Mouthwash Formulation Co-Delivering Quercetin and Mint Oil in Liposomes Improved with Glycol and Ethanol and Tailored for Protecting and Tackling Oral Cavity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Vesicle Preparation
2.3. Vesicle Characterization
2.4. Stability Study
2.5. Vesicle Behaviour at Salivary pH
2.6. Cell Viability and Protection against Oxidative Stress
2.7. Determination of Antibacterial Activity
2.8. Statistical Analysis of Data
3. Results
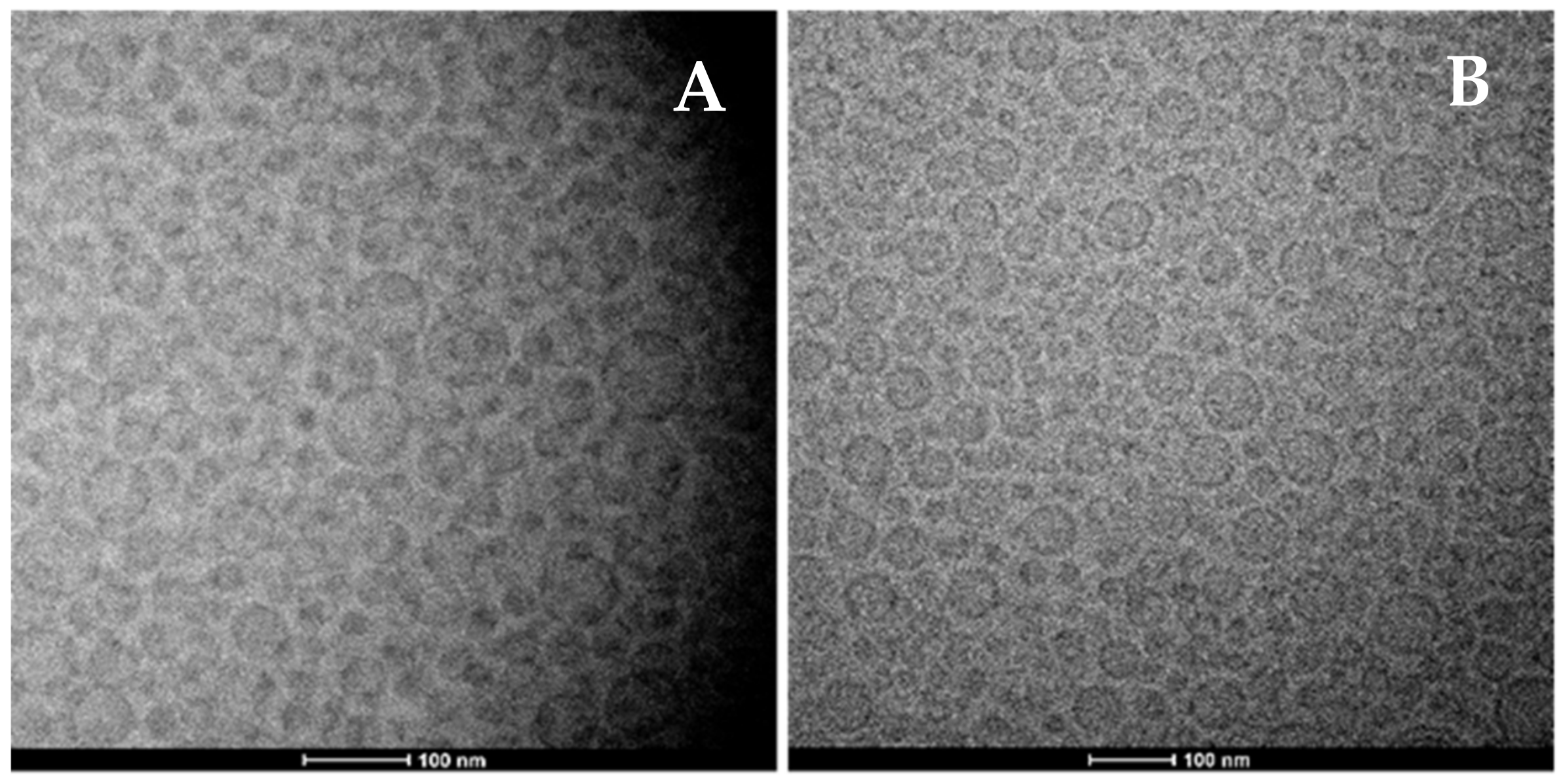
3.1. Physico-Chemical and Technological Characterization of Vesicles
3.2. Evaluation of Biological Properties of Vesicle Formulations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.F.; Flamm, G.W.; Williams, G.M.; Lines, T.C. A Critical Review of the Data Related to the Safety of Quercetin and Lack of Evidence of in Vivo Toxicity, Including Lack of Genotoxic/Carcinogenic Properties. Food Chem. Toxicol. 2007, 45, 2179–2205. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Suman, U.; Rubal; Yadav, S.K. Flavonoid Secondary Metabolite: Biosynthesis and Role in Growth and Development in Plants. In Recent Trends and Techniques in Plant Metabolic Engineering; Springer: Singapore, 2018; pp. 19–45. [Google Scholar] [CrossRef]
- Cook, N. Flavonoids—Chemistry, Metabolism, Cardioprotective Effects, and Dietary Sources. J. Nutr. Biochem. 1996, 7, 66–76. [Google Scholar] [CrossRef]
- Afonso, V.; Champy, R.; Mitrovic, D.; Collin, P.; Lomri, A. Reactive Oxygen Species and Superoxide Dismutases: Role in Joint Diseases. Jt. Bone Spine 2007, 74, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, Oxidative Stress and Autophagy in Skin Aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Shu, Y.; Liu, Y.; Li, L.; Feng, J.; Lou, B.; Zhou, X.; Wu, H. Antibacterial Activity of Quercetin on Oral Infectious Pathogens. Afr. J. Microbiol. Res. 2011, 5, 5358–5361. [Google Scholar] [CrossRef]
- Marsh, P.D. Microbial Ecology of Dental Plaque and Its Significance in Health and Disease. Adv. Dent. Res. 1994, 8, 263–271. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatahet, T.; Morille, M.; Hommoss, A.; Devoisselle, J.M.; Müller, R.H.; Bégu, S. Quercetin Topical Application, from Conventional Dosage Forms to Nanodosage Forms. Eur. J. Pharm. Biopharm. 2016, 108, 41–53. [Google Scholar] [CrossRef]
- Allaw, M.; Manca, M.L.; Castangia, I.; Manconi, M. From Plants to Phospholipid Vesicles: A Comprehensive Review on the Incorporation of Phytochemicals into Phospholipid Vesicles Designed for Skin Applications with Special Focus on Scalability and in Vitro and in Vivo Efficacy. J. Drug Deliv. Sci. Technol. 2022, 67, 103049. [Google Scholar] [CrossRef]
