Potential for Prebiotic Stabilized Cornus mas L. Lyophilized Extract in the Prophylaxis of Diabetes Mellitus in Streptozotocin Diabetic Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Plant Material
2.3. Corni fructus Extract Preparation
2.4. Phytochemical Analysis of Aqueous Extracts
2.4.1. Determination of Anthocyanin Content
2.4.2. Determination of Loganic Acid Content
2.4.3. Determination of Total Phenolic Content (TPC)
2.5. In Vitro Activity of Corni fructus Extracts
2.5.1. α-Glucosidase Inhibitory Assay
2.5.2. DPPH Assay
2.5.3. FRAP Assay
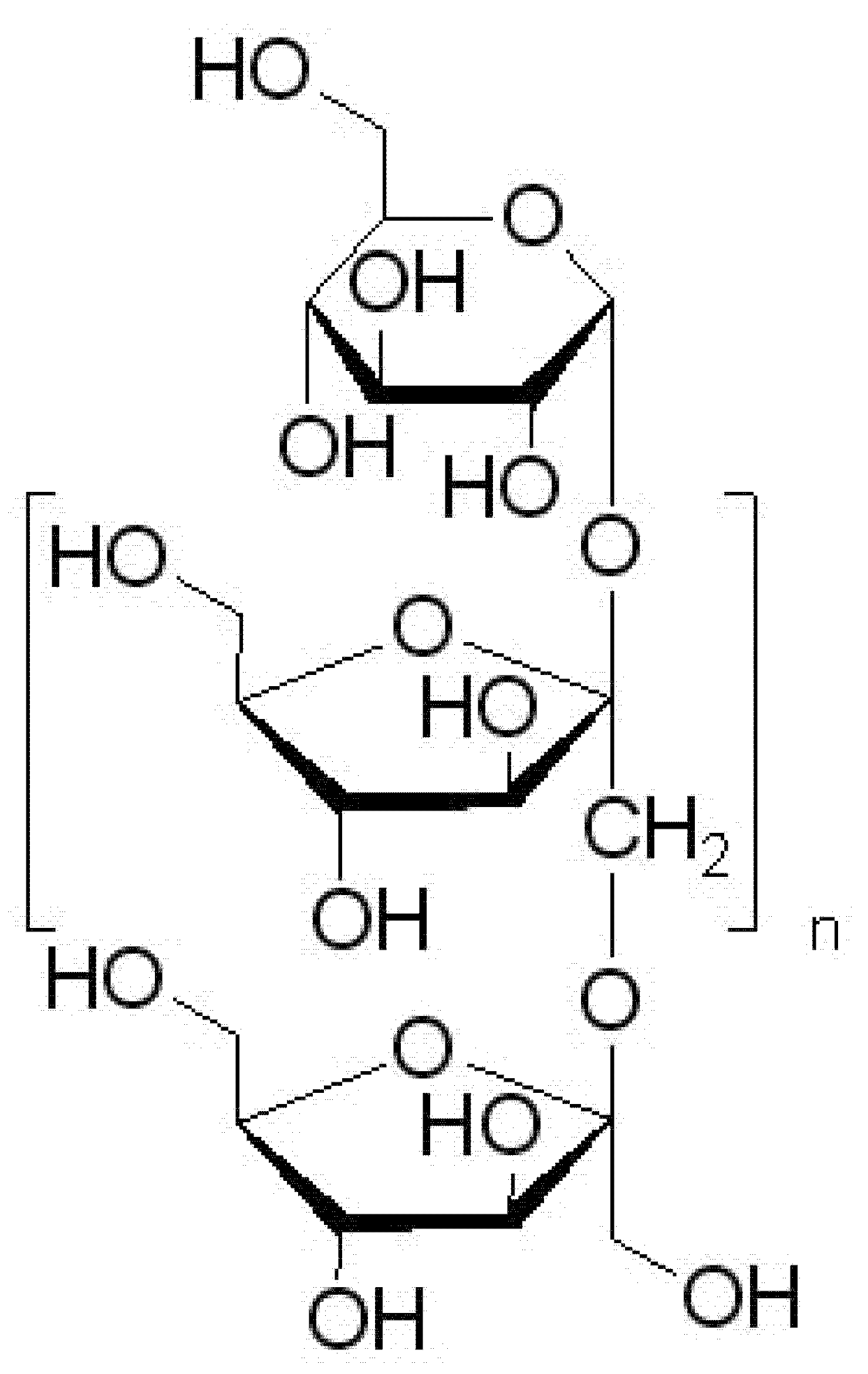
2.6. Preparation of Corni fructus Extract System with Prebiotic Carrier
2.7. In Vivo Antidiabetic Activity of Prebiotic Stabilized Lyophilizate of Corni fructus
2.8. Quantitative and Qualitative Analysis of Microorganisms Present in the Gastrointestinal Tract
2.9. Statistical Analysis
3. Results and Discussion
3.1. Quantitative and Qualitative Analysis of the Examined Extracts
3.2. In Vitro Activity
3.3. In Vivo Activity
3.4. Quantitative and Qualitative Analysis of Changes in the Intestinal Microflora
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Putthapiban, P.; Sukhumthammarat, W.; Sriphrapradang, C. Concealed Use of Herbal and Dietary Supplements among Thai Patients with Type 2 Diabetes Mellitus. J. Diabetes Metab. Disord. 2017, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Yeh, G.Y.; Eisenberg, D.M.; Kaptchuk, T.J.; Phillips, R.S. Systematic Review of Herbs and Dietary Supplements for Glycemic Control in Diabetes. Diabetes Care 2003, 26, 1277–1294. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Santini, A. Plants and Diabetes: Description, Role, Comprehension and Exploitation. Int. J. Mol. Sci. 2021, 22, 3938. [Google Scholar] [CrossRef] [PubMed]
- Thi Bui, D.H.; Nguyen, B.X.; Truong, D.C.; Meyrowitsch, D.W.; Søndergaard, J.; Gammeltoft, T.; Bygbjerg, I.C.; Jannie, N. Polypharmacy among People Living with Type 2 Diabetes Mellitus in Rural Communes in Vietnam. PLoS ONE 2021, 16, e0249849. [Google Scholar] [CrossRef]
- Mahdavi, A.; Bagherniya, M.; Mirenayat, M.S.; Atkin, S.L.; Sahebkar, A. Medicinal Plants and Phytochemicals Regulating Insulin Resistance and Glucose Homeostasis in Type 2 Diabetic Patients: A Clinical Review. Adv. Exp. Med. Biol. 2021, 1308, 161–183. [Google Scholar] [CrossRef]
- Liu, X.; Zeng, X.; Liu, W.; Lu, Y.; Cheng, J.; Chen, Y. An Overview of Dietary Supplements on Obesity and Type 2 Diabetes: Efficacy and Mechanisms. Curr. Drug Metab. 2021, 22, 415–440. [Google Scholar] [CrossRef]
- Kazimierski, M.; Regula, J.; Molska, M. Cornelian Cherry (Cornus mas L.)—Characteristics, Nutritional and pro-Health Properties. Acta Sci. Pol. Technol. Aliment. 2019, 18, 5–12. [Google Scholar] [CrossRef]
- Klimenko, S. The Cornelian cherry (Cornus mas L.): Collection, preservation, and utilization of genetic resources. J. Fruit Ornam. Plant Res. 2004, 12, 93–98. [Google Scholar]
- Blagojević, B.; Agić, D.; Serra, A.T.; Matić, S.; Matovina, M.; Bijelić, S.; Popović, B.M. An in Vitro and in Silico Evaluation of Bioactive Potential of Cornelian Cherry (Cornus mas L.) Extracts Rich in Polyphenols and Iridoids. Food Chem. 2021, 335, 127619. [Google Scholar] [CrossRef]
- Dzydzan, O.; Bila, I.; Kucharska, A.Z.; Brodyak, I.; Sybirna, N. Antidiabetic Effects of Extracts of Red and Yellow Fruits of Cornelian Cherries (Cornus mas L.) on Rats with Streptozotocin-Induced Diabetes Mellitus. Food Funct. 2019, 10, 6459–6472. [Google Scholar] [CrossRef]
- Bajaj, S.; Khan, A. Antioxidants and Diabetes. Indian J. Endocrinol. Metab. 2012, 16, S267–S271. [Google Scholar] [CrossRef] [PubMed]
- Klymenko, S.; Kucharska, A.Z.; Sokół-Łętowska, A.; Piórecki, N.; Przybylska, D.; Grygorieva, O. Iridoids, Flavonoids, and Antioxidant Capacity of Cornus mas, C. officinalis, and C. mas × C. officinalis Fruits. Biomolecules 2021, 11, 776. [Google Scholar] [CrossRef] [PubMed]
