Prenatal Fine Particulate Matter, Maternal Micronutrient Antioxidant Intake, and Early Childhood Repeated Wheeze: Effect Modification by Race/Ethnicity and Sex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Maternal Antioxidant Intake
2.2. Prenatal PM2.5 Exposure
2.3. Repeated Wheeze
2.4. Covariates
2.5. Statistical Analysis
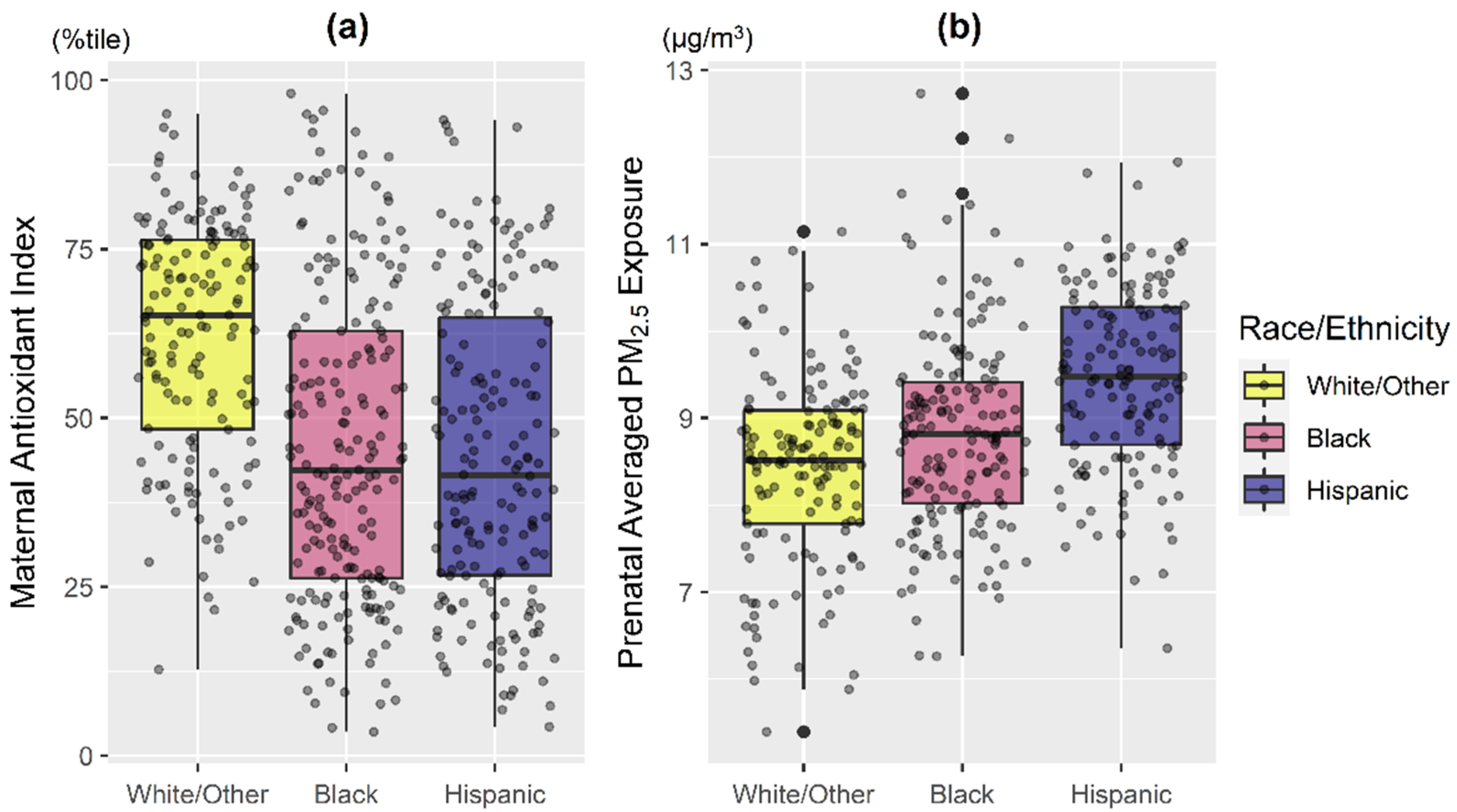
3. Results
3.1. Main Effects of Prenatal Antioxidant Intake and PM2.5 on Repeated Wheeze
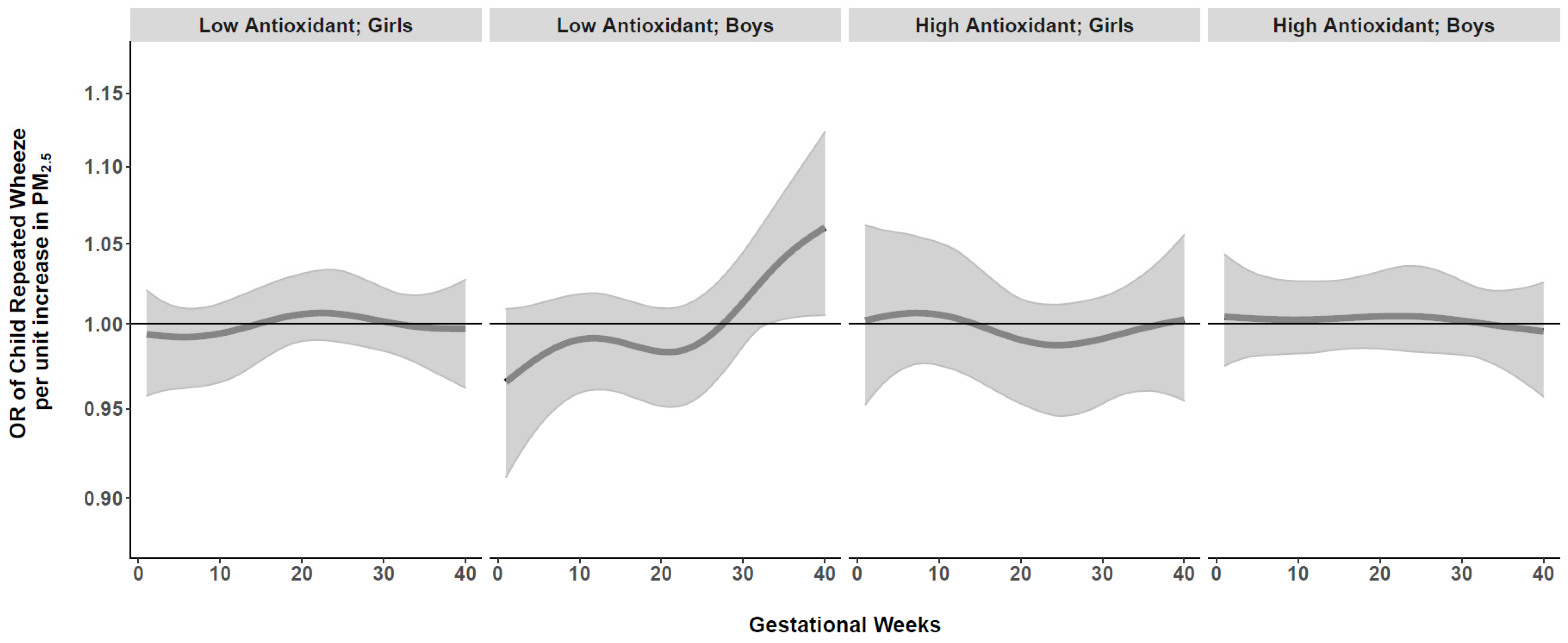
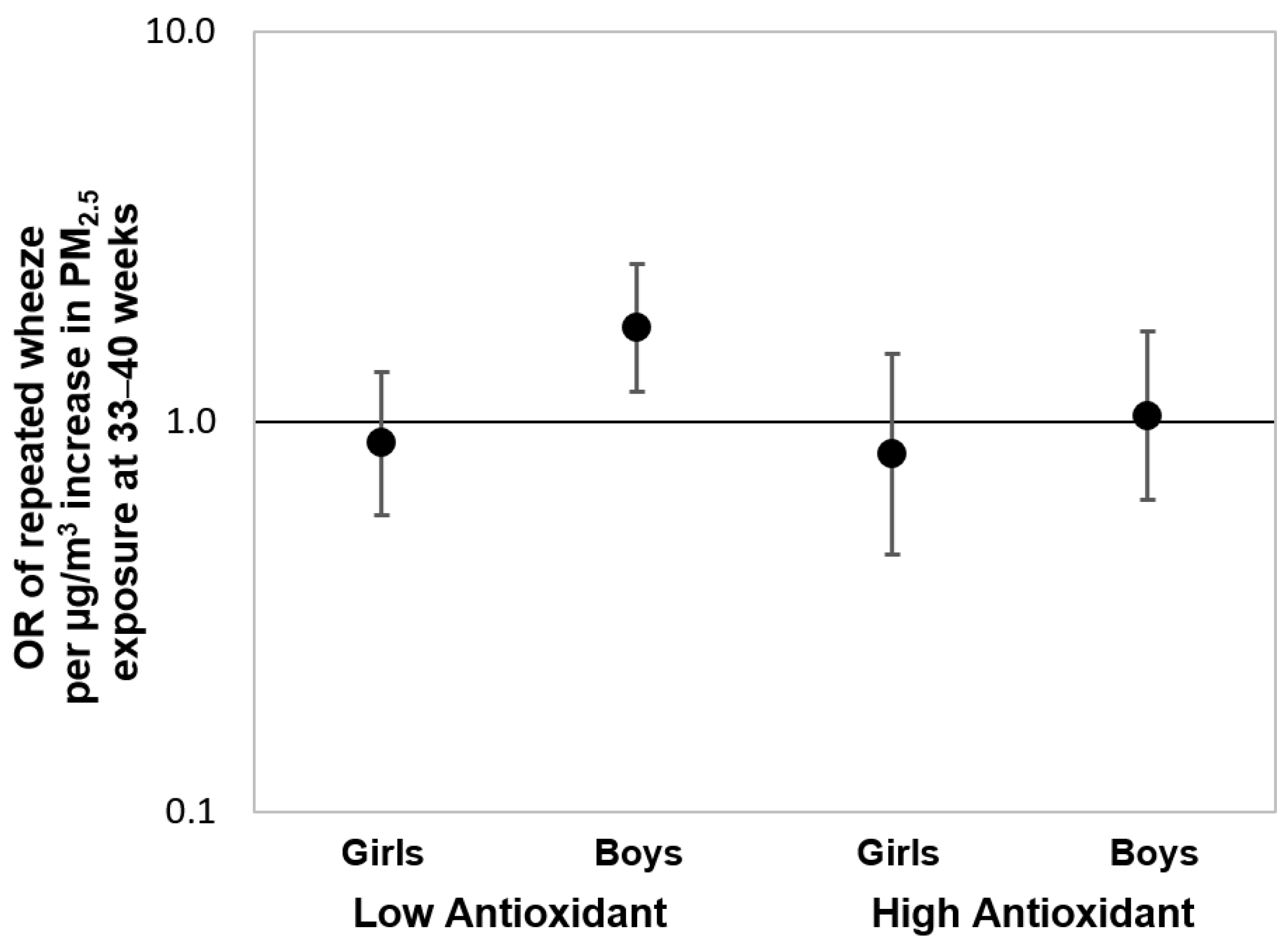
3.2. Prenatal PM2.5 and Repeated Wheeze: Effect Modification by AI, Race/Ethnicity, and Child Sex
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ducharme, F.M.; Tse, S.M.; Chauhan, B. Diagnosis, management, and prognosis of preschool wheeze. Lancet 2014, 383, 1593–1604. [Google Scholar] [CrossRef]
- Stocks, J.; Sonnappa, S. Early life influences on the development of chronic obstructive pulmonary disease. Ther. Adv. Respir. Dis. 2013, 7, 161–173. [Google Scholar] [CrossRef] [Green Version]
- Duijts, L. Fetal and infant origins of asthma. Eur. J. Epidemiol. 2012, 27, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, Y.H.; Coull, B.A.; Sternthal, M.J.; Kloog, I.; Schwartz, J.; Cohen, S.; Wright, R.J. Effects of prenatal community violence and ambient air pollution on childhood wheeze in an urban population. J. Allergy Clin. Immunol. 2014, 133, 713–722.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jedrychowski, W.; Perera, F.; Maugeri, U.; Spengler, J.D.; Mroz, E.; Rauh, V.; Flak, E.; Pac, A.; Jacek, R.; Edwards, S. Effect of prenatal exposure to fine particles and postnatal indoor air quality on the occurrence of respiratory symptoms in the first two years of life. Int. J. Environ. Health Res. 2008, 2, 314–329. [Google Scholar] [CrossRef]
- Peden, D.B. Prenatal exposure to particulate matter air pollution: A preventable risk for childhood asthma. J. Allergy Clin. Immunol. 2021, 148, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.J.; Brunst, K.J. Programming of respiratory health in childhood: Influence of outdoor air pollution. Curr. Opin. Pediatr. 2013, 25, 232–239. [Google Scholar] [CrossRef]
- Yan, W.; Wang, X.; Dong, T.; Sun, M.; Zhang, M.; Fang, K.; Chen, Y.; Chen, R.; Sun, Z.; Xia, Y. The impact of prenatal exposure to PM(2.5) on childhood asthma and wheezing: A meta-analysis of observational studies. Environ. Sci. Pollut. Res. Int. 2020, 27, 29280–29290. [Google Scholar] [CrossRef] [PubMed]