- Paolino, D.; Mancuso, A.; Cristiano, M.C.; Froiio, F.; Lammari, N.; Celia, C.; Fresta, M. Nanonutraceuticals: The New Frontier of Supplementary Food. Nanomaterials 2021, 11, 792. [Google Scholar] [CrossRef]
- Manca, M.L.; Castangia, I.; Caddeo, C.; Pando, D.; Escribano, E.; Valenti, D.; Lampis, S.; Zaru, M.; Fadda, A.M.; Manconi, M. Improvement of Quercetin Protective Effect against Oxidative Stress Skin Damages by Incorporation in Nanovesicles. Colloids Surfaces. B Biointerfaces 2014, 123, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Hu, H.; Lin, Z.; Chen, D.; Zhu, Y.; Hou, S.; Shi, X. Quercetin Deformable Liposome: Preparation and Efficacy against Ultraviolet B Induced Skin Damages In Vitro and In Vivo. J. Photochem. Photobiol. B Biol. 2013, 127, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tao, B.; Wan, Y.; Sun, Y.; Wang, L.; Sun, J.; Li, C. Drug Delivery Based Pharmacological Enhancement and Current Insights of Quercetin with Therapeutic Potential against Oral Diseases. Biomed. Pharmacother. 2020, 128, 110372. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Usach, I.; Peris, J.E.; Ibba, A.; Orrù, G.; Valenti, D.; Escribano-Ferrer, E.; Gomez-Fernandez, J.C.; Aranda, F.J.; Fadda, A.M.; et al. Optimization of Innovative Three-Dimensionally-Structured Hybrid Vesicles to Improve the Cutaneous Delivery of Clotrimazole for the Treatment of Topical Candidiasis. Pharmaceutics 2019, 11, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manca, M.L.; Castangia, I.; Matricardi, P.; Lampis, S.; Fernàndez-Busquets, X.; Fadda, A.M.; Manconi, M. Molecular Arrangements and Interconnected Bilayer Formation Induced by Alcohol or Polyalcohol in Phospholipid Vesicles. Colloids Surf. B Biointerfaces 2014, 117, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Peh, K.; Sci, C.W.-J.P.P. Undefined Polymeric Films as Vehicle for Buccal Delivery: Swelling, Mechanical, and Bioadhesive Properties. Citeseer 1999, 2, 53–61. [Google Scholar]
- Catalán-Latorre, A.; Pleguezuelos-Villa, M.; Castangia, I.; Manca, M.L.; Caddeo, C.; Nácher, A.; Díez-Sales, O.; Peris, J.E.; Pons, R.; Escribano-Ferrer, E.; et al. Nutriosomes: Prebiotic Delivery Systems Combining Phospholipids, a Soluble Dextrin and Curcumin to Counteract Intestinal Oxidative Stress and Inflammation. Nanoscale 2018, 10, 1957–1969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manca, M.L.; Valenti, D.; Sales, O.D.; Nacher, A.; Fadda, A.M.; Manconi, M. Fabrication of Polyelectrolyte Multilayered Vesicles as Inhalable Dry Powder for Lung Administration of Rifampicin. Int. J. Pharm. 