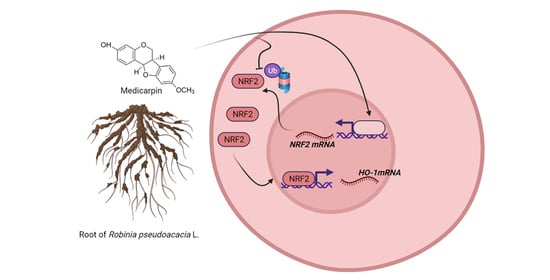
Medicarpin Increases Antioxidant Genes by Inducing NRF2 Transcriptional Level in HeLa Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.3. Cell Toxicity Assay
2.4. Cloning
2.5. ARE Luciferase Assay
2.6. Western Blot Analysis
2.7. Real-Time PCR Analysis
2.8. Protein Stability Assay
2.9. Statistical Analysis
3. Results
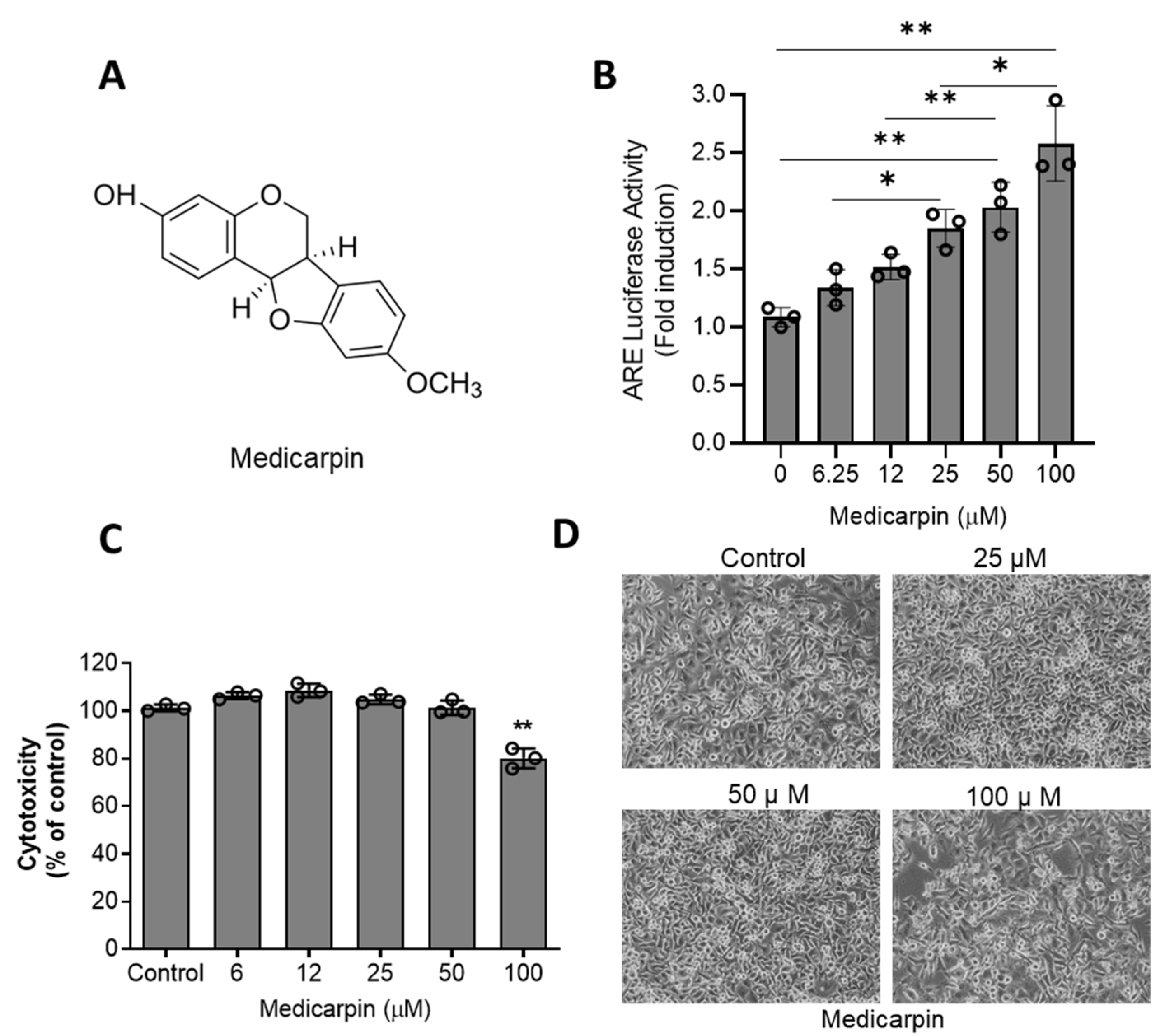
3.1. Medicarpin Increases NRF2 Activity through Are System in HeLa Cells
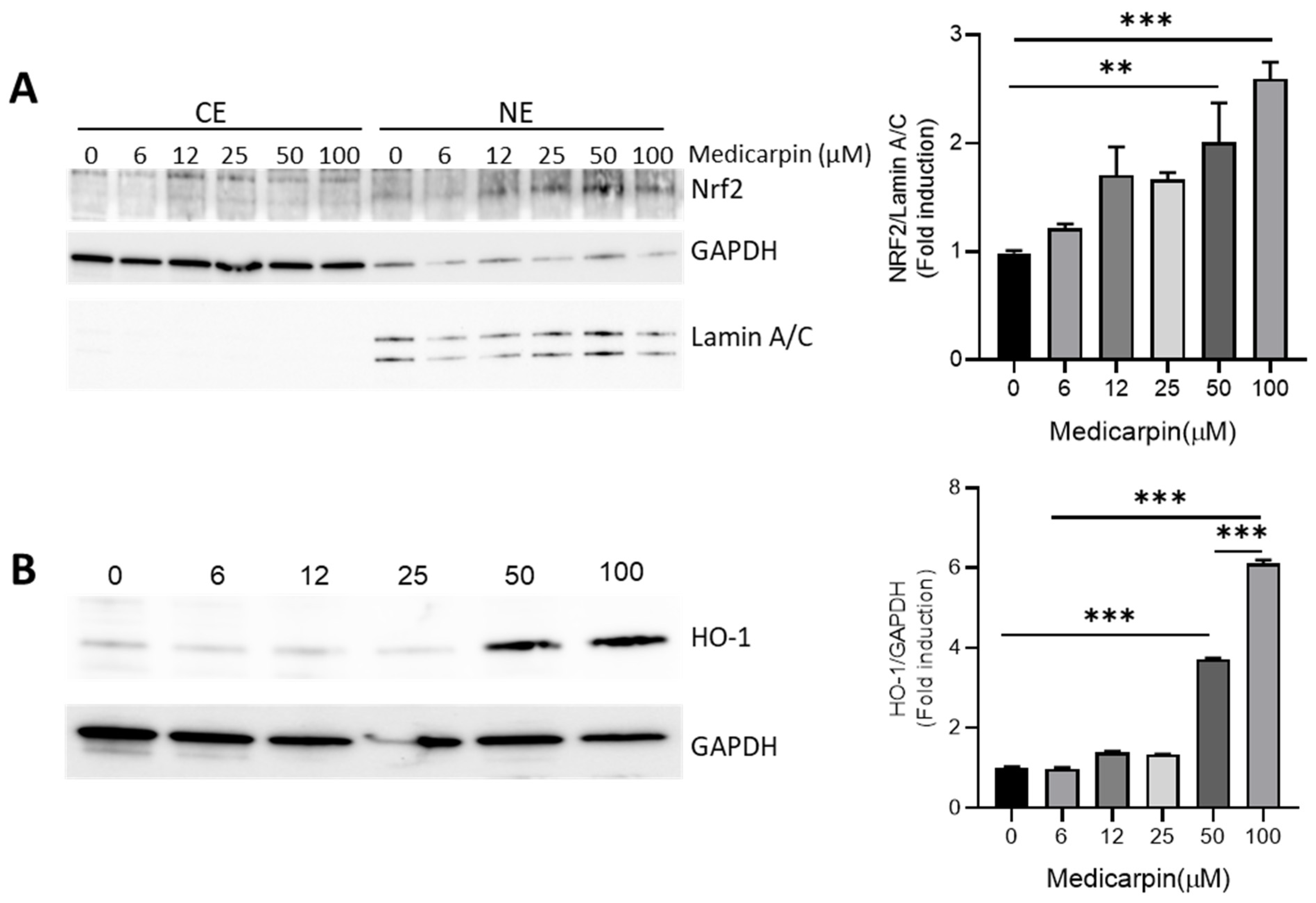
3.2. Medicarpin Increases HO-1 by NRF2 Activation in HeLa Cells
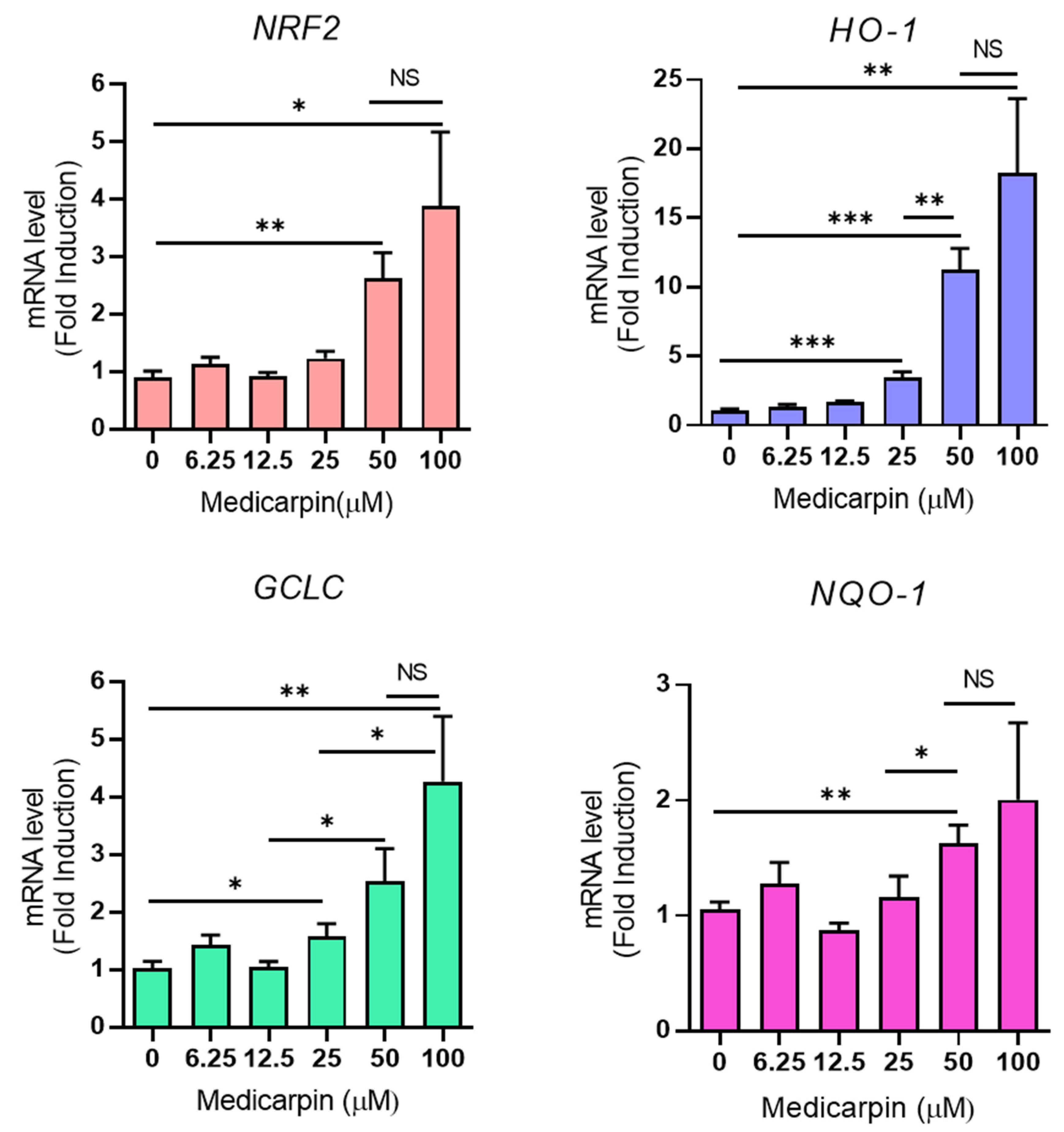
3.3. Medicarpin Increases the Transcriptional Level of NRF2 Target Genes in HeLa Cells
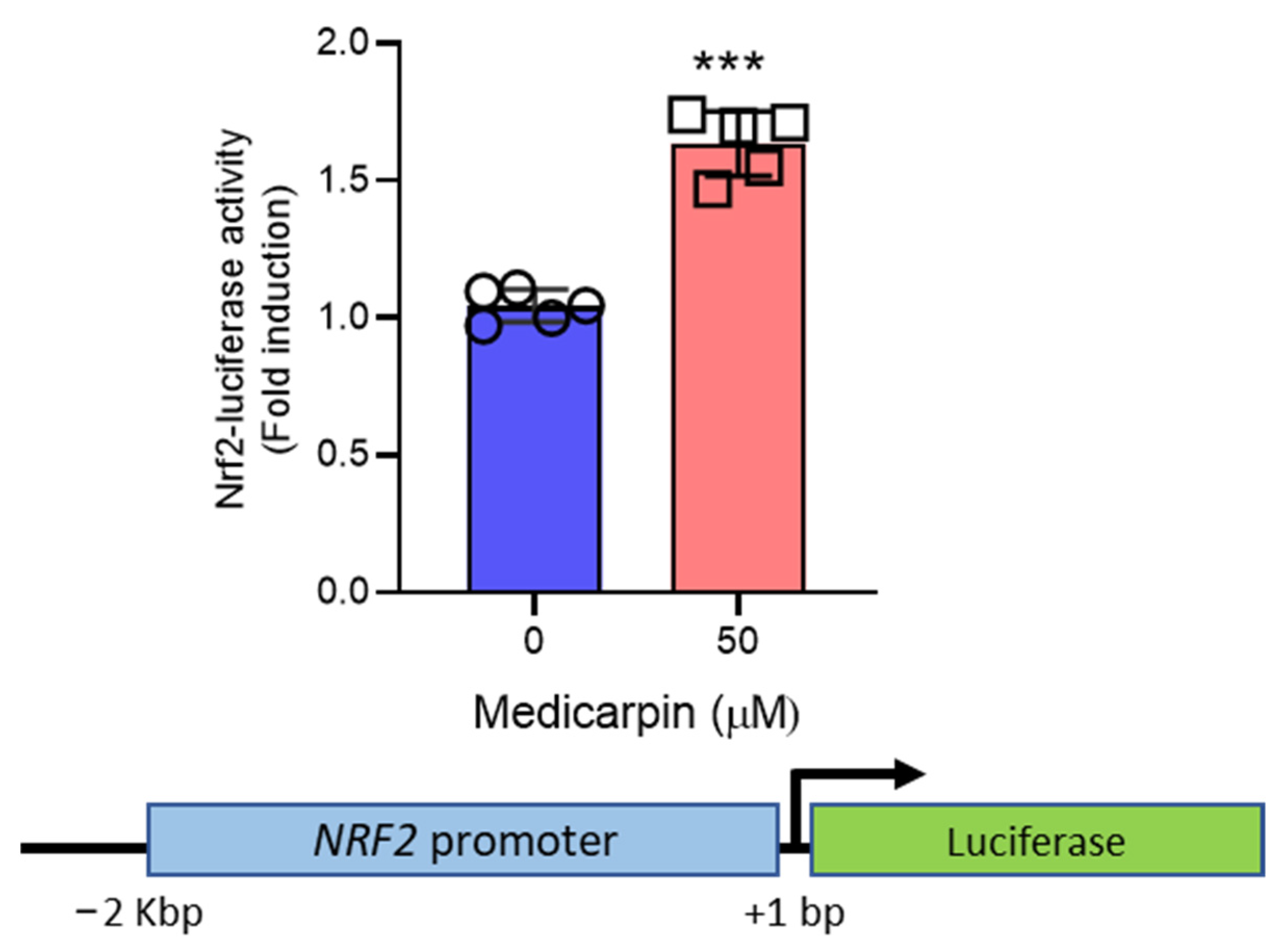
3.4. Medicarpin Increases the Transcriptional Activity NRF2 Gene
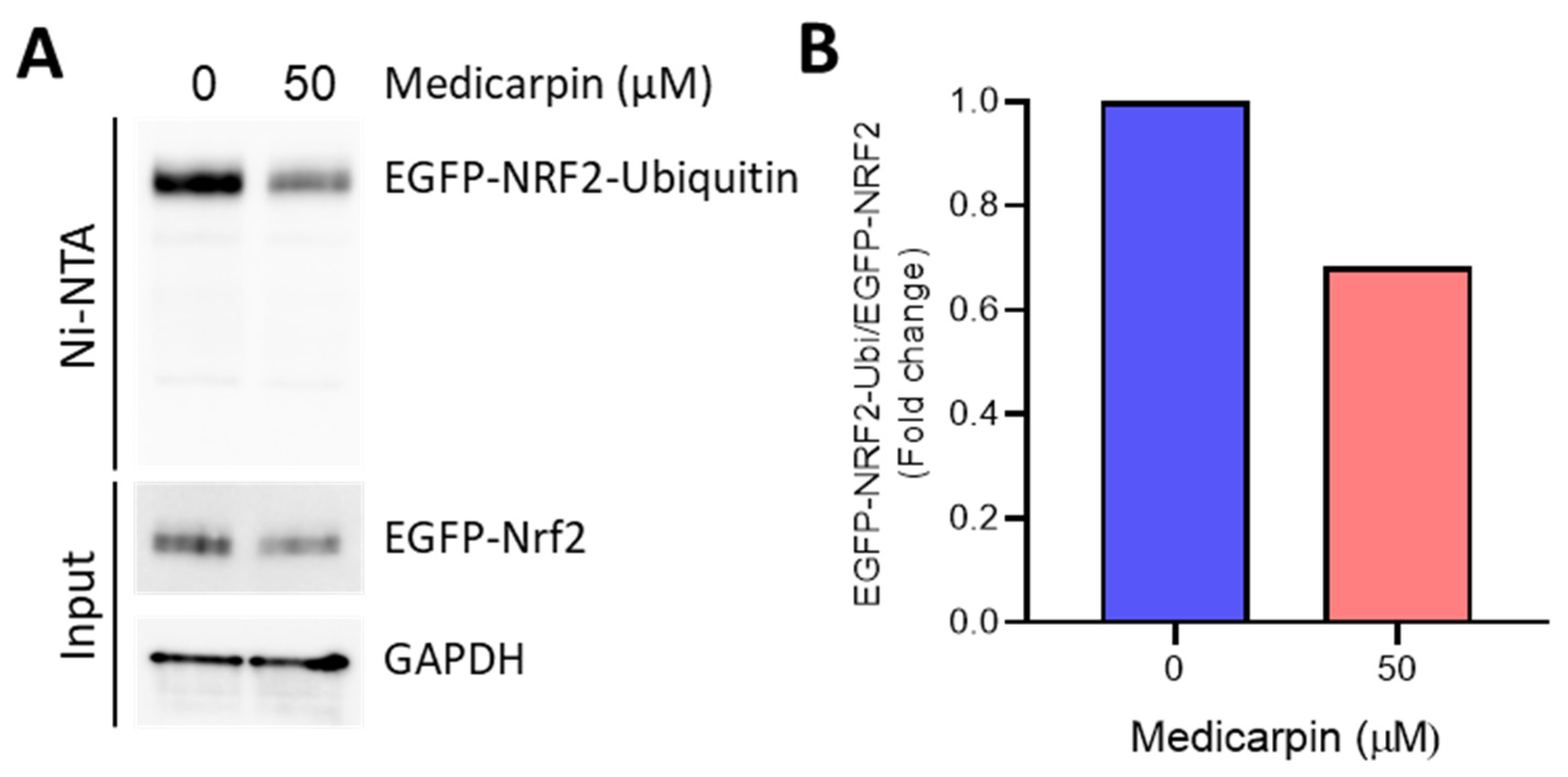
3.5. Medicarpin Potentiates the NRF2 Stability by Inhibiting Ubiquitin-Mediated Degradation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.-L.; Kensler, T.W.; et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019, 18, 295–317. [Google Scholar] [CrossRef] [Green Version]
