Neurolocomotor Behavior and Oxidative Stress Markers of Thiazole and Thiazolidinedione Derivatives against Nauphoeta cinerea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. N. cinerea, Diet Formulation and Treatment
2.3. Behavioral Tests for Locomotor Performance
2.4. Markers of Oxidative Stress
Preparation of N. cinerea Homogenates for Biochemical Tests
2.5. Assessment of Cell Viability
2.6. Measurement of Protein Thiols and Non-Protein Thiol (NPSH) Levels
2.7. Determination of Species Reactive to 2-Thiobarbituric Acid
2.8. Free Fe2+ Content Determination
2.9. Determination of Antioxidant Activity In Vitro by DPPH Method
2.10. Statistical Analysis
3. Results
3.1. Thiazole and Thiazolidinedione—Locomotor Activity
3.2. Cells Viability
3.3. Measurement of Protein and Non-Protein Thiols (NPSH)
3.4. Measurement of Malondialdehyde (MDA) Content
3.5. Determination of Total Free Iron Levels
3.6. Evaluation of Antioxidants by the DPPH Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conti, I.; Morigi, R.; Locatelli, A.; Rambaldi, M.; Bua, G.; Gallinella, G.; Leoni, A. Synthesis of 3-(Imidazo[2,1-b]thiazol-6-yl)-2H-chromen-2-one Derivatives and Study of Their Antiviral Activity against Parvovirus B19. Molecules 2019, 24, 1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousavi, S.M.; Zarei, M.; Hashemi, S.A.; Babapoor, A.; Amani, A.M. A conceptual review of rhodanine: Current applications of antiviral drugs, anticancer and antimicrobial activities. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1132–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takate, S.J.; Shinde, A.D.; Karale, B.K.; Akolkar, H.; Nawale, L.; Sarkar, D.; Mhaske, P.C. Thiazolyl-pyrazole derivatives as potential antimycobacterial agents. Bioorgan. Med. Chem. Lett. 2019, 29, 1199–1202. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.H.; Patel, N.B.; Patel, V.M. Synthesis, In silico Molecular Docking and Pharmacokinetic Studies, in vitro Antimycobacterial and Antimicrobial Studies of New Imidozolones Clubbed with Thiazolidinedione. Curr. Comput. Aided. Drug Des. 2018, 14, 269–283. [Google Scholar] [CrossRef]
- Pérez, M.J.; Quintanilla, R.A. Therapeutic Actions of the Thiazolidinediones in Alzheimer’s Disease. PPAR Res. 2015, 2015, 957248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, L.; Huang, R.; Wu, S.; Chen, Z.; Sun, K.; Jiang, Y.; Cai, X. PPARγ and Its Ligands: Potential Antitumor Agents in the Digestive System. Curr. Stem Cell Res. Ther. 2016, 11, 274–281. [Google Scholar] [CrossRef]
- Tadesse, S.; Yu, M.; Mekonnen, L.B.; Lam, F.; Islam, S.; Tomusange, K.; Rahaman, M.H.; Noll, B.; Basnet, S.K.C.; Teo, T.; et al. Highly Potent, Selective, and Orally Bioavailable 4-Thiazol-N-(pyridin-2-yl)pyrimidin-2-amine Cyclin-Dependent Kinases 4 and 6 Inhibitors as Anticancer Drug Candidates: Design, Synthesis, and Evaluation. J. Med. Chem. 2017, 60, 1892–1915. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-K.; Sun, T.; Li, Y.-J.; Wang, Y.-H.; Li, Y.-J.; Yang, L.-D.; Feng, D.; Zhao, M.-G.; Wu, Y.-M. A novel thiazolidinediones ATZD2 rescues memory deficits in a rat model of type 2 diabetes through antioxidant and antiinflammation. Oncotarget 2017, 8, 107409–107422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramalingan, C.; Balasubramanian, S.; Kabilan, S.; Vasudevan, M. Synthesis and study of antibacterial and antifungal activities of novel 1-[2-(benzoxazol-2-yl)ethoxy]-2,6-diarylpiperidin-4-ones. Eur. J. Med. Chem. 2004, 39, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Karatepe, M.; Karatas, F. Antioxidant, pro-oxidant effect of the thiosemicarbazone derivative Schiff base (4-(1-phenylmethylcyclobutane-3-yl)-2-(2-hydroxybenzylidenehydrazino) thiazole) and its metal complexes on rats. Cell Biochem. Funct. 2006, 24, 547–554. [Google Scholar] [CrossRef]
- Jaishree, V.; Ramdas, N.; Sachin, J.; Ramesh, B. In vitro antioxidant properties of new thiazole derivatives. J. Saudi Chem. Soc. 2012, 16, 371–376. [Google Scholar] [CrossRef] [Green Version]
- Portillo, A.; Vila, R.; Freixa, B.; Adzet, T.; Cañigueral, S. Antifungal activity of Paraguayan plants used in traditional medicine. J. Ethnopharmacol. 2001, 76, 93–98. [Google Scholar] [CrossRef]
- Hua, H.; Xing, F.; Selvaraj, J.N.; Wang, Y.; Zhao, Y.; Zhou, L.; Liu, X.; Liu, Y. Inhibitory Effect of Essential Oils on Aspergillus ochraceus Growth and Ochratoxin A Production. PLoS ONE 2014, 9, e108285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamdem, J.P.; Abolaji, A.O.; Elekofehinti, O.; Omotuyi, I.O.; Ibrahim, M.; Hassan, W.; Barbosa, N.; Souza, D.; Da Rocha, J.B.T. Therapeutic Potential of Plant Extracts and Phytochemicals against Brain Ischemia-Reperfusion Injury: A Review. Nat. Prod. J. 2016, 6, 250–284. [Google Scholar] [CrossRef]
- Pereira, P.S.; de Lima, M.D.C.A.; Neto, P.P.M.; Oliveira-Tintino, C.D.D.M.; Tintino, S.R.; Menezes, I.R.D.A.; de Oliveira, J.F.; Marchand, P.; Coutinho, H.D.M.; Rodrigues, M.D.D.; et al. Thiazolidinedione and thiazole derivatives potentiate norfloxacin activity against NorA efflux pump over expression in Staphylococcus aureus 1199B strains. Bioorgan. Med. Chem. 2019, 27, 3797–3804. [Google Scholar] [CrossRef] [PubMed]
- Araújo, T.G.; Carvalho, B.M.; da Fonseca, C.S.M.; de Lima, M.D.C.A.; Galdino, S.L.; Pitta, I.D.R.; Lima, V.L.D.M. Metabolic effects of benzylidene thiazolidinedione derivatives in high-fat fed mice. Med. Chem. Res. 2012, 21, 2408–2414. [Google Scholar] [CrossRef]
- Blanquicett, C.; Roman, J.; Hart, C.M. Thiazolidinediones as anti-cancer agents. Cancer Ther. 2008, 6, 25. [Google Scholar] [PubMed]
- Mathews, S.T.; Kothari, V.; Galdo, J. Hypoglycemic agents and potential anti-inflammatory activity. J. Inflamm. Res. 2016, 9, 27–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, A.-P.; Li, X.; Zheng, Y.; Liu, B.-L.; Huang, G.; Yan, X.; Liu, Z.; Zhou, Z. Thiazolidinediones protect mouse pancreatic β-cells directly from cytokine-induced cytotoxicity through PPARγ-dependent mechanisms. Acta Diabetol. 2013, 50, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Farina, M.; Aschner, M.; Rocha, J.B. Oxidative stress in MeHg-induced neurotoxicity. Toxicol. Appl. Pharmacol. 2011, 256, 405–417. [Google Scholar] [CrossRef] [Green Version]
- Anantha, N.D. Approaches to pre-formulation R and D for phytopharmaceuticals emanating from herb based traditional Ayurvedic processes. J. Ayurveda Integr. Med. 2013, 4, 4–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhary, N.; Sekhon, B.S. An overview of advances in the standardization of herbal drugs. J. Pharm. Educ. Res. 2011, 2, 55–70. [Google Scholar]
- Aschner, M.; Syversen, T.; Souza, D.; da Rocha, J.B.T.; Farina, M. Involvement of glutamate and reactive oxygen species in methylmercury neurotoxicity. Braz. J. Med. Biol. Res. 2007, 40, 285–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adedara, I.A.; Rosemberg, D.B.; Souza, D.O.; Kamdem, J.P.; Farombi, E.O.; Aschner, M.; Rocha, J.B.T. Biochemical and behavioral deficits in the lobster cockroach Nauphoeta cinerea model of methylmercury exposure. Toxicol. Res. 2015, 4, 442–451. [Google Scholar] [CrossRef]
- Benzidane, Y.; Touinsi, S.; Motte, E.; Jadas-Hécart, A.; Communal, P.-Y.; Leduc, L.; Thany, S.H. Effect of thiamethoxam on cockroach locomotor activity is associated with its metabolite clothianidin. Pest Manag. Sci. 2010, 66, 1351–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrazoni, T.; Heberle, M.D.A.; Perin, A.P.A.; Zanatta, A.P.; Rodrigues, P.V.; dos Santos, F.D.M.; de Almeida, C.G.M.; Breda, R.V.; dos Santos, D.S.; Pinto, P.M.; et al. Central and peripheral neurotoxicity induced by the Jack Bean Urease (JBU) in Nauphoeta cinerea cockroaches. Toxicology 2016, 368, 162–171. [Google Scholar] [CrossRef]
- Stankiewicz, M.; Dąbrowski, M.; de Lima, M.E. Nervous System of Periplaneta americana Cockroach as a Model in Toxinological Studies: A Short Historical and Actual View. J. Toxicol. 2012, 2012, 143740. [Google Scholar] [CrossRef] [Green Version]
- Huber, I. Cockroaches as Models for Neurobiology: Applications in Biomedical Research; Huber, I., Master, E.P., Rao, B.R., Eds.; CRC Press: Boca Raton, FL, USA, 2019; ISBN 9781351070751. [Google Scholar]
- Aseervatham, G.S.B.; Suryakala, U.; Doulethunisha; Sundaram, S.; Bose, P.C.; Sivasudha, T. Expression pattern of NMDA receptors reveals antiepileptic potential of apigenin 8-C-glucoside and chlorogenic acid in pilocarpine induced epileptic mice. Biomed. Pharmacother. 2016, 82, 54–64. [Google Scholar] [CrossRef]
- da Silva, L.M.M.G.; de Oliveira, J.F.; Silva, W.L.; da Silva, A.L.; Júnior, A.A.; dos Santos, V.H.B.; Alves, L.C.; dos Santos, F.A.B.; Costa, V.M.; Aires, A.D.L.; et al. New 1,3-benzodioxole derivatives: Synthesis, evaluation of in vitro schistosomicidal activity and ultrastructural analysis. Chem.-Biol. Interact. 2018, 283, 20–29. [Google Scholar] [CrossRef]
- Holschneider, D.P.; Guo, Y.; Roch, M.; Norman, K.M.; Scremin, O.U. Acetylcholinesterase Inhibition and Locomotor Function after Motor-Sensory Cortex Impact Injury. J. Neurotrauma 2011, 28, 1909–1919. [Google Scholar] [CrossRef]
- Da Silva, C.S.; de Cássia Gonçalves de Lima, R.; Elekofehinti, O.O.; Ogunbolude, Y.; Duarte, A.E.; Rocha, J.B.T.; De Menezes, I.R.A.; Barros, L.M.; Tsopmo, A.; Lukong, K.E.; et al. Caffeine-supplemented diet modulates oxidative stress markers and improves locomotor behavior in the lobster cockroach Nauphoeta cinerea. Chem. Biol. Interact. 2018, 282, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Filho, V.M.B.; Waczuk, E.P.; Kamdem, J.P.; Abolaji, A.O.; Lacerda, S.R.; da Costa, J.G.M.; de Menezes, I.R.A.; Boligon, A.A.; Athayde, M.L.; da Rocha, J.B.T.; et al. Phytochemical constituents, antioxidant activity, cytotoxicity and osmotic fragility effects of Caju (Anacardium microcarpum). Ind. Crop. Prod. 2014, 55, 280–288. [Google Scholar] [CrossRef]
- Kamdem, J.P.; Adeniran, A.; Boligon, A.A.; Klimaczewski, C.V.; Elekofehinti, O.O.; Hassan, W.; Ibrahim, M.; Waczuk, E.P.; Meinerz, D.F.; Athayde, M.L. Antioxidant activity, genotoxicity and cytotoxicity evaluation of lemon balm (Melissa officinalis L.) ethanolic extract: Its potential role in neuroprotection. Ind. Crop. Prod. 2013, 51, 26–34. [Google Scholar] [CrossRef]
- Adedara, I.A.; Rosemberg, D.B.; de Souza, D.; Farombi, E.O.; Aschner, M.; Souza, D.O.; Rocha, J.B. Neurobehavioral and biochemical changes in Nauphoeta cinerea following dietary exposure to chlorpyrifos. Pestic. Biochem. Physiol. 2016, 130, 22–30. [Google Scholar] [CrossRef]
- Adedara, I.A.; Abolaji, A.O.; Rocha, J.B.T.; Farombi, E.O. Diphenyl Diselenide Protects against Mortality, Locomotor Deficits and Oxidative Stress in Drosophila melanogaster Model of Manganese-Induced Neurotoxicity. Neurochem. Res. 2016, 41, 1430–1438. [Google Scholar] [CrossRef]
- Rodrigues, N.; Nunes, M.; Silva, D.; Zemolin, A.; Meinerz, D.; Cruz, L.; Pereira, A.; Rocha, J.; Posser, T.; Franco, J. Is the lobster cockroach Nauphoeta cinerea a valuable model for evaluating mercury induced oxidative stress? Chemosphere 2013, 92, 1177–1182. [Google Scholar] [CrossRef] [Green Version]
- Waczuk, E.P.; Wagner, R.; Klein, B.; da Rocha, J.B.T.; Ardisson-Araújo, D.M.; Barbosa, N.V. Assessing the toxicant effect of spontaneously volatilized 4-vinylcyclohexane exposure in nymphs of the lobster cockroach nauphoeta cinerea. Environ. Toxicol. Pharmacol. 2019, 72, 103264. [Google Scholar] [CrossRef]
- Adedara, I.A.; Awogbindin, I.O.; Afolabi, B.A.; Ajayi, B.O.; Rocha, J.B.; Farombi, E.O. Hazardous impact of diclofenac exposure on the behavior and antioxidant defense system in Nauphoeta cinerea. Environ. Pollut. 2020, 265, 115053. [Google Scholar] [CrossRef]
- Harper, J.M. Body Size and the Righting Response: A Cost of Reproductive Success in Nauphoeta cinerea (Blattodea: Blaberidae)? J. Èntomol. Sci. 2018, 53, 523–532. [Google Scholar] [CrossRef]
- Tikhonova, T.A.; Rassokhina, I.V.; Kondrakhin, E.A.; Fedosov, M.A.; Bukanova, J.; Rossokhin, A.V.; Sharonova, I.N.; Kovalev, G.I.; Zavarzin, I.V.; Volkova, Y.A. Development of 1,3-thiazole analogues of imidazopyridines as potent positive allosteric modulators of GABAA receptors. Bioorgan. Chem. 2020, 94, 103334. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, W.; Li, G.; Chen, J.; Guan, X.; Chen, X.; Guan, Z. Neuroprotective Effect and Mechanism of Thiazolidinedione on Dopaminergic Neurons In Vivo and In Vitro in Parkinson’s Disease. PPAR Res. 2017, 2017, 4089214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb.prot095505. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Fan, J.; Zhang, Q.; Bao, C.; Liu, Z.; Yang, R. Turning natural products into insecticide candidates: Design and semisynthesis of novel fraxinellone-based N-(1,3-thiazol-2-yl)carboxamides against two crop-threatening insect pests. Bioorgan. Med. Chem. Lett. 2019, 29, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Soliman, N.N.; Abd el Salam, M.; Fadda, A.A.; Abdel-Motaal, M. Synthesis, Characterization, and Biochemical Impacts of Some New Bioactive Sulfonamide Thiazole Derivatives as Potential Insecticidal Agents against the Cotton Leafworm, Spodoptera littoralis. J. Agric. Food Chem. 2020, 68, 5790–5805. [Google Scholar] [CrossRef] [PubMed]
- Afolabi, B.A.; Olagoke, O.C.; Souza, D.O.; Aschner, M.; Rocha, J.B.; Segatto, A.L.A. Modified expression of antioxidant genes in lobster cockroach, Nauphoeta cinerea exposed to methylmercury and monosodium glutamate. Chem.-Biol. Interact. 2020, 318, 108969. [Google Scholar] [CrossRef]
- Kopanska, M.; Czech, J.; Zagata, P.; Dobrek, L.; Thor, P.; Formicki, G. Effect of the different doses of acrylamide on acetylocholinoesterase activity, thiol groups, malondialdehyde concentrations in hypothalamus and selected muscles of mice. J. Physiol. Pharmacol. 2017, 68, 565–571. [Google Scholar]
- Ebtesam, M.M.G.; Ibrahim, S.E. Protective and Therapeutic Effects of Propolis against Monosodium Glutamate (MSG) Toxicity in Rat Brain: Histopathological Study. EPH-Int. J. Appl. Sci. 2017, 1, 396–404. [Google Scholar]
- Hasanein, P.; Ghafari-Vahed, M.; Khodadadi, I. Effects of isoquinoline alkaloid berberine on lipid peroxidation, antioxidant defense system, and liver damage induced by lead acetate in rats. Redox Rep. 2017, 22, 42–50. [Google Scholar] [CrossRef]
- Jin, Y.; Huang, Z.-L.; Li, L.; Yang, Y.; Wang, C.-H.; Wang, Z.-T.; Ji, L.-L. Quercetin attenuates toosendanin-induced hepatotoxicity through inducing the Nrf2/GCL/GSH antioxidant signaling pathway. Acta Pharmacol. Sin. 2019, 40, 75–85. [Google Scholar] [CrossRef]
- Kumar, A.S.; Gandhimathi, R. Effect of Guettarda speciosa extracts on antioxidant enzymes levels in rat brain after induction of seizures by MES and PTZ. J. Nat. Prod. 2010, 3, 80–85. [Google Scholar]
- Michel, F.; Bonnefont-Rousselot, D.; Mas, E.; Drai, J.; Thérond, P. Biomarkers of lipid peroxidation: Analytical aspects. Ann. Biol. Clin. 2008, 66, 605–620. [Google Scholar] [CrossRef]
- Ademiluyi, A.O.; Oyeniran, O.H.; Oboh, G. Dietary monosodium glutamate altered redox status and dopamine metabolism in lobster cockroach (Nauphoeta cinerea). J. Food Biochem. 2020, 44, e13451. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, L.S.; Bortolin, R.C.; Kolling, E.A.; Schnorr, C.E.; Filho, A.Z.; Gelain, D.P.; Moreira, J.C.F. Antioxidant Profile Characterization of a Commercial Paullinia cupana (Guarana) Extracts. J. Nat. Prod. Resour. 2016, 2, 47–52. [Google Scholar]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, W.; Zhang, M.; Yu, W.; Gao, F.; Li, C.; Wang, S.-B.; Feng, J.; Zhang, X.-Z. Ferrous-Supply-Regeneration Nanoengineering for Cancer-Cell-Specific Ferroptosis in Combination with Imaging-Guided Photodynamic Therapy. ACS Nano 2018, 12, 12181–12192. [Google Scholar] [CrossRef]
- Sang, M.; Luo, R.; Bai, Y.; Dou, J.; Zhang, Z.; Liu, F.; Feng, F.; Xu, J.; Liu, W. Mitochondrial membrane anchored photosensitive nano-device for lipid hydroperoxides burst and inducing ferroptosis to surmount therapy-resistant cancer. Theranostics 2019, 9, 6209–6223. [Google Scholar] [CrossRef]
- Schneider, T.; Gavrilova, I.; Budisa, N. Synthesis of a new metal chelating amino acid: Terpyridyl-alanine. Tetrahedron Lett. 2019, 60, 906–910. [Google Scholar] [CrossRef]
- Karthik, C.S.; Mallesha, L.; Nagashree, S.; Mallu, P.; Patil, V.; Kumar, S. Schiff bases of 4-(methylthio)benzaldehydes: Synthesis, characterization, antibacterial, antioxidant and cytotoxicity Studies. Curr. Chem. Lett. 2016, 5, 71–82. [Google Scholar] [CrossRef]
- Nagendrappa, P.B.; Naik, M.P.; Payyappallimana, U. Ethnobotanical survey of malaria prophylactic remedies in Odisha, India. J. Ethnopharmacol. 2013, 146, 768–772. [Google Scholar] [CrossRef]
- Kołodziejczyk, K.; Sójka, M.; Abadias, M.; Viñas, I.; Guyot, S.; Baron, A. Polyphenol composition, antioxidant capacity, and antimicrobial activity of the extracts obtained from industrial sour cherry pomace. Ind. Crop. Prod. 2013, 51, 279–288. [Google Scholar] [CrossRef]
- Naik, G.; Priyadarsini, K.; Satav, J.; Banavalikar, M.; Sohoni, D.; Biyani, M.; Mohan, H. Comparative antioxidant activity of individual herbal components used in Ayurvedic medicine. Phytochemistry 2003, 63, 97–104. [Google Scholar] [CrossRef]
- Marius, M.; Amadou, D.; Donatien, A.A.; Gilbert, A.; William, Y.N.; Rauf, K.; Arif, M.; Adeline, F.Y.S.; Saint, N.I.; Dar, H.; et al. In Vitro Antioxidant, Anti-inflammatory, and In Vivo Anticolitis Effects of Combretin A and Combretin B on Dextran Sodium Sulfate-Induced Ulcerative Colitis in Mice. Gastroenterol. Res. Pract. 2020, 2020, 4253174. [Google Scholar] [CrossRef]
- Silva, F.A.; Pizzuti, L.; Quina, F.H.; Souza, S.P.; Rosales, P.F.; Siqueira, G.M.; Pereira, C.M.; Barros, S.B.; Rivelli, D.P. Antioxidant Capacity of 2-(3,5-diaryl-4,5-dihydro-1H-pyrazol-1-yl)-4-phenylthiazoles. Lett. Drug Des. Discov. 2010, 7, 657–660. [Google Scholar] [CrossRef]
- Harini, S.T.; Kumar, H.V.; Rangaswamy, J.; Naik, N. Synthesis of thiazole-based substituted piperidinone oximes: Profiling of antioxidant and antimicrobial activity. Russ. J. Bioorgan. Chem. 2017, 43, 186–196. [Google Scholar] [CrossRef]
- Marc, G.; Stana, A.; Oniga, S.D.; Pîrnău, A.; Vlase, L.; Oniga, O. New Phenolic Derivatives of Thiazolidine-2,4-dione with Antioxidant and Antiradical Properties: Synthesis, Characterization, In Vitro Evaluation, and Quantum Studies. Molecules 2019, 24, 2060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajpai, P.; Jain, S.; Choubey, A. Thiazolidine, a versatile ring in the treatment of various diseases: A systematic review. Asian J. Res. Chem. 2020, 13, 283. [Google Scholar] [CrossRef]



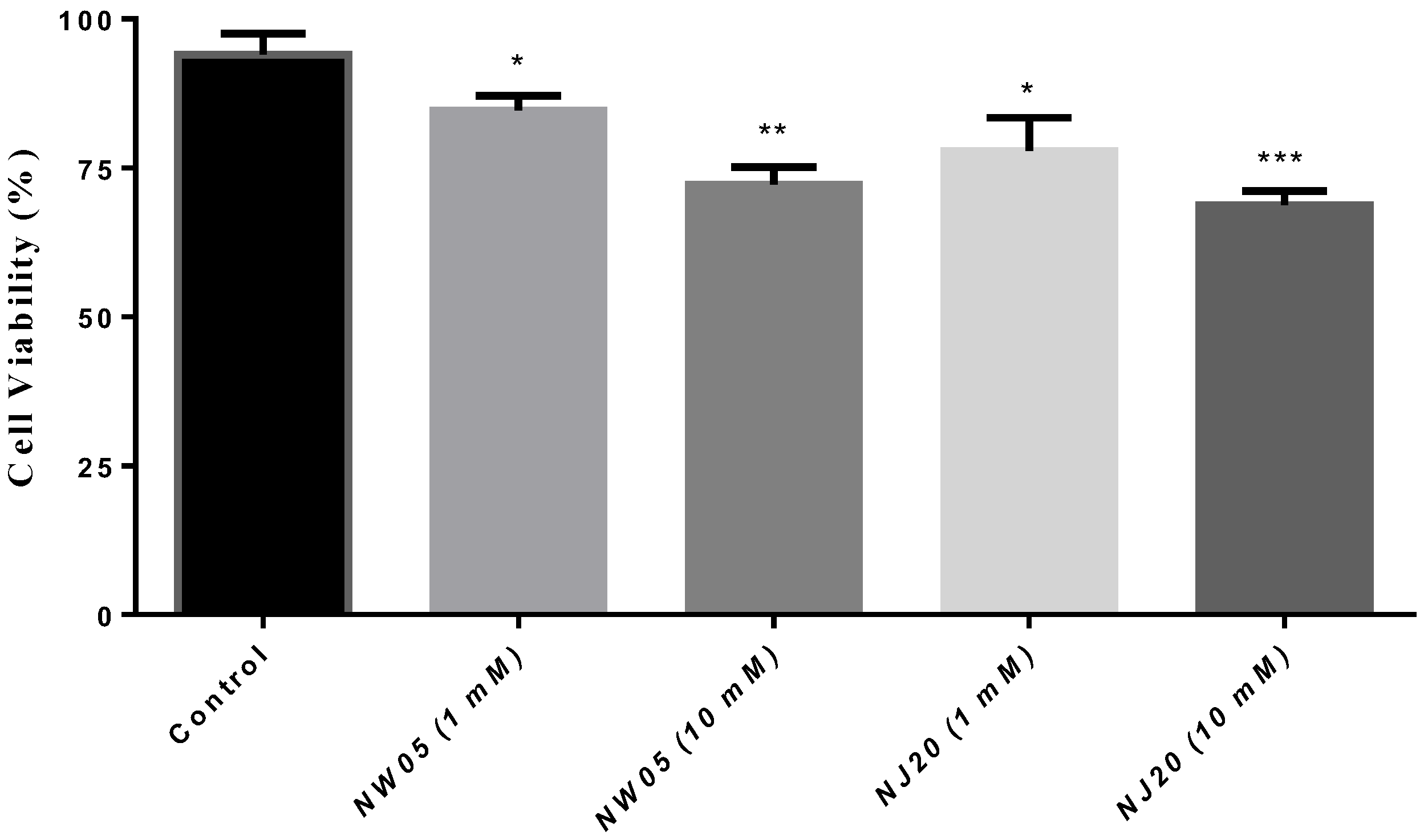

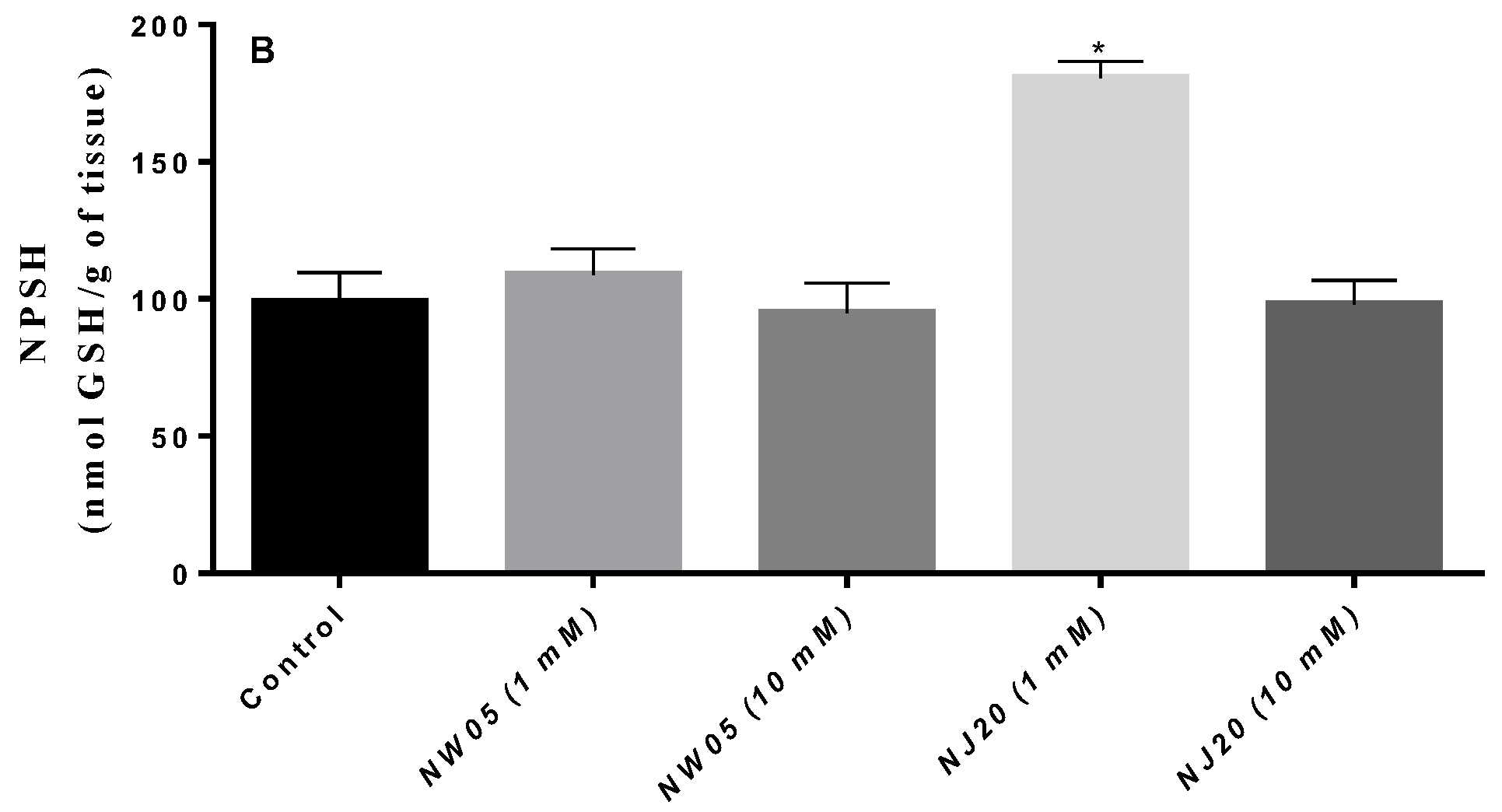
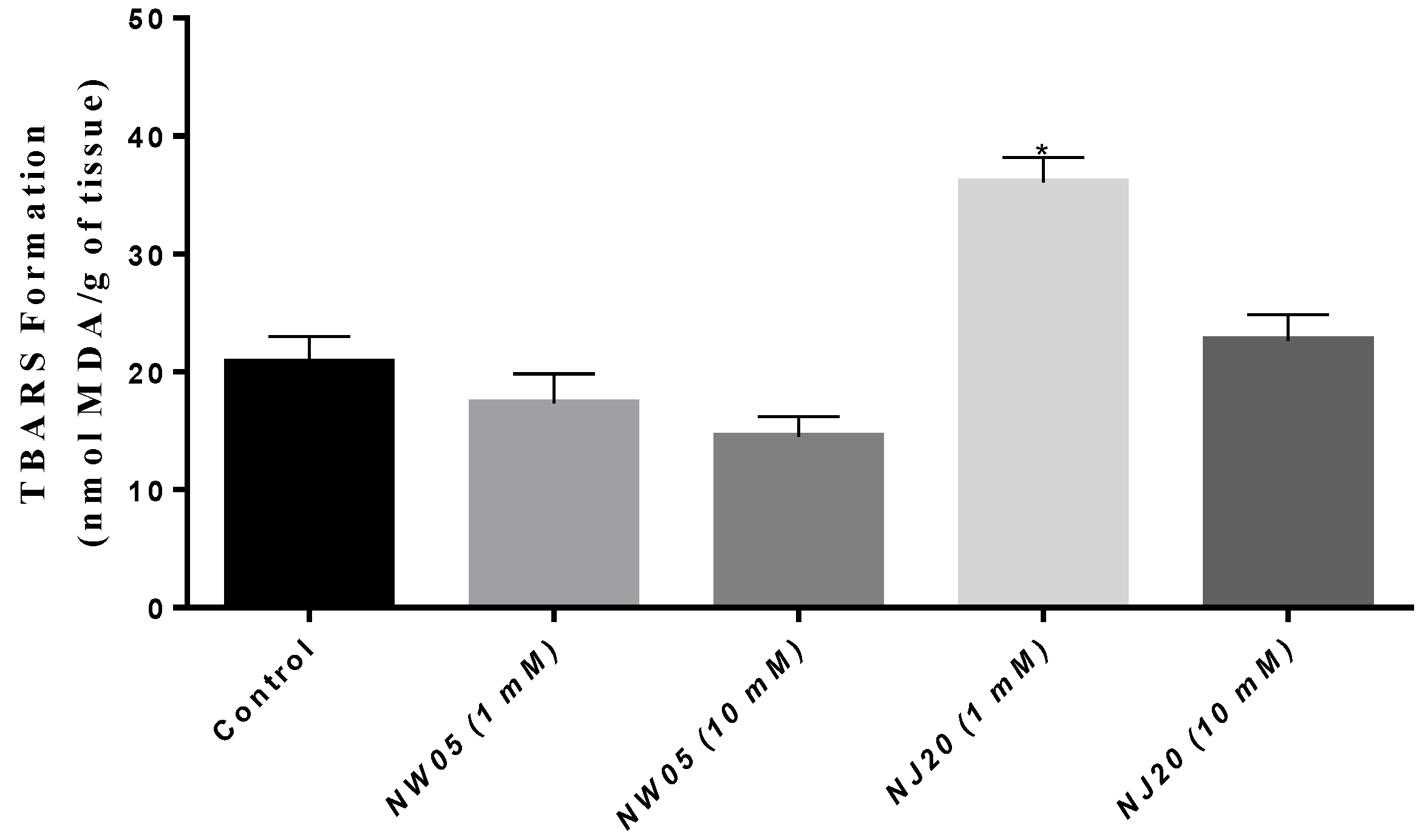

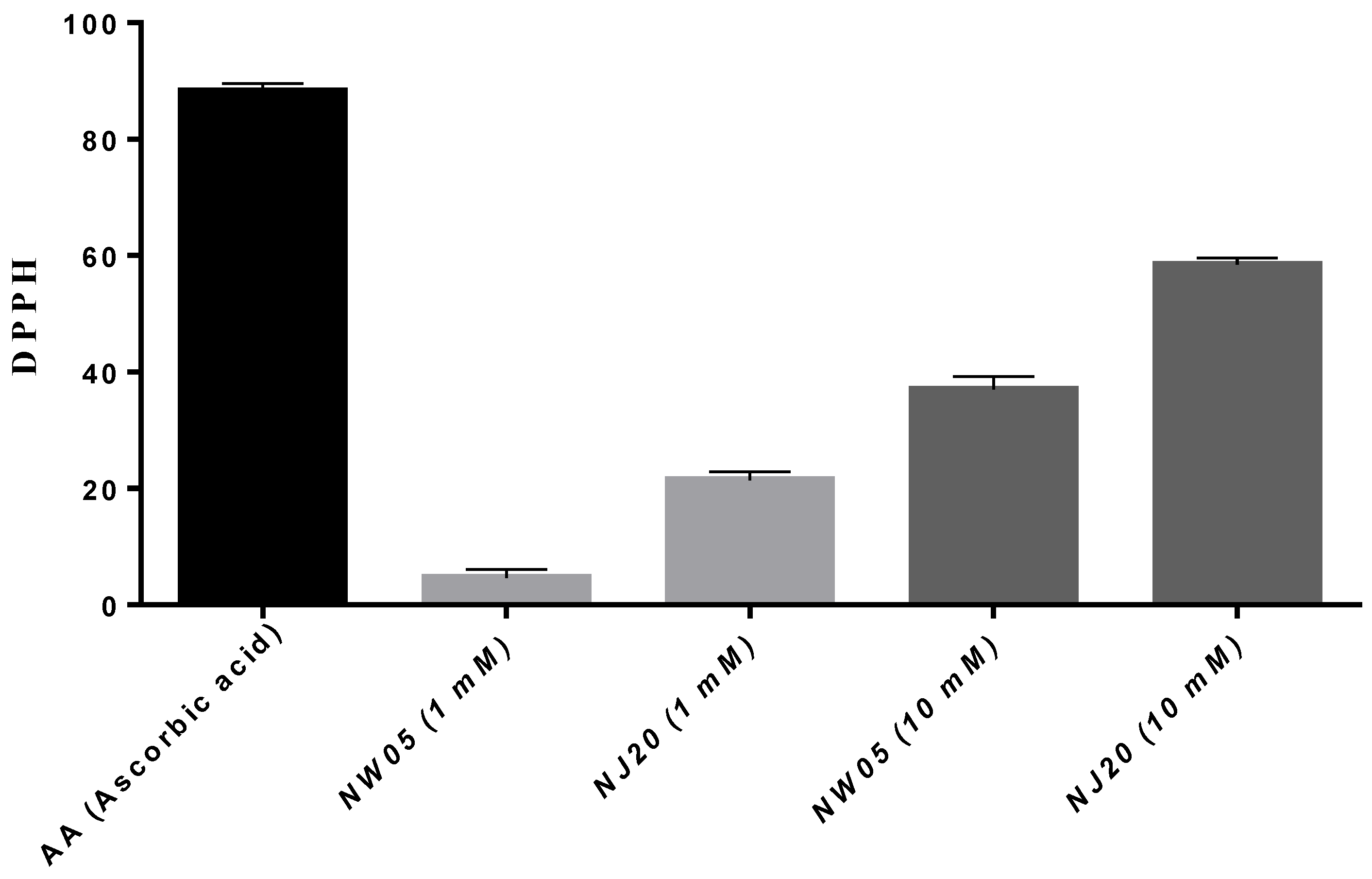
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, P.S.; Costa, A.R.; de Oliveira, T.J.S.; Oliveira, C.V.B.; de Lima, M.d.C.A.; de Oliveira, J.F.; Kim, B.; Coutinho, H.D.M.; Duarte, A.E.; Kamdem, J.P.; et al. Neurolocomotor Behavior and Oxidative Stress Markers of Thiazole and Thiazolidinedione Derivatives against Nauphoeta cinerea. Antioxidants 2022, 11, 420. https://doi.org/10.3390/antiox11020420
Pereira PS, Costa AR, de Oliveira TJS, Oliveira CVB, de Lima MdCA, de Oliveira JF, Kim B, Coutinho HDM, Duarte AE, Kamdem JP, et al. Neurolocomotor Behavior and Oxidative Stress Markers of Thiazole and Thiazolidinedione Derivatives against Nauphoeta cinerea. Antioxidants. 2022; 11(2):420. https://doi.org/10.3390/antiox11020420
Chicago/Turabian StylePereira, Pedro Silvino, Adrielle Rodrigues Costa, Thalyta Julyanne Silva de Oliveira, Carlos Vinícius Barros Oliveira, Maria do Carmo Alves de Lima, Jamerson Ferreira de Oliveira, Bonglee Kim, Henrique D. M. Coutinho, Antonia Eliene Duarte, Jean Paul Kamdem, and et al. 2022. "Neurolocomotor Behavior and Oxidative Stress Markers of Thiazole and Thiazolidinedione Derivatives against Nauphoeta cinerea" Antioxidants 11, no. 2: 420. https://doi.org/10.3390/antiox11020420
APA StylePereira, P. S., Costa, A. R., de Oliveira, T. J. S., Oliveira, C. V. B., de Lima, M. d. C. A., de Oliveira, J. F., Kim, B., Coutinho, H. D. M., Duarte, A. E., Kamdem, J. P., & da Silva, T. G. (2022). Neurolocomotor Behavior and Oxidative Stress Markers of Thiazole and Thiazolidinedione Derivatives against Nauphoeta cinerea. Antioxidants, 11(2), 420. https://doi.org/10.3390/antiox11020420