2014, 472, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Cassano, R.; Valenti, D.; Trombino, S.; Ferrarelli, T.; Picci, N.; Fadda, A.M.; Manconi, M. Isoniazid-Gelatin Conjugate Microparticles Containing Rifampicin for the Treatment of Tuberculosis. J. Pharm. Pharmacol. 2013, 65, 1302–1311. [Google Scholar] [CrossRef]
- Manca, M.L.; Cencetti, C.; Matricardi, P.; Castangia, I.; Zaru, M.; Sales, O.D.; Nacher, A.; Valenti, D.; Maccioni, A.M.; Fadda, A.M.; et al. Glycerosomes: Use of Hydrogenated Soy Phosphatidylcholine Mixture and Its Effect on Vesicle Features and Diclofenac Skin Penetration. Int. J. Pharm. 2016, 511, 198–204. [Google Scholar] [CrossRef]
- Manconi, M.; Petretto, G.; D’hallewin, G.; Escribano, E.; Milia, E.; Pinna, R.; Palmieri, A.; Firoznezhad, M.; Peris, J.E.; Usach, I.; et al. Thymus Essential Oil Extraction, Characterization and Incorporation in Phospholipid Vesicles for the Antioxidant/Antibacterial Treatment of Oral Cavity Diseases. Colloids Surf. B Biointerfaces 2018, 171, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Castangia, I.; Nácher, A.; Caddeo, C.; Valenti, D.; Fadda, A.M.; Díez-Sales, O.; Ruiz-Saurí, A.; Manconi, M. Fabrication of Quercetin and Curcumin Bionanovesicles for the Prevention and Rapid Regeneration of Full-Thickness Skin Defects on Mice. Acta Biomater. 2014, 10, 1292–1300. [Google Scholar] [CrossRef]
- Manconi, M.; Aparicio, J.; Vila, A.O.; Pendás, J.; Figueruelo, J.; Molina, F. Viscoelastic Properties of Concentrated Dispersions in Water of Soy Lecithin. Colloids Surf. A Physicochem. Eng. Asp. 2003, 222, 141–145. [Google Scholar] [CrossRef]
- Keivani Nahr, F.; Ghanbarzadeh, B.; Hamishehkar, H.; Kafil, H.S.; Hoseini, M.; Moghadam, B.E. Investigation of Physicochemical Properties of Essential Oil Loaded Nanoliposome for Enrichment Purposes. LWT 2019, 105, 282–289. [Google Scholar] [CrossRef]
- Sebaaly, C.; Jraij, A.; Fessi, H.; Charcosset, C.; Greige-Gerges, H. Preparation and Characterization of Clove Essential Oil-Loaded Liposomes. Food Chem. 2015, 178, 52–62. [Google Scholar] [CrossRef]