- Tiptiri-Kourpeti, A.; Fitsiou, E.; Spyridopoulou, K.; Vasileiadis, S.; Iliopoulos, C.; Galanis, A.; Vekiari, S.; Pappa, A.; Chlichlia, K. Evaluation of Antioxidant and Antiproliferative Properties of Cornus mas L. Fruit Juice. Antioxidants 2019, 8, 377. [Google Scholar] [CrossRef] [PubMed]
- Szumny, D.; Sozański, T.; Kucharska, A.Z.; Dziewiszek, W.; Piórecki, N.; Magdalan, J.; Chlebda-Sieragowska, E.; Kupczynski, R.; Szeląg, A.; Szumny, A. Application of Cornelian Cherry Iridoid-Polyphenolic Fraction and Loganic Acid to Reduce Intraocular Pressure. Evid. Based Complement. Alternat. Med. 2015, 2015, 939402. [Google Scholar] [CrossRef]
- Sozański, T.; Kucharska, A.Z.; Rapak, A.; Szumny, D.; Trocha, M.; Merwid-Ląd, A.; Dzimira, S.; Piasecki, T.; Piórecki, N.; Magdalan, J.; et al. Iridoid-Loganic Acid versus Anthocyanins from the Cornus mas Fruits (Cornelian Cherry): Common and Different Effects on Diet-Induced Atherosclerosis, PPARs Expression and Inflammation. Atherosclerosis 2016, 254, 151–160. [Google Scholar] [CrossRef]
- Carrillo-Ocampo, D.; Bazaldúa-Gómez, S.; Bonilla-Barbosa, J.R.; Aburto-Amar, R.; Rodríguez-López, V. Anti-Inflammatory Activity of Iridoids and Verbascoside Isolated from Castilleja tenuiflora. Molecules 2013, 18, 12109–12118. [Google Scholar] [CrossRef]
- Sozański, T.; Kucharska, A.Z.; Dzimira, S.; Magdalan, J.; Szumny, D.; Matuszewska, A.; Nowak, B.; Piórecki, N.; Szeląg, A.; Trocha, M. Loganic Acid and Anthocyanins from Cornelian Cherry (Cornus mas L.) Fruits Modulate Diet-Induced Atherosclerosis and Redox Status in Rabbits. Adv. Clin. Exp. Med. 2018, 27, 1505–1513. [Google Scholar] [CrossRef]
- Kanapathy, M.; Portou, M.J.; Tsui, J.; Richards, T. Diabetic Foot Ulcers in Conjunction with Lower Limb Lymphedema: Pathophysiology and Treatment Procedures. CWCMR 2015, 2, 129–136. [Google Scholar] [CrossRef]
- Zhao, Y.-X.; Chen, X.-W. Diabetes and Risk of Glaucoma: Systematic Review and a Meta-Analysis of Prospective Cohort Studies. Int. J. Ophthalmol. 2017, 10, 1430–1435. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.-A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, Stress, and Diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Świerczewska, A.; Buchholz, T.; Melzig, M.F.; Czerwińska, M.E. In Vitro α-Amylase and Pancreatic Lipase Inhibitory Activity of Cornus mas L. and Cornus alba L. Fruit Extracts. J. Food Drug Anal. 2019, 27, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Sozański, T.; Kucharska, A.Z.; Szumny, D.; Magdalan, J.; Merwid-Ląd, A.; Nowak, B.; Piórecki, N.; Dzimira, S.; Jodkowska, A.; Szeląg, A.; et al. Cornelian Cherry Consumption Increases the L-Arginine/ADMA Ratio, Lowers ADMA and SDMA Levels in the Plasma, and Enhances the Aorta Glutathione Level in Rabbits Fed a High-Cholesterol Diet. J. Funct. Foods 2017, 34, 189–196. [Google Scholar] [CrossRef]
- Dinda, B.; Kyriakopoulos, A.M.; Dinda, S.; Zoumpourlis, V.; Thomaidis, N.S.; Velegraki, A.; Markopoulos, C.; Dinda, M. Cornus mas L. (Cornelian Cherry), an Important European and Asian Traditional Food and Medicine: Ethnomedicine, Phytochemistry and Pharmacology for Its Commercial Utilization in Drug Industry. J. Ethnopharmacol. 2016, 193, 670–690. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Olson, L.K.; Schutzki, R.E.; Tai, M.-H.; Nair, M.G. Amelioration of Obesity and Glucose Intolerance in High-Fat-Fed C57BL/6 Mice by Anthocyanins and Ursolic Acid in Cornelian Cherry (Cornus mas). J. Agric. Food Chem. 2006, 54, 243–248. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Wright, R.S.; Farkouh, M.; Plutzky, J. Modulating Peroxisome Proliferator–Activated Receptors for Therapeutic Benefit? Biology, Clinical Experience, and Future Prospects. Am. Heart J. 2012, 164, 672–680. [Google Scholar] [CrossRef]
- White Fat Progenitor Cells Reside in the Adipose Vasculature|Science. Available online: https://science.sciencemag.org/content/322/5901/583 (accessed on 27 March 2020).
