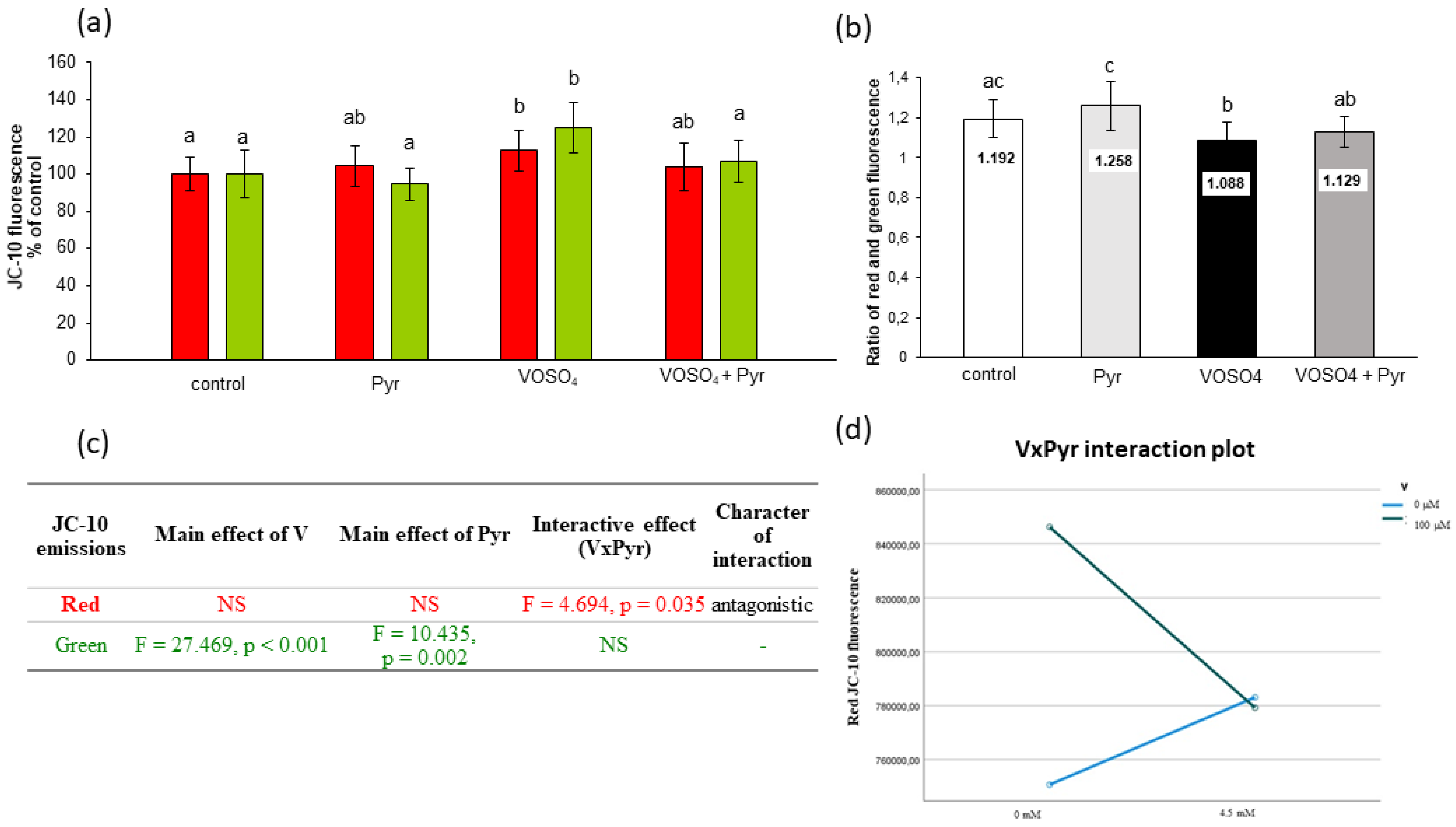
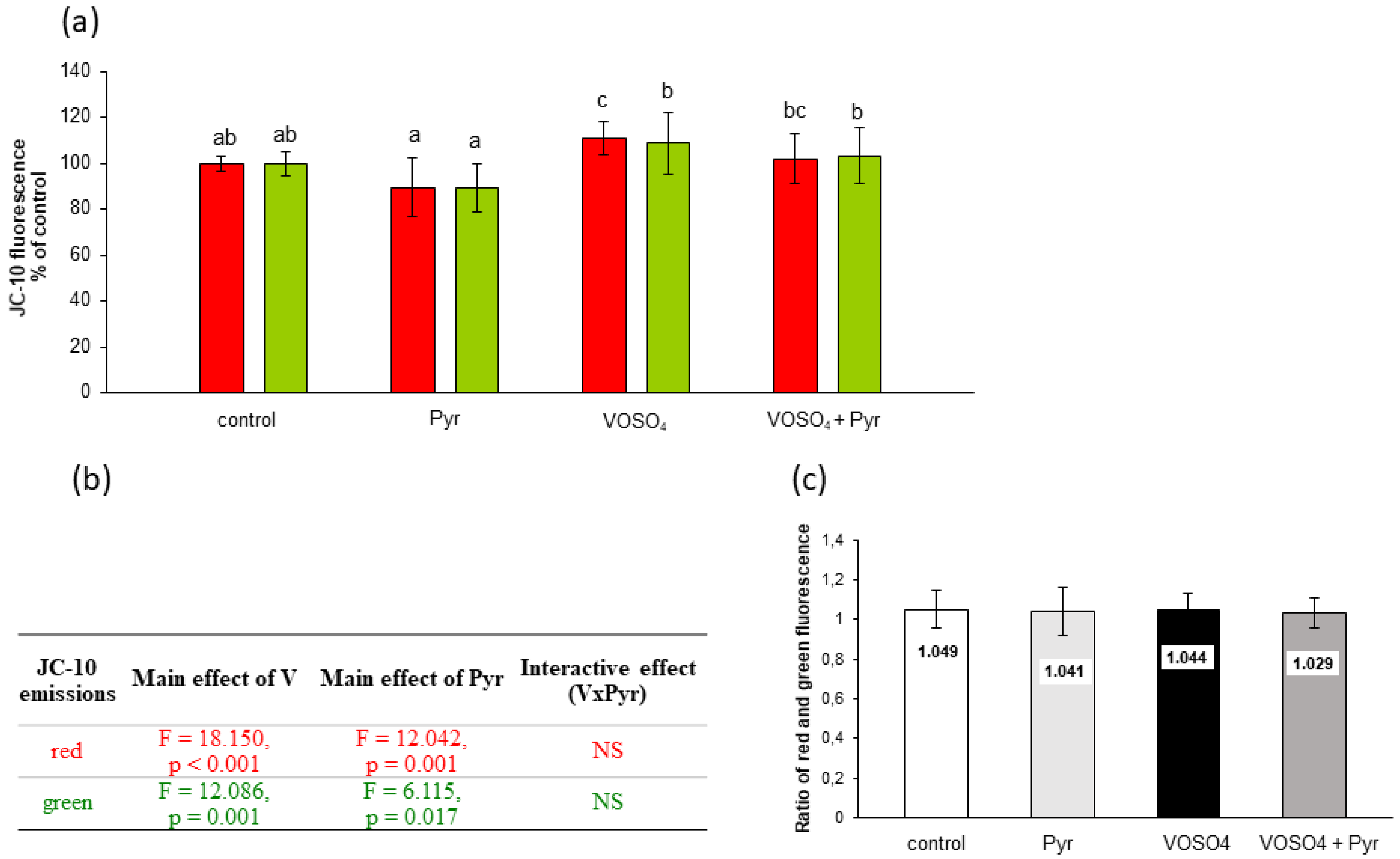
3.5. Mitochondrial Membrane Potential Assessed by JC-10 Staining
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Petranikova, M.; Tkaczyk, A.H.; Bartl, A.; Amato, A.; Lapkovskis, V.; Tunsu, C. Vanadium Sustainability in the Context of Innovative Recycling and Sourcing Development. Waste Manag. 2020, 113, 521–544. [Google Scholar] [CrossRef] [PubMed]
- Ścibior, A.; Pietrzyk, Ł.; Plewa, Z.; Skiba, A. Vanadium: Risks and Possible Benefits in the Light of a Comprehensive Overview of Its Pharmacotoxicological Mechanisms and Multi-Applications with a Summary of Further Research Trends. J. Trace Elem. Med. Biol. 2020, 61, 126508. [Google Scholar] [CrossRef] [PubMed]
- Ścibior, A.; Wnuk, E.; Gołębiowska, D. Wild Animals in Studies on Vanadium Bioaccumulation—Potential Animal Models of Environmental Vanadium Contamination: A Comprehensive Overview with a Polish Accent. Sci. Total Environ. 2021, 785, 147205. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Klein, E.M.; Vengosh, A. Global Biogeochemical Cycle of Vanadium. Proc. Natl. Acad. Sci. USA 2017, 114, E11092–E11100. [Google Scholar] [CrossRef] [Green Version]
- Fortoul, T.I.; Rojas-Lemus, M.; Rodriguez-Lara, V.; Gonzalez-Villalva, A.; Ustarroz-Cano, M.; Cano-Gutierrez, G.; Gonzalez-Rendon, S.E.; Montaño, L.F.; Altamirano-Lozano, M. Overview of Environmental and Occupational Vanadium Exposure and Associated Health Outcomes: An Article Based on a Presentation at the 8th International Symposium on Vanadium Chemistry, Biological Chemistry, and Toxicology, Washington DC, 15–18 August 2012. J. Immunotoxicol. 2014, 11, 13–18. [Google Scholar] [CrossRef]
- Ivancsits, S.; Pilger, A.; Diem, E.; Schaffer, A.; Rüdiger, H.W. Vanadate Induces DNA Strand Breaks in Cultured Human Fibroblasts at Doses Relevant to Occupational Exposure. Mutat. Res. 2002, 519, 25–35. [Google Scholar] [CrossRef]
- Ehrlich, V.A.; Nersesyan, A.K.; Atefie, K.; Hoelzl, C.; Ferk, F.; Bichler, J.; Valic, E.; Schaffer, A.; Schulte-Hermann, R.; Fenech, M.; et al. Inhalative Exposure to Vanadium Pentoxide Causes DNA Damage in Workers: Results of a Multiple End Point Study. Environ. Health Perspect 2008, 116, 1689–1693. [Google Scholar] [CrossRef]