- Kohlert, C.; van Rensen, I.; Marz, R.; Schindler, G.; Graefe, E.U.; Veit, M. Bioavailability and Pharmacokinetics of Natural Volatile Terpenes in Animals and Humans. Planta Med. 2000, 66, 495–505. [Google Scholar] [CrossRef] [Green Version]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W.; Nahar, L.; Basar, N.; Sarker, S.D. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hearnden, V.; Sankar, V.; Hull, K.; Juras, D.V.; Greenberg, M.; Kerr, A.R.; Lockhart, P.B.; Patton, L.L.; Porter, S.; Thornhill, M.H. New Developments and Opportunities in Oral Mucosal Drug Delivery for Local and Systemic Disease. Adv. Drug Deliv. Rev. 2012, 64, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Detoni, C.B.; Cabral-Albuquerque, E.C.M.; Hohlemweger, S.V.A.; Sampaio, C.; Barros, T.F.; Velozo, E.S. Essential Oil from Zanthoxylum Tingoassuiba Loaded into Multilamellar Liposomes Useful as Antimicrobial Agents. J. Microencapsul. 2009, 26, 684–691. [Google Scholar] [CrossRef]
- Molinaro, R.; Gagliardi, A.; Mancuso, A.; Cosco, D.; Soliman, M.E.; Casettari, L.; Paolino, D. Development and In Vivo Evaluation of Multidrug Ultradeformable Vesicles for the Treatment of Skin Inflammation. Pharmaceutics 2019, 11, 644. [Google Scholar] [CrossRef] [Green Version]
- Proctor, G.B. The Physiology of Salivary Secretion. Periodontology 2016, 70, 11–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manca, M.L.; Manconi, M.; Falchi, A.M.; Castangia, I.; Valenti, D.; Lampis, S.; Fadda, A.M. Close-Packed Vesicles for Diclofenac Skin Delivery and Fibroblast Targeting. Colloids Surfaces. B Biointerfaces 2013, 111, 609–617. [Google Scholar] [CrossRef]
- Straubinger, R.M.; Hong, K.; Friend, D.S.; Papahadjopoulos, D. Endocytosis of Liposomes and Intracellular Fate of Encapsulated Molecules: Encounter with a Low PH Compartment after Internalization in Coated Vesicles. Cell 1983, 32, 1069–1079. [Google Scholar] [CrossRef]
- Padmini, E.; Prema, K.; Vijaya Geetha, B.; Rani, M.U. Comparative Study on Composition and Antioxidant Properties of Mint and Black Tea Extract. Int. J. Food Sci. Technol. 2008, 43, 1887–1895. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health Effects of Quercetin: From Antioxidant to Nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Tsourounakis, I.; Palaiologou-Gallis, A.A.; Stoute, D.; Maney, P.; Lallier, T.E. Erratum: Effect of Essential Oil and Chlorhexidine Mouthwashes on Gingival Fibroblast Survival and Migration. J. Periodontol. 2014, 85, 876. [Google Scholar] [CrossRef] [PubMed]
- Durham, J.; Fraser, H.M.; McCracken, G.I.; Stone, K.M.; John, M.T.; Preshaw, P.M. Impact of Periodontitis on Oral Health-Related Quality of Life. J. Dent. 2013, 41, 370–376. [Google Scholar] [CrossRef]