- Pascale, A.; Marchesi, N.; Marelli, C.; Coppola, A.; Luzi, L.; Govoni, S.; Giustina, A.; Gazzaruso, C. Microbiota and Metabolic Diseases. Endocrine 2018, 61, 357–371. [Google Scholar] [CrossRef]
- Utzschneider, K.M.; Kratz, M.; Damman, C.J.; Hullarg, M. Mechanisms Linking the Gut Microbiome and Glucose Metabolism. J. Clin. Endocrinol. Metab. 2016, 101, 1445–1454. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the Normal Gut Microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef] [PubMed]
- D’Argenio, V.; Salvatore, F. The Role of the Gut Microbiome in the Healthy Adult Status. Clin. Chim. Acta 2015, 451, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Godoy-Vitorino, F.; Knight, R.; Blaser, M.J. Role of the Microbiome in Human Development. Gut 2019, 68, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The Healthy Human Microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef]
- Ruan, W.; Engevik, M.A.; Spinler, J.K.; Versalovic, J. Healthy Human Gastrointestinal Microbiome: Composition and Function After a Decade of Exploration. Dig. Dis. Sci. 2020, 65, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Blainski, A.; Lopes, G.C.; De Mello, J.C.P. Application and Analysis of the Folin Ciocalteu Method for the Determination of the Total Phenolic Content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef]
- Telagari, M.; Hullatti, K. In-Vitro α-Amylase and α-Glucosidase Inhibitory Activity of Adiantum caudatum Linn. and Celosia argentea Linn. Extracts and Fractions. Indian J. Pharmacol. 2015, 47, 425–429. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Piotrowska, H.; Kucińska, M.; Murias, M.; Bylka, W. Cytotoxic Activity of Physodic Acid and Acetone Extract from Hypogymnia physodes against Breast Cancer Cell Lines. Pharm. Biol. 2016, 54, 2480–2485. [Google Scholar] [CrossRef]
- Tiveron, A.P.; Melo, P.S.; Bergamaschi, K.B.; Vieira, T.M.F.S.; Regitano-d’Arce, M.A.B.; Alencar, S.M. Antioxidant Activity of Brazilian Vegetables and Its Relation with Phenolic Composition. Int. J. Mol. Sci. 2012, 13, 8943–8957. [Google Scholar] [CrossRef]
- Zha, D.; Yao, T.; Bao, L.; Gao, P.; Wu, X. Telmisartan Attenuates Diabetic Nephropathy Progression by Inhibiting the Dimerization of Angiotensin Type-1 Receptor and Adiponectin Receptor-1. Life Sci. 2019, 221, 109–120. [Google Scholar] [CrossRef]
- Essa, R.; Sadek, A.M.E.; Baset, M.E.; Rawash, M.A.; Sami, D.G.; Badawy, M.T.; Mansour, M.E.; Attia, H.; Saadeldin, M.K.; Abdellatif, A. Effects of Turmeric (Curcuma longa) Extract in Streptozocin-Induced Diabetic Model. J. Food Biochem. 2019, 43, e12988. [Google Scholar] [CrossRef]
- Anonymous ICH Q2 (R1) Validation of Analytical Procedures: Text and Methodology. Available online: https://www.ema.europa.eu/en/ich-q2-r1-validation-analytical-procedures-text-methodology (accessed on 26 November 2021).
- Vieira, L.M.; Marinho, L.M.G.; Rocha, J.d.C.G.; Barros, F.A.R.; Stringheta, P.C. Chromatic Analysis for Predicting Anthocyanin Content in Fruits and Vegetables. Food Sci. Technol. 2019, 39, 415–422. [Google Scholar] [CrossRef]
- Martín, J.; Navas, M.J.; Jiménez-Moreno, A.M.; Asuero, A.G. Anthocyanin Pigments: Importance, Sample Preparation and Extraction. In Phenolic Compounds—Natural Sources, Importance and Applications; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Phenolics in Cereals, Fruits and Vegetables: Occurrence, Extraction and Analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, O.M.; Kobus-Cisowska, J.; Kusek, W.; Przeor, M. Functional Properties of Cornelian Cherry (Cornus mas L.): A Comprehensive Review. Eur. Food Res. Technol. 2019, 245, 2071–2087. [Google Scholar] [CrossRef]
- Enache, I.M.; Vasile, A.M.; Enachi, E.; Barbu, V.; Stănciuc, N.; Vizireanu, C. Co-Microencapsulation of Anthocyanins from Cornelian Cherry Fruits and Lactic Acid Bacteria in Biopolymeric Matrices by Freeze-Drying: Evidences on Functional Properties and Applications in Food. Polymers 2020, 12, 906. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic Andjelkovic, A.; Radovanović, B.; Andjelkovic, M.; Radovanovic, A.; Nikolic, V.; Randjelovic, V. The Anthocyanin Content and Bioactivity of Cornelian Cherry (Cornus mas) and Wild Blackberry (Rubus fruticosus): Fruit Extracts from the Vlasina Region. Adv. Technol. 2015, 4, 26–31. [Google Scholar] [CrossRef]
- Salaritabar, A.; Darvish, B.; Hadjiakhoondi, F.; Manayi, A.; Sureda, A.; Nabavi, S.; Fitzpatrick, L.; Nabavi, S.; Bishayee, A. Therapeutic Potential of Flavonoids in Inflammatory Bowel Disease: A Comprehensive Review. World J. Gastroenterol. 2017, 23, 5097. [Google Scholar] [CrossRef]
- Szczepaniak, O.; Cielecka-Piontek, J.; Kobus-Cisowska, J. Hypoglycaemic, Antioxidative and Phytochemical Evaluation of Cornus mas Varieties. Eur. Food Res. Technol. 2021, 247, 183–191. [Google Scholar] [CrossRef]
- Assefa, S.T.; Yang, E.-Y.; Chae, S.-Y.; Song, M.; Lee, J.; Cho, M.-C.; Jang, S. Alpha Glucosidase Inhibitory Activities of Plants with Focus on Common Vegetables. Plants 2020, 9, 2. [Google Scholar] [CrossRef]