- Rojo de la Vega, M.; Chapman, E.; Zhang, D.D. NRF2 and the Hallmarks of Cancer. Cancer Cell 2018, 34, 21–43. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Khazaei, M. Oxidative Stress and Cancer: The Role of Nrf2. Curr. Cancer Drug Targets 2018, 18, 538–557. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [Green Version]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Kim, J.H.; Xu, E.Y.; Sacks, D.B.; Lee, J.; Shu, L.; Xia, B.; Kong, A.N. Identification and functional studies of a new Nrf2 partner IQGAP1: A critical role in the stability and transactivation of Nrf2. Antioxid. Redox Signal. 2013, 19, 89–101. [Google Scholar] [CrossRef] [Green Version]
- Chun, K.S.; Kundu, J.; Kundu, J.K.; Surh, Y.J. Targeting Nrf2-Keap1 signaling for chemoprevention of skin carcinogenesis with bioactive phytochemicals. Toxicol. Lett. 2014, 229, 73–84. [Google Scholar] [CrossRef]
- Park, J.S.; Kang, D.H.; Lee, D.H.; Bae, S.H. Fenofibrate activates Nrf2 through p62-dependent Keap1 degradation. Biochem. Biophys. Res. Commun. 2015, 465, 542–547. [Google Scholar] [CrossRef]
- Kwak, M.K.; Wakabayashi, N.; Kensler, T.W. Chemoprevention through the Keap1-Nrf2 signaling pathway by phase 2 enzyme inducers. Mutat. Res. 2004, 555, 133–148. [Google Scholar] [CrossRef]