- Rossiter, S.E.; Fletcher, M.H.; Wuest, W.M. Natural Products as Platforms To Overcome Antibiotic Resistance. Chem. Rev. 2017, 117, 12415–12474. [Google Scholar] [CrossRef]




| P90 (mg/mL) | Quercetin (mg/mL) | Tween80 (mg/mL) | Mint oil (mg/mL) | |
|---|---|---|---|---|
| 25MO-vesicles | 240 | 15 | 13 | 25 |
| 50MO-vesicles | 240 | 15 | 13 | 50 |
| 100MO-vesicles | 240 | 15 | 13 | 100 |
| 200MO-vesicles | 240 | 15 | 13 | 200 |
| MD (nm) | PI | ZP (mV) | EE (%) | |
|---|---|---|---|---|
| 25MO-vesicles | * 122 ± 9 | 0.28 | −16 ± 3 | 99 ± 2 |
| 50MO-vesicles | * 119 ± 13 | 0.29 | −17 ± 3 | 100 ± 2 |
| 100MO-vesicles | * 115 ± 8 | 0.29 | −15 ± 4 | 99 ± 1 |
| 200MO-vesicles | ° 145 ± 14 | 0.29 | −25 ± 3 | 100 ± 1 |
| MD (nm) | PI | ZP (mV) | |
|---|---|---|---|
| 25MO-vesicles | 579 ± 14 | 0.49 | −1± 2 |
| 50MO-vesicles | 590 ± 28 | 0.48 | −1 ± 3 |
| 100MO-vesicles | 411 ± 46 | 0.42 | −1 ± 1 |
| 200MO-vesicles | 289 ± 4 | 0.37 | −1 ± 2 |
| Sample | S. mutans IH (mm) | L. acidophilus IH (mm) |
|---|---|---|
| 200MO-dispersion | 10 ± 4 | 8 ± 3 |
| 200MO-vesicles | 12 ± 3 | 9 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castangia, I.; Manconi, M.; Allaw, M.; Perra, M.; Orrù, G.; Fais, S.; Scano, A.; Escribano-Ferrer, E.; Ghavam, M.; Rezvani, M.; et al. Mouthwash Formulation Co-Delivering Quercetin and Mint Oil in Liposomes Improved with Glycol and Ethanol and Tailored for Protecting and Tackling Oral Cavity. Antioxidants 2022, 11, 367. https://doi.org/10.3390/antiox11020367
Castangia I, Manconi M, Allaw M, Perra M, Orrù G, Fais S, Scano A, Escribano-Ferrer E, Ghavam M, Rezvani M, et al. Mouthwash Formulation Co-Delivering Quercetin and Mint Oil in Liposomes Improved with Glycol and Ethanol and Tailored for Protecting and Tackling Oral Cavity. Antioxidants. 2022; 11(2):367. https://doi.org/10.3390/antiox11020367
Chicago/Turabian StyleCastangia, Ines, Maria Manconi, Mohamad Allaw, Matteo Perra, Germano Orrù, Sara Fais, Alessandra Scano, Elvira Escribano-Ferrer, Mansureh Ghavam, Maryam Rezvani, and et al. 2022. "Mouthwash Formulation Co-Delivering Quercetin and Mint Oil in Liposomes Improved with Glycol and Ethanol and Tailored for Protecting and Tackling Oral Cavity" Antioxidants 11, no. 2: 367. https://doi.org/10.3390/antiox11020367
APA StyleCastangia, I., Manconi, M., Allaw, M., Perra, M., Orrù, G., Fais, S., Scano, A., Escribano-Ferrer, E., Ghavam, M., Rezvani, M., & Manca, M. L. (2022). Mouthwash Formulation Co-Delivering Quercetin and Mint Oil in Liposomes Improved with Glycol and Ethanol and Tailored for Protecting and Tackling Oral Cavity. