Assessment of Methodological Pipelines for the Determination of Isothiocyanates Derived from Natural Sources
Abstract
1. Introduction
2. Determination of ITC Content
2.1. Sample Processing and Storage
2.2. Hydrolysis of GSLs
2.2.1. Effect of pH
2.2.2. Effect of Temperature
2.2.3. Effect of EDTA and Ascorbic Acid
3. Extraction of Isothiocyanates
3.1. Extraction of Sulforaphane
3.2. Extraction of Iberin
3.3. Extraction of Allyl Isothiocyanate
3.4. Extraction of Benzyl Isothiocyanate
3.5. Extraction of Phenethyl Isothiocyanate
4. Quantification of the Extracted ITCs Content
4.1. Chemistry and Reactivity of ITCs
4.2. Cyclo-Condensation Assay
4.3. Quantification via ITCs Chemical Derivatization
4.4. Quantification via Analytical Instrumentation
4.4.1. Attenuated Total Reflectance Infrared Fourier Transform (ATR-FT-IR) Spectroscopy
4.4.2. Chromatographic Approaches
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AITC | allyl isothiocyanate |
| APCI | atmospheric pressure chemical ionization |
| ATR-FT-IR | attenuated total reflectance infrared Fourier transform spectroscopy |
| BDT | 1,2-benzenedithiol |
| BITC | benzyl isothiocyanate |
| CDK | cyclic depended kinase |
| DAD | diode-array detection |
| EDTA | ethylenediaminetetraacetic acid |
| ESI | electron spray ionisation |
| ESP | epithiospecifier protein-like factors |
| FID | flame ionization detection |
| GC | gas chromatography |
| GLs | glucosinolates |
| GSH | glutathione |
| GST | glutathione S-transferase |
| HPLC | high performance liquid chromatography |
| IBN | iberin |
| ITC | isothiocyanates |
| MRM | multiple reaction monitoring |
| MS | mass spectrometry |
| MS/MS | tandem mass spectrometry |
| m/z | mass to charge ratio |
| NAC | N-acetyl cysteine |
| PDA | photodiode-array detection |
| PEITC | phenethyl isothiocyanate |
| ROS | reactive oxygen species |
| SFE | supercritical fluid extraction |
| SFN | sulforaphane |
| SPE | solid phase extraction |
| UV | ultra-violate |
References
- Rodriguez-Casado, A. The Health Potential of Fruits and Vegetables Phytochemicals: Notable Examples. Crit. Rev. Food Sci. Nutr. 2016, 56, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Bolling, B.W. Review of Nut Phytochemicals, Fat-Soluble Bioactives, Antioxidant Components and Health Effects. Br. J. Nutr. 2015, 113, S68–S78. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, Antioxidant Efficacies, and Health Effects—A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1580–1604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Daglia, M.; Braidy, N.; Nabavi, S.F. Natural Products, Micronutrients, and Nutraceuticals for the Treatment of Depression: A Short Review. Nutr. Neurosci. 2017, 20, 180–194. [Google Scholar] [CrossRef]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef]
- Baby, B.; Antony, P.; Vijayan, R. Antioxidant and Anticancer Properties of Berries. Crit. Rev. Food Sci. Nutr. 2018, 58, 2491–2507. [Google Scholar] [CrossRef]
- Azzini, E.; Giacometti, J.; Russo, G.L. Antioxidant Phytochemicals at the Pharma-Nutrition Interface. Oxidative Med. Cell. Longev. 2017, 2017, 6986143. [Google Scholar] [CrossRef]
- Lu, B.; Zhao, Y. Photooxidation of Phytochemicals in Food and Control: A Review. Ann. N. Y. Acad. Sci. 2017, 1398, 72–82. [Google Scholar] [CrossRef]
- Satija, A.; Hu, F.B. Plant-Based Diets and Cardiovascular Health. Trends Cardiovasc. Med. 2018, 28, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Miller, V.; Mente, A.; Dehghan, M.; Rangarajan, S.; Zhang, X.; Swaminathan, S.; Dagenais, G.; Gupta, R.; Mohan, V.; Lear, S.; et al. Fruit, Vegetable, and Legume Intake, and Cardiovascular Disease and Deaths in 18 Countries (PURE): A Prospective Cohort Study. Lancet 2017, 390, 2037–2049. [Google Scholar] [CrossRef]
- Vieira, A.R.; Abar, L.; Vingeliene, S.; Chan, D.S.M.; Aune, D.; Navarro-Rosenblatt, D.; Stevens, C.; Greenwood, D.; Norat, T. Fruits, Vegetables and Lung Cancer Risk: A Systematic Review and Meta-Analysis. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and Vegetable Intake and the Risk of Cardiovascular Disease, Total Cancer and All-Cause Mortality-a Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Liu, C.; Gao, Z.; He, Y. Study on Effects and Mechanisms of Phytochemicals in Vegetables and Fruits in Preventing and Treating Lung Cancer. Chin. J. Lung Cancer 2017, 20, 841–846. [Google Scholar] [CrossRef]
- Sayem, A.S.M.; Arya, A.; Karimian, H.; Krishnasamy, N.; Ashok Hasamnis, A.; Hossain, C.F. Action of Phytochemicals on Insulin Signaling Pathways Accelerating Glucose Transporter (GLUT4) Protein Translocation. Molecules 2018, 23, 258. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Colletti, A. Role of Phytochemicals in the Management of Metabolic Syndrome. Phytomedicine 2016, 23, 1134–1144. [Google Scholar] [CrossRef]
- Ansari, P.; Flatt, P.R.; Harriott, P.; Abdel-Wahab, Y.H.A. Evaluation of the antidiabetic and insulin releasing effects of A. Squamosa including isolation and characterization of active phytochemicals. Plants 2020, 9, 1348. [Google Scholar] [CrossRef]
- Bacanli, M.; Dilsiz, S.A.; Başaran, N.; Başaran, A.A. Effects of Phytochemicals against Diabetes. Adv. Food Nutr. Res. 2019, 89, 209–238. [Google Scholar] [CrossRef]
- Onaolapo, A.Y.; Onaolapo, O.J. Nutraceuticals and diet-based phytochemicals in Type 2 diabetes mellitus: From whole food to components with defined roles and mechanisms. Curr. Diabetes Rev. 2019, 16, 12–25. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, C.; Wai, S.T.C.; Zhang, Y.; Portillo, M.P.; Paoli, P.; Wu, Y.; San Cheang, W.; Liu, B.; Carpéné, C.; et al. Regulation of Glucose Metabolism by Bioactive Phytochemicals for the Management of Type 2 Diabetes Mellitus. Crit. Rev. Food Sci. Nutr. 2019, 59, 830–847. [Google Scholar] [CrossRef]
- La Rosa, F.; Clerici, M.; Ratto, D.; Occhinegro, A.; Licito, A.; Romeo, M.; Di Iorio, C.; Rossi, P. The Gut-Brain Axis in Alzheimer’s Disease and Omega-3. A Critical Overview of Clinical Trials. Nutrients 2018, 10, 1267. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, C.; Moccia, S.; Russo, G.L. Anti-Inflammatory Effects of Flavonoids in Neurodegenerative Disorders. Eur. J. Med. Chem. 2018, 153, 105–115. [Google Scholar] [CrossRef]
- Uddin, M.S.; Kabir, M.T.; Niaz, K.; Jeandet, P.; Clément, C.; Mathew, B.; Rauf, A.; Rengasamy, K.R.R.; Sobarzo-Sánchez, E.; Ashraf, G.M.; et al. Molecular Insight into the Therapeutic Promise of Flavonoids against Alzheimer’s Disease. Molecules 2020, 25, 1267. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, M.; Li, C.; Jiang, X.; Su, Y.; Zhang, Y. Benefits of Vitamins in the Treatment of Parkinson’s Disease. Oxid. Med. Cell. Longev. 2019, 2019, 9426867. [Google Scholar] [CrossRef] [PubMed]
- Palliyaguru, D.L.; Yuan, J.-M.; Kensler, T.W.; Fahey, J.W. Isothiocyanates: Translating the Power of Plants to People. Mol. Nutr. Food Res. 2018, 62, e1700965. [Google Scholar] [CrossRef] [PubMed]
- Assony, S.J. Chapter 28-The Chemistry of Isothiocyanates. In Organic Sulfur Compounds; Kharasch, N., Ed.; Pergamon: Oxfrord, UK, 1961; pp. 326–338. [Google Scholar] [CrossRef]
- Ghawi, S.K.; Methven, L.; Niranjan, K. The Potential to Intensify Sulforaphane Formation in Cooked Broccoli (Brassica oleracea var. italica) Using Mustard Seeds (Sinapis alba). Food Chem. 2013, 138, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Y.; Fang, Z.; Yang, L.; Zhuang, M.; Zhang, Y.; Zhao, W.; Sun, P. Variation of Sulforaphane Levels in Broccoli (Brassica Oleracea var. Italica) during Flower Development and the Role of Gene AOP2. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 1199–1211. [Google Scholar] [CrossRef]
- Vanduchova, A.; Anzenbacher, P.; Anzenbacherova, E. Isothiocyanate from Broccoli, Sulforaphane, and Its Properties. J. Med. Food 2019, 22, 121–126. [Google Scholar] [CrossRef]
- Gupta, P.; Wright, S.E.; Kim, S.-H.; Srivastava, S.K. Phenethyl Isothiocyanate: A Comprehensive Review of Anti-Cancer Mechanisms. Biochim. Biophys. Acta 2014, 1846, 405–424. [Google Scholar] [CrossRef]
- Liang, H.; Yuan, Q.P.; Dong, H.R.; Liu, Y.M. Determination of Sulforaphane in Broccoli and Cabbage by High-Performance Liquid Chromatography. J. Food Compos. Anal. 2006, 19, 473–476. [Google Scholar] [CrossRef]
- Koo, S.Y.; Cha, K.H.; Song, D.-G.; Lee, D.-U.; Pan, C.-H. Increased Sulforaphane Concentration in Brussels Sprout Following High Hydrostatic Pressure Treatment. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 685–687. [Google Scholar] [CrossRef]
- Jakobsen, T.H.; Bragason, S.K.; Phipps, R.K.; Christensen, L.D.; van Gennip, M.; Alhede, M.; Skindersoe, M.; Larsen, T.O.; Høiby, N.; Bjarnsholt, T.; et al. Food as a Source for Quorum Sensing Inhibitors: Iberin from Horseradish Revealed as a Quorum Sensing Inhibitor of Pseudomonas Aeruginosa. Appl. Environ. Microbiol. 2012, 78, 2410–2421. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, G.-A.; Zeng, S.; Lin, K. Extraction of Allyl Isothiocyanate from Horseradish (Armoracia Rusticana) and Its Fumigant Insecticidal Activity on Four Stored-Product Pests of Paddy. Pest Manag. Sci. 2009, 65, 1003–1008. [Google Scholar] [CrossRef]
- Dixon, M.J.; Shaw, P.J. Watercress and Water Quality: The Effect of Phenethyl Isothiocyanate on the Mating Behaviour of Gammarus pulex. Int. J. Zool. 2011, 2011, 328749. [Google Scholar] [CrossRef][Green Version]
- Li, L.; Lee, W.; Lee, W.J.; Auh, J.H.; Kim, S.S.; Yoon, J. Extraction of Allyl Isothiocyanate from Wasabi (Wasabia Japonica Matsum) Using Supercritical Carbon Dioxide. Food Sci. Biotechnol. 2010, 19, 405–410. [Google Scholar] [CrossRef]
- Márton, M.; Lavric, V. A Simple Method for the Quantification of Isothiocyanates from Mustard. U.P.B. Sci. Bull. Ser. B 2013, 75, 63–72. [Google Scholar]
- Ma, Y.; Wen, Y.; Chen, J.; Zhang, Y.; Zhang, H.; Sui, J.; Yi, G.; He, X. Rapid and sensitive analysis of benzyl isothiocyanate in peel, pulp, and seeds of Carica papaya linn. by headspace gas chromatography-mass spectrometry. SN Appl. Sci. 2021, 374, 1–9. [Google Scholar] [CrossRef]
- Jeschke, V.; Gershenzon, J.; Vassão, D.G. A Mode of Action of Glucosinolate-Derived Isothiocyanates: Detoxification Depletes Glutathione and Cysteine Levels with Ramifications on Protein Metabolism in Spodoptera littoralis. Insect Biochem. Mol. Biol. 2016, 71, 37–48. [Google Scholar] [CrossRef]
- Wittstock, U.; Agerbirk, N.; Stauber, E.J.; Olsen, C.E.; Hippler, M.; Mitchell-Olds, T.; Gershenzon, J.; Vogel, H. Successful Herbivore Attack Due to Metabolic Diversion of a Plant Chemical Defense. Proc. Natl. Acad. Sci. USA 2004, 101, 4859–4864. [Google Scholar] [CrossRef]
- Bhat, R.; Vyas, D. Myrosinase: Insights on Structural, Catalytic, Regulatory, and Environmental Interactions. Crit. Rev. Biotechnol. 2019, 39, 508–523. [Google Scholar] [CrossRef] [PubMed]
- Chhajed, S.; Misra, B.B.; Tello, N.; Chen, S. Chemodiversity of the Glucosinolate-Myrosinase System at the Single Cell Type Resolution. Front. Plant Sci. 2019, 10, 618. [Google Scholar] [CrossRef] [PubMed]
- Peñas, E.; Frias, J.; Martínez-Villaluenga, C.; Vidal-Valverde, C. Bioactive compounds, myrosinase activity and antioxidant capacity of white cabbages grown in different locations of Spain. J. Agric. Food Chem. 2011, 59, 3772–3779. [Google Scholar] [CrossRef] [PubMed]
- Bellostas, N.; Sørensen, J.; Sørensen, H. Qualitative and Quantitative Evaluation of Glucosinolates in Cruciferous Plants during Their Life Cycles. Agroindustria 2004, 3, 5–10. [Google Scholar]
- Kim, M.J.; Chiu, Y.-C.; Ku, K.-M. Glucosinolates, Carotenoids, and Vitamins E and K Variation from Selected Kale and Collard Cultivars. J. Food Qual. 2017, 2017, 5123572. [Google Scholar] [CrossRef]
- Alvarez, S.; He, Y.; Chen, S. Comparative Investigations of the Glucosinolate-Myrosinase System in Arabidopsis Suspension Cells and Hypocotyls. Plant Cell Physiol. 2008, 49, 324–333. [Google Scholar] [CrossRef]
- Wang, J.; Yu, H.; Zhao, Z.; Sheng, X.; Shen, Y.; Gu., H. Natural Variation of Glucosinolates and Their Breakdown Products in Broccoli (Brassica oleracea var. italica) Seeds. J. Agric. Food Chem. 2019, 67, 12528–12537. [Google Scholar] [CrossRef]
- Sánchez-Pujante, P.J.; Borja-Martínez, M.; Pedreño, M.Á.; Almagro, L. Biosynthesis and Bioactivity of Glucosinolates and Their Production in Plant in Vitro Cultures. Planta 2017, 246, 19–32. [Google Scholar] [CrossRef]
- Guerrero-Alonso, A.; Antunez-Mojica, M.; Medina-Franco, J.L. Chemoinformatic Analysis of Isothiocyanates: Their Impact in Nature and Medicine. Mol. Inf. 2021, 40, 2411–2502. [Google Scholar] [CrossRef]
- Iriti, M.; Faoro, F. Chemical Diversity and Defence Metabolism: How Plants Cope with Pathogens and Ozone Pollution. Int. J. Mol. Sci. 2009, 10, 3371–3399. [Google Scholar] [CrossRef]
- Sarikamis, G.; Marquez, J.; Maccormack, R.; Bennett, R.; Roberts, J.; Mithen, R. High Glucosinolate Broccoli: A Delivery System for Sulforaphane. Mol. Breed. 2006, 18, 219–228. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and Isothiocyanates in Health and Disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Radojcic Redovnikovic, I.; Glivetic, T.; Delonga, K.; Vorkapic-Furac, J. Glucosinolates and Their Potential Role in Plant. Period. Biol. 2008, 110, 297–309. [Google Scholar]
- Lee, J.W.; Kim, I.H.; Woyengo, T.A. Toxicity of Canola-Derived Glucosinolate Degradation Products in Pigs—A Review. Animals 2020, 10, 2337. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Klopsch, R.; Oliviero, T.; Schreiner, M.; Verkerk, R.; Dekker, M. Optimizing Isothiocyanate Formation during Enzymatic Glucosinolate Breakdown by Adjusting pH Value, Temperature and Dilution in Brassica Vegetables and Arabidopsis thaliana. Sci. Rep. 2017, 7, 40807. [Google Scholar] [CrossRef]
- Williams, D.J.; Critchley, C.; Pun, S.; Chaliha, M.; O’Hare, T.J. Differing Mechanisms of Simple Nitrile Formation on Glucosinolate Degradation in Lepidium sativum and Nasturtium officinale Seeds. Phytochemistry 2009, 70, 1401–1409. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Schreiner, M. Isothiocyanates, Nitriles, and Epithionitriles from Glucosinolates Are Affected by Genotype and Developmental Stage in Brassica Oleracea Varieties. Front. Plant Sci. 2017, 8, 1095. [Google Scholar] [CrossRef]
- Prieto, M.A.; López, C.J.; Simal-Gandara, J. Glucosinolates: Molecular Structure, Breakdown, Genetic, Bioavailability, Properties and Healthy and Adverse Effects. Adv. Food Nutr. Res. 2019, 90, 305–350. [Google Scholar] [CrossRef]
- Cole, R.A. Isothiocyanates, Nitriles and Thiocyanates as Products of Autolysis of Glucosinolates in Cruciferae. Phytochemistry 1976, 15, 759–762. [Google Scholar] [CrossRef]
- Lambrix, V.; Reichelt, M.; Mitchell-Olds, T.; Kliebenstein, D.J.; Gershenzon, J. The Arabidopsis Epithiospecifier Protein Promotes the Hydrolysis of Glucosinolates to Nitriles and Influences Trichoplusia ni Herbivory. Plant Cell 2001, 13, 2793–2807. [Google Scholar] [CrossRef]
- Kissen, R.; Bones, A.M. Nitrile-Specifier Proteins Involved in Glucosinolate Hydrolysis in Arabidopsis thaliana. J. Biol. Chem. 2009, 284, 12057–12070. [Google Scholar] [CrossRef] [PubMed]
- Kissen, R.; Hyldbakk, E.; Wang, C.-W.V.; Sørmo, C.G.; Rossiter, J.T.; Bones, A.M. Ecotype Dependent Expression and Alternative Splicing of Epithiospecifier Protein (ESP) in Arabidopsis thaliana. Plant Mol. Biol. 2012, 78, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Gao, T.Y.; Shu, X.O.; Cai, Q.; Li, G.L.; Ji, B.T.; Rothman, D.; Dyba, M.; Xiang, Y.B.; Chung, F.L.; et al. Isothiocyanate exposure, glutathione S-transferase polymorphisms, and colorectal cancer risk. Am. J. Clin. Nutr. 2009, 91, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhu, Y.; Yan, H.; Liu, B.; Li, Y.; Zhou, Q.; Xu, K. Isothiocyanates Induce Oxidative Stress and Suppress the Metastasis Potential of Human Non-Small Cell Lung Cancer Cells. BMC Cancer 2010, 10, 269. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, S.; Yan, S.; Wang, J.; Zhang, L.; Li, X.; Wen, L.; Wu, J. Phenylethyl Isothiocyanate Induces Oxidative Damage of Porcine Kidney Cells Mediated by Reactive Oxygen Species. J. Biochem. Mol. Toxicol. 2020, 34, e22428. [Google Scholar] [CrossRef]
- Seow, A.; Yuan, J.-M.; Sun, C.-L.; Van Den Berg, D.; Lee, H.-P.; Yu, M.C. Dietary Isothiocyanates, Glutathione S -Transferase Polymorphisms and Colorectal Cancer Risk in the Singapore Chinese Health Study. Carcinogenesis 2002, 23, 2055–2061. [Google Scholar] [CrossRef]
- Nakamura, Y. Chemoprevention by Isothiocyanates: Molecular Basis of Apoptosis Induction. Forum Nutr. 2009, 61, 170–181. [Google Scholar] [CrossRef]
- Huang, L.; Cai, C.; Dang, W.; Lu, J.; Hu, G.; Gu, J. Propyl Isothiocyanate Induces Apoptosis in Gastric Cancer Cells by Oxidative Stress via Glutathione Depletion. Oncol. Lett. 2019, 18, 5490–5498. [Google Scholar] [CrossRef]
- Han, K.W.W.; Po, W.W.; Sohn, U.D.; Kim, H.-J. Benzyl Isothiocyanate Induces Apoptosis via Reactive Oxygen Species-Initiated Mitochondrial Dysfunction and DR4 and DR5 Death Receptor Activation in Gastric Adenocarcinoma Cells. Biomolecules 2019, 9, 839. [Google Scholar] [CrossRef]
- Hu, J.; Straub, J.; Xiao, D.; Singh, S.V.; Yang, H.-S.; Sonenberg, N.; Vatsyayan, J. Phenethyl Isothiocyanate, a Cancer Chemopreventive Constituent of Cruciferous Vegetables, Inhibits Cap-Dependent Translation by Regulating the Level and Phosphorylation of 4E-BP1. Cancer Res. 2007, 67, 3569–3573. [Google Scholar] [CrossRef][Green Version]
- Tang, L.; Zhang, Y. Dietary Isothiocyanates Inhibit the Growth of Human Bladder Carcinoma Cells. J. Nutr. 2004, 134, 2004–2010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, L.; Gonzalez, V. Selected Isothiocyanates Rapidly Induce Growth Inhibition of Cancer Cells. Mol. Cancer Ther. 2003, 2, 1045–1052. [Google Scholar] [PubMed]
- Mantso, T.; Anestopoulos, I.; Lamprianidou, E.; Kotsianidis, I.; Pappa, A.; Panayiotidis, M.I. Isothiocyanate-Induced Cell Cycle Arrest in a Novel In Vitro Exposure Protocol of Human Malignant Melanoma (A375) Cells. Anticancer Res. 2019, 39, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Cavell, B.E.; Syed Alwi, S.S.; Donlevy, A.; Packham, G. Anti-Angiogenic Effects of Dietary Isothiocyanates: Mechanisms of Action and Implications for Human Health. Biochem. Pharmacol. 2011, 81, 327–336. [Google Scholar] [CrossRef]
- Keum, Y.-S.; Jeong, W.-S.; Kong, A.-N.T. Chemopreventive Functions of Isothiocyanates. Drug News Perspect. 2005, 18, 445–451. [Google Scholar] [CrossRef]
- Sundaram, M.K.; Preetha, R.; Haque, S.; Akhter, N.; Khan, S.; Ahmed, S.; Hussain, A. Dietary Isothiocyanates Inhibit Cancer Progression by Modulation of Epigenome. Semin. Cancer Biol. 2021, in press, S1044-579X(20)30281-9. [Google Scholar] [CrossRef]
- Mitsiogianni, M.; Amery, T.; Franco, R.; Zoumpourlis, V.; Pappa, A.; Panayiotidis, M.I. From chemoprevention to epigenetic regulation: The role of isothiocyanates in skin cancer prevention. Pharmacol. Ther. 2018, 190, 187–201. [Google Scholar] [CrossRef]
- Novío, S.; Núñez-Iglesias, M.J.; Freire-Garabal, M. Chapter 7-Isothiocyanates, Epigenetics, and Cancer Prevention. In Translational Epigenetics; Bishayee, A., Bhatia, D., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 8, pp. 149–168. [Google Scholar] [CrossRef]
- Wang, Q.; Bao, Y. Nanodelivery of natural isothiocyanates as a cancer therapeutic. Free Radic. Biol. Med. 2021, 167, 125–140. [Google Scholar] [CrossRef]
- Oliviero, T.; Verkerk, R.; Dekker, M. Isothiocyanates from Brassica Vegetables—Effects of Processing, Cooking, Mastication, and Digestion. Mol. Nutr. Food Res. 2018, 62, 1701069. [Google Scholar] [CrossRef]
- Song, L.; Thornalley, P.J. Effect of Storage, Processing and Cooking on Glucosinolate Content of Brassica Vegetables. Food Chem. Toxicol. 2007, 45, 216–224. [Google Scholar] [CrossRef]
- Rungapamestry, V.; Duncan, A.J.; Fuller, Z.; Ratcliffe, B. Effect of Cooking Brassica Vegetables on the Subsequent Hydrolysis and Metabolic Fate of Glucosinolates. Proc. Nutr. Soc. 2007, 66, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Kosson, R.; Horbowicz, M. Effect of Long-Term Storage on Some Nutritive Components and Isothiocyanates Content in Roots of Two Horseradish Types. Veg. Crop. Res. Bull. 2008, 69, 155–164. [Google Scholar] [CrossRef][Green Version]
- Vaughn, S.F.; Berhow, M.A. Glucosinolate Hydrolysis Products from Various Plant Sources: pH Effects, Isolation, and Purification. Ind. Crops Prod. 2005, 21, 193–202. [Google Scholar] [CrossRef]
- Luang-In, V.; Rossiter, J.T. Stability Studies of Isothiocyanates and Nitriles in Aqueous Media. Songklanakarin J. Sci. Technol. 2015, 37, 625–630. [Google Scholar]
- Surugau, N.; Aripin, N. Effects of Temperature and pH on Myrosinase Activity and Gluconasturtiin Hydrolysis Products in Watercress. Trans. Sci. Technol. 2016, 3, 449–454. [Google Scholar]