- Malandrino, P.; Russo, M.; Ronchi, A.; Minoia, C.; Cataldo, D.; Regalbuto, C.; Giordano, C.; Attard, M.; Squatrito, S.; Trimarchi, F.; et al. Increased Thyroid Cancer Incidence in a Basaltic Volcanic Area Is Associated with Non-Anthropogenic Pollution and Biocontamination. Endocrine 2016, 53, 471–479. [Google Scholar] [CrossRef]
- Li, H.; Zhou, D.; Zhang, Q.; Feng, C.; Zheng, W.; He, K.; Lan, Y. Vanadium Exposure-Induced Neurobehavioral Alterations among Chinese Workers. Neurotoxicology 2013, 36, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Bell, M.L.; Ebisu, K.; Peng, R.D.; Samet, J.M.; Dominici, F. Hospital Admissions and Chemical Composition of Fine Particle Air Pollution. Am. J. Respir. Crit. Care Med. 2009, 179, 1115–1120. [Google Scholar] [CrossRef]
- Hu, J.; Xia, W.; Pan, X.; Zheng, T.; Zhang, B.; Zhou, A.; Buka, S.L.; Bassig, B.A.; Liu, W.; Wu, C.; et al. Association of Adverse Birth Outcomes with Prenatal Exposure to Vanadium: A Population-Based Cohort Study. Lancet Planet. Health 2017, 1, e230–e241. [Google Scholar] [CrossRef]
- Hu, J.; Peng, Y.; Zheng, T.; Zhang, B.; Liu, W.; Wu, C.; Jiang, M.; Braun, J.M.; Liu, S.; Buka, S.L.; et al. Effects of Trimester-Specific Exposure to Vanadium on Ultrasound Measures of Fetal Growth and Birth Size: A Longitudinal Prospective Prenatal Cohort Study. Lancet Planet. Health 2018, 2, e427–e437. [Google Scholar] [CrossRef] [Green Version]
- Ścibior, A.; Kurus, J. Vanadium and Oxidative Stress Markers—In Vivo Model: A Review. Curr. Med. Chem. 2019, 26, 5456–5500. [Google Scholar] [CrossRef]
- Folarin, O.R.; Adaramoye, O.A.; Akanni, O.O.; Olopade, J.O. Changes in the Brain Antioxidant Profile after Chronic Vanadium Administration in Mice. Metab. Brain. Dis. 2018, 33, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, S.; Francés, D.; García, G.B. ROS Formation and Antioxidant Status in Brain Areas of Rats Exposed to Sodium Metavanadate. Neurotoxicol. Teratol. 2011, 33, 297–302. [Google Scholar] [CrossRef]
- Samira, M.; Mounira, T.; Kamel, K.; Yacoubi, M.T.; Ben Rhouma, K.; Sakly, M.; Tebourbi, O. Hepatotoxicity of Vanadyl Sulfate in Nondiabetic and Streptozotocin-Induced Diabetic Rats. Can J. Physiol. Pharmacol. 2018, 96, 1076–1083. [Google Scholar] [CrossRef]
- Zwolak, I. Protective Effects of Dietary Antioxidants against Vanadium-Induced Toxicity: A Review. Oxid. Med. Cell Longev. 2020, 2020, 1490316. [Google Scholar] [CrossRef]
- Gummow, B.; Botha, C.J.; Williams, M.C. Chronic Vanadium Poisoning in Calves and Its Treatment with Calcium Disodium Ethylenediaminetetraacetate. Vet. Res. Commun. 2006, 30, 807–822. [Google Scholar] [CrossRef]
- Sanchez, D.J.; Colomina, M.T.; Domingo, J.L.; Corbella, J. Prevention by Sodium 4,5-Dihydroxybenzene1,3-Disulfonate (Tiron) of Vanadium-Induced Behavioral Toxicity in Rats. Biol. Trace Elem. Res. 1999, 69, 249–259. [Google Scholar] [CrossRef]
- Flora, S.J.S.; Pachauri, V. Chelation in Metal Intoxication. Int. J. Environ. Res. Public Health 2010, 7, 2745–2788. [Google Scholar] [CrossRef] [Green Version]
- Poteet, E.; Winters, A.; Xie, L.; Ryou, M.-G.; Liu, R.; Yang, S.-H. In Vitro Protection by Pyruvate against Cadmium-Induced Cytotoxicity in Hippocampal HT-22 Cells. J. Appl. Toxicol. 2014, 34, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Chavali, V.D.; Agarwal, M.; Vyas, V.K.; Saxena, B. Neuroprotective Effects of Ethyl Pyruvate against Aluminum Chloride-Induced Alzheimer’s Disease in Rats via Inhibiting Toll-Like Receptor 4. J. Mol. Neurosci. 2020, 70, 836–850. [Google Scholar] [CrossRef] [PubMed]
- Sul, J.-W.; Kim, T.-Y.; Yoo, H.J.; Kim, J.; Suh, Y.-A.; Hwang, J.J.; Koh, J.-Y. A Novel Mechanism for the Pyruvate Protection against Zinc-Induced Cytotoxicity: Mediation by the Chelating Effect of Citrate and Isocitrate. Arch. Pharm. Res. 2016, 39, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Schillinger, W.; Hünlich, M.; Sossalla, S.; Hermann, H.-P.; Hasenfuss, G. Intracoronary Pyruvate in Cardiogenic Shock as an Adjunctive Therapy to Catecholamines and Intra-Aortic Balloon Pump Shows Beneficial Effects on Hemodynamics. Clin. Res. Cardiol. 2011, 100, 433–438. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zhou, S.; Chen, C.; Ma, L.; Luo, D.; Tian, X.; Dong, X.; Zhou, Y.; Yang, Y.; Cui, Y. Therapeutic Potential of Pyruvate Therapy for Patients with Mitochondrial Diseases: A Systematic Review. Ther. Adv. Endocrinol. 2020, 11, 2042018820938240. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Briefing Information for the June 23, 2016 Meeting of the Pharmacy Compounding Advisory Committee (PCAC); FDA: Silver Spring, MD, USA, 2019. [Google Scholar]
- Sharma, P.; Mongan, P.D. Hypertonic Sodium Pyruvate Solution Is More Effective than Ringer’s Ethyl Pyruvate in the Treatment of Hemorrhagic Shock. Shock 2010, 33, 532–540. [Google Scholar] [CrossRef]
- Zwolak, I.; Gołębiowska, D. Protective Activity of Pyruvate against Vanadium-Dependent Cytotoxicity in Chinese Hamster Ovary (CHO-K1) Cells. Toxicol. Ind. Health 2018, 34, 283–292. [Google Scholar] [CrossRef]
- Koprivica, I.; Vujičić, M.; Gajić, D.; Saksida, T.; Stojanović, I. Ethyl Pyruvate Stimulates Regulatory T Cells and Ameliorates Type 1 Diabetes Development in Mice. Front Immunol. 2018, 9, 3130. [Google Scholar] [CrossRef]
- Turkyilmaz, S.; Cekic, A.B.; Usta, A.; Alhan, E.; Kural, B.V.; Ercin, C.; Sağlam, K. Ethyl Pyruvate Treatment Ameliorates Pancreatic Damage: Evidence from a Rat Model of Acute Necrotizing Pancreatitis. Arch. Med. Sci. 2019, 15, 232–239. [Google Scholar] [CrossRef] [Green Version]
- Mallet, R.T.; Olivencia-Yurvati, A.H.; Bünger, R. Pyruvate Enhancement of Cardiac Performance: Cellular Mechanisms and Clinical Application. Exp. Biol. Med. 2018, 243, 198–210. [Google Scholar] [CrossRef]
- Ramos-Ibeas, P.; Barandalla, M.; Colleoni, S.; Lazzari, G. Pyruvate Antioxidant Roles in Human Fibroblasts and Embryonic Stem Cells. Mol. Cell. Biochem. 2017, 429, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, E.; Juan-García, A.; Font, G.; Ruiz, M.J. Reactive Oxygen Species Induced by Beauvericin, Patulin and Zearalenone in CHO-K1 Cells. Toxicol. Vitr. 2009, 23, 1504–1509. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Foldbjerg, R.; Miclaus, T.; Wang, L.; Singh, R.; Hayashi, Y.; Sutherland, D.; Chen, C.; Autrup, H.; Beer, C. Multi-Platform Genotoxicity Analysis of Silver Nanoparticles in the Model Cell Line CHO-K1. Toxicol. Lett. 2013, 222, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-K.; Park, C.-G.; Shin, H.-J.; Park, K.-H.; Lim, H.-B. Comparison of the in Vitro Toxicological Activity of Various Particulate Matter. Toxicol. Ind. Health 2018, 34, 99–109. [Google Scholar] [CrossRef]
- García-Fernández, A.J.; Bayoumi, A.E.; Pérez-Pertejo, Y.; Motas, M.; Reguera, R.M.; Ordóñez, C.; Balaña-Fouce, R.; Ordóñez, D. Alterations of the Glutathione–Redox Balance Induced by Metals in CHO-K1 Cells. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 132, 365–373. [Google Scholar] [CrossRef]
- Bayoumi, A.-E.; Garcia-Fernández, A.J.; Navas, I.; Balaña-Fouce, R.; Ordoñez, D. Cytotoxic Evaluation of Heavy Metals on Mammalian and Fish Cellular Models. Toxicol. Lett. 1996, 88, 39. [Google Scholar] [CrossRef]
- Sukanya, P.; Reddy, C.V.R. Structural Investigation, DNA Interactions and in Vitro Anticancer Studies of Transition Metal Complexes of 3-(2-(2,4-Dihydroxy Benzylidene) Hydrazinyl) Quinoxalin-2(1H) -One. J. Biomol. Struct. Dyn. 2021, 1–12. [Google Scholar] [CrossRef]
- Francisco, L.F.V.; Baldivia, D.d.; Crispim, B.d.A.; Klafke, S.M.F.F.; de Castilho, P.F.; Viana, L.F.; Santos, E.L.D.; de Oliveira, K.M.P.; Barufatti, A. Acute Toxic and Genotoxic Effects of Aluminum and Manganese Using In Vitro Models. Toxics 2021, 9, 153. [Google Scholar] [CrossRef]
- Owusu-Yaw, J.; Cohen, M.D.; Fernando, S.Y.; Wei, C.I. An Assessment of the Genotoxicity of Vanadium. Toxicol. Lett. 1990, 50, 327–336. [Google Scholar] [CrossRef]
- Olin, K.L.; Cherr, G.N.; Rifkin, E.; Keen, C.L. The Effects of Some Redox-Active Metals and Reactive Aldehydes on DNA-Protein Cross-Links in Vitro. Toxicology 1996, 110, 1–8. [Google Scholar] [CrossRef]
- Shukla, R.; Barve, V.; Padhye, S.; Bhonde, R. Reduction of Oxidative Stress Induced Vanadium Toxicity by Complexing with a Flavonoid, Quercetin: A Pragmatic Therapeutic Approach for Diabetes. Biometals 2006, 19, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Tyszka-Czochara, M.; Adach, A.; Grabowski, T.; Konieczny, P.; Pasko, P.; Ortyl, J.; Świergosz, T.; Majka, M. Selective Cytotoxicity of Complexes with N,N,N-Donor Dipodal Ligand in Tumor Cells. Int. J. Mol. Sci. 2021, 22, 1802. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, M.Z.; Srivastava, A.K. Organo-Vanadium Compounds Are Potent Activators of the Protein Kinase B Signaling Pathway and Protein Tyrosine Phosphorylation: Mechanism of Insulinomimesis. Arch. Biochem. Biophys. 2005, 440, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ye, L.; Liu, H.; Xia, Q.; Zhang, Y.; Yang, X.; Wang, K. Vanadium Compounds Induced Mitochondria Permeability Transition Pore (PTP) Opening Related to Oxidative Stress. J. Inorg. Biochem. 2010, 104, 371–378. [Google Scholar] [CrossRef]
- Zwolak, I. Comparison of Three Different Cell Viability Assays for Evaluation of Vanadyl Sulphate Cytotoxicity in a Chinese Hamster Ovary K1 Cell Line. Toxicol. Ind. Health 2016, 32, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Palomo, G.; Rendón-Huerta, E.P.; Montaño, L.F.; Fortoul, T.I. Vanadium Compounds and Cellular Death Mechanisms in the A549 Cell Line: The Relevance of the Compound Valence. J. Appl. Toxicol. 2019, 39, 540–552. [Google Scholar] [CrossRef]
- Wozniak, K.; Blasiak, J. Vanadyl Sulfate Can Differentially Damage DNA in Human Lymphocytes and HeLa Cells. Arch. Toxicol. 2004, 78, 7–15. [Google Scholar] [CrossRef]
- Fernandez-Gomez, F.J.; Pastor, M.D.; Garcia-Martinez, E.M.; Melero-Fernandez de Mera, R.; Gou-Fabregas, M.; Gomez-Lazaro, M.; Calvo, S.; Soler, R.M.; Galindo, M.F.; Jordán, J. Pyruvate Protects Cerebellar Granular Cells from 6-Hydroxydopamine-Induced Cytotoxicity by Activating the Akt Signaling Pathway and Increasing Glutathione Peroxidase Expression. Neurobiol. Dis. 2006, 24, 296–307. [Google Scholar] [CrossRef]
- Wang, X.; Perez, E.; Liu, R.; Yan, L.-J.; Mallet, R.T.; Yang, S.-H. Pyruvate Protects Mitochondria from Oxidative Stress in Human Neuroblastoma SK-N-SH Cells. Brain Res. 2007, 1132, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Elizondo, M.B.; Barenholz-Cohen, T.; Weihs, D. Sodium Pyruvate Pre-Treatment Prevents Cell Death Due to Localised, Damaging Mechanical Strains in the Context of Pressure Ulcers. Int. Wound. J. 2019, 16, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Slinker, B.K. The Statistics of Synergism. J. Mol. Cell. Cardiol. 1998, 30, 723–731. [Google Scholar] [CrossRef]
- Zwolak, I. Comparison of Five Different in Vitro Assays for Assessment of Sodium Metavanadate Cytotoxicity in Chinese Hamster Ovary Cells (CHO-K1 Line). Toxicol. Ind. Health 2015, 31, 677–690. [Google Scholar] [CrossRef]
- Di Pietro, A.; Visalli, G.; Baluce, B.; Micale, R.T.; La Maestra, S.; Spataro, P.; De Flora, S. Multigenerational Mitochondrial Alterations in Pneumocytes Exposed to Oil Fly Ash Metals. Int. J. Hyg. Environ. Health 2011, 214, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, Z.; Ding, M.; Li, J.; Ye, J.; Leonard, S.S.; Shen, H.M.; Butterworth, L.; Lu, Y.; Costa, M.; et al. Vanadate Induces P53 Transactivation through Hydrogen Peroxide and Causes Apoptosis. J. Biol. Chem. 2000, 275, 32516–32522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Medan, D.; Mercer, R.; Overmiller, D.; Leornard, S.; Castranova, V.; Shi, X.; Ding, M.; Huang, C.; Rojanasakul, Y. Vanadium-Induced Apoptosis and Pulmonary Inflammation in Mice: Role of Reactive Oxygen Species. J. Cell Physiol. 2003, 195, 99–107. [Google Scholar] [CrossRef]
- Crow, J.P. Dichlorodihydrofluorescein and Dihydrorhodamine 123 Are Sensitive Indicators of Peroxynitritein Vitro:Implications for Intracellular Measurement of Reactive Nitrogen and Oxygen Species. Nitric. Oxide. 1997, 1, 145–157. [Google Scholar] [CrossRef]
- Wrona, M.; Patel, K.; Wardman, P. Reactivity of 2’,7’-Dichlorodihydrofluorescein and Dihydrorhodamine 123 and Their Oxidized Forms toward Carbonate, Nitrogen Dioxide, and Hydroxyl Radicals. Free Radic. Biol. Med. 2005, 38, 262–270. [Google Scholar] [CrossRef]
- Glebska, J.; Koppenol, W.H. Peroxynitrite-Mediated Oxidation of Dichlorodihydrofluorescein and Dihydrorhodamine. Free Radic. Biol. Med. 2003, 35, 676–682. [Google Scholar] [CrossRef]
- Kooy, N.W.; Royall, J.A.; Ischiropoulos, H.; Beckman, J.S. Peroxynitrite-Mediated Oxidation of Dihydrorhodamine 123. Free Radic. Biol. Med. 1994, 16, 149–156. [Google Scholar] [CrossRef]
- Capella, L.S.; Gefé, M.R.; Silva, E.F.; Affonso-Mitidieri, O.; Lopes, A.G.; Rumjanek, V.M.; Capella, M.A.M. Mechanisms of Vanadate-Induced Cellular Toxicity: Role of Cellular Glutathione and NADPH. Arch. Biochem. Biophys. 2002, 406, 65–72. [Google Scholar] [CrossRef]
- Holleman, A.F. Notice Sur l’action de l’eau Oxygénée Sur Les Acides α-Cétoniques et Sur Les Dicétones 1. 2. Recl. Trav. Chim. Pays-Bas. Belg. 1904, 23, 169–172. [Google Scholar] [CrossRef]
- Battaglia, S.; De Santis, S.; Rutigliano, M.; Sallustio, F.; Picerno, A.; Frassanito, M.A.; Schaefer, I.; Vacca, A.; Moschetta, A.; Seibel, P.; et al. Uridine and Pyruvate Protect T Cells’ Proliferative Capacity from Mitochondrial Toxic Antibiotics: A Clinical Pilot Study. Sci. Rep. 2021, 11, 12841. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, G.; Ramos, M.; Ruiz, F.; Satrústegui, J.; Bogónez, E. Pyruvate Protection against Beta-Amyloid-Induced Neuronal Death: Role of Mitochondrial Redox State. J. Neurosci. Res. 2003, 73, 260–269. [Google Scholar] [CrossRef]
- Kang, Y.H.; Chung, S.J.; Kang, I.J.; Park, J.H.; Bünger, R. Intramitochondrial Pyruvate Attenuates Hydrogen Peroxide-Induced Apoptosis in Bovine Pulmonary Artery Endothelium. Mol. Cell Biochem. 2001, 216, 37–46. [Google Scholar] [CrossRef]
- Adler, L.; Chen, C.; Koutalos, Y. Mitochondria Contribute to NADPH Generation in Mouse Rod Photoreceptors. J. Biol. Chem. 2014, 289, 1519–1528. [Google Scholar] [CrossRef] [Green Version]
- Long, L.; Halliwell, B. Artefacts in Cell Culture: Pyruvate as a Scavenger of Hydrogen Peroxide Generated by Ascorbate or Epigallocatechin Gallate in Cell Culture Media. Biochem. Biophys. Res. Commun. 2009, 388, 700–704. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zwolak, I.; Wnuk, E. Effects of Sodium Pyruvate on Vanadyl Sulphate-Induced Reactive Species Generation and Mitochondrial Destabilisation in CHO-K1 Cells. Antioxidants 2022, 11, 909. https://doi.org/10.3390/antiox11050909
Zwolak I, Wnuk E. Effects of Sodium Pyruvate on Vanadyl Sulphate-Induced Reactive Species Generation and Mitochondrial Destabilisation in CHO-K1 Cells. Antioxidants. 2022; 11(5):909. https://doi.org/10.3390/antiox11050909
Chicago/Turabian StyleZwolak, Iwona, and Ewa Wnuk. 2022. "Effects of Sodium Pyruvate on Vanadyl Sulphate-Induced Reactive Species Generation and Mitochondrial Destabilisation in CHO-K1 Cells" Antioxidants 11, no. 5: 909. https://doi.org/10.3390/antiox11050909
APA StyleZwolak, I., & Wnuk, E. (2022). Effects of Sodium Pyruvate on Vanadyl Sulphate-Induced Reactive Species Generation and Mitochondrial Destabilisation in CHO-K1 Cells. Antioxidants, 11(5), 909. https://doi.org/10.3390/antiox11050909