- Lee, J.M.; Johnson, J.A. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J. Biochem. Mol. Biol. 2004, 37, 139–143. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.; Sherratt, P.J.; Pickett, C.B. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 233–260. [Google Scholar] [CrossRef]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef]
- Ryter, S.W.; Alam, J.; Choi, A.M. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol. Rev. 2006, 86, 583–650. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, A.; Kang, M.I.; Okawa, H.; Ohtsuji, M.; Zenke, Y.; Chiba, T.; Igarashi, K.; Yamamoto, M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004, 24, 7130–7139. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.H.; Sharrocks, A.D.; Whitmarsh, A.J. MAP kinase signalling cascades and transcriptional regulation. Gene 2013, 513, 1–13. [Google Scholar] [CrossRef]
- Bloom, D.A.; Jaiswal, A.K. Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:quinone oxidoreductase-1 gene expression. J. Biol. Chem. 2003, 278, 44675–44682. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.; Sherratt, P.J.; Huang, H.C.; Yang, C.S.; Pickett, C.B. Increased protein stability as a mechanism that enhances Nrf2-mediated transcriptional activation of the antioxidant response element. Degradation of Nrf2 by the 26 S proteasome. J. Biol. Chem. 2003, 278, 4536–4541. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.D.; Hannink, M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell. Biol. 2003, 23, 8137–8151. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.C.; Nguyen, T.; Pickett, C.B. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J. Biol. Chem. 2002, 277, 42769–42774. [Google Scholar] [CrossRef] [Green Version]
- Joo, M.S.; Kim, W.D.; Lee, K.Y.; Kim, J.H.; Koo, J.H.; Kim, S.G. AMPK Facilitates Nuclear Accumulation of Nrf2 by Phosphorylating at Serine 550. Mol. Cell. Biol. 2016, 36, 1931–1942. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Khalil, A.A.K.; Kim, H.J.; Kim, S.E.; Ahn, M.J. 2-Methoxy-7-Acetonyljuglone Isolated from Reynoutria japonica Increases the Activity of Nuclear Factor Erythroid 2-Related Factor-2 through Inhibition of Ubiquitin Degradation in HeLa Cells. Antioxidants 2019, 8, 398. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.; Wahyudi, L.D.; Keum, Y.S.; Yang, H.; Kim, J.H. E-p-Methoxycinnamoyl-alpha-l-rhamnopyranosyl Ester, a Phenylpropanoid Isolated from Scrophularia buergeriana, Increases Nuclear Factor Erythroid-Derived 2-Related Factor 2 Stability by Inhibiting Ubiquitination in Human Keratinocytes. Molecules 2018, 23, 768. [Google Scholar] [CrossRef] [Green Version]
- Vigliante, I.; Mannino, G.; Maffei, M.E. OxiCyan (R), a phytocomplex of bilberry (Vaccinium myrtillus) and spirulina (Spirulina platensis), exerts both direct antioxidant activity and modulation of ARE/Nrf2 pathway in HepG2 cells. J. Funct. Foods 2019, 61, 103508. [Google Scholar] [CrossRef]
- Husain, I.; Akhtar, M.; Madaan, T.; Abdin, M.Z.; Islamuddin, M.; Najmi, A.K. Rosuvastatin alleviates high-salt and cholesterol diet-induced cognitive impairment in rats via Nrf2-ARE pathway. Redox Rep. 2018, 23, 168–179. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Blunder, M.; Liu, X.; Malainer, C.; Blazevic, T.; Schwaiger, S.; Rollinger, J.M.; Heiss, E.H.; et al. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARgamma): A review. Biochem. Pharmacol. 2014, 92, 73–89. [Google Scholar] [CrossRef] [Green Version]
- Imran, K.M.; Yoon, D.; Lee, T.J.; Kim, Y.S. Medicarpin induces lipolysis via activation of Protein Kinase A in brown adipocytes. BMB Rep. 2018, 51, 249–254. [Google Scholar] [CrossRef] [Green Version]
- Dixit, M.; Raghuvanshi, A.; Gupta, C.P.; Kureel, J.; Mansoori, M.N.; Shukla, P.; John, A.A.; Singh, K.; Purohit, D.; Awasthi, P.; et al. Medicarpin, a Natural Pterocarpan, Heals Cortical Bone Defect by Activation of Notch and Wnt Canonical Signaling Pathways. PLoS ONE 2015, 10, e0144541. [Google Scholar] [CrossRef]
- Trivedi, R.; Maurya, R.; Mishra, D.P. Medicarpin, a legume phytoalexin sensitizes myeloid leukemia cells to TRAIL-induced apoptosis through the induction of DR5 and activation of the ROS-JNK-CHOP pathway. Cell Death Dis. 2014, 5, e1465. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, A.M.; Gautam, A.K.; Kumar, A.; Srivastava, K.; Bhargavan, B.; Trivedi, R.; Saravanan, S.; Yadav, D.K.; Singh, N.; Pollet, C.; et al. Medicarpin inhibits osteoclastogenesis and has nonestrogenic bone conserving effect in ovariectomized mice. Mol. Cell. Endocrinol. 2010, 325, 101–109. [Google Scholar] [CrossRef]
- Kim, J.H.; Chen, C.; Tony Kong, A.N. Resveratrol inhibits genistein-induced multi-drug resistance protein 2 (MRP2) expression in HepG2 cells. Arch. Biochem. Biophys. 2011, 512, 160–166. [Google Scholar] [CrossRef]
- Kim, J.H.; Yu, S.; Chen, J.D.; Kong, A.N. The nuclear cofactor RAC3/AIB1/SRC-3 enhances Nrf2 signaling by interacting with transactivation domains. Oncogene 2013, 32, 514–527. [Google Scholar] [CrossRef] [Green Version]
- Wahyudi, L.D.; Jeong, J.; Yang, H.; Kim, J.H. Amentoflavone-induced oxidative stress activates NF-E2-related factor 2 via the p38 MAP kinase-AKT pathway in human keratinocytes. Int. J. Biochem. Cell Biol. 2018, 99, 100–108. [Google Scholar] [CrossRef]
- Sun, Y.X.; Xu, A.H.; Yang, Y.; Li, J. Role of Nrf2 in bone metabolism. J. Biomed. Sci. 2015, 22, 101. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Zhong, H.; Wei, J.; Lin, S.; Zong, Z.; Gong, F.; Huang, X.; Sun, J.; Li, P.; Lin, H.; et al. Inhibition of Nrf2/HO-1 signaling leads to increased activation of the NLRP3 inflammasome in osteoarthritis. Arthritis Res. Ther. 2019, 21, 300. [Google Scholar] [CrossRef] [Green Version]
- Chadha, S.; Behl, T.; Kumar, A.; Khullar, G.; Arora, S. Role of Nrf2 in rheumatoid arthritis. Curr. Res. Transl. Med. 2020, 68, 171–181. [Google Scholar] [CrossRef]
| Gene | Foward | Reverse |
|---|---|---|
| NRF2 | 5′-TCT TGC CTC CAA AGT ATG TCA A-3′ | 5′-ACA CGG TCC ACA GCT CAT C-3′ |
| HO-1 | 5′-GAG TGT AAG GAC CCA TCG GA-3′ | 5′-GCC AGC AAC AAA GTG CAA G-3′ |
| NQO-1 | 5′-TCC TTT CTT CTT CAA AGC CG-3′ | 5′-GGA CTG CAC CAG AGC CAT-3′ |
| GCLC | 5′-CTT TCT CCC CAG ACA GGA CC-3′ | 5′-CAA GGA CGT TCT CAA GTG GG-3′ |
| GAPDH | 5′-AAG GTG AAG GTC GGA GTC AA-3′ | 5′-AAT GAA GGG GTC ATT GAT GG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Kang, D.-M.; Cho, Y.-J.; Hyun, J.-W.; Ahn, M.-J. Medicarpin Increases Antioxidant Genes by Inducing NRF2 Transcriptional Level in HeLa Cells. Antioxidants 2022, 11, 421. https://doi.org/10.3390/antiox11020421
Kim J-H, Kang D-M, Cho Y-J, Hyun J-W, Ahn M-J. Medicarpin Increases Antioxidant Genes by Inducing NRF2 Transcriptional Level in HeLa Cells. Antioxidants. 2022; 11(2):421. https://doi.org/10.3390/antiox11020421
Chicago/Turabian StyleKim, Jung-Hwan, Dong-Min Kang, Young-Jin Cho, Jin-Won Hyun, and Mi-Jeong Ahn. 2022. "Medicarpin Increases Antioxidant Genes by Inducing NRF2 Transcriptional Level in HeLa Cells" Antioxidants 11, no. 2: 421. https://doi.org/10.3390/antiox11020421
APA StyleKim, J.-H., Kang, D.-M., Cho, Y.-J., Hyun, J.-W., & Ahn, M.-J. (2022). Medicarpin Increases Antioxidant Genes by Inducing NRF2 Transcriptional Level in HeLa Cells. Antioxidants, 11(2), 421. https://doi.org/10.3390/antiox11020421