- Flom, J.D.; Chiu, Y.M.; Cowell, W.; Kannan, S.; Ganguri, H.B.; Coull, B.A.; Wright, R.J.; Carroll, K. Maternal active asthma in pregnancy influences associations between polyunsaturated fatty acid intake and child asthma. Ann. Allergy Asthma Immunol. 2021, 127, 553–561.e3. [Google Scholar] [CrossRef] [PubMed]
- Lee-Sarwar, K.; Litonjua, A.A. As You Eat It: Effects of Prenatal Nutrition on Asthma. J. Allergy Clin. Immunol. Pract. 2018, 6, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.J.; Hartman, T.J.; Adgent, M.; Gardner, K.; Gebretsadik, T.; Moore, P.E.; Davis, R.L.; LeWinn, K.Z.; Bush, N.R.; Tylavsky, F.; et al. Prenatal polyunsaturated fatty acids and child asthma: Effect modification by maternal asthma and child sex. J. Allergy Clin. Immunol. 2020, 145, 800–807.e4. [Google Scholar] [CrossRef] [PubMed]
- Hansell, A.L.; Bakolis, I.; Cowie, C.T.; Belousova, E.G.; Ng, K.; Weber-Chrysochoou, C.; Britton, W.J.; Leeder, S.R.; Tovey, E.R.; Webb, K.L.; et al. Childhood fish oil supplementation modifies associations between traffic related air pollution and allergic sensitisation. Environ. Health 2018, 17, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, N.M.; Hoffmann, A.R.; Behlen, J.C.; Lau, C.; Pendleton, D.; Harvey, N.; Shore, R.; Li, Y.; Chen, J.; Tian, Y.; et al. Air pollution and children’s health-a review of adverse effects associated with prenatal exposure from fine to ultrafine particulate matter. Environ. Health Prev. Med. 2021, 26, 72. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Wijegunawardana, D. Redox Role of ROS and Inflammation in Pulmonary Diseases. Adv. Exp. Med. Biol. 2021, 1304, 187–204. [Google Scholar]
- Péter, S.; Holguin, F.; Wood, L.G.; Clougherty, J.E.; Raederstorff, D.; Antal, M.; Weber, P.; Eggersdorfer, M. Nutritional Solutions to Reduce Risks of Negative Health Impacts of Air Pollution. Nutrients 2015, 7, 10398–10416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Chan, Y.-L.; Li, G.; Ho, K.F.; Anwer, A.G.; Smith, B.J.; Guo, H.; Jalaludin, B.; Herbert, C.; Thomas, P.S.; et al. Maternal Particulate Matter Exposure Impairs Lung Health and Is Associated with Mitochondrial Damage. Antioxidants 2021, 10, 1029. [Google Scholar] [CrossRef]
- Sordillo, J.E.; Rifas-Shiman, S.L.; Switkowski, K.; Coull, B.; Gibson, H.; Rice, M.; Platts-Mills, T.A.E.; Kloog, I.; Litonjua, A.A.; Gold, D.R.; et al. Prenatal oxidative balance and risk of asthma and allergic disease in adolescence. J. Allergy Clin. Immunol. 2019, 144, 1534–1541.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, K.; Thakur, N. Structural and Social Determinants of Health in Asthma in Developed Economies: A Scoping Review of Literature Published Between 2014 and 2019. Curr. Allergy Asthma Rep. 2020, 20, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, D.R.; Sternthal, M.; Wright, R.J. Social determinants: Taking the social context of asthma seriously. Pediatrics 2009, 123 (Suppl. S3), S174–S184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, H.H.; Chiu, Y.H.; Coull, B.A.; Kloog, I.; Schwartz, J.; Lee, A.; Wright, R.O.; Wright, R.J. Prenatal Particulate Air Pollution and Asthma Onset in Urban Children. Identifying Sensitive Windows and Sex Differences. Am. J. Respir. Crit. Care Med. 2015, 192, 1052–1059. [Google Scholar] [PubMed]
- Lee, A.G.; Leon Hsu, H.H.; Mathilda Chiu, Y.H.; Bose, S.; Rosa, M.J.; Kloog, I.; Wilson, A.; Schwartz, J.; Cohen, S.; Coull, B.A.; et al. Prenatal fine particulate exposure and early childhood asthma: Effect of maternal stress and fetal sex. J. Allergy Clin. Immunol. 2018, 141, 1880–1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Xia, W.; Jiang, Y.; Liu, W.; Zhang, B.; Xu, S.; Li, Y. Low level prenatal exposure to a mixture of Sr, Se and Mn and neurocognitive development of 2-year-old children. Sci. Total. Environ. 2020, 735, 139403. [Google Scholar] [CrossRef] [PubMed]
- Block, G.; Hartman, A.M.; Dresser, C.M.; Carroll, M.D.; Gannon, J.; Gardner, L. A data-based approach to diet questionnaire design and testing. Am. J. Epidemiol. 1986, 124, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Boucher, B.; Cotterchio, M.; Kreiger, N.; Nadalin, V.; Block, T.; Block, G. Validity and reliability of the Block98 food-frequency questionnaire in a sample of Canadian women. Public Health Nutr. 2006, 9, 84–93. [Google Scholar] [CrossRef]
- Institute of Medicine (IOM) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes: Proposed Definition and Plan for Review of Dietary Antioxidants and Related Compounds; National Academies Press: Washington, DC, USA, 1998. [Google Scholar]
- Institute of Medicine (IOM) Panel on Dietary Antioxidants Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Brunst, K.J.; Kannan, S.; Ni, Y.M.; Gennings, C.; Ganguri, H.B.; Wright, R.J. Validation of a Food Frequency Questionnaire for Estimating Micronutrient Intakes in an Urban US Sample of Multi-Ethnic Pregnant Women. Matern. Child Health J. 2016, 20, 250–260. [Google Scholar] [CrossRef] [Green Version]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65 (Suppl. S4), 1220S–1228S, discussion 9S–31S. [Google Scholar] [CrossRef]
- Cowell, W.; Colicino, E.; Tanner, E.; Amarasiriwardena, C.; Andra, S.S.; Bollati, V.; Kannan, S.; Ganguri, H.; Gennings, C.; Wright, R.O.; et al. Prenatal toxic metal mixture exposure and newborn telomere length: Modification by maternal antioxidant intake. Environ. Res. 2020, 190, 110009. [Google Scholar] [CrossRef]
- Just, A.C.; Arfer, K.B.; Rush, J.; Dorman, M.; Shtein, A.; Lyapustin, A.; Kloog, I. Advancing methodologies for applying machine learning and evaluating spatiotemporal models of fine particulate matter (PM2.5) using satellite data over large regions. Atmos. Environ. 2020, 239, 117649. [Google Scholar] [CrossRef]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef] [Green Version]
- Kloog, I.; Nordio, F.; Coull, B.A.; Schwartz, J. Predicting spatiotemporal mean air temperature using MODIS satellite surface temperature measurements across the Northeastern USA. Remote Sens. Environ. 2014, 150, 132–139. [Google Scholar] [CrossRef]
- Wilson, A.; Chiu, Y.H.M.; Hsu, H.L.; Wright, R.O.; Wright, R.J.; Coull, B.A. Potential for Bias When Estimating Critical Windows for Air Pollution in Children’s Health. Am. J. Epidemiol. 2017, 186, 1281–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, A.; Chiu, Y.H.M.; Hsu, H.L.; Wright, R.O.; Wright, R.J.; Coull, B.A. Bayesian distributed lag interaction models to identify perinatal windows of vulnerability in children’s health. Biostatistics 2017, 18, 537–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, M.J.; Just, A.C.; Kloog, I.; Pantic, I.; Schnaas, L.; Lee, A.; Bose, S.; Chiu, Y.M.; Hsu, H.L.; Coull, B.; et al. Prenatal particulate matter exposure and wheeze in Mexican children: Effect modification by prenatal psychosocial stress. Ann. Allergy Asthma Immunol. 2017, 119, 232–237.e1. [Google Scholar] [CrossRef] [PubMed]
- Jedrychowski, W.A.; Perera, F.P.; Maugeri, U.; Mrozek-Budzyn, D.; Mroz, E.; Klimaszewska-Rembiasz, M.; Flak, E.; Edwards, S.; Spengler, J.; Jacek, R.; et al. Intrauterine exposure to polycyclic aromatic hydrocarbons, fine particulate matter and early wheeze. Prospective birth cohort study in 4-year olds. Pediatr. Allergy Immunol. 2010, 21 Pt 2, e723–e732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zajac, D. Mineral Micronutrients in Asthma. Nutrients 2021, 13, 4001. [Google Scholar] [CrossRef] [PubMed]
- Whyand, T.; Hurst, J.R.; Beckles, M.; Caplin, M.E. Pollution and respiratory disease: Can diet or supplements help? A review. Respir. Res. 2018, 19, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.G.; Cowell, W.; Kannan, S.; Ganguri, H.B.; Nentin, F.; Wilson, A.; Coull, B.A.; Wright, R.O.; Baccarelli, A.; Bollati, V.; et al. Prenatal particulate air pollution and newborn telomere length: Effect modification by maternal antioxidant intakes and infant sex. Environ. Res. 2020, 187, 109707. [Google Scholar] [CrossRef]
- Guxens, M.; Aguilera, I.; Ballester, F.; Estarlich, M.; Fernandez-Somoano, A.; Lertxundi, A.; Lertxundi, N.; Mendez, M.A.; Tardon, A.; Vrijheid, M.; et al. Prenatal exposure to residential air pollution and infant mental development: Modulation by antioxidants and detoxification factors. Environ. Health Perspect. 2012, 120, 144–149. [Google Scholar] [CrossRef] [Green Version]
- Sordillo, J.E.; Switkowski, K.M.; Coull, B.A.; Schwartz, J.; Kloog, I.; Gibson, H.; Litonjua, A.A.; Bobb, J.; Koutrakis, P.; Rifas-Shiman, S.L.; et al. Relation of Prenatal Air Pollutant and Nutritional Exposures with Biomarkers of Allergic Disease in Adolescence. Sci. Rep. 2018, 8, 10578. [Google Scholar] [CrossRef] [Green Version]
- Panebianco, C.; Eddine, F.B.N.; Forlani, G.; Palmieri, G.; Tatangelo, L.; Villani, A.; Xu, L.; Accolla, R.; Pazienza, V. Probiotic Bifidobacterium lactis, anti-oxidant vitamin E/C and anti-inflammatory dha attenuate lung inflammation due to pm2.5 exposure in mice. Benef. Microbes. 2019, 10, 69–75. [Google Scholar] [CrossRef]
- Wright, R.J.; Hsu, H.L.; Chiu, Y.M.; Coull, B.A.; Simon, M.C.; Hudda, N.; Schwartz, J.; Kloog, I.; Durant, J.L. Prenatal Ambient Ultrafine Particle Exposure and Childhood Asthma in the Northeastern United States. Am. J. Respir. Crit. Care Med. 2021, 204, 788–796. [Google Scholar] [CrossRef]
- Minghetti, L.; Greco, A.; Zanardo, V.; Suppiej, A. Early-life sex-dependent vulnerability to oxidative stress: The natural twining model. J. Matern. Fetal. Neonatal. Med. 2013, 26, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Brunst, K.J.; Sanchez-Guerra, M.; Chiu, Y.-H.M.; Wilson, A.; Coull, B.A.; Kloog, I.; Schwartz, J.; Brennan, K.J.; Bosquet Enlow, M.; Wright, R.O.; et al. Prenatal particulate matter exposure and mitochondrial dysfunction at the maternal-fetal interface: Effect modification by maternal lifetime trauma and child sex. Environ. Int. 2018, 112, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Martens, D.S.; Cox, B.; Janssen, B.G.; Clemente, D.B.P.; Gasparrini, A.; Vanpoucke, C.; Lefebvre, W.; Roels, H.A.; Plusquin, M.; Nawrot, T.S. Prenatal Air Pollution and Newborns’ Predisposition to Accelerated Biological Aging. JAMA Pediatr. 2017, 171, 1160–1167. [Google Scholar] [CrossRef]
- Pomatto, L.C.D.; Carney, C.; Shen, B.; Wong, S.; Halaszynski, K.; Salomon, M.P.; Davies, K.J.A.; Tower, J. The Mitochondrial Lon Protease Is Required for Age-Specific and Sex-Specific Adaptation to Oxidative Stress. Curr. Biol. 2017, 27, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Saenen, N.D.; Martens, D.S.; Neven, K.Y.; Alfano, R.; Bové, H.; Janssen, B.G.; Roels, H.A.; Plusquin, M.; Vrijens, K.; Nawrot, T.S. Air pollution-induced placental alterations: An interplay of oxidative stress, epigenetics, and the aging phenotype? Clin. Epigenetics 2019, 11, 124. [Google Scholar] [CrossRef]
- Rosenfeld, C.S. Sex-Specific Placental Responses in Fetal Development. Endocrinology 2015, 156, 3422–3434. [Google Scholar] [CrossRef] [Green Version]
- Emeny, R.T.; Carpenter, D.O.; Lawrence, D.A. Health disparities: Intracellular consequences of social determinants of health. Toxicol. Appl. Pharmacol. 2021, 416, 115444. [Google Scholar] [CrossRef] [PubMed]
- De Martinis, M.; Sirufo, M.M.; Suppa, M.; Di Silvestre, D.; Ginaldi, L. Sex and Gender Aspects for Patient Stratification in Allergy Prevention and Treatment. Int. J. Mol. Sci. 2020, 21, 1535. [Google Scholar] [CrossRef] [Green Version]
- Laffont, S.; Guéry, J.C. Deconstructing the sex bias in allergy and autoimmunity: From sex hormones and beyond. Adv. Immunol. 2019, 142, 35–64. [Google Scholar]
- Martindale, S.; McNeill, G.; Devereux, G.; Campbell, D.; Russell, G.; Seaton, A. Antioxidant intake in pregnancy in relation to wheeze and eczema in the first two years of life. Am. J. Respir. Crit. Care Med. 2005, 171, 121–128. [Google Scholar] [CrossRef] [PubMed]
- West, C.E.; Dunstan, J.; McCarthy, S.; Metcalfe, J.; D’Vaz, N.; Meldrum, S.; Oddy, W.H.; Tulic, M.K.; Prescott, S.L. Associations between maternal antioxidant intakes in pregnancy and infant allergic outcomes. Nutrients 2012, 4, 1747–1758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litonjua, A.A.; Rifas-Shiman, S.L.; Ly, N.P.; Tantisira, K.G.; Rich-Edwards, J.W.; Camargo, C.A., Jr.; Weiss, S.T.; Gillman, M.W.; Gold, D.R. Maternal antioxidant intake in pregnancy and wheezing illnesses in children at 2 y of age. Am. J. Clin. Nutr. 2006, 84, 903–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baïz, N.; Chastang, J.; Ibanez, G.; Annesi-Maesano, I. Prenatal exposure to selenium may protect against wheezing in children by the age of 3. Immun. Inflamm Dis. 2017, 5, 37–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devereux, G.; McNeill, G.; Newman, G.; Turner, S.; Craig, L.; Martindale, S.; Helms, P.; Seaton, A. Early childhood wheezing symptoms in relation to plasma selenium in pregnant mothers and neonates. Clin. Exp. Allergy 2007, 37, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Bergen, S.; Sheppard, L.; Sampson, P.D.; Kim, S.Y.; Richards, M.; Vedal, S.; Kaufman, J.D.; Szpiro, A.A. A national prediction model for PM2.5 component exposures and measurement error-corrected health effect inference. Environ. Health Perspect. 2013, 121, 1017–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Q.Y.; Spector, D.; Colome, S.; Turpin, B. Determinants of Indoor and Personal Exposure to PM(2.5) of Indoor and Outdoor Origin during the RIOPA Study. Atmos. Environ. 2009, 43, 5750–5758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Partti-Pellinen, K.; Marttila, O.; Ahonen, A.; Suominen, O.; Haahtela, T. Penetration of nitrogen oxides and particles from outdoor into indoor air and removal of the pollutants through filtration of incoming air. Indoor Air. 2000, 10, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Sarnat, J.A.; Brown, K.W.; Schwartz, J.; Coull, B.A.; Koutrakis, P. Ambient gas concentrations and personal particulate matter exposures: Implications for studying the health effects of particles. Epidemiology 2005, 16, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Koefoed, H.J.L.; Zwitserloot, A.M.; Vonk, J.M.; Koppelman, G.H. Asthma, bronchial hyperresponsiveness, allergy and lung function development until early adulthood: A systematic literature review. Pediatr. Allergy Immunol. 2021, 32, 1238–1254. [Google Scholar] [CrossRef]
- Okyere, D.O.; Bui, D.S.; Washko, G.R.; Lodge, C.J.; Lowe, A.J.; Cassim, R.; Perret, J.L.; Abramson, M.J.; Walters, E.H.; Waidyatillake, N.T.; et al. Predictors of lung function trajectories in population-based studies: A systematic review. Respirology 2021, 26, 938–959. [Google Scholar] [CrossRef] [PubMed]
- Erkkola, M.; Karppinen, M.; Javanainen, J.; Räsänen, L.; Knip, M.; Virtanen, S.M. Validity and reproducibility of a food frequency questionnaire for pregnant Finnish women. Am. J. Epidemiol. 2001, 154, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Vioque, J.; Navarrete-Muñoz, E.M.; Gimenez-Monzó, D.; García-de-la-Hera, M.; Granado, F.; Young, I.S.; Ramón, R.; Ballester, F.; Murcia, M.; Rebagliato, M.; et al. Reproducibility and validity of a food frequency questionnaire among pregnant women in a Mediterranean area. Nutr. J. 2013, 12, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, E.K.; Gardner, J.; Field, A.E.; Rosner, B.A.; Colditz, G.A.; Suitor, C.W. Validity of a food frequency questionnaire in assessing nutrient intakes of low-income pregnant women. Matern. Child Health J. 1999, 3, 241–246. [Google Scholar] [CrossRef]
- World Health Organisation (WHO) Media Centre. Ambient (Outdoor) Air Pollution. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 7 December 2021).