- Bhatia, A.; Singh, B.; Arora, R.; Arora, S. In Vitro Evaluation of the α-Glucosidase Inhibitory Potential of Methanolic Extracts of Traditionally Used Antidiabetic Plants. BMC Complement. Altern. Med. 2019, 19, 74. [Google Scholar] [CrossRef]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. α-Glucosidase Inhibitors from Plants: A Natural Approach to Treat Diabetes. Pharmacogn. Rev. 2011, 5, 19–29. [Google Scholar] [CrossRef]
- Asgary, S.; Sahebkar, A.; Afshani, M.R.; Keshvari, M.; Haghjooyjavanmard, S.; Rafieian-Kopaei, M. Clinical Evaluation of Blood Pressure Lowering, Endothelial Function Improving, Hypolipidemic and Anti-Inflammatory Effects of Pomegranate Juice in Hypertensive Subjects. Phytother. Res. 2014, 28, 193–199. [Google Scholar] [CrossRef]
- De Biaggi, M.; Donno, D.; Mellano, M.G.; Riondato, I.; Rakotoniaina, E.N.; Beccaro, G.L. Cornus mas (L.) Fruit as a Potential Source of Natural Health-Promoting Compounds: Physico-Chemical Characterisation of Bioactive Components. Plant Foods Hum. Nutr. 2018, 73, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; West, B.J.; Jensen, C.J. UPLC-TOF-MS Characterization and Identification of Bioactive Iridoids in Cornus mas Fruit. J. Anal. Methods Chem. 2013, 2013, 710972. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Tang, S.; Zhang, L.; Xiao, J.; Luo, Q.; Chen, Y.; Zhou, M.; Feng, N.; Wang, C. The Inhibitory Effect of the Catechin Structure on Advanced Glycation End Product Formation in Alcoholic Media. Food Funct. 2020, 11, 5396–5408. [Google Scholar] [CrossRef]
- Oboh, G.; Ogunsuyi, O.B.; Ogunbadejo, M.D.; Adefegha, S.A. Influence of Gallic Acid on α-Amylase and α-Glucosidase Inhibitory Properties of Acarbose. J. Food Drug Anal. 2016, 24, 627–634. [Google Scholar] [CrossRef]
- Tan, C.; Wang, Q.; Luo, C.; Chen, S.; Li, Q.; Li, P. Yeast α-Glucosidase Inhibitory Phenolic Compounds Isolated from Gynura Medica Leaf. Int. J. Mol. Sci. 2013, 14, 2551–2558. [Google Scholar] [CrossRef]
- Bashan, N.; Kovsan, J.; Kachko, I.; Ovadia, H.; Rudich, A. Positive and Negative Regulation of Insulin Signaling by Reactive Oxygen and Nitrogen Species. Physiol. Rev. 2009, 89, 27–71. [Google Scholar] [CrossRef]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Oxidative Stress and Stress-Activated Signaling Pathways: A Unifying Hypothesis of Type 2 Diabetes. Endocr. Rev. 2002, 23, 599–622. [Google Scholar] [CrossRef]
- Gan, B.; Hu, J.; Jiang, S.; Liu, Y.; Sahin, E.; Zhuang, L.; Fletcher-Sananikone, E.; Colla, S.; Wang, Y.A.; Chin, L.; et al. Lkb1 Regulates Quiescence and Metabolic Homeostasis of Haematopoietic Stem Cells. Nature 2010, 468, 701–704. [Google Scholar] [CrossRef]
- Chakraborthy, A.; Ramani, P.; Sherlin, H.J.; Premkumar, P.; Natesan, A. Antioxidant and Pro-Oxidant Activity of Vitamin C in Oral Environment. Indian J. Dent. Res. 2014, 25, 499. [Google Scholar] [CrossRef] [PubMed]
- Nizioł-Łukaszewska, Z.; Wasilewski, T.; Bujak, T.; Osika, P. Iridoids from Cornus mas L. and Their Potential as Innovative Ingredients in Cosmetics. Pol. J. Chem. Technol. 2017, 19, 122–127. [Google Scholar] [CrossRef]
- Roberto, D.; Micucci, P.; Sebastian, T.; Graciela, F.; Anesini, C. Antioxidant Activity of Limonene on Normal Murine Lymphocytes: Relation to H2O2 Modulation and Cell Proliferation. Basic Clin. Pharmacol. Toxicol. 2010, 106, 38–44. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Falony, G.; Lazidou, K.; Verschaeren, A.; Weckx, S.; Maes, D.; Vuyst, L.D. In Vitro Kinetic Analysis of Fermentation of Prebiotic Inulin-Type Fructans by Bifidobacterium Species Reveals Four Different Phenotypes. Appl. Environ. Microbiol. 2009. [Google Scholar] [CrossRef]
- Ramirez-Farias, C.; Slezak, K.; Fuller, Z.; Duncan, A.; Holtrop, G.; Louis, P. Effect of Inulin on the Human Gut Microbiota: Stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br. J. Nutr. 2009, 101, 541–550. [Google Scholar] [CrossRef]
- Harmsen, H.J.M.; Raangs, G.C.; Franks, A.H.; Wildeboer-Veloo, A.C.M.; Welling, G.W. The Effect of the Prebiotic Inulin and the Probiotic Bifidobacterium longum on the Fecal Microflora of Healthy Volunteers Measured by FISH and DGGE. Microb. Ecol. Health Dis. 2002, 14, 212–220. [Google Scholar] [CrossRef]
- Staffolo, M.D.; Bevilacqua, A.E.; Rodríguez, M.S.; Albertengo, L. Dietary Fiber and Availability of Nutrients: A Case Study on Yoghurt as a Food Model; IntechOpen: London, UK, 2012; ISBN 978-953-51-0819-1. [Google Scholar]
- Navarro, D.M.D.L.; Abelilla, J.J.; Stein, H.H. Structures and Characteristics of Carbohydrates in Diets Fed to Pigs: A Review. J. Anim. Sci. Biotechnol. 2019, 10, 39. [Google Scholar] [CrossRef]
- Gao, D.; Li, Q.; Gao, Z.; Wang, L. Antidiabetic Effects of Corni Fructus Extract in Streptozotocin-Induced Diabetic Rats. Yonsei Med. J. 2012, 53, 691–700. [Google Scholar] [CrossRef]
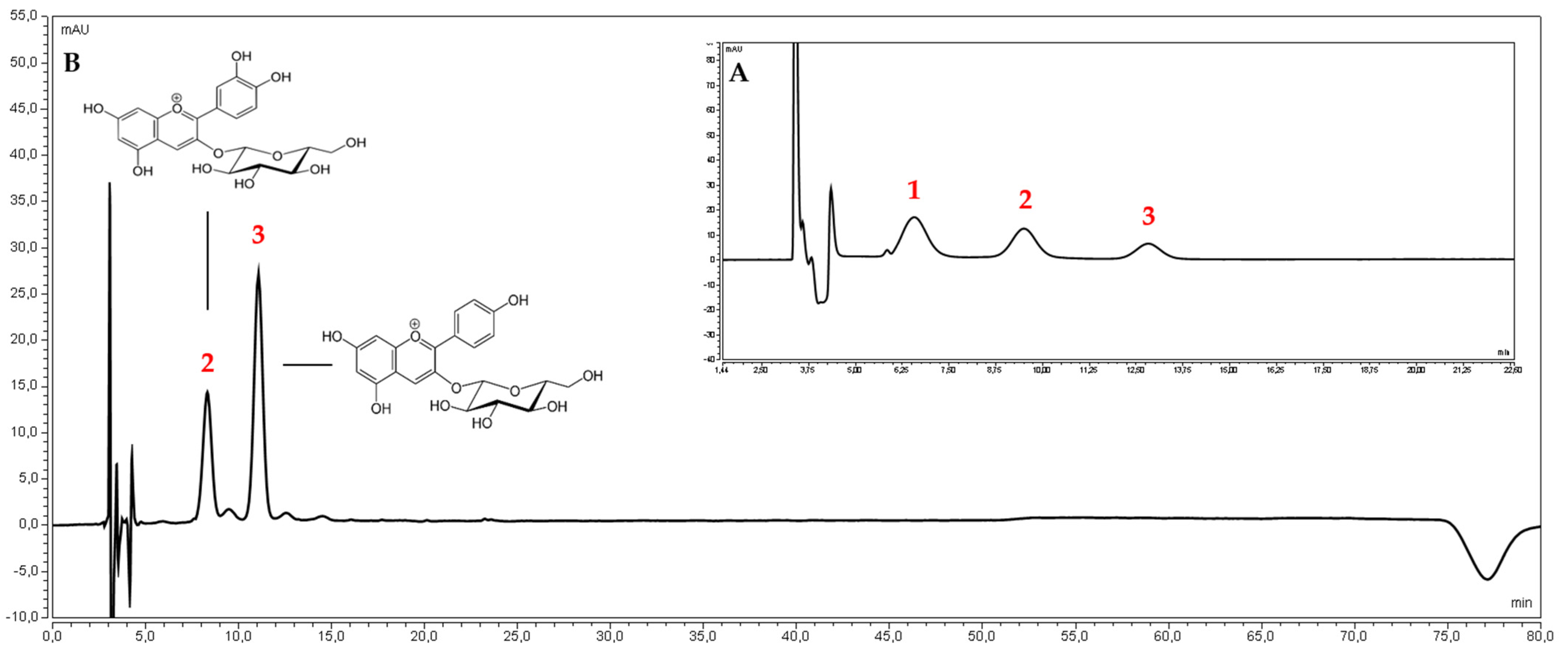
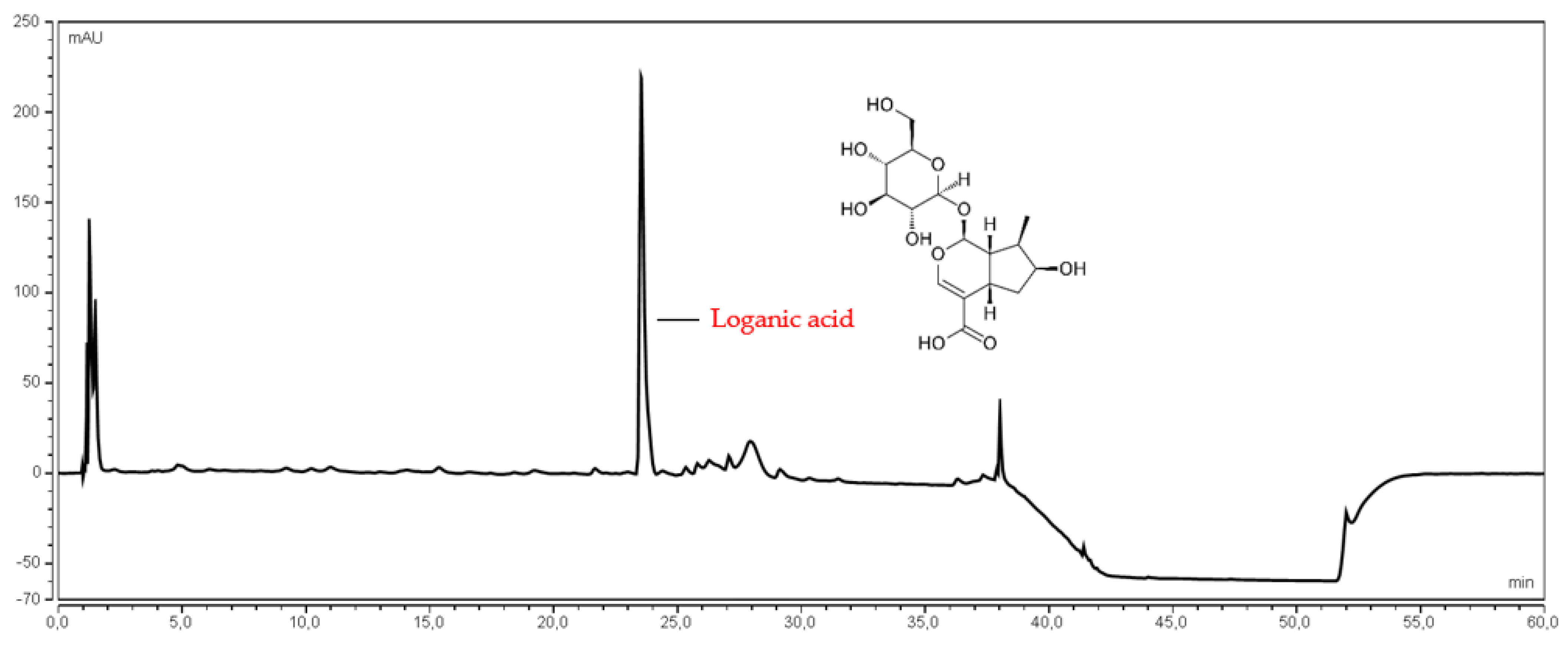
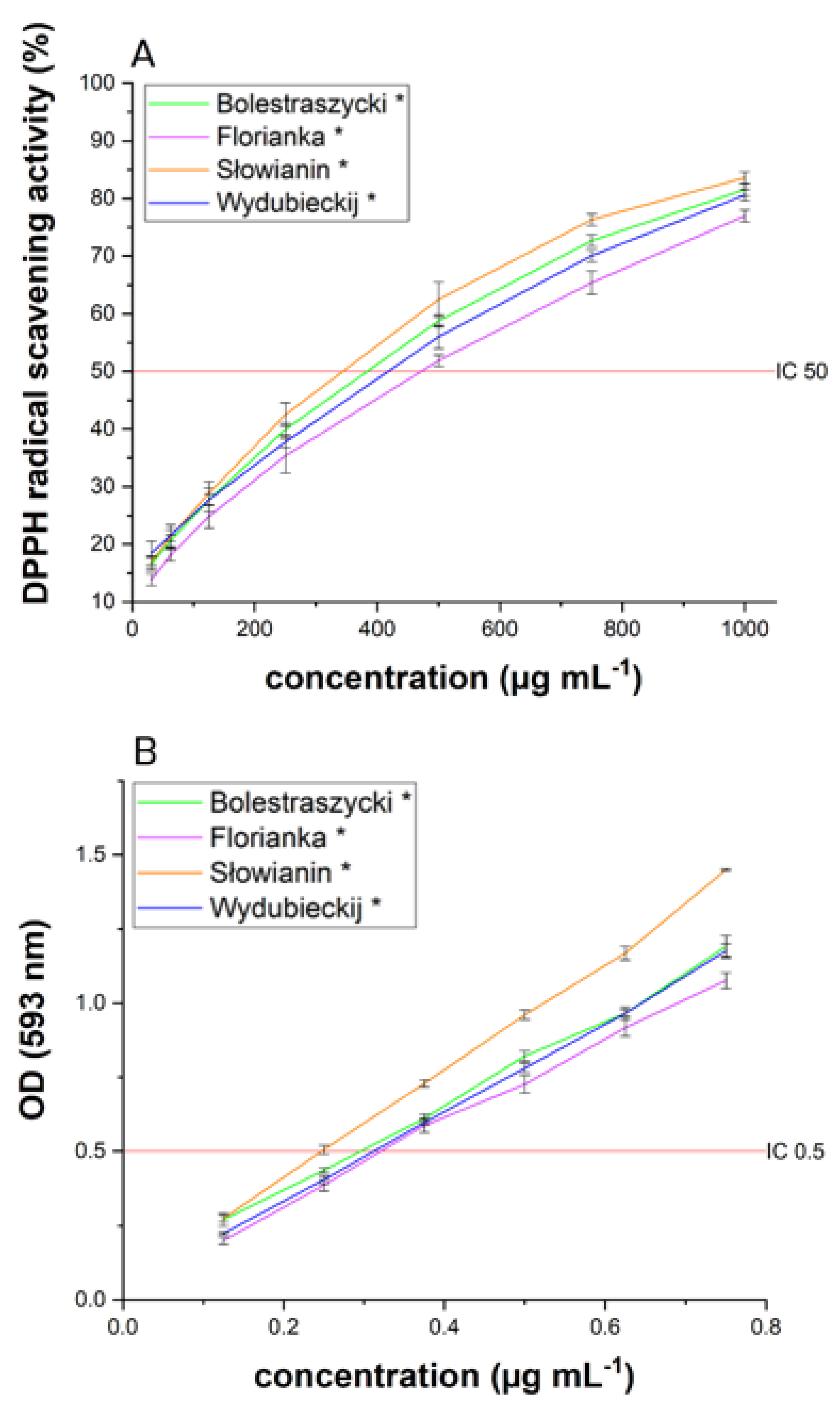
| Cultivar | Content | |||
|---|---|---|---|---|
| mg GAE/g | mg/g | |||
| TPC | Loganic Acid | Pelargonidin 3-O-Glucoside | Cyanidin-3-O-Glucoside | |
| Bolestraszycki | 8.94 ± 0.83 * | 5.38 ± 0.52 * | 2.21 ± 0.19 * | 1.08 ± 0.13 * |
| Florianka | 7.34 ± 0.66 * | 4.71 ± 0.45 * | 3.09 ± 0.25 * | 0.05 ± 0.00 * |
| Słowianin | 8.42 ± 0.79 * | 4.96 ± 0.12 * | 2.28 ± 0.11 * | 0.94 ± 0.09 * |
| Wydubieckij | 16.03 ± 1.28 * | 2.82 ± 0.27 * | 0.10 ± 0.02 * | 0.10 ± 0.00 * |
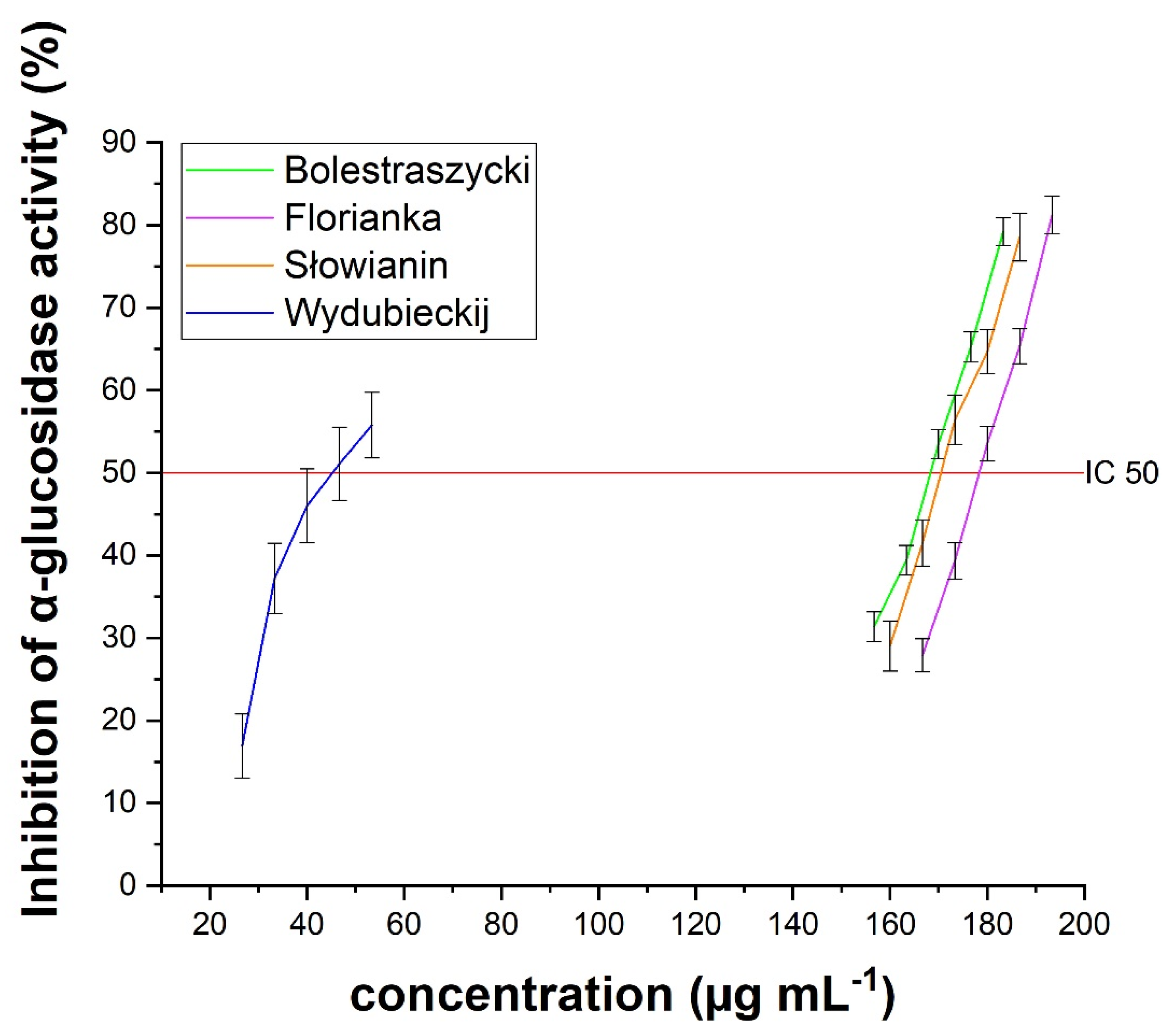
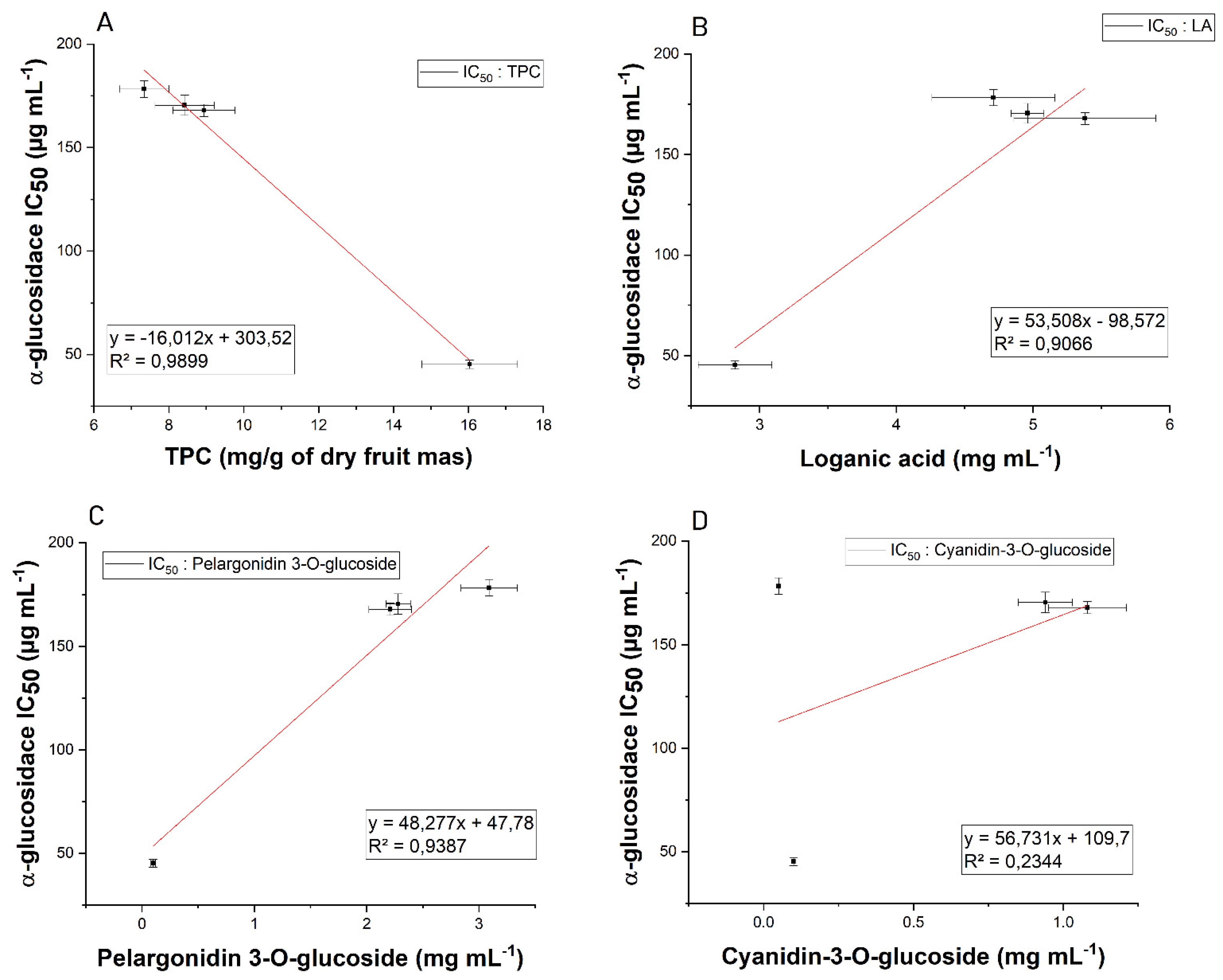
| Cultivar | IC50 (µg/mL) | IC0.5 (µg/mL) | |
|---|---|---|---|
| Inhibition of α-Glucosidase | DPPH | FRAP | |
| Bolestraszycki | 167.94 ± 2.97 * | 383.49 ± 1.54 * | 0.30 ± 0.02 * |
| Florianka | 178.26 ± 3.64 * | 471.25 ± 5.20 * | 0.32 ± 0.02 * |
| Słowianin | 170.47 ± 4.92 * | 343.63 ± 1.29 * | 0.25 ± 0.01 * |
| Wydubieckij | 45.23 ± 1.96 * | 417.56 ± 0.81 * | 0.31 ± 0.02 * |
| Content | ||||
|---|---|---|---|---|
| mg GAE/g | mg/g | |||
| TPC | Loganic Acid | Pelargonidin 3-O-Glucoside | Cyanidin-3-O-Glucoside | |
| Prebiotic system | 119.0 ± 2.34 | 5.33 ± 0.48 | 1.30 ± 0.11 | 0.32 ± 0.05 |
| Cultivar | IC50 (mg/mL) | IC0.5 (mg/mL) | |
|---|---|---|---|
| Inhibition of α-Glucosidase | DPPH | FRAP | |
| Prebiotic system | 0.173 ± 0.025 | 1.176 ± 0.066 | 0.905 ± 0.07 |
| STZ + Extract with Inulin | T0 | T7 | T14 | T21 |
| CFU/g | ||||
| Total number of microorganisms | 5.00 × 1011 | 1.90 × 1010 | 6.70 × 1011 | 7.60 × 1012 |
| Bifidobacterium | 6.70 × 106 | 2.30 × 106 | 7.70 × 107 | 2.30 × 107 |
| Lactobacillus | 5.00 × 107 | 4.50 × 107 | 5.40 × 108 | 6.40 × 108 |
| Enterococcus | 1.20 × 106 | 7.60 × 107 | 6.50 × 108 | 4.30 × 108 |
| E. coli | 1.00 × 103 | 5.40 × 105 | 3.20 × 105 | 1.30 × 106 |
| E. coli Biovare | 3.10 × 105 | 2.30 × 105 | 3.20 × 104 | 3.20 × 104 |
| Clostridium | 4.00 × 108 | 6.70 × 105 | 3.20 × 105 | 1.20 × 104 |
| Proteus | <10 | <10 | <10 | <10 |
| Pseudomonas | <10 | <10 | <10 | <10 |
| Candida | <10 | <10 | <10 | <10 |
| Healthy + Extract with Inulin | T0 | T7 | T14 | T21 |
| CFU/g | ||||
| Total number of microorganisms | 5.00 × 1011 | 5.60 × 1010 | 5.60 × 1012 | 9.40 × 1011 |
| Bifidobacterium | 6.70 × 106 | 6.50 × 107 | 5.40 × 107 | 3.40 × 108 |
| Lactobacillus | 5.00 × 107 | 1.90 × 107 | 7.60 × 107 | 2.30 × 107 |
| Enterococcus | 1.20 × 106 | 6.60 × 106 | 2.30 × 108 | 5.40 × 108 |
| E. coli | 1.00 × 103 | 5.40 × 104 | 5.40 × 104 | 3.40 × 104 |
| E. coli Biovare | 3.10 × 105 | 3.20 × 104 | 2.10 × 104 | 3.20 × 104 |
| Clostridium | 4.00 × 108 | 4.50 × 107 | 3.50 × 105 | 8.70 × 105 |
| Proteus | <10 | <10 | <10 | <10 |
| Pseudomonas | <10 | <10 | <10 | <10 |
| Candida | <10 | <10 | <10 | <10 |
| STZ + Saline | T0 | T7 | T14 | T21 |
| CFU/g | ||||
| Total number of microorganisms | 5.00 × 1011 | 4.30 × 1010 | 5.40 × 1011 | 2.30 × 1011 |
| Bifidobacterium | 6.70 × 106 | 4.50 × 106 | 4.50 × 106 | 7.60 × 106 |
| Lactobacillus | 5.00 × 107 | 5.40 × 106 | 3.30 × 107 | 6.70 × 106 |
| Enterococcus | 1.20 × 106 | 5.20 × 106 | 3.20 × 107 | 4.50 × 107 |
| E. coli | 1.00 × 103 | 2.20 × 106 | 1.20 × 107 | 8.90 × 107 |
| E. coli Biovare | 3.10 × 105 | 2.30 × 103 | 2.50 × 104 | 2.30 × 105 |
| Clostridium | 4.00 × 108 | 1.50 × 107 | 2.30 × 107 | 2.30 × 105 |
| Proteus | <10 | <10 | <10 | <10 |
| Pseudomonas | <10 | <10 | <10 | <10 |
| Candida | <10 | 2.20 × 103 | 2.30 × 104 | 2.10 × 104 |
| Healthy + Saline | T0 | T7 | T14 | T21 |
| CFU/g | ||||
| Total number of microorganisms | 5.00 × 1011 | 1.40 × 1010 | 2.30 × 1010 | 4.30 × 1010 |
| Bifidobacterium | 6.70 × 106 | 4.50 × 106 | 1.20 × 106 | 2.40 × 105 |
| Lactobacillus | 5.00 × 107 | 4.50 × 106 | 4.50 × 106 | 3.80 × 106 |
| Enterococcus | 1.20 × 106 | 2.30 × 105 | 2.30 × 105 | 4.50 × 105 |
| E. coli | 1.00 × 103 | 1.20 × 104 | 4.30 × 104 | 1.30 × 105 |
| E. coli Biovare | 3.10 × 105 | 1.80 × 104 | 4.50 × 104 | 8.40 × 104 |
| Clostridium | 4.00 × 108 | 3.40 × 108 | 4.50 × 107 | 9.80 × 108 |
| Proteus | <10 | <10 | <10 | <10 |
| Pseudomonas | <10 | <10 | <10 | <10 |
| Candida | <10 | 4.50 × 102 | 5.20 × 103 | 7.60 × 103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sip, S.; Szymanowska, D.; Chanaj-Kaczmarek, J.; Skalicka-Woźniak, K.; Budzyńska, B.; Wronikowska-Denysiuk, O.; Słowik, T.; Szulc, P.; Cielecka-Piontek, J. Potential for Prebiotic Stabilized Cornus mas L. Lyophilized Extract in the Prophylaxis of Diabetes Mellitus in Streptozotocin Diabetic Rats. Antioxidants 2022, 11, 380. https://doi.org/10.3390/antiox11020380
Sip S, Szymanowska D, Chanaj-Kaczmarek J, Skalicka-Woźniak K, Budzyńska B, Wronikowska-Denysiuk O, Słowik T, Szulc P, Cielecka-Piontek J. Potential for Prebiotic Stabilized Cornus mas L. Lyophilized Extract in the Prophylaxis of Diabetes Mellitus in Streptozotocin Diabetic Rats. Antioxidants. 2022; 11(2):380. https://doi.org/10.3390/antiox11020380
Chicago/Turabian StyleSip, Szymon, Daria Szymanowska, Justyna Chanaj-Kaczmarek, Krystyna Skalicka-Woźniak, Barbara Budzyńska, Olga Wronikowska-Denysiuk, Tymoteusz Słowik, Piotr Szulc, and Judyta Cielecka-Piontek. 2022. "Potential for Prebiotic Stabilized Cornus mas L. Lyophilized Extract in the Prophylaxis of Diabetes Mellitus in Streptozotocin Diabetic Rats" Antioxidants 11, no. 2: 380. https://doi.org/10.3390/antiox11020380
APA StyleSip, S., Szymanowska, D., Chanaj-Kaczmarek, J., Skalicka-Woźniak, K., Budzyńska, B., Wronikowska-Denysiuk, O., Słowik, T., Szulc, P., & Cielecka-Piontek, J. (2022). Potential for Prebiotic Stabilized Cornus mas L. Lyophilized Extract in the Prophylaxis of Diabetes Mellitus in Streptozotocin Diabetic Rats. Antioxidants, 11(2), 380. https://doi.org/10.3390/antiox11020380