- Li, Z. Development and Verification of Sulforaphane Extraction Method in Cabbage (Brassica oleracea L. Var. capitata) and Broccoli (Brassica oleracea L. Var. italica Planch.). J. Med. Plants Res. 2012, 6, 4796–4803. [Google Scholar] [CrossRef]
- Uda, Y.; Kurata, T.; Arakawa, N. Effects of pH and Ferrous Ion on the Degradation of Glucosinolates by Myrosinase. Agric. Biol. Chem. 1986, 50, 2735–2740. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Fang, Z.; Yang, L.; Zhuang, M.; Zhang, Y.; Lv, H. Natural Sulforaphane from Broccoli Seeds against Influenza A Virus Replication in MDCK Cells. Nat. Prod. Commun. 2019, 14, 1–8. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Hoeflinger, J.L.; Neme, B.P.; Jeffery, E.H.; Miller, M.J. Dietary Broccoli Alters Rat Cecal Microbiota to Improve Glucoraphanin Hydrolysis to Bioactive Isothiocyanates. Nutrients 2017, 9, 262. [Google Scholar] [CrossRef]
- Dufour, V.; Stahl, M.O.; Baysse, C. The antibacterial properties of isothiocyanates. Microbiology. 2015, 161, 229–243. [Google Scholar] [CrossRef]
- Li, X.; Kushad, M.M. Purification and Characterization of Myrosinase from Horseradish (Armoracia rusticana) Roots. Plant Physiol. Biochem. 2005, 43, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Kassie, F.; Rabot, S.; Uhl, M.; Huber, W.; Qin, H.M.; Helma, C.; Schulte-Hermann, R.; Knasmüller, S. Chemoprotective Effects of Garden Cress (Lepidium sativum) and Its Constituents towards 2-Amino-3-Methyl-Imidazo [4,5-f] Quinoline (IQ)-Induced Genotoxic Effects and Colonic Preneoplastic Lesions. Carcinogenesis 2002, 23, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.-I.; Kodera, M.; Hirai, A.; Nakada, M.; Ueno, Y.; Osawa, T. Benzyl isothiocyanate produced by garden cress (Lepidium sativum) prevents accumulation of hepatic lipids. J. Nutr. Sci. Vitaminol. 2020, 66, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Yoshimoto, M.; Murata, Y.; Shimoishi, Y.; Asai, Y.; Park, E.Y.; Sato, K.; Nakamura, Y. Papaya Seed Represents a Rich Source of Biologically Active Isothiocyanate. J. Agric. Food Chem. 2007, 55, 4407–4413. [Google Scholar] [CrossRef]
- Guzmán-Pérez, V.; Bumke-Vogt, C.; Schreiner, M.; Mewis, I.; Borchert, A.; Pfeiffer, A.F.H. Benzylglucosinolate Derived Isothiocyanate from Tropaeolum majus Reduces Gluconeogenic Gene and Protein Expression in Human Cells. PLoS ONE 2016, 11, e0162397. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Reis, C.A.; Pintado, M. Phenylethyl Isothiocyanate Extracted from Watercress By-Products with Aqueous Micellar Systems: Development and Optimisation. Antioxidants 2020, 9, 698. [Google Scholar] [CrossRef]
- Prakash, O.; Rai, A.K.; Singh, J.; Singh, P.M. Partial purification and kinetic properties of myrosinase from cauliflower (Brassica oleracea var. botrytis). Indian J. Agric. Biochem. 2013, 26, 190–194. [Google Scholar]
- Román, J.; Castillo, A.; Cottet, L.; Mahn, A. Kinetic and Structural Study of Broccoli Myrosinase and Its Interaction with Different Glucosinolates. Food Chem. 2018, 254, 87–94. [Google Scholar] [CrossRef]
- Monsterrat, E. Mechanisms Underlying Biological Effects of Cruciferous Glucosinolate-Derived Isothiocyanates/Indoles: A Focus on Metabolic Syndrome. Front. Nutr. 2020, 7, 111. [Google Scholar] [CrossRef]
- Omri Hichri, A.; Mosbah, H.; Majouli, K.; Besbes Hlila, M.; Ben Jannet, H.; Flamini, G.; Aouni, M.; Selmi, B. Chemical Composition and Biological Activities of Eruca vesicaria Subsp. longirostris Essential Oils. Pharm. Biol. 2016, 54, 2236–2243. [Google Scholar] [CrossRef]
- Miyazawa, M.; Kawata, J. Identification of the Main Aroma Compounds in Dried Seeds of Brassica Hirta. J. Nat. Med. 2006, 60, 89–92. [Google Scholar] [CrossRef]
- Matusheski, N.V.; Jeffery, E.H. Comparison of the Bioactivity of Two Glucoraphanin Hydrolysis Products Found in Broccoli, Sulforaphane and Sulforaphane Nitrile. J. Agric. Food Chem. 2001, 49, 5743–5749. [Google Scholar] [CrossRef] [PubMed]
- Van Ommen Kloeke, A.E.E.; Jager, T.; van Gestel, C.A.M.; Ellers, J.; Pomeren, M.; van Krommenhoek, T.; Styrishave, B.; Hansen, M.; Roelofs, D. Time-Related Survival Effects of Two Gluconasturtiin Hydrolysis Products on the Terrestrial Isopod Porcellio Scaber. Chemosphere 2012, 89, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Kyriakou, S.; Tragkola, V.; Alghol, H.; Antestopoulos, I.; Amery, T.; Stewart, K.; Winyard, G.P.; Trafalis, T.D.; Franco, R.; Pappa, A.; et al. Evaluation of Bioctive Properties of Lipophilic Fractions of Edible and Non-Edible Parts of Nasturtium officinale (Watercress) in a Model of Human Malignant Melanoma Cells. Pharmaceuticals 2022, 15, 141. [Google Scholar] [CrossRef]
- Wielanek, M.; Urbanek, H. Enhanced Glucotropaeolin Production in Hairy Root Cultures of Tropaeolum Majus L. by Combining Elicitation and Precursor Feeding. Plant Cell. Tissue Organ Cult. 2006, 86, 177–186. [Google Scholar] [CrossRef]
- Herzallah, S.; Lledó, M.L.; Holley, R. Influence of NaCl and NaNO3 on Sinigrin Hydrolysis by Foodborne Bacteria. J. Food Prot. 2011, 74, 2162–2168. [Google Scholar] [CrossRef]
- Rouzaud, G.; Young, S.A.; Duncan, A.J. Hydrolysis of glucosinolates to isothiocyanates after ingestion of raw or microwaved cabbage by human volunteers. Cancer Epidemiol. Biomark. Prev. 2004, 13, 125–131. [Google Scholar] [CrossRef]
- Connolly, E.L.; Sim, M.; Travica, N.; Marx, W.; Beasy, G.; Lynch, G.S.; Bondonno, C.P.; Lewis, J.R.; Hodgson, J.M.; Blekkenhorst, L.C. Glucosinolates from Cruciferous Vegetables and Their Potential Role in Chronic Disease: Investigating the Preclinical and Clinical Evidence. Front. Pharmacol. 2021, 12, 2964. [Google Scholar] [CrossRef]
- Tarar, A.; Alyami, E.M.; Peng, C.-A. Eradication of Myrosinase-Tethered Cancer Cells by Allyl Isothiocyanate Derived from Enzymatic Hydrolysis of Sinigrin. Pharmaceutics 2022, 14, 144. [Google Scholar] [CrossRef]
- Okunade, O.A.; Ghawi, S.K.; Methven, L.; Niranjan, K. Thermal and Pressure Stability of Myrosinase Enzymes from Black Mustard (Brassica nigra L. W.D.J. Koch. Var. nigra), Brown Mustard (Brassica juncea L. Czern. Var. juncea) and Yellow Mustard (Sinapsis alba L. Subsp. maire) Seeds. Food Chem. 2015, 187, 485–490. [Google Scholar] [CrossRef]
- Pérez, C.; Barrientos, H.; Roman, J.; Mahn, A. Optimization of a Blanching Step to Maximize Sulforaphane Synthesis in Broccoli Florets. Food Chem. 2014, 145, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Van Eylen, D.; Oey, I.; Hendrickx, M.; Van Loey, A. Kinetics of the Stability of Broccoli (Brassica oleracea Cv italica) Myrosinase and Isothiocyanates in Broccoli Juice during Pressure/Temperature Treatments. J. Agric. Food Chem. 2007, 55, 2163–2170. [Google Scholar] [CrossRef] [PubMed]
- Mahn, A.; Angulo, A.; Cabañas, F. Purification and Characterization of Broccoli (Brassica oleracea Var italica) Myrosinase (β-Thioglucosidase Glucohydrolase). J. Agric. Food Chem. 2014, 62, 11666–11671. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, T.; Verkerk, R.; Van Boekel, M.A.J.S.; Dekker, M. Effect of Water Content and Temperature on Inactivation Kinetics of Myrosinase in Broccoli (Brassica oleracea Var. italica). Food Chem. 2014, 163, 197–201. [Google Scholar] [CrossRef]
- Akpolat, H.; Barringer, S. The Effect of pH and Temperature on Cabbage Volatiles During Storage. J. Food Sci. 2015, 80, S1878–S1884. [Google Scholar] [CrossRef]
- Barba, F.J.; Nikmaram, N.; Roohinejad, S.; Khelfa, A.; Zhu, Z.; Koubaa, M. Bioavailability of Glucosinolates and their Breakdown Products: Impact of processing. Front. Nutr. 2016, 3, 24. [Google Scholar] [CrossRef]
- Kong, X.Y.; Kissen, R.; Bones, A.M. Characterization of Recombinant Nitrile-Specifier Proteins (NSPs) of Arabidopsis thaliana: Dependency on Fe(II) Ions and the Effect of Glucosinolate Substrate and Reaction Conditions. Phytochemistry 2012, 84, 7–17. [Google Scholar] [CrossRef]
- Van Dam, N.M.; Tytgat, T.O.G.; Kirkegaard, J.A. Root and Shoot Glucosinolates: A Comparison of Their Diversity, Function and Interactions in Natural and Managed Ecosystems. Phytochem. Rev. 2009, 8, 171–186. [Google Scholar] [CrossRef]
- Van Eylen, D.; Hendrickx, M.; Van Loey, A. Temperature and Pressure Stability of Mustard Seed (Sinapis alba L.) Myrosinase. Food Chem. 2006, 97, 263–271. [Google Scholar] [CrossRef]
- Nakamura, T.; Murata, Y.; Nakamura, Y. Characterization of Benzyl Isothiocyanate Extracted from Mashed Green Papaya by Distillation. Food Chem. 2019, 299, 125118. [Google Scholar] [CrossRef]
- Wittstock, U.; Meier, K.; Dörr, F.; Ravindran, B.M. NSP-Dependent Simple Nitrile Formation Dominates upon Breakdown of Major Aliphatic Glucosinolates in Roots, Seeds, and Seedlings of Arabidopsis thaliana Columbia-0. Front. Plant Sci. 2016, 7, 1821. [Google Scholar] [CrossRef] [PubMed]
- Burow, M.; Losansky, A.; Müller, R.; Plock, A.; Kliebenstein, D.J.; Wittstock, U. The genetic basis of constitutive and herbivore-induced ESP-independent nitrile formation in Arabidopsis. Plant Physiol. 2009, 149, 561–574. [Google Scholar] [CrossRef]
- Eisenschmidt-Bönn, D.; Schneegans, N.; Backenköhler, A.; Wittstock, U.; Brandt, W. Structural Diversification during Glucosinolate Breakdown: Mechanisms of Thiocyanate, Epithionitrile and Simple Nitrile Formation. Plant J. 2019, 99, 329–343. [Google Scholar] [CrossRef] [PubMed]
- de Torres Zabala, M.; Grant, M.; Bones, A.M.; Bennett, R.; Lim, Y.S.; Kissen, R.; Rossiter, J.T. Characterisation of Recombinant Epithiospecifier Protein and Its Over-Expression in Arabidopsis thaliana. Phytochemistry 2005, 66, 859–867. [Google Scholar] [CrossRef]
- Burow, M.; Markert, J.; Gershenzon, J.; Wittstock, U. Comparative Biochemical Characterization of Nitrile-Forming Proteins from Plants and Insects That Alter Myrosinase-Catalysed Hydrolysis of Glucosinolates. FEBS J. 2006, 273, 2432–2446. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Guo, L.; Wang, Z.; Zhuang, Y.; Gu, Z. Response Surface Optimization and Identification of Isothiocyanates Produced from Broccoli Sprouts. Food Chem. 2013, 141, 1580–1586. [Google Scholar] [CrossRef]
- Doheny-Adams, T.; Redeker, K.; Kittipol, V.; Bancroft, I.; Hartley, S.E. Development of an Efficient Glucosinolate Extraction Method. Plant Methods 2017, 13, 17. [Google Scholar] [CrossRef]
- Mohd Zul, S.; Surugau, N. Effects of Ascorbic Acid and Ferum Ions Concentration on the Hydrolysis of Glucosinolate and Myrosinase Activity in the Watercress (Nasturtium officinale sp.). J. Teknol. 2016, 78, 133–138. [Google Scholar] [CrossRef][Green Version]
- Novotny, C.; Schulzova, V.; Krmela, A.; Hajslova, J.; Svobodova, K.; Koudela, M. Ascorbic Acid and Glucosinolate Levels in New Czech Cabbage Cultivars: Effect of Production System and Fungal Infection. Molecules 2018, 23, 1855. [Google Scholar] [CrossRef]
- Liang, H.; Yuan, Q.; Xiao, Q. Effects of Metal Ions on Myrosinase Activity and the Formation of Sulforaphane in Broccoli Seed. J. Mol. Catal. B Enzym. 2006, 43, 19–22. [Google Scholar] [CrossRef]
- Gu, Z.; GUO, Q.; GU, Y. Factors Influencing Glucoraphanin and Sulforaphane Formation in Brassica Plants: A Review. J. Integr. Agric. 2012, 11, 1804–1816. [Google Scholar] [CrossRef]
- Gu, Y.; Guo, Q.; Zhang, L.; Chen, Z.; Han, Y.; Gu, Z. Physiological and Biochemical Metabolism of Germinating Broccoli Seeds and Sprouts. J. Agric. Food Chem. 2012, 60, 209–213. [Google Scholar] [CrossRef]
- Ludikhuyze, L.; Rodrigo, L.; Hendrickx, M. The Activity of Myrosinase from Broccoli (Brassica oleracea L. cv Italica): Influence of Intrinsic and Extrinsic Factors. J Food Prot. 2000, 63, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Bones, A.M.; Rossiter, J.T. The Enzymic and Chemically Induced Decomposition of Glucosinolates. Phytochemistry 2006, 67, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Kleinwachter, M.; Selmar, D. A novel approach for reliable activity determination of ascorbic acid depending myrosinases. Biochem. Biophys. Methods 2004, 59, 253–265. [Google Scholar] [CrossRef]
- Tian, S.; Liu, X.; Lei, P.; Zhang, X.; Shan, Y. Microbiota: A Mediator to Transform Glucosinolate Precursors in Cruciferous Vegetables to the Active Isothiocyanates. J. Sci. Food Agric. 2018, 98, 1255–1260. [Google Scholar] [CrossRef]
- Han, D.; Row, K.H. Separation and Purification of Sulforaphane from Broccoli by Solid Phase Extraction. Int. J. Mol. Sci. 2011, 12, 1854–1861. [Google Scholar] [CrossRef]
- Ares, A.M.; Bernal, J.; Martín, M.T.; Bernal, J.L.; Nozal, M.J. Optimized Formation, Extraction, and Determination of Sulforaphane in Broccoli by Liquid Chromatography with Diode Array Detection. Food Anal. Methods 2014, 7, 730–740. [Google Scholar] [CrossRef]
- Campas-Baypoli, O.N.; Sánchez-Machado, D.I.; Bueno-Solano, C.; Ramírez-Wong, B.; López-Cervantes, J. HPLC Method Validation for Measurement of Sulforaphane Level in Broccoli By-Products. Biomed. Chromatogr. 2010, 24, 387–392. [Google Scholar] [CrossRef]
- Suresh, S.; Waly, M.I.; Rahman, M.S. Broccoli (Brassica oleracea) as a Preventive Biomaterial for Cancer. In Bioactive Components, Diet and Medical Treatment in Cancer Prevention; Waly, M.I., Rahman, M.S., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 75–87. [Google Scholar] [CrossRef]
- Abdulah, R.; Faried, A.; Kobayashi, K.; Yamazaki, C.; Suradji, E.W.; Ito, K.; Suzuki, K.; Murakami, M.; Kuwano, H.; Koyama, H. Selenium Enrichment of Broccoli Sprout Extract Increases Chemosensitivity and Apoptosis of LNCaP Prostate Cancer Cells. BMC Cancer 2009, 9, 414. [Google Scholar] [CrossRef]
- Martínez-Hernández, G.B.; Artés-Hernández, F.; Gómez, P.A.; Artés, F. Induced Changes in Bioactive Compounds of Kailan-Hybrid Broccoli after Innovative Processing and Storage. J. Funct. Foods 2013, 5, 133–143. [Google Scholar] [CrossRef]
- Guo, R.; Yuan, G.; Wang, Q. Effect of Sucrose and Mannitol on the Accumulation of Health-Promoting Compounds and the Activity of Metabolic Enzymes in Broccoli Sprouts. Sci. Hortic. 2011, 128, 159–165. [Google Scholar] [CrossRef]
- Arora, R.; Vig, A.; Arora, S. Glucosinolates: Transposing Trends of Identification Methods from Paper Chromatography to Microchip Analysis. Int. J. Life Sc. Bt Pharma Res. 2014, 3, 42–61. [Google Scholar]
- Hanschen, F.S.; Brüggemann, N.; Brodehl, A.; Mewis, I.; Schreiner, M.; Rohn, S.; Kroh, L.W. Characterization of Products from the Reaction of Glucosinolate-Derived Isothiocyanates with Cysteine and Lysine Derivatives Formed in Either Model Systems or Broccoli Sprouts. J. Agric. Food Chem. 2012, 60, 7735–7745. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.F.; Yuan, G.F.; Wang, Q.M. Effect of NaCl Treatments on Glucosinolate Metabolism in Broccoli Sprouts. J. Zhejiang Univ. Sci. B 2013, 14, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, V.; Luciano, F.; Meca, G.; Ritieni, A.; Mañes, J. Bioaccessibility of Glucoraphanin from Broccoli Using an in Vitro Gastrointestinal Digestion Model. CyTA-J. Food. 2014, 13, 361–365. [Google Scholar] [CrossRef]
- Vergara, F.; Wenzler, M.; Hansen, B.G.; Kliebenstein, D.J.; Halkier, B.A.; Gershenzon, J.; Schneider, B. Determination of the Absolute Configuration of the Glucosinolate Methyl Sulfoxide Group Reveals a Stereospecific Biosynthesis of the Side Chain. Phytochemistry 2008, 69, 2737–2742. [Google Scholar] [CrossRef]
- Van Eylen, D.; Bellostas, N.; Strobel, B.W.; Oey, I.; Hendrickx, M.; Van Loey, A.; Sørensen, H.; Sørensen, J.C. Influence of Pressure/Temperature Treatments on Glucosinolate Conversion in Broccoli (Brassica oleraceae L. Cv italica) Heads. Food Chem. 2009, 112, 646–653. [Google Scholar] [CrossRef]
- Liang, H.; Li, C.; Yuan, Q.; Vriesekoop, F. Separation and Purification of Sulforaphane from Broccoli Seeds by Solid Phase Extraction and Preparative High-Performance Liquid Chromatography. J. Agric. Food Chem. 2007, 55, 8047–8053. [Google Scholar] [CrossRef]
- Farag, M.A.; Motal, A.A.A. Sulforaphane Composition, Cytotoxic and Antioxidant Activity of Crucifer Vegetables. J. Adv. Res. 2010, 1, 65–70. [Google Scholar] [CrossRef]
- Ares, A.M.; Nozal, M.J.; Bernal, J. Extraction, Chemical Characterization and Biological Activity Determination of Broccoli Health Promoting Compounds. J. Chromatogr. A. 2013, 1313, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Iori, R.; Thornalley, P.J. Purification of Major Glucosinolates from Brassicaceae Seeds and Preparation of Isothiocyanate and Amine Metabolites. J. Sci. Food Agric. 2006, 86, 1271–1280. [Google Scholar] [CrossRef]
- Sangkret, S.; Pongmalai, P.; Devahastin, S.; Chiewchan, N. Enhanced Production of Sulforaphane by Exogenous Glucoraphanin Hydrolysis Catalyzed by Myrosinase Extracted from Chinese Flowering Cabbage (Brassica rapa var. parachinensis). Sci. Rep. 2019, 9, 9882. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, X.; Yang, Y.; Zhang, X. Extraction and Identification of Isothiocyanates from Broccolini Seeds. Nat. Prod. Commun. 2011, 6, 65–66. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Wang, J.; Cai, C.; Chang, J.; Zhao, Y.; Wang, Q. Accumulation of Glucosinolates in Broccoli. In Glucosinolates; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–30. [Google Scholar] [CrossRef]
- Campas-Baypoli, O.N.; Bueno-Solano, C.; Martínez-Ibarra, D.M.; Camacho-Gil, F.; Villa-Lerma, A.G.; Rodríguez-Núñez, J.R.; Lóez-Cervantes, J.; Sánchez-Machado, D.I. Sulforaphane (1-isothiocyanato-4-(methylsulfinyl)-butane) content in cruciferous vegetables. Arch. Latinoam. Nutr. 2009, 59, 95–100. [Google Scholar] [PubMed]
- Totušek, J.; Tříska, J.; Lefnerová, D.; Strohalm, J.; Vrchotová, N.; Zendulka, O.; Průchová, J.; Chaloupková, J.; Novotná, P.; Houška, M. Contents of Sulforaphane and Total Isothiocyanates, Antimutagenic Activity, and Inhibition of Clastogenicity in Pulp Juices from Cruciferous Plants. Czech J. Food Sci. 2011, 29, 548–556. [Google Scholar] [CrossRef]
- Langston-Cox, A.; Anderson, D.; Creek, D.J.; Palmer, K.; Wallace, E.M.; Marshall, S.A. Measuring Sulforaphane and Its Metabolites in Human Plasma: A High Throughput Method. Molecules. 2020, 25, 829. [Google Scholar] [CrossRef]
- Hafezian, S.M.; Azizi, S.N.; Biparva, P.; Bekhradnia, A. High-Efficiency Purification of Sulforaphane from the Broccoli Extract by Nanostructured SBA-15 Silica Using Solid-Phase Extraction Method. J. Chromatogr. B. 2019, 1108, 1–10. [Google Scholar] [CrossRef]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate Structural Diversity, Identification, Chemical Synthesis and Metabolism in Plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The Chemical Diversity and Distribution of Glucosinolates and Isothiocyanates among Plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Kassie, F.; Uhl, M.; Rabot, S.; Grasl-Kraupp, B.; Verkerk, R.; Kundi, M.; Chabicovsky, M.; Schulte-Hermann, R.; Knasmüller, S. Chemoprevention of 2-Amino-3-Methylimidazo[4,5- f ]Quinoline (IQ)-Induced Colonic and Hepatic Preneoplastic Lesions in the F344 Rat by Cruciferous Vegetables Administered Simultaneously with the Carcinogen. Carcinogenesis 2003, 24, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, U.; Ezhilarasan, R.; Vaughn, S.F.; Berhow, M.A.; Mohanam, S. Iberin Induces Cell Cycle Arrest and Apoptosis in Human Neuroblastoma Cells. Int. J. Mol. Med. 2007, 19, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, U.; Ezhilarasan, R.; Vaughn, S.; Berhow, M.; Mohanam, S. Dietary Isothiocyanate Iberin Inhibits Growth and Induces Apoptosis in Human Glioblastoma Cells. J. Pharmacol. Sci. 2007, 103, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.-Y.; Liu, Y.; Chua, S.L.; Vejborg, R.M.; Jakobsen, T.H.; Chew, S.C.; Li, Y.; Nielsen, T.E.; Tolker-Nielsen, T.; Yang, L.; et al. Comparative Systems Biology Analysis to Study the Mode of Action of the Isothiocyanate Compound Iberin on Pseudomonas Aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 6648–6659. [Google Scholar] [CrossRef]
- Mitsiogianni, M.; Trafalis, D.; Franco, R.; Zoumpourlis, V.; Pappa, A.; Panayiotidis, M. Sulforaphane and Iberin Are Potent Epigenetic Modulators of Histone Acetylation and Methylation in Malignant Melanoma. Eur. J. Nutr. 2021, 60, 147–158. [Google Scholar] [CrossRef]
- Cirilli, R.; Gallo, F.R.; Multari, G.; Palazzino, G.; Mustazza, C.; Panusa, A. Study of Solvent Effect on the Stability of Isothiocyanate Iberin, a Breakdown Product of Glucoiberin. J. Food Compos. Anal. 2020, 92, 103515. [Google Scholar] [CrossRef]
- Deng, Q.; Zinoviadou, K.G.; Galanakis, C.M.; Orlien, V.; Grimi, N.; Vorobiev, E.; Lebovka, N.; Barba, F.J. The Effects of Conventional and Non-Conventional Processing on Glucosinolates and Its Derived Forms, Isothiocyanates: Extraction, Degradation, and Applications. Food Eng. Rev. 2015, 7, 357–381. [Google Scholar] [CrossRef]