- Wright, R.J. Influences of climate change on childhood asthma and allergy risk. Lancet Child Adolesc. Health 2020, 4, 859–860. [Google Scholar] [CrossRef]
- Pfeffer, P.E.; Mudway, I.S.; Grigg, J. Air Pollution and Asthma: Mechanisms of Harm and Considerations for Clinical Interventions. Chest 2021, 159, 1346–1355. [Google Scholar] [CrossRef]

| Analytic Sample Overall | Antioxidant Intake b | |||||
|---|---|---|---|---|---|---|
| (n = 530) | Low (n = 269) | High (n = 271) | ||||
| Child sex (n, %) | ||||||
| Girls | 254 | 47.9 | 133 | 50.2 | 121 | 45.7 |
| Boys | 276 | 52.1 | 132 | 49.8 | 144 | 54.3 |
| Child repeated wheeze (n, %) | ||||||
| No | 420 | 79.3 | 205 | 77.4 | 215 | 81.1 |
| Yes | 110 | 20.8 | 60 | 22.6 | 50 | 18.9 |
| Maternal age at delivery | ||||||
| Age in years (median, IQR a) | 30.2 | (25.3–34.2) | 27.6 | (23.7–32) | 32.2 | (28.6–35.5) |
| Maternal education (n, %) | ||||||
| >12 years (more than high school) | 342 | 64.5 | 143 | 54.0 | 199 | 75.1 |
| ≤12 years (high school or less) | 188 | 35.5 | 122 | 46.0 | 66 | 24.9 |
| Maternal race/ethnicity (n, %) | ||||||
| Black (Black/Hispanic Black) | 205 | 38.7 | 121 | 45.7 | 84 | 31.7 |
| Hispanic (non-Black Hispanic) | 175 | 33.0 | 105 | 39.6 | 70 | 26.4 |
| White (non-Hispanic White) | 120 | 22.6 | 26 | 9.8 | 94 | 35.5 |
| Other c | 30 | 5.7 | 13 | 4.9 | 17 | 6.4 |
| Mother asthma (n, %) | ||||||
| No | 372 | 70.2 | 183 | 69.1 | 189 | 71.3 |
| Yes | 158 | 29.8 | 82 | 30.9 | 76 | 28.7 |
| Maternal antioxidant intake | ||||||
| Antioxidant index (AI; median, IQR a) | 49.5 | (31.3–70.6) | 31.3 | (21.9–39.9) | 70.6 | (58.7–77.9) |
| Prenatal daily PM2.5 exposure | ||||||
| Prenatal average (µg/m3; median, IQR a) | 8.9 | (8.2–9.6) | 9.1 | (8.3–9.9) | 8.7 | (8–9.4) |
| Prenatal daily temperature | ||||||
| Prenatal average (°C; median, IQR a) | 12.1 | (9.6–14.5) | 12.4 | (9.9–14.7) | 11.8 | (9.3–14.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, Y.-H.M.; Carroll, K.N.; Coull, B.A.; Kannan, S.; Wilson, A.; Wright, R.J. Prenatal Fine Particulate Matter, Maternal Micronutrient Antioxidant Intake, and Early Childhood Repeated Wheeze: Effect Modification by Race/Ethnicity and Sex. Antioxidants 2022, 11, 366. https://doi.org/10.3390/antiox11020366
Chiu Y-HM, Carroll KN, Coull BA, Kannan S, Wilson A, Wright RJ. Prenatal Fine Particulate Matter, Maternal Micronutrient Antioxidant Intake, and Early Childhood Repeated Wheeze: Effect Modification by Race/Ethnicity and Sex. Antioxidants. 2022; 11(2):366. https://doi.org/10.3390/antiox11020366
Chicago/Turabian StyleChiu, Yueh-Hsiu Mathilda, Kecia N. Carroll, Brent A. Coull, Srimathi Kannan, Ander Wilson, and Rosalind J. Wright. 2022. "Prenatal Fine Particulate Matter, Maternal Micronutrient Antioxidant Intake, and Early Childhood Repeated Wheeze: Effect Modification by Race/Ethnicity and Sex" Antioxidants 11, no. 2: 366. https://doi.org/10.3390/antiox11020366
APA StyleChiu, Y.-H. M., Carroll, K. N., Coull, B. A., Kannan, S., Wilson, A., & Wright, R. J. (2022). Prenatal Fine Particulate Matter, Maternal Micronutrient Antioxidant Intake, and Early Childhood Repeated Wheeze: Effect Modification by Race/Ethnicity and Sex. Antioxidants, 11(2), 366. https://doi.org/10.3390/antiox11020366






