Exploring the Diversity of the Thioredoxin Systems in Cyanobacteria
Abstract
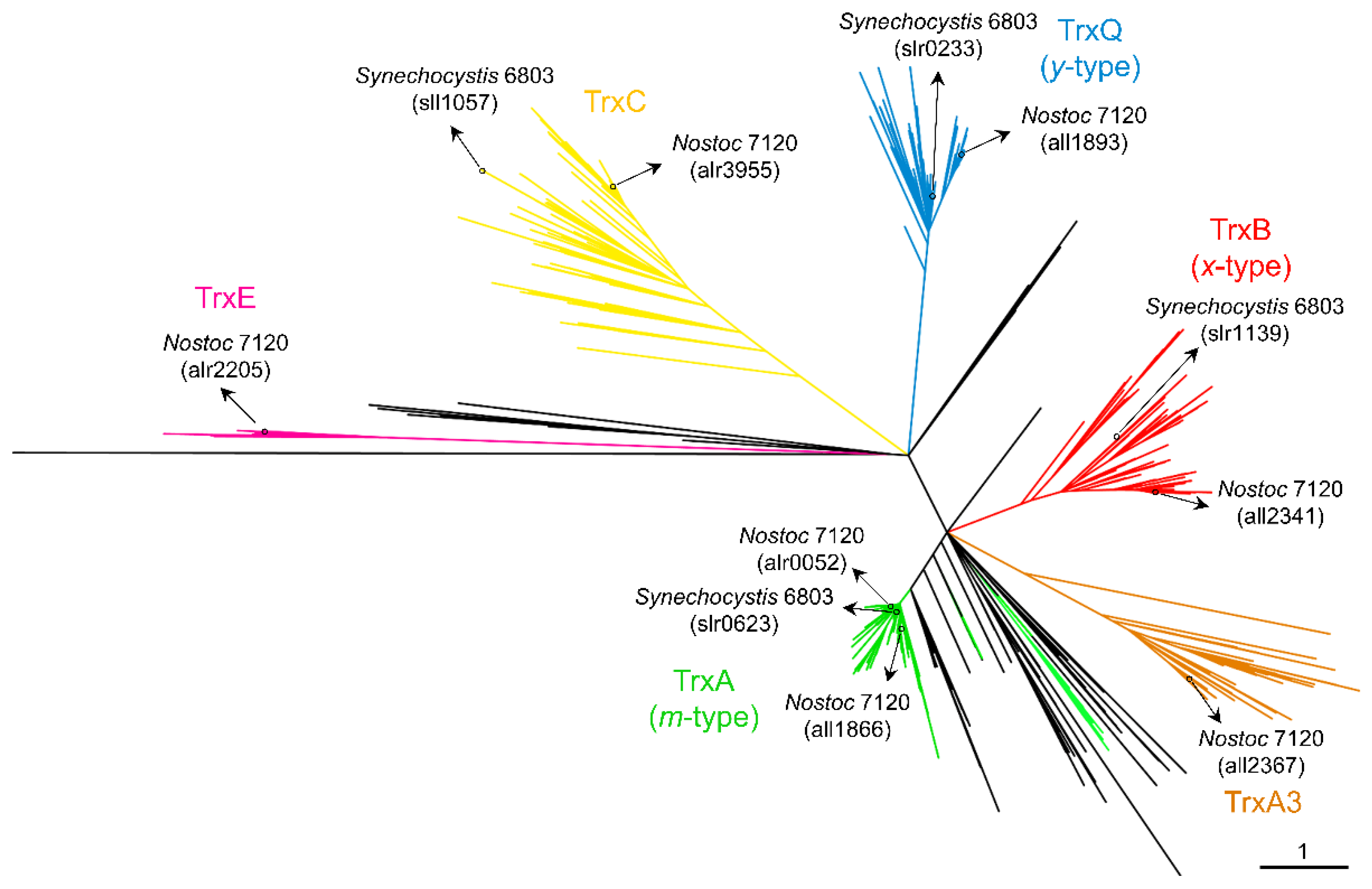
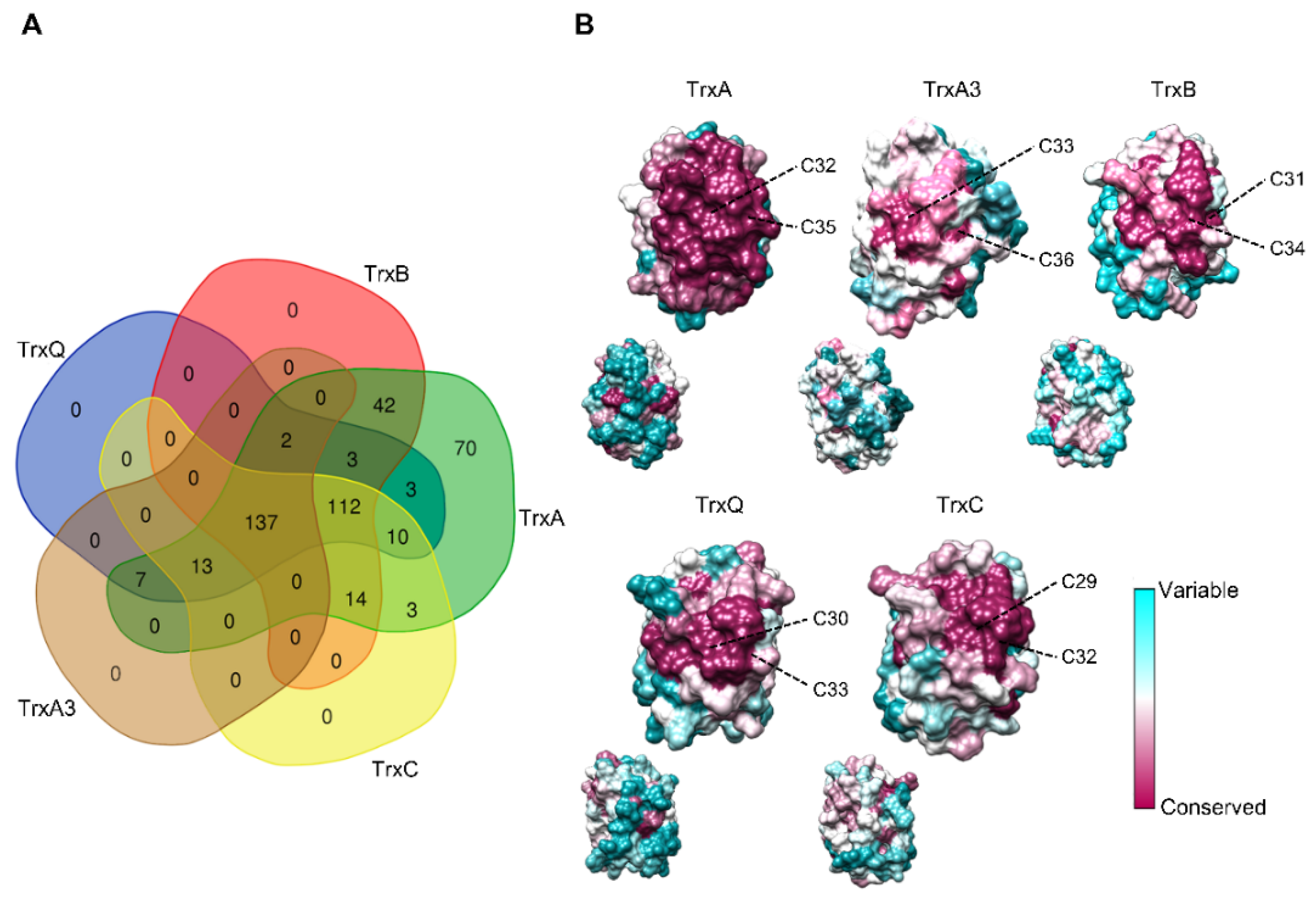
1. Distribution of Cyanobacterial Thioredoxins
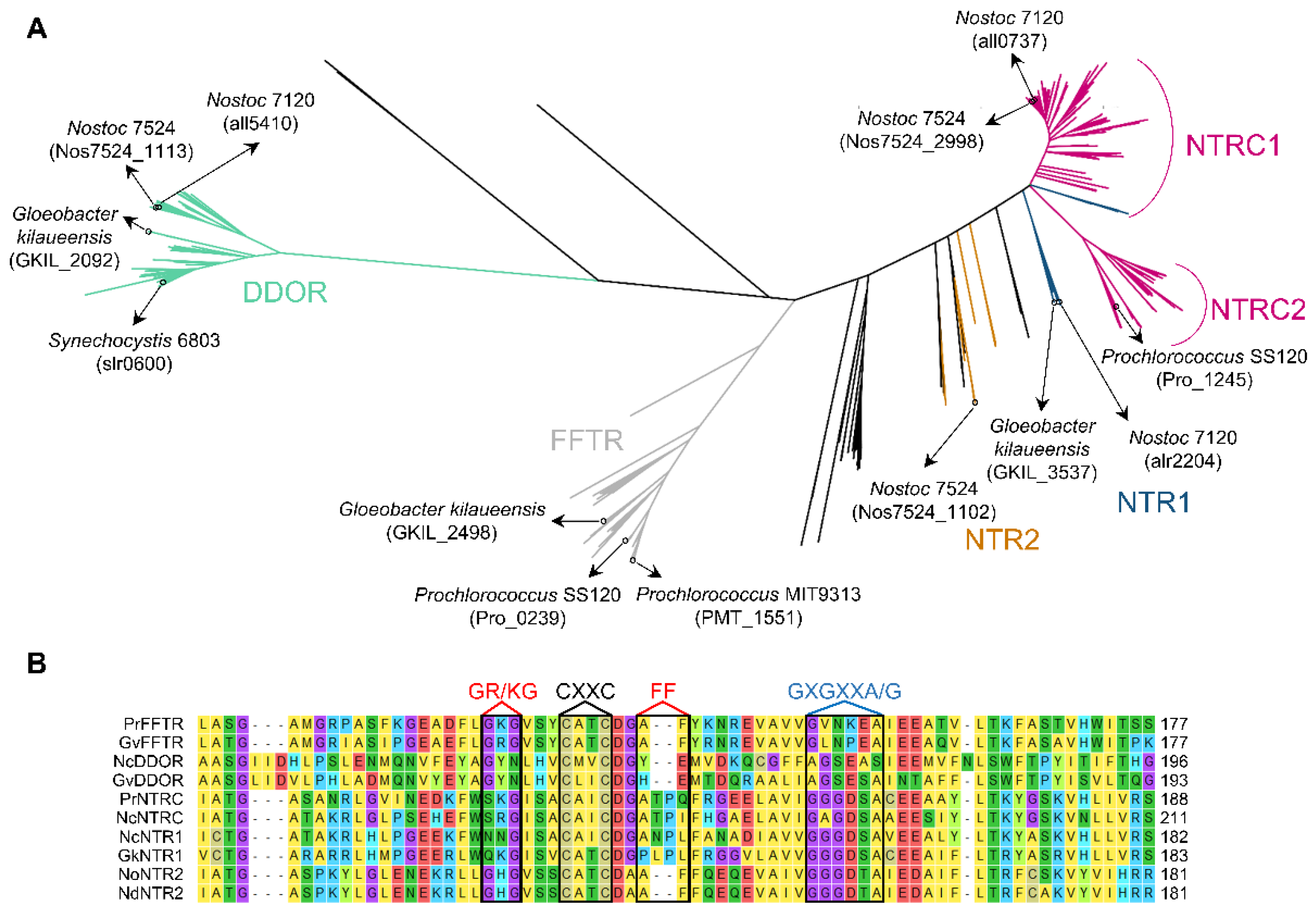
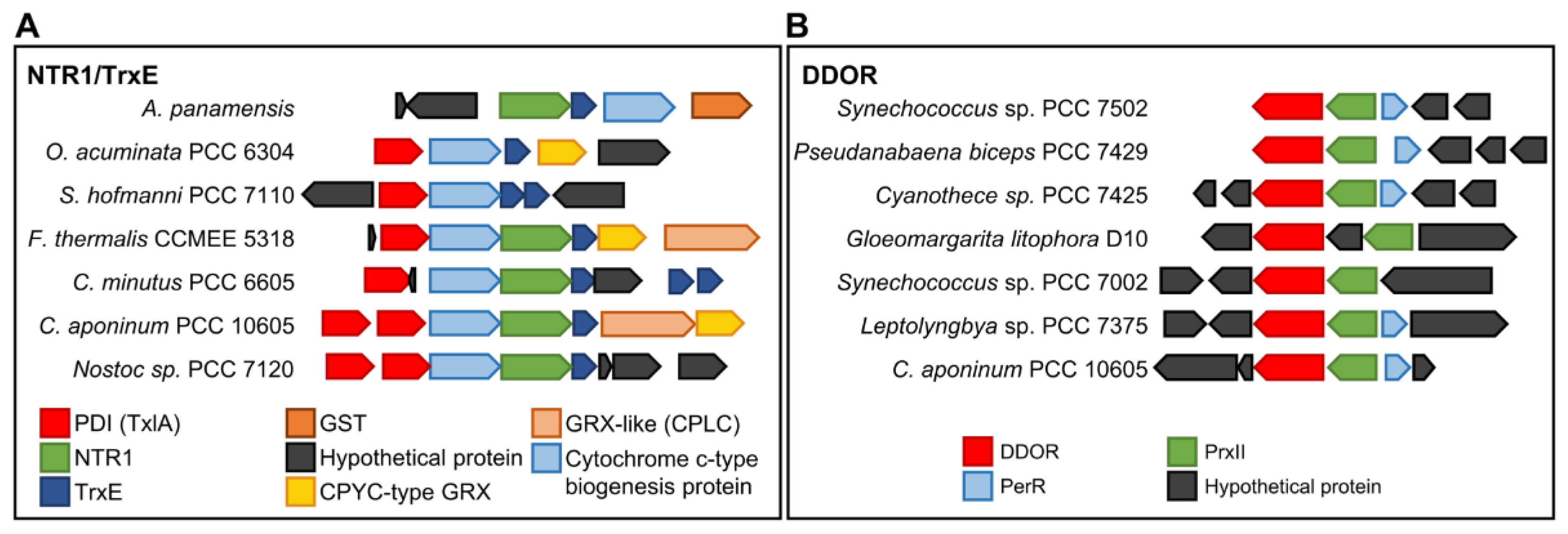
2. Diversity and Evolution of Cyanobacterial Thioredoxin Reductases
3. Timing the Expansion of TRX and TR in Cyanobacteria
4. Early Signs and In Vitro Approaches to Identify Functions of Thioredoxins
| Cellular Processes | Target Protein | Regulation In Vitro by | References |
|---|---|---|---|
| CBB cycle | FBP/SBPase | SynTrxA | [10,58,59,60,61] |
| PGK | SynTrxA | [74] | |
| CP12 | AnTrxA1 | [75,76,77,78,79] | |
| PRK | NmTrxA/MaTrxA | [59,79] | |
| OPP pathway | G6PDH | SynTrxA/SynTrxB | [62,63] |
| OpcA | AnTrxA1/AnTrxA2 | [64,65] | |
| Nitrogen fixation | NifU | AnTrxA1 | [68] |
| Glycogen metabolism | AGP | SynTrxA | [71] |
| PGM | SynTrxA | [67] | |
| Antioxidant defense | 2-Cys Prx | SynTrxA/SynTrxQ/AnTrxA1 | [35,72] |
| 1-Cys Prx | SynTrxA/SynTrxQ | [72,80] | |
| PrxQ1 | SynTrxA/SynTrxB | [72] | |
| PrxQ2 | SynTrxA/SynTrxQ | [72] | |
| PrxII | SynTrxA/SynTrxQ/SynTrxB | [72,80] | |
| Transcriptional regulation | RpaB | SynTrxA | [81] |
| RpaA | SynTrxA | [81] | |
| ManR | SynTrxA | [82] | |
| RexT | AnTrxA2 | [83] | |
| PedR | SynTrxA/SynTrxB | [84] | |
| FurA | AnTrxA | [85] | |
| GntR-like (Sll1961) | SynTrxA | [86] | |
| Protein synthesis | EF-Tu | SynTrxA | [70] |
| EF-G | SynTrxA | [69] |
5. The Role of Thioredoxins in Day-Night Cycles in Cyanobacteria
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wolosiuk, R.A.; Buchanan, B.B. Thioredoxin and glutathione regulate photosynthesis in chloroplasts. Nature 1977, 266, 565–567. [Google Scholar] [CrossRef]
- Buchanan, B.B. Role of light in the regulation of chloroplast enzymes. Annu. Rev. Plant Physiol. 1980, 31, 341–374. [Google Scholar] [CrossRef]
- Meyer, Y.; Reichheld, J.P.; Vignols, F. Thioredoxins in Arabidopsis and other plants. Photosynth. Res. 2005, 86, 419–433. [Google Scholar] [CrossRef]
- Florencio, F.J.; Pérez-Pérez, M.E.; López-Maury, L.; Mata-Cabana, A.; Lindahl, M. The diversity and complexity of the cyanobacterial thioredoxin systems. Photosynth. Res. 2006, 89, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Geigenberger, P.; Thormählen, I.; Daloso, D.M.; Fernie, A.R. The unprecedented versatility of the plant thioredoxin system. Trends Plant Sci. 2017, 22, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, B.; Wolosiuk, R.A.; Holmgren, A. Photosynthetic regulatory protein found in animal and bacterial cells. Nature 1976, 264, 669–670. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, B.B.; Schürmann, P.; Wolosiuk, R.A.; Jacquot, J.P. The ferredoxin/thioredoxin system: From discovery to molecular structures and beyond. Photosynth. Res. 2002, 73, 215–222. [Google Scholar] [CrossRef]
- Udvardy, J.; Godeh, M.M.; Farkas, G.L. Regulatory properties of a fructose 1,6-bisphosphatase from the cyanobacterium Anacystis nidulans. J. Bacteriol. 1982, 151, 203–208. [Google Scholar] [CrossRef]
- Yee, B.C.; de la Torre, A.; Crawford, N.A.; Lara, C.; Carlson, D.E.; Buchanan, B.B. The ferredoxin/thioredoxin system of enzyme regulation in a cyanobacterium. Arch. Microbiol. 1981, 130, 14–18. [Google Scholar] [CrossRef]
- Schmidt, A. Isolation of two thioredoxins from the cyanobacterium Synechococcus 6301. Arch. Microbiol. 1980, 127, 259–265. [Google Scholar] [CrossRef]
- Gleason, F.K.; Holmgren, A. Isolation and characterization of thioredoxin from the cyanobacterium, Anabaena sp. Biol. Chem. 1981, 256, 8306–8309. [Google Scholar] [CrossRef]
- Rahmatpour, N.; Hauser, D.A.; Nelson, J.M.; Chen, P.Y.; Villarreal, A.J.C.; Ho, M.-Y.; Li, F.-W. A novel thylakoid-less isolate fills a billion-year gap in the evolution of Cyanobacteria. Curr. Biol. 2021, 31, 2857–2867. [Google Scholar] [CrossRef]
- Navarro, F.; Florencio, F.J. The cyanobacterial thioredoxin gene is required for both photoautotrophic and heterotrophic growth. Plant Physiol. 1996, 111, 1067–1075. [Google Scholar] [CrossRef]
- Muller, E.G.; Buchanan, B.B. Thioredoxin is essential for photosynthetic growth. The thioredoxin m gene of Anacystis nidulans. J. Biol. Chem. 1989, 264, 4008–4014. [Google Scholar] [CrossRef]
- Mallén-Ponce, M.J.; Huertas, M.J.; Sánchez-Riego, A.M.; Florencio, F.J. Depletion of m-type thioredoxin impairs photosynthesis, carbon fixation, and oxidative stress in cyanobacteria. Plant Physiol. 2021, 187, 1325–1340. [Google Scholar] [CrossRef]
- Balsera, M.; Uberegui, E.; Susanti, D.; Schmitz, R.A.; Mukhopadhyay, B.; Schürmann, P.; Buchanan, B.B. Ferredoxin:thioredoxin reductase (FTR) links the regulation of oxygenic photosynthesis to deeply rooted bacteria. Planta 2013, 237, 619–635. [Google Scholar] [CrossRef]
- Dai, S.; Schwendtmayer, C.; Schürmann, P.; Ramaswamy, S.; Eklund, H. Redox signaling in chloroplasts: Cleavage of disulfides by an iron-sulfur cluster. Science 2000, 287, 655–658. [Google Scholar] [CrossRef]
- Jacquot, J.-P.; Eklund, H.; Rouhier, N.; Schürmann, P. Structural and evolutionary aspects of thioredoxin reductases in photosynthetic organisms. Trends Plant Sci. 2009, 14, 336–343. [Google Scholar] [CrossRef]
- Schürmann, P.; Buchanan, B.B. The ferredoxin/thioredoxin system of oxygenic photosynthesis. Antioxid. Redox Signal. 2008, 10, 1235–1274. [Google Scholar] [CrossRef]
- Dai, S.; Friemann, R.; Glauser, D.A.; Bourquin, F.; Manieri, W.; Schürmann, P.; Eklund, H. Structural snapshots along the reaction pathway of ferredoxin-thioredoxin reductase. Nature 2007, 448, 92–96. [Google Scholar] [CrossRef]
- Juniar, L.; Tanaka, H.; Yoshida, K.; Hisabori, T.; Kurisu, G. Structural basis for thioredoxin isoform-based fine-tuning of ferredoxin-thioredoxin reductase activity. Protein Sci. 2020, 29, 2538–2545. [Google Scholar] [CrossRef]
- Hosoya-Matsuda, N.; Inoue, K.; Hisabori, T. Roles of thioredoxins in the obligate anaerobic green sulfur photosynthetic bacterium Chlorobaculum tepidum. Mol. Plant 2009, 2, 336–343. [Google Scholar] [CrossRef]
- Buey, R.M.; Galindo-Trigo, S.; López-Maury, L.; Velázquez-Campoy, A.; Revuelta, J.L.; Florencio, F.J.; de Pereda, J.M.; Schürmann, P.; Buchanan, B.B.; Balsera, M. A new member of the thioredoxin reductase family from early oxygenic photosynthetic organisms. Mol. Plant 2017, 10, 212–215. [Google Scholar] [CrossRef]
- Buey, R.M.; Fernández-Justel, D.; González-Holgado, G.; Martínez-Júlvez, M.; González-López, A.; Velázquez-Campoy, A.; Medina, M.; Buchanan, B.B.; Balsera, M. Unexpected diversity of ferredoxin-dependent thioredoxin reductases in cyanobacteria. Plant Physiol. 2021, 186, 285–296. [Google Scholar] [CrossRef]
- Hammel, K.E.; Cornwell, K.L.; Buchanan, B.B. Ferredoxin/flavoprotein-linked pathway for the reduction of thioredoxin. Proc. Natl. Acad. Sci. USA 1983, 80, 3681–3685. [Google Scholar] [CrossRef]
- Buey, R.M.; Fernández-Justel, D.; de Pereda, J.M.; Revuelta, J.L.; Schürmann, P.; Buchanan, B.B.; Balsera, M. Ferredoxin-linked flavoenzyme defines a family of pyridine nucleotide-independent thioredoxin reductases. Proc. Natl. Acad. Sci. USA 2018, 115, 12967–12972. [Google Scholar] [CrossRef] [PubMed]
- Buey, R.M.; Arellano, J.B.; López-Maury, L.; Galindo-Trigo, S.; Velázquez-Campoy, A.; Revuelta, J.L.; de Pereda, J.M.; Florencio, F.J.; Schürmann, P.; Buchanan, B.B.; et al. Unprecedented pathway of reducing equivalents in a diflavin-linked disulfide oxidoreductase. Proc. Natl. Acad. Sci. USA 2017, 114, 12725–12730. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Nishizawa, A.; Kikuchi, M.; Nonaka, C.; Komuro, M.; Nakayama, M.; Kashino, Y.; Fukuda, M.; Kimura, S. Biphenyl degradation by recombinant photosynthetic cyanobacterium Synechocystis sp. PCC6803 in an oligotrophic environment using unphysiological electron transfer. Biochem. J. 2019, 476, 3615–3630. [Google Scholar] [CrossRef] [PubMed]
- Mihara, S.; Sugiura, K.; Yoshida, K.; Hisabori, T. Thioredoxin targets are regulated in heterocysts of cyanobacterium Anabaena sp. PCC 7120 in a light-independent manner. J. Exp. Bot. 2020, 71, 2018–2027. [Google Scholar] [CrossRef] [PubMed]
- Serrato, A.J.; Pérez-Ruiz, J.M.; Spínola, M.C.; Cejudo, F.J. A novel NADPH thioredoxin reductase, localized in the chloroplast, which deficiency causes hypersensitivity to abiotic stress in Arabidopsis thaliana. J. Biol. Chem. 2004, 279, 43821–43827. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ruiz, J.M.; Spínola, M.C.; Kirchsteiger, K.; Moreno, J.; Sahrawy, M.; Cejudo, F.J. Rice NTRC is a high-efficiency redox system for chloroplast protection against oxidative damage. Plant Cell 2006, 18, 2356–2368. [Google Scholar] [CrossRef]
- Sánchez-Riego, A.M.; Mata-Cabana, A.; Galmozzi, C.V.; Florencio, F.J. NADPH-thioredoxin reductase C mediates the response to oxidative stress and thermotolerance in the cyanobacterium Anabaena sp. PCC7120. Front. Microbiol. 2016, 7, 1283. [Google Scholar] [CrossRef]
- Mihara, S.; Yoshida, K.; Higo, A.; Hisabori, T. Functional significance of NADPH-thioredoxin reductase C in the antioxidant defense system of cyanobacterium Anabaena sp. PCC 7120. Plant Cell Physiol. 2016, 58, 86–94. [Google Scholar]
- Banerjee, M.; Chakravarty, D.; Ballal, A. Redox-dependent chaperone/peroxidase function of 2-Cys-Prx from the cyanobacterium Anabaena PCC7120: Role in oxidative stress tolerance. BMC Plant Biol. 2015, 15, 60. [Google Scholar] [CrossRef]
- Pascual, M.B.; Mata-Cabana, A.; Florencio, F.J.; Lindahl, M.; Cejudo, F.J. A comparative analysis of the NADPH thioredoxin reductase C-2-Cys peroxiredoxin system from plants and Cyanobacteria. Plant Physiol. 2011, 155, 1806–1816. [Google Scholar] [CrossRef]
- Hishiya, S.; Hatakeyama, W.; Mizota, Y.; Hosoya-Matsuda, N.; Motohashi, K.; Ikeuchi, M.; Hisabori, T. Binary reducing equivalent pathways using NADPH-thioredoxin reductase and ferredoxin-thioredoxin reductase in the cyanobacterium Synechocystis sp. strain PCC 6803. Plant Cell Physiol. 2008, 49, 11–18. [Google Scholar] [CrossRef][Green Version]
- Kobayashi, M.; Ishizuka, T.; Katayama, M.; Kanehisa, M. Response to oxidative stress involves a novel peroxiredoxin gene in the unicellular cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 2004, 45, 290–299. [Google Scholar] [CrossRef]
- Fischer, W.W.; Hemp, J.; Johnson, J.E. Evolution of Oxygenic Photosynthesis. Annu. Rev. Earth Planet. Sci. 2016, 44, 647–683. [Google Scholar] [CrossRef]
- Konhauser, K.O.; Lalonde, S.V.; Planavsky, N.J.; Pecoits, E.; Lyons, T.W.; Mojzsis, S.J.; Rouxel, O.J.; Barley, M.E.; Rosìere, C.; Fralick, P.W.; et al. Aerobic bacterial pyrite oxidation and acid rock drainage during the Great Oxidation Event. Nature 2011, 478, 369–373. [Google Scholar] [CrossRef]
- Warke, M.R.; Di Rocco, T.; Zerkle, A.L.; Lepland, A.; Prave, A.R.; Martin, A.P.; Ueno, Y.; Condon, D.J.; Claire, M.W. The Great Oxidation Event preceded a Paleoproterozoic “snowball Earth”. Proc. Natl. Acad. Sci. USA 2020, 117, 13314–13320. [Google Scholar] [CrossRef]
- Blank, C.E.; Sánchez-Baracaldo, P. Timing of morphological and ecological innovations in the cyanobacteria—A key to understanding the rise in atmospheric oxygen. Geobiology 2010, 8, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Saw, J.H.; Cardona, T.; Montejano, G. Complete genome sequencing of a novel Gloeobacter species from a waterfall cave in Mexico. Genome Biol. Evol. 2021, 13, evab264. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Baracaldo, P. Origin of marine planktonic cyanobacteria. Sci. Rep. 2015, 5, 17418. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, A.; Salanoubat, M.; Partensky, F.; Artiguenave, F.; Axmann, I.M.; Barbe, V.; Duprat, S.; Galperin, M.Y.; Koonin, E.V.; Le Gall, F.; et al. Genome sequence of the cyanobacterium Prochlorococcus marinus SS120, a nearly minimal oxyphototrophic genome. Proc. Natl. Acad. Sci. USA 2003, 100, 10020–10025. [Google Scholar] [CrossRef]
- Luo, H.; Friedman, R.; Tang, J.; Hughes, A.L. Genome reduction by deletion of paralogs in the marine cyanobacterium Prochlorococcus. Mol. Biol. Evol. 2011, 28, 2751–2760. [Google Scholar] [CrossRef]
- Rocap, G.; Larimer, F.W.; Lamerdin, J.; Malfatti, S.; Chain, P.; Ahlgren, N.A.; Arellano, A.; Coleman, M.; Hauser, L.; Hess, W.R.; et al. Genome divergence in two Prochlorococcus ecotypes reflects oceanic niche differentiation. Nature 2003, 424, 1042–1047. [Google Scholar] [CrossRef]
- Sun, Z.; Blanchard, J.L. Strong genome-wide selection early in the evolution of Prochlorococcus resulted in a reduced genome through the loss of a large number of small effect genes. PLoS ONE 2014, 9, e88837. [Google Scholar] [CrossRef]
- Thompson, A.W.; Huang, K.; Saito, M.A.; Chisholm, S.W. Transcriptome response of high- and low-light-adapted Prochlorococcus strains to changing iron availability. ISME J. 2011, 5, 1580–1594. [Google Scholar] [CrossRef]
- Rusch, D.B.; Martiny, A.C.; Dupont, C.L.; Halpern, A.L.; Venter, J.C. Characterization of Prochlorococcus clades from iron-depleted oceanic regions. Proc. Natl. Acad. Sci. USA 2010, 107, 16184–16189. [Google Scholar] [CrossRef]
- Bertilsson, S.; Berglund, O.; Karl, D.M.; Chisholm, S.W. Elemental composition of marine Prochlorococcus and Synechococcus: Implications for the ecological stoichiometry of the sea. Limnol. Oceanogr. 2003, 48, 1721–1731. [Google Scholar] [CrossRef]
- Biller, S.J.; Berube, P.M.; Lindell, D.; Chisholm, S.W. Prochlorococcus: The structure and function of collective diversity. Nat. Rev. Microbiol. 2015, 13, 13–27. [Google Scholar] [CrossRef]
- Schirrmeister, B.E.; Antonelli, A.; Bagheri, H.C. The origin of multicellularity in cyanobacteria. BMC Evol. Biol. 2011, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Larsson, J.; Nylander, J.A.A.; Bergman, B. Genome fluctuations in cyanobacteria reflect evolutionary, developmental and adaptive traits. BMC Evol. Biol. 2011, 11, 187. [Google Scholar] [CrossRef]
- Tomitani, A.; Knoll, A.H.; Cavanaugh, C.M.; Ohno, T. The evolutionary diversification of cyanobacteria: Molecular–phylogenetic and paleontological perspectives. Proc. Natl. Acad. Sci. USA 2006, 103, 5442–5447. [Google Scholar] [CrossRef]
- Lim, C.J.; Gleason, F.K.; Fuchs, J.A. Cloning, expression, and characterization of the Anabaena thioredoxin gene in Escherichia coli. J. Bacteriol. 1986, 168, 1258–1264. [Google Scholar] [CrossRef]
- Alam, J.; Curtis, S.; Gleason, F.K.; Gerami-Nejad, M.; Fuchs, J.A. Isolation, sequence, and expression in Escherichia coli of an unusual thioredoxin gene from the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 1989, 171, 162–171. [Google Scholar] [CrossRef]
- Pérez-Pérez, M.E.; Florencio, F.J.; Lindahl, M. Selecting thioredoxins for disulphide proteomics: Target proteomes of three thioredoxins from the cyanobacterium Synechocystis sp. PCC 6803. Proteomics 2006, 6, S186–S195. [Google Scholar] [CrossRef]
- Schmidt, A. A thioredoxin-activated fructose-1, 6-bisphosphatase from the cyanobacterium Synechococcus 6301. Planta 1981, 152, 101–104. [Google Scholar] [CrossRef]
- Crawford, N.A.; Sutton, C.W.; Yee, B.C.; Johnson, T.C.; Carlson, D.C.; Buchanan, B.B. Contrasting modes of photosynthetic enzyme regulation in oxygenic and anoxygenic prokaryotes. Arch. Microbiol. 1984, 139, 124–129. [Google Scholar] [CrossRef]
- Ip, S.-M.; Rowell, P.; Aitken, A.; Stewart, W.D.P. Purification and characterization of thioredoxin from the N2-fixing cyanobacterium Anabaena cylindrica. Eur. J. Biochem. 1984, 141, 497–504. [Google Scholar] [CrossRef]
- Gerbling, K.-P.; Steup, M.; Latzko, E. Fructose 1,6-bisphosphatase form B from Synechococcus leopoliensis hydrolyzes both fructose and sedoheptulose bisphosphate. Plant Physiol. 1986, 80, 716–720. [Google Scholar] [CrossRef]
- Cossar, J.D.; Rowell, P.; Stewart, W.D.P. Thioredoxin as a modulator of glucose-6-phosphate dehydrogenase in a N2-fixing cyanobacterium. Microbiology 1984, 130, 991–998. [Google Scholar] [CrossRef][Green Version]
- Gleason, F.K. Glucose-6-phosphate dehydrogenase from the cyanobacterium, Anabaena sp. PCC 7120: Purification and kinetics of redox modulation. Arch. Biochem. Biophys. 1996, 334, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Hagen, K.D.; Meeks, J.C. The unique cyanobacterial protein OpcA is an allosteric effector of glucose-6-phosphate dehydrogenase in Nostoc punctiforme ATCC 29133. J. Biol. Chem. 2001, 276, 11477–11486. [Google Scholar] [CrossRef]
- Mihara, S.; Wakao, H.; Yoshida, K.; Higo, A.; Sugiura, K.; Tsuchiya, A.; Nomata, J.; Wakabayashi, K.; Hisabori, T. Thioredoxin regulates G6PDH activity by changing redox states of OpcA in the nitrogen-fixing cyanobacterium Anabaena sp. PCC 7120. Biochem. J. 2018, 475, 1091–1105. [Google Scholar] [CrossRef]
- Mata-Cabana, A.; Florencio, F.J.; Lindahl, A.M. Membrane proteins from the cyanobacterium Synechocystis sp. PCC 6803 interacting with thioredoxin. Proteomics 2007, 7, 3953–3963. [Google Scholar] [CrossRef]
- Lindahl, M.; Florencio, F.J. Thioredoxin-linked processes in cyanobacteria are as numerous as in chloroplasts, but targets are different. Proc. Natl. Acad. Sci. USA 2003, 100, 16107–16112. [Google Scholar] [CrossRef]
- Nomata, J.; Maeda, M.; Isu, A.; Inoue, K.; Hisabori, T. Involvement of thioredoxin on the scaffold activity of NifU in heterocyst cells of the diazotrophic cyanobacterium Anabaena sp. strain PCC 7120. J. Biochem. 2015, 158, 253–261. [Google Scholar] [CrossRef]
- Kojima, K.; Motohashi, K.; Morota, T.; Oshita, M.; Hisabori, T.; Hayashi, H.; Nishiyama, Y. Regulation of translation by the redox state of elongation factor G in the cyanobacterium Synechocystis sp. PCC 6803. J. Biol. Chem. 2009, 284, 18685–18691. [Google Scholar] [CrossRef]
- Yutthanasirikul, R.; Nagano, T.; Jimbo, H.; Hihara, Y.; Kanamori, T.; Ueda, T.; Haruyama, T.; Konno, H.; Yoshida, K.; Hisabori, T.; et al. Oxidation of a cysteine residue in elongation factor EF-Tu reversibly inhibits translation in the cyanobacterium Synechocystis sp. PCC 6803. J. Biol. Chem. 2016, 291, 5860–5870. [Google Scholar] [CrossRef]
- Díaz-Troya, S.; López-Maury, L.; Sánchez-Riego, A.M.; Roldán, M.; Florencio, F.J. Redox Regulation of Glycogen Biosynthesis in the Cyanobacterium Synechocystis sp. PCC 6803: Analysis of the AGP and Glycogen Synthases. Mol. Plant 2014, 7, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, M.E.; Mata-Cabana, A.; Sánchez-Riego, A.M.; Lindahl, M.; Florencio, F.J. A comprehensive analysis of the peroxiredoxin reduction system in the cyanobacterium Synechocystis sp. strain PCC 6803 reveals that all five peroxiredoxins are thioredoxin dependent. J. Bacteriol. 2009, 191, 7477–7489. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.-J. Peroxiredoxins in plants and cyanobacteria. Antioxid. Redox Signal. 2011, 15, 1129–1159. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, Y.; Fukushima, Y.; Hara, S.; Hisabori, T. Redox Control of the Activity of Phosphoglycerate Kinase in Synechocystis sp. PCC6803. Plant Cell Physiol. 2013, 54, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Serra, J.L.; Llama, M.J.; Rowell, P.; Stewart, W.D.P. Purification and characterization of phosphoribulokinase from the N2-fixing cyanobacterium Anabaena cylindrica. Plant Sci. 1989, 59, 1–9. [Google Scholar] [CrossRef]
- McFarlane, C.R.; Shah, N.R.; Kabasakal, B.V.; Echeverria, B.; Cotton, C.A.R.; Bubeck, D.; Murray, J.W. Structural basis of light-induced redox regulation in the Calvin–Benson cycle in cyanobacteria. Proc. Natl. Acad. Sci. USA 2019, 116, 20984–20990. [Google Scholar] [CrossRef]
- Wilson, R.H.; Hayer-Hartl, M.; Bracher, A. Crystal structure of phosphoribulokinase from Synechococcus sp. strain PCC 6301. Acta Crystallogr. Sect. F 2019, 75, 278–289. [Google Scholar] [CrossRef]
- Yu, A.; Xie, Y.; Pan, X.; Zhang, H.; Cao, P.; Su, X.; Chang, W.; Li, M. Photosynthetic phosphoribulokinase structures: Enzymatic mechanisms and the redox regulation of the Calvin-Benson-Bassham cycle. Plant Cell 2020, 32, 1556–1573. [Google Scholar] [CrossRef]
- Hackenberg, C.; Hakanpää, J.; Cai, F.; Antonyuk, S.; Eigner, C.; Meissner, S.; Laitaoja, M.; Jänis, J.; Kerfeld, C.A.; Dittmann, E.; et al. Structural and functional insights into the unique CBS–CP12 fusion protein family in cyanobacteria. Proc. Natl. Acad. Sci. USA 2018, 115, 7141–7146. [Google Scholar] [CrossRef]
- Hosoya-Matsuda, N.; Motohashi, K.; Yoshimura, H.; Nozaki, A.; Inoue, K.; Ohmori, M.; Hisabori, T. Anti-oxidative Stress System in Cyanobacteria. Significance of type II peroxiredoxin and the role of 1-Cys peroxiredoxin in Synechocystis sp. strain PCC 6803. J. Biol. Chem. 2005, 280, 840–846. [Google Scholar] [CrossRef]
- Kadowaki, T.; Nishiyama, Y.; Hisabori, T.; Hihara, Y. Identification of OmpR-family response regulators interacting with thioredoxin in the cyanobacterium Synechocystis sp. PCC 6803. PLoS ONE 2015, 10, e0119107. [Google Scholar]
- Yamaguchi, K.; Suzuki, I.; Yamamoto, H.; Lyukevich, A.; Bodrova, I.; Los, D.A.; Piven, I.; Zinchenko, V.; Kanehisa, M.; Murata, N. A two-component Mn2+-sensing aystem negatively regulates expression of the mntCAB operon in Synechocystis. Plant Cell 2002, 14, 2901–2913. [Google Scholar] [CrossRef] [PubMed]
- Ehira, S.; Ohmori, M. The redox-sensing transcriptional regulator RexT controls expression of thioredoxin A2 in the cyanobacterium Anabaena sp. strain PCC 7120. J. Biol. Chem. 2012, 287, 40433–40440. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, M.; Nakamura, K.; Kojima, K.; Nishiyama, Y.; Hatakeyama, W.; Hisabori, T.; Hihara, Y. The PedR transcriptional regulator interacts with thioredoxin to connect photosynthesis with gene expression in cyanobacteria. Biochem. J. 2010, 431, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Guío, J.; Bes, M.T.; Balsera, M.; Calvo-Begueria, L.; Sevilla, E.; Peleato, M.L.; Fillat, M.F. Thioredoxin dependent changes in the redox states of FurA from Anabaena sp. PCC 7120. Antioxidants 2021, 10, 913. [Google Scholar] [CrossRef] [PubMed]
- Kujirai, J.; Nanba, S.; Kadowaki, T.; Oka, Y.; Nishiyama, Y.; Hayashi, Y.; Arai, M.; Hihara, Y. Interaction of the GntR-family transcription factor Sll1961 with thioredoxin in the cyanobacterium Synechocystis sp. PCC 6803. Sci. Rep. 2018, 8, 6666. [Google Scholar] [CrossRef]
- Nakamura, K.; Hihara, Y. Photon flux density-dependent gene expression in Synechocystis sp. PCC 6803 is regulated by a small, redox-responsive, LuxR-type regulator. J. Biol. Chem. 2006, 281, 36758–36766. [Google Scholar] [CrossRef] [PubMed]
- Welkie, D.G.; Rubin, B.E.; Diamond, S.; Hood, R.D.; Savage, D.F.; Golden, S.S. A hard day’s night: Cyanobacteria in diel cycles. Trends Microbiol. 2019, 27, 231–242. [Google Scholar] [CrossRef]
- Wilde, A.; Hihara, Y. Transcriptional and posttranscriptional regulation of cyanobacterial photosynthesis. Biochim. Biophys. Acta-Bioenerg. 2016, 1857, 296–308. [Google Scholar] [CrossRef]
- Pérez-Pérez, M.E.; Martín-Figueroa, E.; Florencio, F.J. Photosynthetic regulation of the cyanobacterium Synechocystis sp. PCC 6803 thioredoxin system and functional analysis of TrxB (Trx x) and TrxQ (Trx y) thioredoxins. Mol. Plant 2009, 2, 270–283. [Google Scholar] [CrossRef]
- López-Maury, L.; Heredia-Martínez, L.G.; Florencio, F.J. Characterization of TrxC, an atypical thioredoxin exclusively present in cyanobacteria. Antioxidants 2018, 7, 164. [Google Scholar] [CrossRef]
- Pulido, P.; Spínola, M.C.; Kirchsteiger, K.; Guinea, M.; Pascual, M.B.; Sahrawy, M.; Sandalio, L.M.; Dietz, K.-J.; González, M.; Cejudo, F.J. Functional analysis of the pathways for 2-Cys peroxiredoxin reduction in Arabidopsis thaliana chloroplasts. J. Exp. Bot. 2010, 61, 4043–4054. [Google Scholar] [CrossRef]
- Jurado-Flores, A.; Delgado-Requerey, V.; Gálvez-Ramírez, A.; Puerto-Galán, L.; Pérez-Ruiz, J.M.; Cejudo, F.J. Exploring the functional relationship between y-Type thioredoxins and 2-Cys peroxiredoxins in Arabidopsis chloroplasts. Antioxidants 2020, 9, 1072. [Google Scholar] [CrossRef]
- Vanacker, H.; Guichard, M.; Bohrer, A.-S.; Issakidis-Bourguet, E. Redox regulation of monodehydroascorbate reductase by thioredoxin y in plastids revealed in the context of water stress. Antioxidants 2018, 7, 183. [Google Scholar] [CrossRef]
- Deschoenmaeker, F.; Mihara, S.; Niwa, T.; Taguchi, H.; Wakabayashi, K.-I.; Hisabori, T. The absence of thioredoxin m1 and thioredoxin c in Anabaena sp. PCC 7120 leads to oxidative stress. Plant Cell Physiol. 2018, 59, 2432–2441. [Google Scholar] [CrossRef]
- Pelroy, R.A.; Rippka, R.; Stanier, R.Y. Metabolism of glucose by unicellular blue-green algae. Arch. Mikrobiol. 1972, 87, 303–322. [Google Scholar] [CrossRef]
- Knowles, V.L.; Plaxton, W.C. From genome to enzyme: Analysis of key glycolytic and oxidative pentose-phosphate pathway enzymes in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 2003, 44, 758–763. [Google Scholar] [CrossRef]
- Summers, M.L.; Wallis, J.G.; Campbell, E.L.; Meeks, J.C. Genetic evidence of a major role for glucose-6-phosphate dehydrogenase in nitrogen fixation and dark growth of the cyanobacterium Nostoc sp. strain ATCC 29133. J. Bacteriol. 1995, 177, 6184–6194. [Google Scholar] [CrossRef]
- Colón-López, M.S.; Sherman, D.M.; Sherman, L.A. Transcriptional and translational regulation of nitrogenase in light-dark- and continuous-light-grown cultures of the unicellular cyanobacterium Cyanothece sp. strain ATCC 51142. J. Bacteriol. 1997, 179, 4319–4327. [Google Scholar] [CrossRef]
- Toepel, J.; Welsh, E.; Summerfield, T.C.; Pakrasi, H.B.; Sherman, L.A. Differential transcriptional analysis of the cyanobacterium Cyanothece sp. strain ATCC 51142 during light-dark and continuous-light growth. J. Bacteriol. 2008, 190, 3904–3913. [Google Scholar] [CrossRef]
- Kumar, K.; Mella-Herrera, R.A.; Golden, J.W. Cyanobacterial heterocysts. Cold Spring Harb. Perspect. Biol. 2010, 2, a000315. [Google Scholar] [CrossRef] [PubMed]
- Nicolaisen, K.; Hahn, A.; Schleiff, E. The cell wall in heterocyst formation by Anabaena sp. PCC 7120. J. Basic Microbiol. 2009, 49, 5–24. [Google Scholar] [CrossRef]
- Cumino, A.C.; Marcozzi, C.; Barreiro, R.; Salerno, G.L. Carbon cycling in Anabaena sp. PCC 7120. Sucrose synthesis in the heterocysts and possible role in nitrogen fixation. Plant Physiol. 2007, 143, 1385–1397. [Google Scholar] [CrossRef]
- Tamoi, M.; Takeda, T.; Shigeoka, S. Functional analysis of fructose-1,6-bisphosphatase isozymes (fbp-I and fbp-II gene products) in cyanobacteria. Plant Cell Physiol. 1999, 40, 257–261. [Google Scholar] [CrossRef]
- Yan, C.; Xu, X. Bifunctional enzyme FBPase/SBPase is essential for photoautotrophic growth in cyanobacterium Synechocystis sp. PCC 6803. Prog. Nat. Sci. 2008, 18, 149–153. [Google Scholar] [CrossRef]
- Feng, L.; Sun, Y.; Deng, H.; Li, D.; Wan, J.; Wang, X.; Wang, W.; Liao, X.; Ren, Y.; Hu, X. Structural and biochemical characterization of fructose 1,6/sedoheptulose 1,7 bisphosphatase from the cyanobacterium Synechocystis strain 6803. FEBS J. 2014, 281, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Cotton, C.A.R.; Kabasakal, B.V.; Miah, N.A.; Murray, J.W. Structure of the dual-function fructose-1, 6/sedoheptulose-1, 7-bisphosphatase from Thermosynechococcus elongatus bound with sedoheptulose-7-phosphate. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2015, 71, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Prieto, A.; Bhattacharya, D. Phylogeny of Calvin cycle enzymes supports plantae monophyly. Mol. Phylogenet. Evol. 2007, 45, 384–391. [Google Scholar] [CrossRef]
- Jiang, Y.-H.; Wang, D.-Y.; Wen, J.-F. The independent prokaryotic origins of eukaryotic fructose-1, 6-bisphosphatase and sedoheptulose-1, 7-bisphosphatase and the implications of their origins for the evolution of eukaryotic Calvin cycle. BMC Evol. Biol. 2012, 12, 208. [Google Scholar] [CrossRef]
- Gütle, D.D.; Roret, T.; Müller, S.J.; Couturier, J.; Lemaire, S.D.; Hecker, A.; Dhalleine, T.; Buchanan, B.B.; Reski, R.; Einsle, O.; et al. Chloroplast FBPase and SBPase are thioredoxin-linked enzymes with similar architecture but different evolutionary histories. Proc. Natl. Acad. Sci. USA 2016, 113, 6779–6784. [Google Scholar] [CrossRef]
- Porter, M.A.; Stringer, C.D.; Hartman, F.C. Characterization of the regulatory thioredoxin site of phosphoribulokinase. J. Biol. Chem. 1988, 263, 123–129. [Google Scholar] [CrossRef]
- Brandes, H.K.; Larimer, F.W.; Hartman, F.C. The molecular pathway for the regulation of phosphoribulokinase by thioredoxin f. J. Biol. Chem. 1996, 271, 3333–3335. [Google Scholar] [CrossRef]
- Pohlmeyer, K.; Paap, B.K.; Soll, J.; Wedel, N. CP12: A small nuclear-encoded chloroplast protein provides novel insights into higher-plant GAPDH evolution. Plant Mol. Biol. 1996, 32, 969–978. [Google Scholar] [CrossRef]
- Wedel, N.; Soll, J.; Paap, B.K. CP12 provides a new mode of light regulation of Calvin cycle activity in higher plants. Proc. Natl. Acad. Sci. USA 1997, 94, 10479–10484. [Google Scholar] [CrossRef]
- Groben, R.; Kaloudas, D.; Raines, C.A.; Offmann, B.; Maberly, S.C.; Gontero, B. Comparative sequence analysis of CP12, a small protein involved in the formation of a Calvin cycle complex in photosynthetic organisms. Photosynth. Res. 2010, 103, 183–194. [Google Scholar] [CrossRef]