- Matusheski, N.V.; Wallig, M.A.; Juvik, J.A.; Klein, B.P.; Kushad, M.M.; Jeffery, E.H. Preparative HPLC Method for the Purification of Sulforaphane and Sulforaphane Nitrile from Brassica oleracea. J. Agric. Food Chem. 2001, 49, 1867–1872. [Google Scholar] [CrossRef]
- Tian, G.; Tang, P.; Xie, R.; Cheng, L.; Yuan, Q.; Hu, J. The Stability and Degradation Mechanism of Sulforaphene in Solvents. Food Chem. 2016, 199, 301–306. [Google Scholar] [CrossRef]
- Tian, G.; Li, Y.; Cheng, L.; Yuan, Q.; Tang, P.; Kuang, P.; Hu, J. The Mechanism of Sulforaphene Degradation to Different Water Contents. Food Chem. 2016, 194, 1022–1027. [Google Scholar] [CrossRef]
- Conaway, C.C.; Chung, Y.Y. and F. Isothiocyanates as Cancer Chemopreventive Agents: Their Biological Activities and Metabolism in Rodents and Humans. Curr. Drug Metabol. 2002, 3, 233–255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, S.; Chen, Q.S.; Chai, Y.F.; Sun, D.X.; Zhang, G.Q. Analysis of Sulforaphane by HPLC-MS/MS in Vitro and in Vivo: Chemical Stability, Metabolic Rate and Metabolites. Asian J. Chem. 2015, 27, 1045–1048. [Google Scholar] [CrossRef]
- Franklin, S.J.; Dickinson, S.E.; Karlage, K.L.; Bowden, G.T.; Myrdal, P.B. Stability of Sulforaphane for Topical Formulation. Drug Dev. Ind. Pharm. 2014, 40, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M. Separation of Functional Macromolecules and Micromolecules: From Ultrafiltration to the Border of Nanofiltration. Trends Food Sci. Technol. 2015, 42, 44–63. [Google Scholar] [CrossRef]
- Galanakis, C.M. Phenols Recovered from Olive Mill Wastewater as Additives in Meat Products. Trends Food Sci. Technol. 2018, 79, 98–105. [Google Scholar] [CrossRef]
- Langeveld, M.; Tan, C.Y.; Soeters, M.R.; Virtue, S.; Watson, L.P.; Murgatroyd, P.R.; Ambler, G.K.; Vidal-Puig, S.; Chatterjee, K.V.; Vidal-Puig, A. No Metabolic Effects of Mustard Allyl-Isothiocyanate Compared with Placebo in Men. Am. J. Clin. Nutr. 2017, 106, 1197–1205. [Google Scholar] [CrossRef]
- Subedi, L.; Venkatesan, R.; Kim, S.Y. Neuroprotective and Anti-Inflammatory Activities of Allyl Isothiocyanate through Attenuation of JNK/NF-κB/TNF-α Signaling. Int. J. Mol. Sci. 2017, 18, 1423. [Google Scholar] [CrossRef]
- Yun, Y.-K.; Kim, H.-K.; Kim, J.-R.; Hwang, K.; Ahn, Y.-J. Contact and Fumigant Toxicity of Armoracia rusticana Essential Oil, Allyl Isothiocyanate and Related Compounds to Dermatophagoides Farinae. Pest Manag. Sci. 2012, 68, 788–794. [Google Scholar] [CrossRef]
- Zhang, Y. Allyl Isothiocyanate as a Cancer Chemopreventive Phytochemical. Mol. Nutr. Food Res. 2010, 54, 127–135. [Google Scholar] [CrossRef]
- Bo, P.; Lien, J.-C.; Chen, Y.-Y.; Yu, F.-S.; Lu, H.-F.; Yu, C.-S.; Chou, Y.-C.; Yu, C.-C.; Chung, J.-G. Allyl Isothiocyanate Induces Cell Toxicity by Multiple Pathways in Human Breast Cancer Cells. Am. J. Chin. Med. 2016, 44, 415–437. [Google Scholar] [CrossRef]
- Chiang, J.-H.; Tsai, F.-J.; Hsu, Y.-M.; Yin, M.-C.; Chiu, H.-Y.; Yang, J.-S. Sensitivity of Allyl Isothiocyanate to Induce Apoptosis via ER Stress and the Mitochondrial Pathway upon ROS Production in Colorectal Adenocarcinoma Cells. Oncol. Rep. 2020, 44, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Sávio, A.L.V.; da Silva, G.N.; Salvadori, D.M.F. Inhibition of Bladder Cancer Cell Proliferation by Allyl Isothiocyanate (Mustard Essential Oil). Mutat. Res. 2015, 771, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Li, Y.; Wade, K.L.; Paonessa, J.D.; Fahey, J.W.; Zhang, Y. Allyl Isothiocyanate-Rich Mustard Seed Powder Inhibits Bladder Cancer Growth and Muscle Invasion. Carcinogenesis 2010, 31, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Li, P.; Xue, Z. Effect of Allyl Isothiocyanate on the Viability and Apoptosis of the Human Cervical Cancer HeLa Cell Line in Vitro. Oncol. Lett. 2018, 15, 8756–8760. [Google Scholar] [CrossRef]
- Dufour, V.; Alazzam, B.; Ermel, G.; Thepaut, M.; Rossero, A.; Tresse, O.; Baysse, C. Antimicrobial Activities of Isothiocyanates against Campylobacter Jejuni Isolates. Front. Cell. Infect. Microbiol. 2012, 2, 53. [Google Scholar] [CrossRef]
- Olivier, C.; Vaughn, S.F.; Mizubuti, E.S.G.; Loria, R. Variation in Allyl Isothiocyanate Production within Brassica Species and Correlation with Fungicidal Activity. J. Chem. Ecol. 1999, 25, 2687–2701. [Google Scholar] [CrossRef]
- Ahmed, R. Evaluation of Antimicrobial Activity of Allyl Isothiocyanate (AITC) Adsorbed in Oyster Shell on Food-Borne Bacteria. Clean Technol. 2015, 21, 241–247. [Google Scholar] [CrossRef][Green Version]
- Kara, M.; Soylu, E.M. Assessment of Glucosinolate-Derived Isothiocyanates as Potential Natural Antifungal Compounds against Citrus Sour Rot Disease Agent Geotrichum Citri-Aurantii. J. Phytopathol. 2020, 168, 279–289. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, X.; Zhang, G.-A.; Zeng, S.-Y.; Lin, K.-C. Antifungal Vapour-phase Activity of a Combination of Allyl Isothiocyanate and Ethyl Isothiocyanate Against Botrytis cinerea and Penicillium expansum Infection on Apples. J. Phytopathol. 2011, 159, 450–455. [Google Scholar] [CrossRef]
- Azaiez, I.; Meca, G.; Manyes, L.; Fernández-Franzón, M. Antifungal Activity of Gaseous Allyl, Benzyl and Phenyl Isothiocyanate in Vitro and Their Use for Fumonisins Reduction in Bread. Food Control 2013, 32, 428–434. [Google Scholar] [CrossRef]
- Manyes, L.; Luciano, F.B.; Mañes, J.; Meca, G. In Vitro Antifungal Activity of Allyl Isothiocyanate (AITC) against Aspergillus parasiticus and Penicillium expansum and Evaluation of the AITC Estimated Daily Intake. Food Chem. Toxicol. 2015, 83, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Wang, H.D.; Wang, X.L.; Qiao, L.; Yin, H.X. Sulforaphane Attenuates Matrix Metalloproteinase-9 Expression Following Spinal Cord Injury in Mice. Ann. Clin. Lab. Sci. 2010, 40, 354–360. [Google Scholar] [PubMed]
- Latronico, T.; Larocca, M.; Milella, S.; Fasano, A.; Rossano, R.; Liuzzi, G.M. Neuroprotective Potential of Isothiocyanates in an in Vitro Model of Neuroinflammation. Inflammopharmacology 2020, 29, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Lim, L.T. Release of Allyl Isothiocyanate from Mustard Seed Meal Powder. J. Food Sci. 2014, 79, E47–E53. [Google Scholar] [CrossRef] [PubMed]
- Cools, K.; Terry, L.A. Comparative Study between Extraction Techniques and Column Separation for the Quantification of Sinigrin and Total Isothiocyanates in Mustard Seed. J. Chromatogr. B. 2012, 901, 115–118. [Google Scholar] [CrossRef]
- Sharna, H.K.; Ingle, S.; Singh, C.; Sarkar, B.C.; Upadhyay, A. Effect of various process treatment conditions on the allyl isothiocyanate extraction rate from mustard meal. J. Food Sci. Technol. 2012, 49, 368–372. [Google Scholar] [CrossRef][Green Version]
- Tripathi, M.K.; Mishra, A. Glucosinolates in Animal Nutrition: A Review. Anim. Feed Sci. Technol. 2007, 132, 1–27. [Google Scholar] [CrossRef]
- Li, Y.; Teng, Z.; Chen, P.; Song, Y.; Luo, Y.; Wang, Q. Enhancement of aqueous stability of allyl isothiocyanate using nanoemulsions prepared by an emulsion inversion point method. J. Colloid Interface Sci. 2014, 15, 130–137. [Google Scholar] [CrossRef]
- Liu, T.T.; Yang, T.S. Stability and antimicrobial activity of allyl isothiocyanate during long-term storage in an oil-in-water emulsion. J. Food Sci. 2010, 75, 445–451. [Google Scholar] [CrossRef]
- Nahar, L.; Sarker, S.D. Supercritical fluid extraction in natural products analyses. Methods Mol. Biol. 2012, 864, 43–74. [Google Scholar] [CrossRef]
- Moyler, D.A. Extraction of Flavours and Fragrances with Compressed CO2. In Extraction of Natural Products Using Near-Critical Solvents; King, M.B., Bott, T.R., Eds.; Springer: Dordrecht, The Netherlands, 1993; pp. 140–183. [Google Scholar] [CrossRef]
- Kraujalis, P.; Venskutonis, P.R. Optimisation of Supercritical Carbon Dioxide Extraction of Amaranth Seeds by Response Surface Methodology and Characterization of Extracts Isolated from Different Plant Cultivars. J. Supercrit. Fluids 2013, 73, 80–86. [Google Scholar] [CrossRef]
- Gracia, I.; Rodríguez, J.F.; García, M.T.; Alvarez, A.; García, A. Isolation of Aroma Compounds from Sugar Cane Spirits by Supercritical CO2. J. Supercrit. Fluids 2007, 43, 37–42. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, A.; Liu, H.; Liu, L.; Zhang, Y.; Li, N.; Gong, K.; Yu, M.; Zheng, L. Peanut By-Products Utilization Technology. In Peanuts: Processing Technology and Product Development; Wang, Q., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 211–325. [Google Scholar] [CrossRef]
- Lizcano, S.C.; Dávila, J.A.; Hernández, V. Fruit Agroindustrial Wastes for Preparing Beverages for Medicinal Purposes by Supercritical Fluid Extraction Technology: Andes Berry (Rubus glaucus benth) Case. In Production and Management of Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 151–177. [Google Scholar] [CrossRef]
- Kim, S.-J.; Lee, M.-K.; Back, S.-S.; Chun, B.-S. Extraction and Identification of Volatile Isothiocyanates from Wasabi Using Supercritical Carbon Dioxide. Korean J. Biotechnol. Bioeng 2007, 22, 174–178. [Google Scholar]
- Jain, A.; Ong, V.; Jayaraman, S.; Balasubramanian, R.; Srinivasan, M.P. Supercritical fluid immobilization of horseradish peroxidase on high surface area mesoporous activated carbon. J Supercrit Fluids 2016, 107, 513–518. [Google Scholar] [CrossRef]
- Szmigielska, A.M.; Schoenau, J.J. Use of Anion-Exchange Membrane Extraction for the High-Performance Liquid Chromatographic Analysis of Mustard Seed Glucosinolates. J. Agric. Food Chem. 2000, 48, 5190–5194. [Google Scholar] [CrossRef]
- Bennett, R.N.; Carvalho, R.; Mellon, F.A.; Eagles, J.; Rosa, E.A.S. Identification and Quantification of Glucosinolates in Sprouts Derived from Seeds of Wild Eruca sativa L. (Salad Rocket) and Diplotaxis tenuifolia L. (Wild Rocket) from Diverse Geographical Locations. J. Agric. Food Chem. 2007, 55, 67–74. [Google Scholar] [CrossRef]
- Powell, E.E.; Hill, G.A.; Juurlink, B.H.J.; Carrier, D.J. Glucoraphanin Extraction from Cardaria Draba: Optimization of Batch Extraction. J. Chem. Technol. Biotechnol. 2005, 80, 985–991. [Google Scholar] [CrossRef]
- Mohn, T.; Cutting, B.; Ernst, B.; Hamburger, M. Extraction and Analysis of Intact Glucosinolates—A Validated Pressurized Liquid Extraction/Liquid Chromatography–Mass Spectrometry Protocol for Isatis tinctoria, and Qualitative Analysis of Other Cruciferous Plants. J. Chromatogr. A 2007, 1166, 142–151. [Google Scholar] [CrossRef]
- Wang, T.; Liang, H.; Yuan, Q. Optimization of Ultrasonic-Stimulated Solvent Extraction of Sinigrin from Indian Mustard Seed (Brassica juncea L.) Using Response Surface Methodology. Phytochem. Anal. 2011, 22, 205–213. [Google Scholar] [CrossRef]
- Soares Melecchi, M.I.; Péres, V.F.; Dariva, C.; Zini, C.A.; Abad, F.C.; Martinez, M.M.; Caramão, E.B. Optimization of the sonication extraction method of Hibiscus tiliaceus L. flowers. Ultrason. Sonochem. 2006, 13, 242–250. [Google Scholar] [CrossRef]
- Huang, W.; Xue, A.; Niu, H.; Jia, Z.; Wang, J. Optimised Ultrasonic-Assisted Extraction of Flavonoids from Folium Eucommiae and Evaluation of Antioxidant Activity in Multi-Test Systems In Vitro. Food Chem. 2009, 114, 1147–1154. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D. Ultrasound-Assisted Extraction Coupled with under Vacuum Distillation of Flavour Compounds from Spearmint (Carvone-Rich) Plants: Comparison with Conventional Hydrodistillation. Ultrason. Sonochem. 2009, 16, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Toma, M.; Vinatoru, M.; Paniwnyk, L.; Mason, T.J. Investigation of the Effects of Ultrasound on Vegetal Tissues during Solvent Extraction. Ultrason. Sonochem. 2001, 8, 137–142. [Google Scholar] [CrossRef]
- Sivakumar, V.; Ravi Verma, V.; Rao, P.G.; Swaminathan, G. Studies on the Use of Power Ultrasound in Solid–Liquid Myrobalan Extraction Process. J. Clean. Prod. 2007, 15, 1813–1818. [Google Scholar] [CrossRef]
- Boonkird, S.; Phisalaphong, C.; Phisalaphong, M. Ultrasound-Assisted Extraction of Capsaicinoids from Capsicum Frutescens on a Lab- and Pilot-Plant Scale. Ultrason. Sonochem. 2008, 15, 1075–1079. [Google Scholar] [CrossRef]
- Variyar, P.S.; Banerjee, A.; Akkarakaran, J.J.; Suprasanna, P. Role of Glucosinolates in Plant Stress Tolerance. In Emerging Technologies and Management of Crop Stress Tolerance; Ahmad, P., Rasool, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 271–291. [Google Scholar] [CrossRef]
- Jensen, J.; Styrishave, B.; Gimsing, A.L.; Bruun Hansen, H.C. The Toxic Effects of Benzyl Glucosinolate and Its Hydrolysis Product, the Biofumigant Benzyl Isothiocyanate, to Folsomia fimetaria. Environ. Toxicol. Chem. 2010, 29, 359–364. [Google Scholar] [CrossRef]
- Platz, S.; Kühn, C.; Schiess, S.; Schreiner, M.; Mewis, I.; Kemper, M.; Pfeiffer, A.; Rohn, S. Determination of Benzyl Isothiocyanate Metabolites in Human Plasma and Urine by LC-ESI-MS/MS after Ingestion of Nasturtium (Tropaeolum majus L.). Anal. Bioanal. Chem. 2013, 405, 7427–7436. [Google Scholar] [CrossRef]
- Zakaria, S.; Helmy, M.W.; Salahuddin, A.; Omran, G. Chemopreventive and Antitumor Effects of Benzyl Isothiocynate on HCC Models: A Possible Role of HGF /PAkt/ STAT3 Axis and VEGF. Biomed. Pharmacother. 2018, 108, 65–75. [Google Scholar] [CrossRef]
- Zhu, M.; Li, W.; Guo, J.; Lu, Y.; Dong, X.; Lin, B.; Chen, Y.; Zhang, X.; Li, M. Alpha Fetoprotein Antagonises Benzyl Isothiocyanate Inhibition of the Malignant Behaviors of Hepatocellular Carcinoma Cells. Oncotarget 2016, 7, 75749–75762. [Google Scholar] [CrossRef]
- Boreddy, S.R.; Sahu, R.P.; Srivastava, S.K. Benzyl Isothiocyanate Suppresses Pancreatic Tumor Angiogenesis and Invasion by Inhibiting HIF-α/VEGF/Rho-GTPases: Pivotal Role of STAT-3. PLoS ONE 2011, 6, e25799. [Google Scholar] [CrossRef]
- Sahu, R.P.; Srivastava, S.K. The Role of STAT-3 in the Induction of Apoptosis in Pancreatic Cancer Cells by Benzyl Isothiocyanate. J. Natl. Cancer Inst. 2009, 101, 176–193. [Google Scholar] [CrossRef] [PubMed]
- Kamii, E.; Isshiki, K. Antimicrobial Efficacy of Benzyl Isothiocyanate. Shokuhin Eiseigaku Zasshi. 2009, 50, 311–314. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, T.; Li, Y.; Bi, Y.; Zhang, M.; Zhang, T.; Zheng, X.; Dong, Y.; Huang, Y. Benzyl Isothiocyanate Fumigation Inhibits Growth, Membrane Integrity and Mycotoxin Production in Alternaria alternata. RSC Adv. 2020, 10, 1829–1837. [Google Scholar] [CrossRef]
- Pereira, C.; Calado, A.M.; Sampaio, A.C. The Effect of Benzyl Isothiocyanate on Candida albicans Growth, Cell Size, Morphogenesis, and Ultrastructure. World J. Microbiol. Biotechnol. 2020, 36, 153. [Google Scholar] [CrossRef] [PubMed]
- Afsharypuor, S.; Hadi, M.-E. Volatile Constituents of the Seeds, Roots and Non-Flowering Aerial Parts of Lepidium satvium L. J. Essent. Oil Res. 2006, 18, 495–496. [Google Scholar] [CrossRef]
- Németh, A.G.; Keserű, G.M.; Ábrányi-Balogh, P. A Novel Three-Component Reaction between Isocyanides, Alcohols or Thiols and Elemental Sulfur: A Mild, Catalyst-Free Approach towards O-Thiocarbamates and Dithiocarbamates. Beilstein J. Org. Chem. 2019, 15, 1523–1533. [Google Scholar] [CrossRef]
- Nakamura, Y.; Miyoshi, N. Electrophiles in Foods: The Current Status of Isothiocyanates and Their Chemical Biology. Biosci. Biotechnol. Biochem. 2010, 74, 242–255. [Google Scholar] [CrossRef]
- Abdel-Kader, M.S.; Alam, P.; Kamal, Y.T.; Alkharfy, K.M.; Foudah, A.I.; Alqasoumi, S.I. Optimization of the Extraction Condition for Benzyl Isothiocyanate Contents in Salvadora persica Roots “Siwak”. Saudi Pharm. J. 2019, 27, 753–755. [Google Scholar] [CrossRef]
- Abdel-Kader, M.S.; Al Shahrani, K.S.; Alqarni, M.H.; Salkini, M.A.; Khamis, E.H.; Ghabbour, H.A.; Alqasoumi, S.I. Effect of Hydroxylated Solvents on the Active Constituents of Salvadora persica Root “Siwak”. Saudi Pharm. J. 2019, 27, 220–224. [Google Scholar] [CrossRef]
- Hegazi, G.; El-Hanafy, N.; Abu-Elkheir, Z.; Hussein, I. Benzyl Isothiocyanate Production from Salvadora persica L. Callus Cultures. IOSR J. Biotechnol. Biochem. 2016, 2, 19–25. [Google Scholar]
- De Nicola, G.R.; Nyegue, M.; Montaut, S.; Iori, R.; Menut, C.; Tatibouët, A.; Rollin, P.; Ndoyé, C.; Zollo, P.-H.A. Profile and Quantification of Glucosinolates in Pentadiplandra Brazzeana Baillon. Phytochemistry 2012, 73, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Conaway, C.C.; Krzeminski, J.; Amin, S.; Chung, F.-L. Decomposition Rates of Isothiocyanate Conjugates Determine Their Activity as Inhibitors of Cytochrome P450 Enzymes. Chem. Res. Toxicol. 2001, 14, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- De Nicola, G.R.; Montaut, S.; Rollin, P.; Nyegue, M.; Menut, C.; Iori, R.; Tatibouët, A. Stability of Benzylic-Type Isothiocyanates in Hydrodistillation-Mimicking Conditions. J. Agric. Food Chem. 2013, 61, 137–142. [Google Scholar] [CrossRef]
- Oerlemans, K.; Barrett, D.M.; Suades, C.B.; Verkerk, R.; Dekker, M. Thermal Degradation of Glucosinolates in Red Cabbage. Food Chem. 2006, 95, 19–29. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Rohn, S.; Mewis, I.; Schreiner, M.; Kroh, L.W. Influence of the Chemical Structure on the Thermal Degradation of the Glucosinolates in Broccoli Sprouts. Food Chem. 2012, 130, 1–8. [Google Scholar] [CrossRef]
- Hasapis, X.; MacLeod, A.J. Benzylglucosinolate Degradation in Heat-Treated Lepidium sativum Seeds and Detection of a Thiocyanate-Forming Factor. Phytochemistry 1982, 21, 1009–1013. [Google Scholar] [CrossRef]
- Li, N.; Xu, L. Thermal Analysis of β-Cyclodextrin/Berberine Chloride Inclusion Compounds. Thermochim. Acta 2010, 499, 166–170. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Yang, Q.; Zhang, N.; Du, Y.; Zhu, H. Preparation and Characterization of Inclusion Complex of Benzyl Isothiocyanate Extracted from Papaya Seed with β-Cyclodextrin. Food Chem. 2015, 184, 99–104. [Google Scholar] [CrossRef]
- Uppal, S.; Kaur, K.; Kumar, R.; Kahlon, N.K.; Singh, R.; Mehta, S.K. Encompassment of Benzyl Isothiocyanate in Cyclodextrin Using Ultrasonication Methodology to Enhance Its Stability for Biological Applications. Ultrason. Sonochem. 2017, 39, 25–33. [Google Scholar] [CrossRef]
- Dayalan Naidu, S.; Suzuki, T.; Yamamoto, M.; Fahey, J.W.; Dinkova-Kostova, A.T. Phenethyl Isothiocyanate, a Dual Activator of Transcription Factors NRF2 and HSF1. Mol. Nutr. Food Res. 2018, 62, 1700908. [Google Scholar] [CrossRef]
- Sarkar, R.P.; Mukherjee, S.; Biswas, J.; Roy, M. Phenethyl Isothiocyanate, by Virtue of Its Antioxidant Activity, Inhibits Invasiveness and Metastatic Potential of Breast Cancer Cells: HIF-1α as a Putative Target. Free Radic. Res. 2016, 50, 84–100. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.D.; Ward, W.M.; Loo, G. Effect of Antioxidants on the Genotoxicity of Phenethyl Isothiocyanate. Mutagenesis 2015, 30, 421–430. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yazdanparast, R.; Bahramikia, S.; Ardestani, A. Nasturtium officinale Reduces Oxidative Stress and Enhances Antioxidant Capacity in Hypercholesterolaemic Rats. Chem. Biol. Interact. 2008, 172, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-Y.; Lin, K.-C.; Lin, J.-P.; Tang, N.-Y.; Yang, J.-S.; Lu, K.-W.; Chung, J.-G. Phenethyl Isothiocyanate (PEITC) Inhibits the Growth of Human Oral Squamous Carcinoma HSC-3 Cells through G 0/G 1 Phase Arrest and Mitochondria-Mediated Apoptotic Cell Death. Evidence-Based Complement. Altern. Med. 2012, 2012, 718320. [Google Scholar] [CrossRef] [PubMed]
- Koschorke, A.; Faraci, S.; Giani, D.; Chiodoni, C.; Iorio, E.; Canese, R.; Colombo, M.P.; Lamolinara, A.; Iezzi, M.; Ladomery, M.; et al. Phenethyl Isothiocyanate Hampers Growth and Progression of HER2-Positive Breast and Ovarian Carcinoma by Targeting Their Stem Cell Compartment. Cell. Oncol. 2019, 42, 815–828. [Google Scholar] [CrossRef]
- Lawson, A.P.; Long, M.J.C.; Coffey, R.T.; Qian, Y.; Weerapana, E.; El Oualid, F.; Hedstrom, L. Naturally Occurring Isothiocyanates Exert Anticancer Effects by Inhibiting Deubiquitinating Enzymes. Cancer Res. 2015, 75, 5130–5142. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, W.; Hao, M. Phenethyl Isothiocyanate Reduces Breast Cancer Stem Cell-like Properties by Epigenetic Reactivation of CDH1. Oncol Rep. 2021, 45, 337–348. [Google Scholar] [CrossRef]
- Bommareddy, A.; Hahm, E.-R.; Xiao, D.; Powolny, A.A.; Fisher, A.L.; Jiang, Y.; Singh, S.V. Atg5 Regulates Phenethyl Isothiocyanate–Induced Autophagic and Apoptotic Cell Death in Human Prostate Cancer Cells. Cancer Res. 2009, 69, 3704–3712. [Google Scholar] [CrossRef]
- Boggs, D.A.; Palmer, J.R.; Wise, L.A.; Spiegelman, D.; Stampfer, M.J.; Adams-Campbell, L.L.; Rosenberg, L. Fruit and Vegetable Intake in Relation to Risk of Breast Cancer in the Black Women’s Health Study. Am. J. Epidemiol. 2010, 172, 1268–1279. [Google Scholar] [CrossRef]
- Gupta, P.; Kim, B.; Kim, S.-H.; Srivastava, S.K. Molecular Targets of Isothiocyanates in Cancer: Recent Advances. Mol. Nutr. Food Res. 2014, 58, 1685–1707. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Wang, X.; Meng, Y.; Zhang, Q.; Zhu, J.; Chen, J.; Cao, W.; Wang, X.; Xie, C.; et al. Phenethyl Isothiocyanate Inhibits Colorectal Cancer Stem Cells by Suppressing Wnt/β-Catenin Pathway. Phyther. Res. 2018, 32, 2447–2455. [Google Scholar] [CrossRef] [PubMed]
- Jaya Seema, D.M.; Saifullah, B.; Selvanayagam, M.; Gothai, S.; Hussein, M.Z.; Subbiah, S.K.; Mohd Esa, N.; Arulselvan, P. Designing of the Anticancer Nanocomposite with Sustained Release Properties by Using Graphene Oxide Nanocarrier with Phenethyl Isothiocyanate as Anticancer Agent. Pharmaceutics 2018, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-X.; Wu, H.-T.; Li, X.-X.; Wu, H.-Y.; Niu, T.-X.; Wang, X.-N.; Lian, R.; Zhang, G.-L.; Hou, H.-M. Comparison of the Inhibitory Potential of Benzyl Isothiocyanate and Phenethyl Isothiocyanate on Shiga Toxin-Producing and Enterotoxigenic Escherichia coli. LWT 2020, 118, 108806. [Google Scholar] [CrossRef]
- Kim, M.G.; Lee, H.S. Growth-Inhibiting Activities of Phenethyl Isothiocyanate and Its Derivatives against Intestinal Bacteria. J. Food Sci. 2009, 74, M467–M471. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, C.; Ye, J.; Zhou, H.; Chen, X. Antimicrobial Activities of Phenethyl Isothiocyanate Isolated from Horseradish. Nat. Prod. Res. 2012, 26, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, D.; Maciąg-Dorszyńska, M.; Bogucka, K.; Szalewska-Pałasz, A.; Herman-Antosiewicz, A. Various Modes of Action of Dietary Phytochemicals, Sulforaphane and Phenethyl Isothiocyanate, on Pathogenic Bacteria. Sci. Rep. 2019, 9, 13677. [Google Scholar] [CrossRef] [PubMed]
- Van Eylen, D.; Oey, I.; Hendrickx, M.; Van Loey, A. Effects of Pressure/Temperature Treatments on Stability and Activity of Endogenous Broccoli (Brassica oleracea L. Cv italica) Myrosinase and on Cell Permeability. J. Food Eng. 2008, 89, 178–186. [Google Scholar] [CrossRef]
- Fusari, C.M.; Ramirez, D.A.; Camargo, A.B. Simplified analytical methodology for glucosinolate hydrolysis products: A mniaturized extraction technique and multivariate optimization. Anal. Methods 2019, 11, 309–316. [Google Scholar] [CrossRef]
- Øverby, A.; Stokland, R.A.; Åsberg, S.E.; Sporsheim, B.; Bones, A.M. Allyl isothiocyanate depletes glutathione and upregulates expression of glutathione S-transferases in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 277. [Google Scholar] [CrossRef]
- Fahey, J.W. Method of Extraction of Isothiocyanates into Oil from Glucosinolsate-Containing Plants. US20060127996A1, 15 June 2006. WO 2006/065736 A3. [Google Scholar]
- Rodrigues, L.; Silva, I.; Poejo, J.; Serra, A.T.; Matias, A.A.; Simplício, A.L.; Bronze, M.R.; Duarte, C.M.M. Recovery of Antioxidant and Antiproliferative Compounds from Watercress Using Pressurized Fluid Extraction. RSC Adv. 2016, 6, 30905–30918. [Google Scholar] [CrossRef]
- Ji, Y.; Morris, M.E. Determination of Phenethyl Isothiocyanate in Human Plasma and Urine by Ammonia Derivatization and Liquid Chromatography–Tandem Mass Spectrometry. Anal. Biochem. 2003, 323, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Chatzilazarou, A. Application of Cloud Point Extraction Using Surfactants in the Isolation of Physical Antioxidants (Phenols) from Olive Mill Wastewater. Fresenius Environ. Bull. 2006, 15, 1122–1125. [Google Scholar]
- Vieira, F.A.; Guilherme, R.J.R.; Neves, M.C.; Abreu, H.; Rodrigues, E.R.O.; Maraschin, M.; Coutinho, J.A.P.; Ventura, S.P.M. Single-Step Extraction of Carotenoids from Brown Macroalgae Using Non-Ionic Surfactants. Sep. Purif. Technol. 2017, 172, 268–276. [Google Scholar] [CrossRef]
- Tani, H.; Kamidate, T.; Watanabe, H. Aqueous Micellar Two-Phase Systems for Protein Separation. Anal. Sci. 1998, 14, 875–888. [Google Scholar] [CrossRef]
- Molina-Bolívar, J.A.; Aguiar, J.; Ruiz, C.C. Light Scattering and Fluorescence Probe Studies on Micellar Properties of Triton X-100 in KCl Solutions. Mol. Phys. 2001, 99, 1729–1741. [Google Scholar] [CrossRef]
- Raja, S.; Murty, V.R.; Thivaharan, V.; Rajasekar, V.; Ramesh, V. Aqueous Two-Phase Systems for the Recovery of Biomolecules—A Review. Sci. Technol. 2012, 1, 7–16. [Google Scholar] [CrossRef]
- Cordisco, E.; Haidar, C.N.; Goñi, R.; Nerli, B.B.; Malpiedi, L.P. Physicochemical Characterization of Aqueous Micellar Systems Formed by Environmentally Friendly Salts. Fluid Phase Equilib. 2015, 393, 111–116. [Google Scholar] [CrossRef]
- Xian, M.; Wawrzyniak, P.; Rückert, B.; Duan, S.; Meng, Y.; Sokolowska, M.; Globinska, A.; Zhang, L.; Akdis, M.; Akdis, C.A. Anionic surfactants and commercial detergents decrease tight junction barrier integrity in human keratinocytes. J. Allergy Clin. Immunol. 2016, 138, 890–893. [Google Scholar] [CrossRef]
- Alibrahim, M. Cloud Point Extraction of Polycyclic Aromatic Hydrocarbons in Aqueous Solution with Nonionic Surfactants. Tenside Surfactants Deterg. 2014, 51, 333–338. [Google Scholar] [CrossRef]
- Yamaguchi, T. Mutagenicity of Isothiocyanates, Isocyanates and Thioureas on Salmonella typhimurium. Agric. Biol. Chem. 2014, 44, 3017–3018. [Google Scholar] [CrossRef]
- Kaschula, C.H.; Hunter, R. Synthesis and Structure–Activity Relations in Allylsulfide and Isothiocyanate Compounds From Garlic and Broccoli Against In Vitro Cancer Cell Growth. In Studies in Natural Products Chemistry; Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 50, pp. 1–43. [Google Scholar] [CrossRef]
- Schlesinger, D.H. PROTEINS | Traditional Methods of Sequence Determination. In Encyclopedia of Analytical Sciences; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Academic Press: Oxford, UK, 2005; pp. 420–424. [Google Scholar] [CrossRef]
- Oe, T.; Maekawa, M.; Satoh, R.; Lee, S.H.; Goto, T. Combining [C-13]-Phenylisothiocyanate and the Edman Degradation Reaction: A Possible Breakthrough for Absolute Quantitative Proteomics Together with Protein Identification. Rapid Commun. Mass Spectrom. 2010, 24, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Hanna, P.E.; Anders, M.W. The Mercapturic Acid Pathway. Crit. Rev. Toxicol. 2019, 49, 819–929. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, C.; Kupke, F.; Baldermann, S.; Klopsch, R.; Lamy, E.; Hornemann, S.; Pfeiffer, F.H.A.; Schreiner, M.; Hanschen, S.F.; Rohn, S. Diverse Excretion Pathways of Benzyl Glucosinolate in Humans after Consumption of Nasturtium (Tropaeolum majus L.) – A Pilot Study. Mol. Nutr. Food. Res 2018, 62, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Lee, D.-H.; Ahn, J.; Chung, W.-J.; Jang, Y.J.; Seong, K.-S.; Moon, J.-H.; Ha, T.Y.; Jung, C.H. Pharmacokinetics, Tissue Distribution, and Anti-Lipogenic/Adipogenic Effects of Allyl-Isothiocyanate Metabolites. PLoS ONE 2015, 10, e0132151. [Google Scholar] [CrossRef] [PubMed]
- Charron, C.S.; Vinyard, B.T.; Ross, S.A.; Seifried, H.E.; Jeffery, E.H.; Novotny, J.A. Absorption and Metabolism of Isothiocyanates Formed from Broccoli Glucosinolates: Effects of BMI and Daily Consumption in a Randomised Clinical Trial. Br. J. Nutr. 2018, 120, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Dyba, M.; Wang, A.; Noone, A.-M.; Goerlitz, D.; Shields, P.; Zheng, Y.-L.; Rivlin, R.; Chung, F.-L. Metabolism of Isothiocyanates in Individuals with Positive and Null GSTT1 and M1 Genotypes after Drinking Watercress Juice. Clin. Nutr. 2010, 29, 813–818. [Google Scholar] [CrossRef]
- Fusari, C.M.; Locatelli, D.A.; Altamirano, J.C.; Camargo, A.B. UAE-HPLC-UV: New Contribution for Fast Determination of Total Isothiocyanates in Brassicaceae Vegetables. J. Chem. 2015, 2015, 294601. [Google Scholar] [CrossRef]
- Zhang, Y.; Cho, C.G.; Posner, G.H.; Talalay, P. Spectroscopic Quantitation of Organic Isothiocyanates by Cyclocondensation with Vicinal Dithiols. Anal. Biochem. 1992, 205, 100–107. [Google Scholar] [CrossRef]
- Zhang, Y. The 1,2-Benzenedithiole-Based Cyclocondensation Assay: A Valuable Tool for the Measurement of Chemopreventive Isothiocyanates. Crit. Rev. Food Sci. Nutr. 2012, 52, 525–532. [Google Scholar] [CrossRef]
- Mukerjee, A.K.; Ashare, R. Isothiocyanates in the Chemistry of Heterocycles. Chem. Rev. 1991, 91, 1–24. [Google Scholar] [CrossRef]
- Cho, C.-G.; Posner, G.H. Alkyl and Aryl Isothiocyanates as Masked Primary Amines. Tetrahedron Lett. 1992, 33, 3599–3602. [Google Scholar] [CrossRef]
- Zhang, Y.; Talalay, P. Mechanism of Differential Potencies of Isothiocyanates as Inducers of Anticarcinogenic Phase 2 Enzymes. Cancer Res. 1998, 58, 4632–4639. [Google Scholar] [PubMed]
- Masutomi, N.; Toyoda, K.; Shibutani, M.; Niho, N.; Uneyama, C.; Takahashi, N.; Masao, H. Toxic Effects of Benzyl and Allyl Isothiocyanates and Benzyl-Isoform Specific Metabolites in the Urinary Bladder After a Single Intravesical Application to Rats. Toxicol. Pathol. 2001, 29, 617–622. [Google Scholar] [CrossRef]
- Shapiro, T.A.; Fahey, J.W.; Wade, K.L.; Stephenson, K.K.; Talalay, P. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: Metabolism and excretion in humans. Cancer Epidemiol Biomark. Prev. 2001, 10, 501–508. [Google Scholar]
- Kassahun, K.; Davis, M.; Hu, P.; Martin, B.; Baillie, T. Biotransformation of the Naturally Occurring Isothiocyanate Sulforaphane in the Rat: Identification of Phase I Metabolites and Glutathione Conjugates. Chem. Res. Toxicol. 1997, 10, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wade, K.L.; Prestera, T.; Talalay, P. Quantitative Determination of Isothiocyanates, Dithiocarbamates, Carbon Disulfide, and Related Thiocarbonyl Compounds by Cyclocondensation with 1,2-Benzenedithiol. Anal. Biochem. 1996, 239, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Dinkova-Kostova, A.T.; Wade, K.L.; Zhang, Y.; Shapiro, T.A.; Talalay, P. Quantitative Determination of Dithiocarbamates in Human Plasma, Serum, Erythrocytes and Urine: Pharmacokinetics of Broccoli Sprout Isothiocyanates in Humans. Clin. Chim. Acta. 2002, 316, 43–53. [Google Scholar] [CrossRef]
- Moy, K.A.; Yuan, J.-M.; Chung, F.-L.; Wang, X.-L.; Van Den Berg, D.; Wang, R.; Gao, Y.-T.; Yu, M.C. Isothiocyanates, Glutathione S-Transferase M1 and T1 Polymorphisms and Gastric Cancer Risk: A Prospective Study of Men in Shanghai, China. Int. J. Cancer 2009, 125, 2652–2659. [Google Scholar] [CrossRef]
- London, S.J.; Yuan, J.M.; Chung, F.L.; Gao, Y.T.; Coetzee, G.A.; Ross, R.K.; Yu, M.C. Isothiocyanates, Glutathione S-Transferase M1 and T1 Polymorphisms, and Lung-Cancer Risk: A Prospective Study of Men in Shanghai, China. Lancet 2000, 356, 724–729. [Google Scholar] [CrossRef]
- Ina, K.; Nobukuni, M.; Sano, A.; Kishima, I. Stability of Allyl Isothiocyanate. Nippon Shokuhin Kogyo Gakkaishi 1981, 28, 627–631. [Google Scholar] [CrossRef]
- Luo, B.; Wang, J.; Li, X.; Lu, W.; Yang, J.; Hu, Y.; Huang, P.; Wen, S. ; New Mild and Simple Approach to Isothiocyanates: A Class of Potent Anticancer Agents. Molecules 2017, 22, 773. [Google Scholar] [CrossRef] [PubMed]
- Agerbirk, N.; De Nicola, G.R.; Olsen, C.E.; Müller, C.; Iori, R. Derivatization of Isothiocyanates and Their Reactive Adducts for Chromatographic Analysis. Phytochemistry 2015, 118, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Morrison, J.J.; Botting, N.P.; Thornalley, P.J. Analysis of gucosinolates, isothiocyanates, and amine degradation products in vegetable extracts and blood plasma by LC-MS/MS. Anal. Biochem. 2005, 347, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.A.; Ennahar, S.; Zhao, M.; Bergaentzle, M.; Marchioni, E.; Bindler, F. Simultaneous Determination of Various Isothiocyanates by RP-LC Following Precolumn Derivatization with Mercaptoethanol. Chromatographia 2011, 73, 137–142. [Google Scholar] [CrossRef][Green Version]
- Revelou, P.K.; Kokotou, M.G.; Pappas, C.S.; Constantinou-Kokotou, V. Direct Determination of Total Isothiocyanate Content in Broccoli Using Attenuated Total Reflectance Infrared Fourier Transform Spectroscopy. J. Food Compos. Anal. 2017, 61, 47–51. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies. J. Raman Spectrosc. 2004, 35, 905. [Google Scholar] [CrossRef]
- Pocasap, P.; Weerapreeyakul, N. ; Sulforaphene and Sulforaphane in commonly consumed cruciferous plants contributed to antiproliferation in HCT116 colon cancer cells. Asian Pac. J. Trop. Biomed. 2016, 6, 119–124. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, M.; Rosen, R.T.; Ho, C.T. Thermal Degradation of Sulforaphane in Aqueous Solution. J. Agric. Food Chem. 1999, 47, 3121–3123. [Google Scholar] [CrossRef]
- Choi, M.M.F.; Shuang, S.; Lai, H.Y.; Cheng, S.C.; Cheng, R.C.W.; Cheung, B.K.B.; Lee, A.W.M. Gas Chromatography-Mass Spectrometric Determination of Total Isothiocyanates in Chinese Medicinal Herbs. Anal. Chim. Acta. 2004, 516, 155–163. [Google Scholar] [CrossRef]
- Hong, E.; Kim, G.H. GC-MS Analysis of the Extracts from Korean Cabbage (Brassica campestris L. ssp. pekinensis) and Its Seed. Prev. Nutr. Food Sci. 2013, 18, 218–221. [Google Scholar] [CrossRef]
- Abdel-Kader, M.S.; Khamis, E.H.; Foudah, A.I.; Alqarni, M.H. GC Quantitative Analysis of Benzyl Isothiocyanate in Salvadora persica Roots Extract and Dental Care Herbal Products. Saudi Pharm. J. 2018, 26, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Sofrata, A.; Santangelo, E.M.; Azeem, M.; Borg-Karlson, A.-K.; Gustafsson, A.; Pütsep, K. Benzyl Isothiocyanate, a Major Component from the Roots of Salvadora Persica Is Highly Active against Gram-Negative Bacteria. PLoS ONE 2011, 6, e23045. [Google Scholar] [CrossRef] [PubMed]
- Karanikolopoulou, S.; Revelou, P.-K.; Xagoraris, M.; Kokotou, M.G.; Constantinou-Kokotou, V. Current Methods for the Extraction and Analysis of Isothiocyanates and Indoles in Cruciferous Vegetables. Analytica 2021, 2, 93–120. [Google Scholar] [CrossRef]
- Zheng, L.; Zheng, F. Development and Validation of an LC-APCI-MS/MS Method for the Determination of Phenethyl Isothiocyanate in Human Plasma. Biomed. Chromatogr. 2015, 29, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ma, F.; Zhang, L.; Li, P. Extraction and Quantification of Sulforaphane and Indole-3-Carbinol from Rapeseed Tissues Using QuEChERS Coupled with UHPLC-MS/MS. Molecules 2020, 25, 2149. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Smyth, T.J.; Valverde, J.; Rai, D.K.; Barry-Ryan, C. Simultaneous Determination of Sulphoraphane and Sulphoraphane Nitrile in Brassica Vegetables Using Ultra-Performance Liquid Chromatography with Tandem Mass Spectrometry. Phytochem. Anal. 2014, 25, 141–146. [Google Scholar] [CrossRef]
- Liang, H.; Li, C.; Yuan, Q.; Vriesekoop, F. Application of High-Speed Countercurrent Chromatography for the Isolation of Sulforaphane from Broccoli Seed Meal. J. Agric. Food Chem. 2008, 56, 7746–7749. [Google Scholar] [CrossRef]
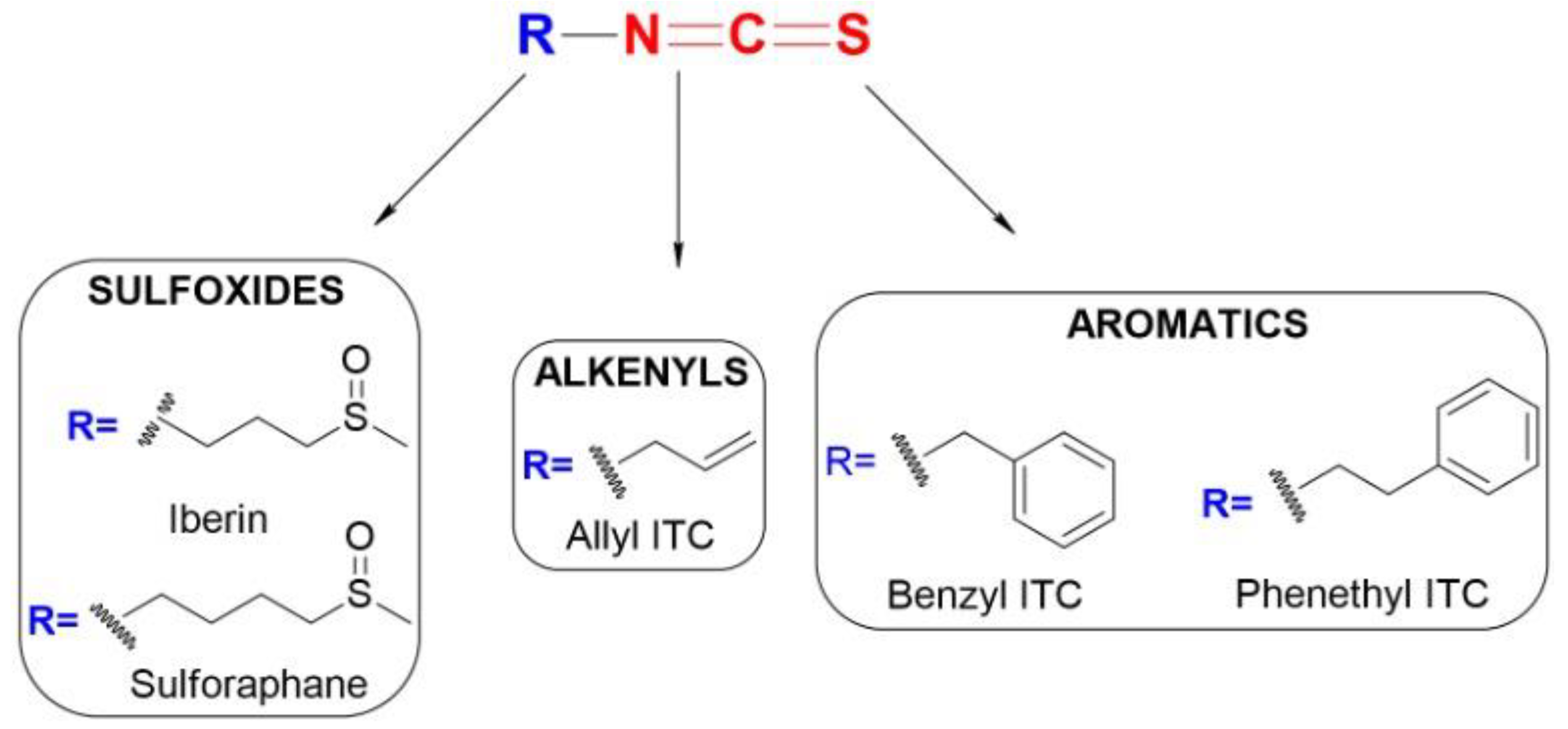
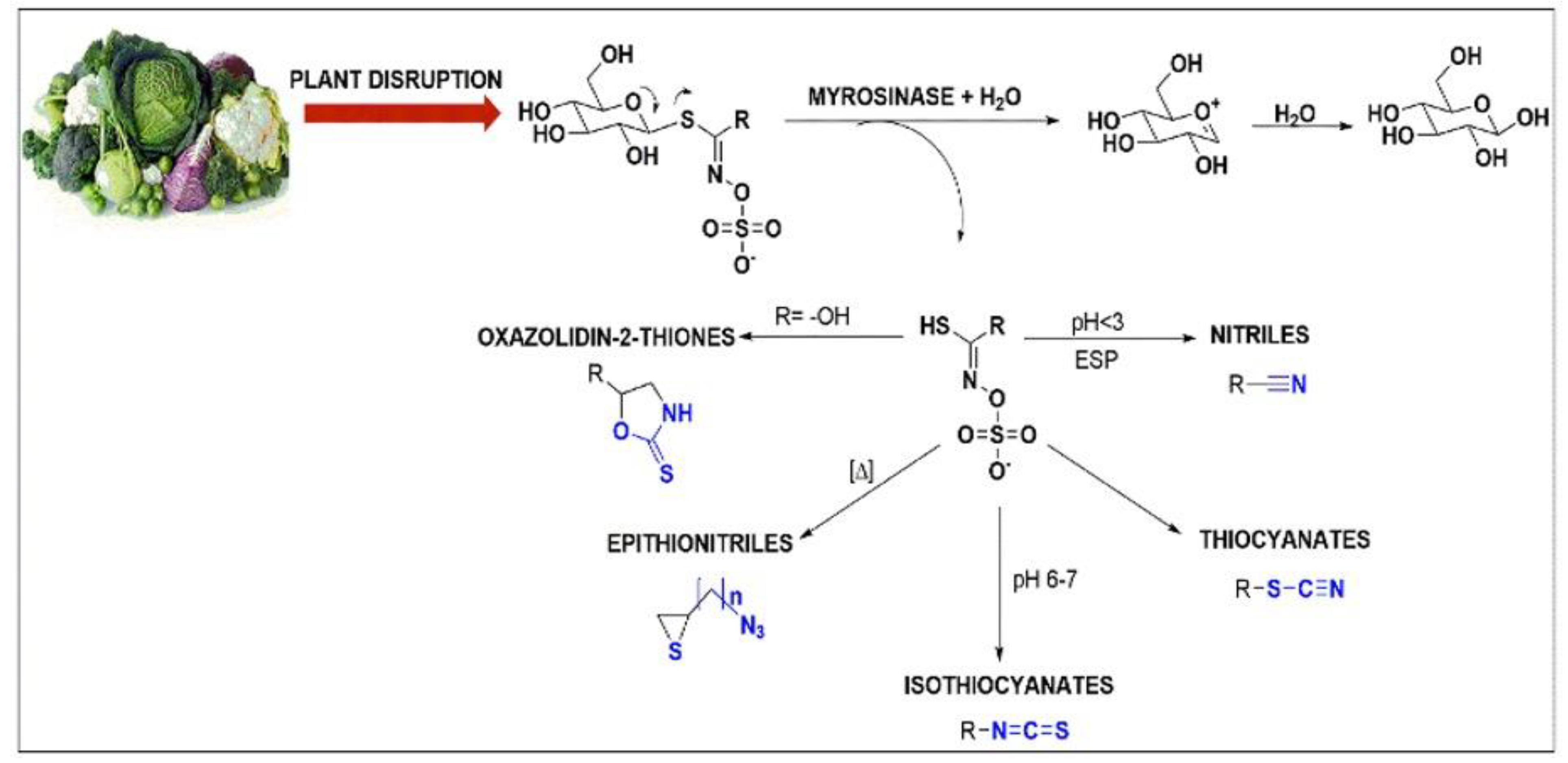
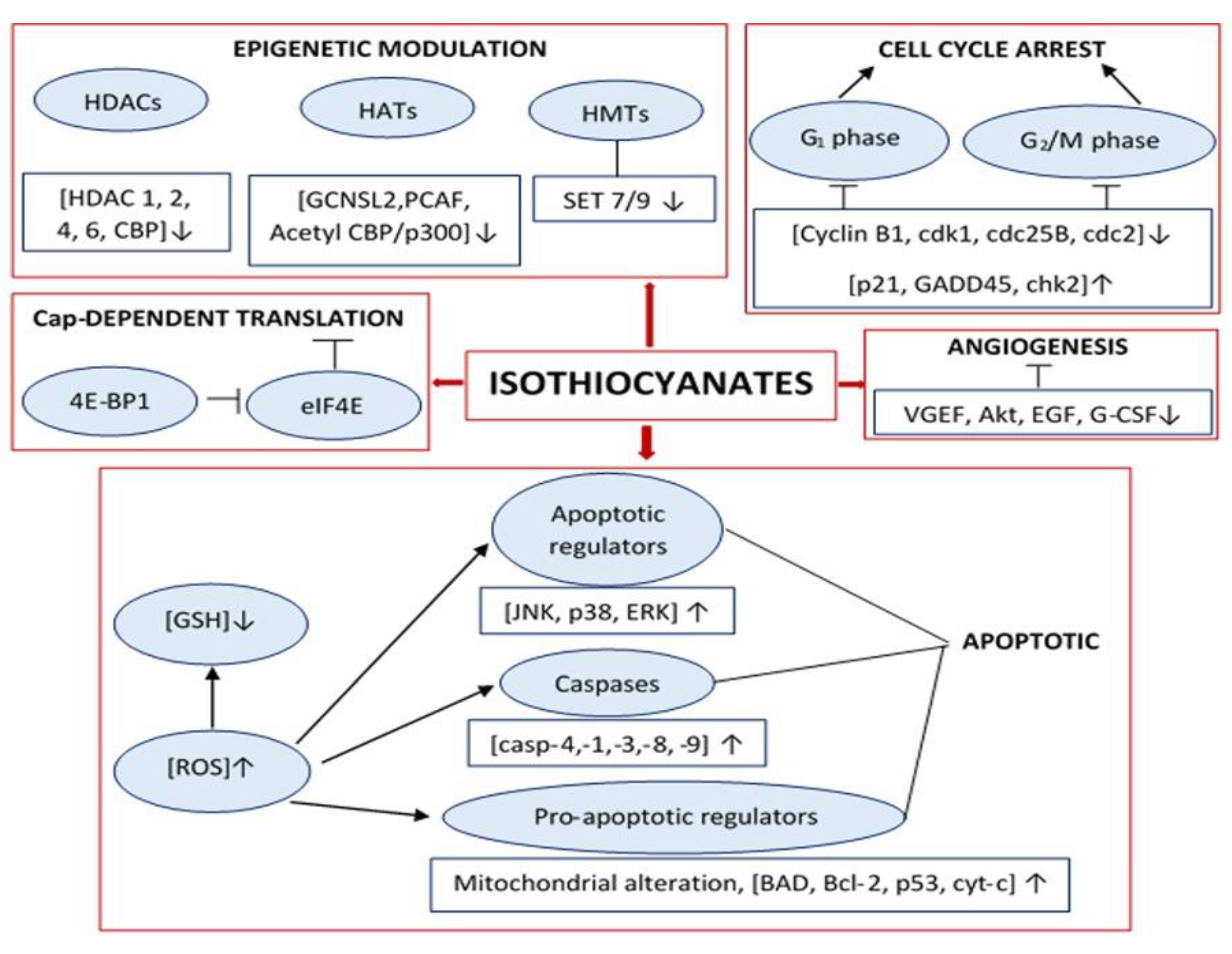
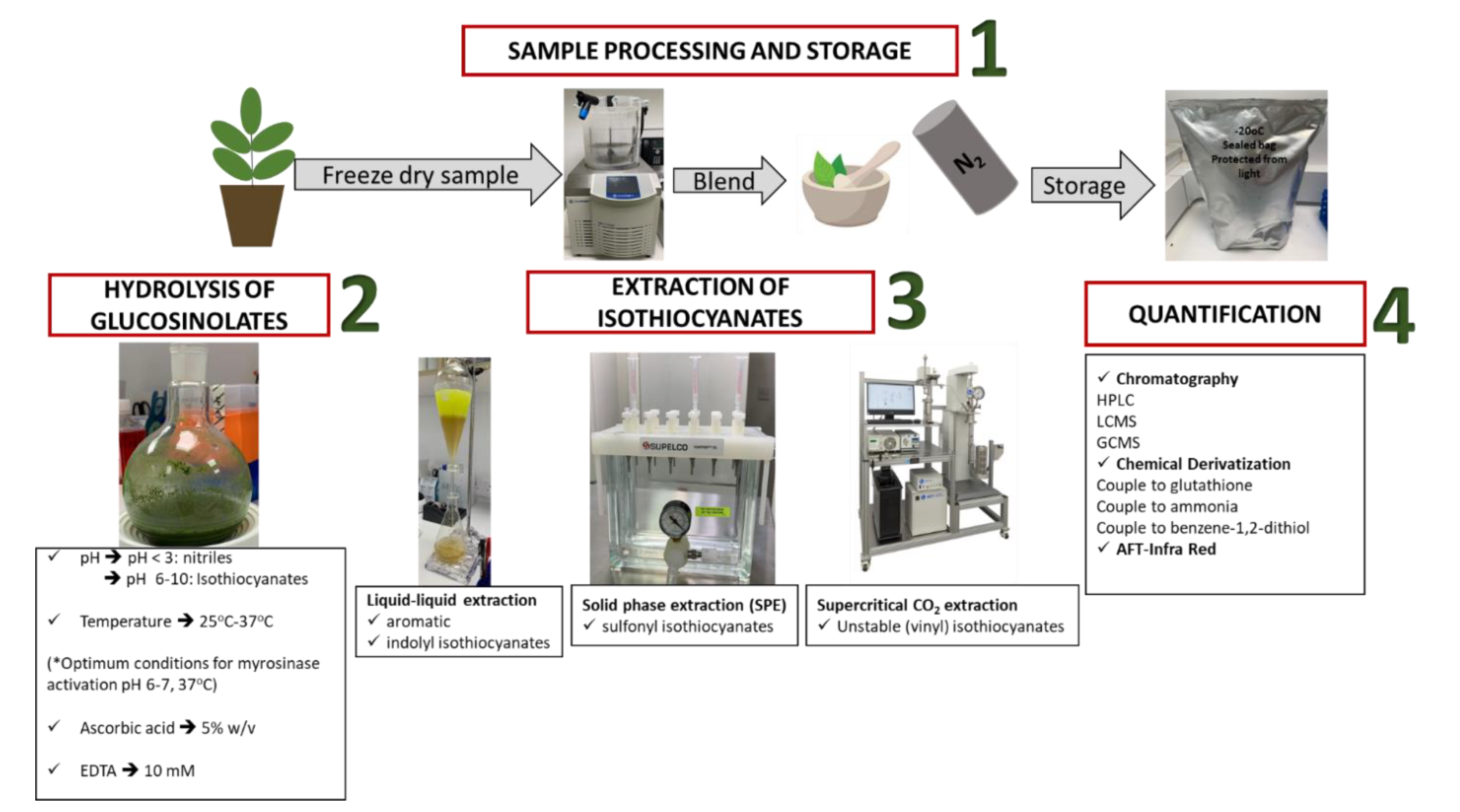
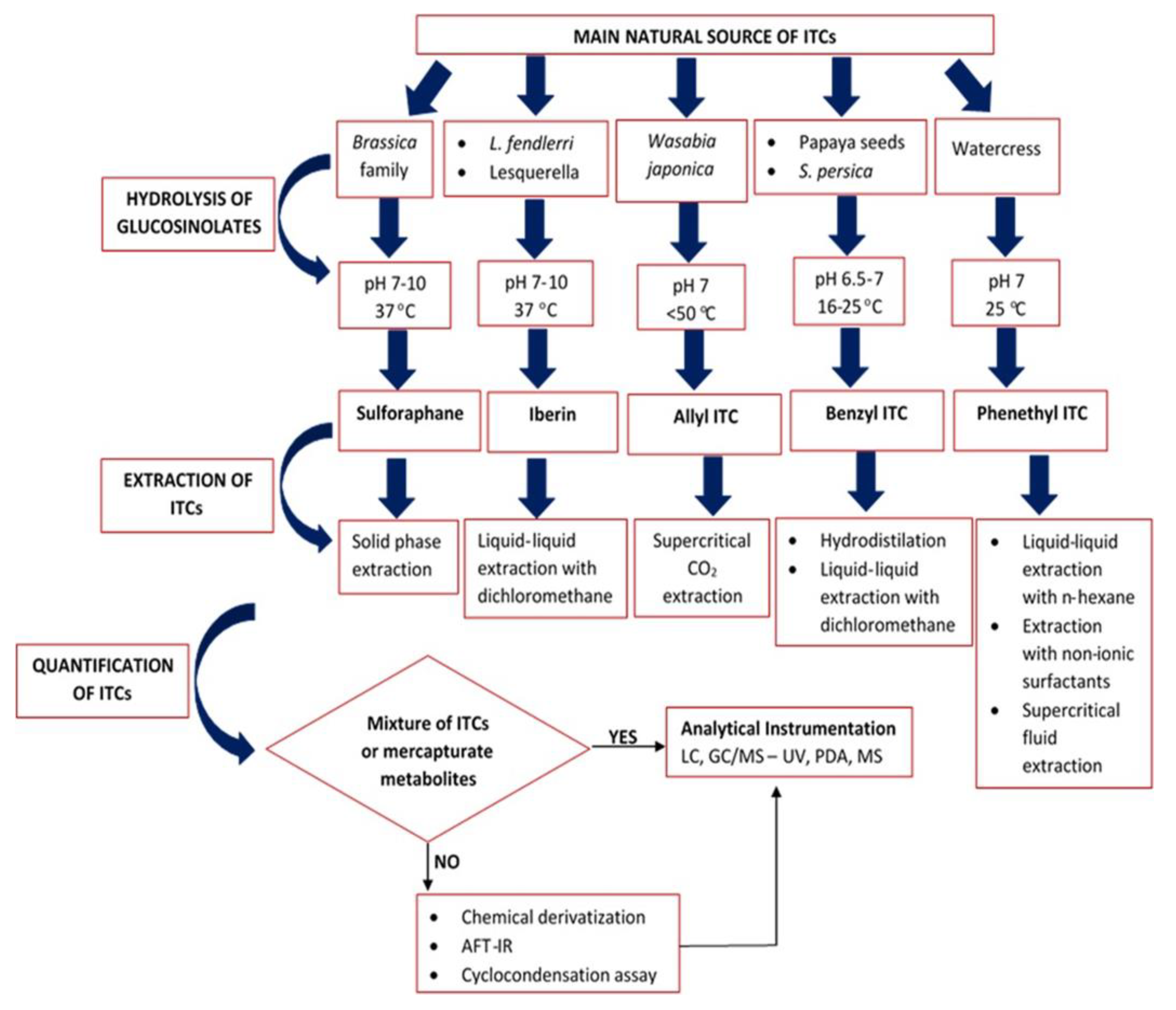
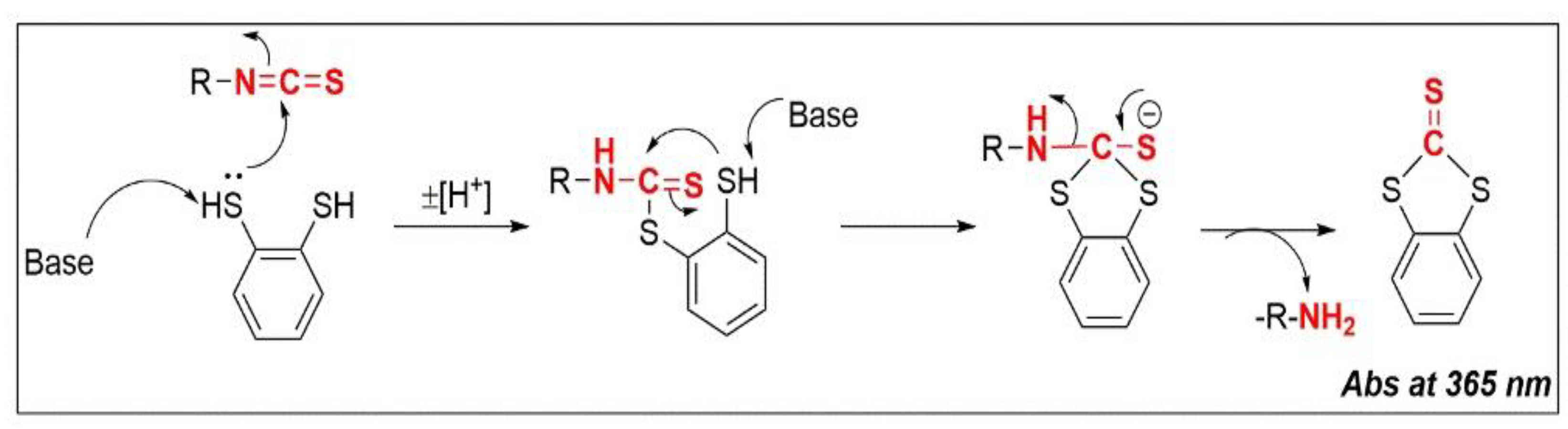
| ITCs Source | Genus Species (Sub Species) | Isothiocyanates (ITCs) | Ref |
|---|---|---|---|
| Broccoli | Brassica oleracea var. italica | sulforaphane (SFN) | [28,29] |
| Curly Kale | Brassica oleracea var. laciniata (L.) | SFN | [30] |
| Cauliflower | Brassica oleracea var. cauliflora | SFNphenethyl isothiocyanate (PEITC) | [30,31] |
| Cabbage | Brassica rapa var. pekinensis | SFN | [32] |
| Brussel sprout | Brassica oleracea var. gemmifera | SFN | [33] |
| Horseradish | Armoracia lapathifolia (L.) Gilib | iberin (IBN) allyl isothiocyanate (AITC) | [34,35] |
| Radish | Raphanus sativus (L.) | IBN | [34] |
| Watercress | Nasturtium officinale | PEITC | [36] |
| Wasabi roots | Eutrema japonicum (L.) Koidz. | AITC | [37] |
| Mustard seeds | Sinaptis alba | benzyl isothiocyanate (BITC) | [38] |
| Papaya seeds | Carica papaya (L.) | BITC | [39] |
| Plant Species | pH | Glucosinolate (GSL) | Isothiocyanate (ITC) | Extraction Yield (%) | Refs |
|---|---|---|---|---|---|
| Eruca sativa var. sativa Erysimum allionii | 10.0 | glucoerucin | erucin | 98.7 96.6 | [61,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102] |
| Brassica hirta (L.) Moench | 7.0, 10.0 | 4-hydroxybenzyl | 4-hydroxybenzyl | 29.6 | [103] |
| Lesquerella fendleri | 7.0, 10.0 | glucoiberin | IBN | 90.0 | [85] |
| Brassica vegetables Mattiola longipetala | 7.0, 10.0 | glucoraphin | SFN | 53.0 15.0 | [61,91,92,93,94,95,96,97,98,99,100,101,102,103,104] |
| Lobularia maritima (L.) Desv Capsella bursa-pastoris (L.) Medik | 7.0, 10.0 | 3-butenyl | 3-butenyl | ~100.0 | [85] |
| Erysimum cheiri (L.) Crantz | 7.0, 10.0 | glucocheirolin | cheirolin | 98.3 | [85] |
| Lobularia maritima (L.) Desv | 7.0, 10.0 | lesquerellin | lesquerellin | 96.3 | [85] |
| Nasturtium officinale | 7.0 | gluconasturtiin | PEITC | 89.095.0 | [87,105,106] |
| Carica papaya (L.) Tropaeolum majus (L.) tropaeolaceae Lepidium sativum (L.) | 6.5–7.0 | glucotropaeolin | BITC | n.d. | [107] |
| Brassica rapa (L.) oleifera | 8.0 | 3-butenyl glucosinolate | 3-butenyl ITC | 40.0 | [56] |
| Arabidopsis thaliana (L.) Heynh | 6.5 | 2-propenyl glucosinolate | 2-propenyl ITC | 32.0 | [56] |
| Brassica oleracra var. italica | 5.5 | 4-(methylsulphinyl) butyl glucosinolate | 4-(methylsulphinyl) butyl isothiocyanate | 82.0 | [56] |
| Armoracia rusticana | 7.0 | sinigrin | AITC | 61.0 | [108] |
| Plant Species | Temperature | Glucosinolate (GSL) | Isothiocyanate(ITC) | Extraction Yield (%) | Refs |
|---|---|---|---|---|---|
| Brassica vegetables | 37 °C | glucoraphane | SFN | 53.0 | [91] |
| Nasturtium officinale | 25 °C | gluconasturtiin | PEITC | 89.0 | [87] [106] |
| Brassica rapa var. oleifera | 25 °C | 3-butenyl glucosinolate | 3-butenyl ITC | 40.0 | [55] |
| Arabidopsis thaliana (L.) Heynh | 37 °C | 2-propenyl glucosinolate | 2-propenyl ITC | 32.0 | [55] |
| 3-butenyl glucosinolate 2- buteny l glucosinolate | 3-Butenyl ITC 2-Butenyl ITC | n.d. n.d. | [62] | ||
| Brassica oleracra var. italica | 37 °C | 4-(methylsulphinyl)butyl glucosinolate | 4-(methylsulphinyl)butyl isothiocyanate | 82.0 | [56] |
| Carica papaya (L.) | 16–18 °C | glucotropaeolin | BEITC | 85.6 | [119] |
| Salix alba (L.) maire | <50 °C | sinigrin | AITC | n.d. | [112] |
| Brassica nigra (L.) nigra | <60 °C | sinigrin | AITC | n.d. | [112] |
| Brassica juncea (L.) Czern. | <50 °C | sinigrin | AITC | n.d. | [112] |
| Plant Source | Isothiocyanates (ITCs) | Extraction Methodology | Extraction Yield (%) | Refs |
|---|---|---|---|---|
| Broccoli | SFN | Solid phase extraction | 94 | [139] |
| Matthiolalongipetala | Liquid-liquid extraction (with n-hexane) | 31.9 | [85] | |
| Lesquerella fendleri (L.) | IBN | Liquid-liquid extraction (with dichloromethane) | 48.6 | [85] |
| Physaria fendleri | 56.7 | [210] | ||
| Wasabia Japonica (L.) matsum | AITC | Suprecritical fluid (CO2) extraction | 79.1 | [35] |
| Armoracia rusticana | Hydrodistillation | 61 | [35] | |
| Liquid-liquid extraction (with diethyl ether) | 96.5 | [35] | ||
| Green papaya | BITC | Hydrodistillation | 80 | [122] |
| Salvadora persica (L.) | Liquid-liquid extraction (with dichloromethane) | 75 | [236] | |
| Liquid-liquid extraction (with chloroform) | 40 | [237] | ||
| Nasturdium officinale | PEITC | Extraction with non-anionic surfactants | 94 | [98] |
| Liquid-liquid extraction (with n-hexane) | 98.7 | [106] | ||
| Suprecritical fluid (CO2) extraction | 87.4, ND | [206,271] | ||
| Lobularia maritima | 6-methylthiohexyl isothiocyanate | Liquid-liquid extraction (with n-hexane) | 96.3 | [85] |
| Matthiolalongipetala | erysolin | Liquid-liquid extraction (with n-hexane) | 38.7 | [85] |
| cherolin | 18.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyriakou, S.; Trafalis, D.T.; Deligiorgi, M.V.; Franco, R.; Pappa, A.; Panayiotidis, M.I. Assessment of Methodological Pipelines for the Determination of Isothiocyanates Derived from Natural Sources. Antioxidants 2022, 11, 642. https://doi.org/10.3390/antiox11040642
Kyriakou S, Trafalis DT, Deligiorgi MV, Franco R, Pappa A, Panayiotidis MI. Assessment of Methodological Pipelines for the Determination of Isothiocyanates Derived from Natural Sources. Antioxidants. 2022; 11(4):642. https://doi.org/10.3390/antiox11040642
Chicago/Turabian StyleKyriakou, Sotiris, Dimitrios T. Trafalis, Maria V. Deligiorgi, Rodrigo Franco, Aglaia Pappa, and Mihalis I. Panayiotidis. 2022. "Assessment of Methodological Pipelines for the Determination of Isothiocyanates Derived from Natural Sources" Antioxidants 11, no. 4: 642. https://doi.org/10.3390/antiox11040642
APA StyleKyriakou, S., Trafalis, D. T., Deligiorgi, M. V., Franco, R., Pappa, A., & Panayiotidis, M. I. (2022). Assessment of Methodological Pipelines for the Determination of Isothiocyanates Derived from Natural Sources. Antioxidants, 11(4), 642. https://doi.org/10.3390/antiox11040642