- Marri, L.; Zaffagnini, M.; Collin, V.; Issakidis-Bourguet, E.; Lemaire, S.D.; Pupillo, P.; Sparla, F.; Miginiac-Maslow, M.; Trost, P. Prompt and easy activation by specific thioredoxins of Calvin cycle enzymes of Arabidopsis thaliana associated in the GAPDH/CP12/PRK supramolecular complex. Mol. Plant 2009, 2, 259–269. [Google Scholar] [CrossRef]
- Howard, T.P.; Metodiev, M.; Lloyd, J.C.; Raines, C.A. Thioredoxin-mediated reversible dissociation of a stromal multiprotein complex in response to changes in light availability. Proc. Natl. Acad. Sci. USA 2008, 105, 4056–4061. [Google Scholar] [CrossRef]
- Fermani, S.; Trivelli, X.; Sparla, F.; Thumiger, A.; Calvaresi, M.; Marri, L.; Falini, G.; Zerbetto, F.; Trost, P. Conformational selection and folding-upon-binding of intrinsically disordered protein CP12 regulate photosynthetic enzymes assembly. J. Biol. Chem. 2012, 287, 21372–21383. [Google Scholar] [CrossRef]
- Matsumura, H.; Kai, A.; Maeda, T.; Tamoi, M.; Satoh, A.; Tamura, H.; Hirose, M.; Ogawa, T.; Kizu, N.; Wadano, A.; et al. Structure basis for the regulation of glyceraldehyde-3-phosphate dehydrogenase activity via the intrinsically disordered protein CP12. Structure 2011, 19, 1846–1854. [Google Scholar] [CrossRef]
- Wedel, N.; Soll, J. Evolutionary conserved light regulation of Calvin cycle activity by NADPH-mediated reversible phosphoribulokinase/CP12/ glyceraldehyde-3-phosphate dehydrogenase complex dissociation. Proc. Natl. Acad. Sci. USA 1998, 95, 9699–9704. [Google Scholar] [CrossRef]
- Sugiura, K.; Yokochi, Y.; Fu, N.; Fukaya, Y.; Yoshida, K.; Mihara, S.; Hisabori, T. The thioredoxin (Trx) redox state sensor protein can visualize Trx activities in the light/dark response in chloroplasts. J. Biol. Chem. 2019, 294, 12091–12098. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.M.; Plummer, S. Production of extracellular reactive oxygen species by phytoplankton: Past and future directions. J. Plankton Res. 2018, 40, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Rose, A. The influence of extracellular superoxide on iron redox chemistry and bioavailability to aquatic microorganisms. Front. Microbiol. 2012, 3, 124. [Google Scholar] [CrossRef] [PubMed]
- Klughammer, B.; Baier, M.; Dietz, K.-J. Inactivation by gene disruption of 2-cysteine-peroxiredoxin in Synechocystis sp. PCC 6803 leads to increased stress sensitivity. Physiol. Plant. 1998, 104, 699–706. [Google Scholar] [CrossRef]
- Latifi, A.; Ruiz, M.; Jeanjean, R.; Zhang, C.-C. PrxQ-A, a member of the peroxiredoxin Q family, plays a major role in defense against oxidative stress in the cyanobacterium Anabaena sp. strain PCC7120. Free Radic. Biol. Med. 2007, 42, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Alexander, P.; Avraham, U.; Dalia, H.; Rakefet, S. Oxidative stress in Synechococcus sp. strain PCC 7942: Various mechanisms for H2O2 detoxification with different physiological roles. J. Bacteriol. 2003, 185, 3654–3660. [Google Scholar]

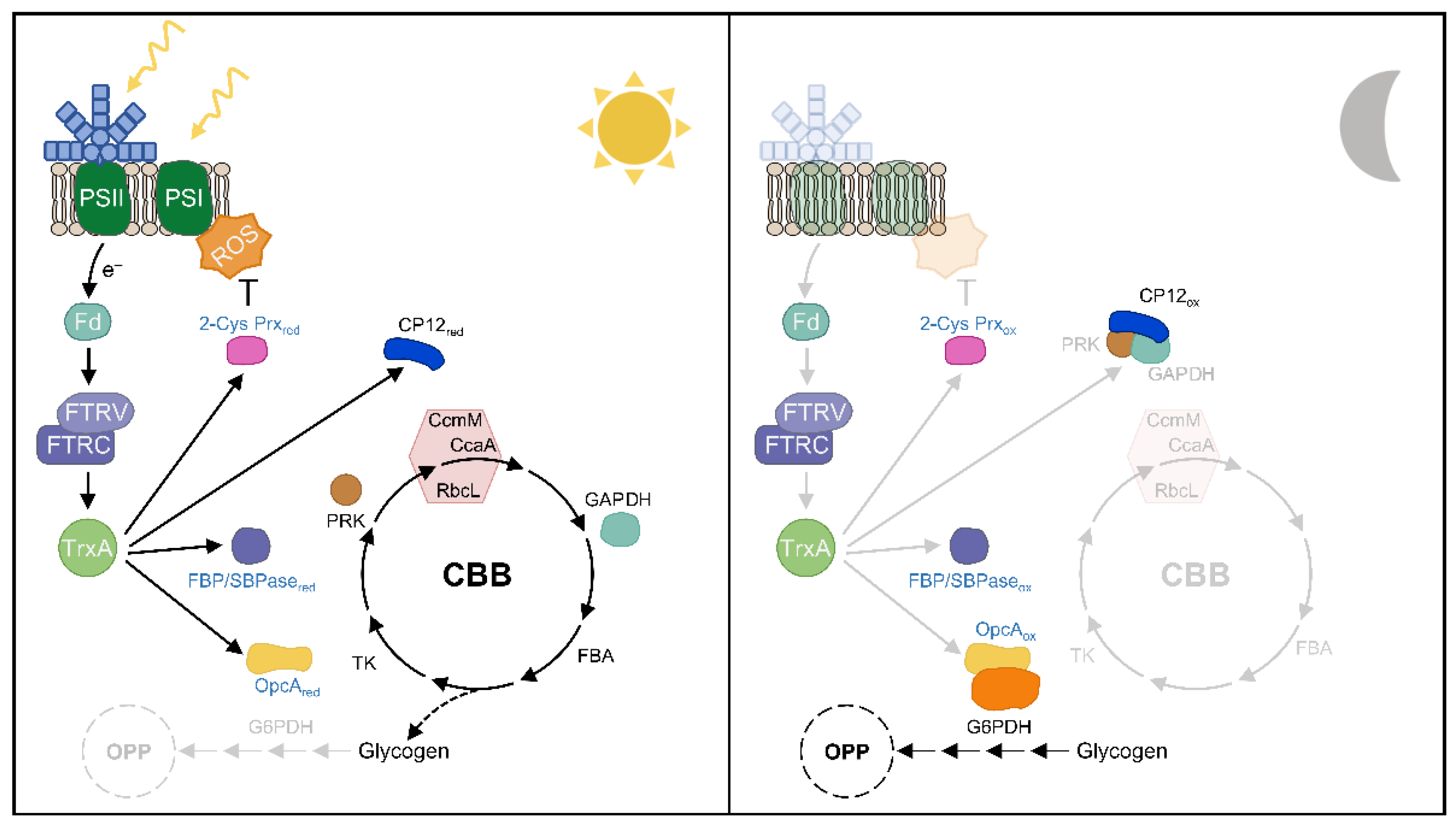
| Cellular Processes | Target Protein | Regulation In Vivo by | References |
|---|---|---|---|
| CBB cycle | FBP/SBPase | SynTrxA | [15] |
| OPP pathway | OpcA | AnTrxA1 | [29] |
| Antioxidant defense | 2-Cys Prx | SynTrxA | [15] |
| Transcriptional regulation | PedR | SynFTRV | [84] |
| Protein synthesis | EF-Tu | SynFTRV | [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallén-Ponce, M.J.; Huertas, M.J.; Florencio, F.J. Exploring the Diversity of the Thioredoxin Systems in Cyanobacteria. Antioxidants 2022, 11, 654. https://doi.org/10.3390/antiox11040654
Mallén-Ponce MJ, Huertas MJ, Florencio FJ. Exploring the Diversity of the Thioredoxin Systems in Cyanobacteria. Antioxidants. 2022; 11(4):654. https://doi.org/10.3390/antiox11040654
Chicago/Turabian StyleMallén-Ponce, Manuel J., María José Huertas, and Francisco J. Florencio. 2022. "Exploring the Diversity of the Thioredoxin Systems in Cyanobacteria" Antioxidants 11, no. 4: 654. https://doi.org/10.3390/antiox11040654
APA StyleMallén-Ponce, M. J., Huertas, M. J., & Florencio, F. J. (2022). Exploring the Diversity of the Thioredoxin Systems in Cyanobacteria. Antioxidants, 11(4), 654. https://doi.org/10.3390/antiox11040654






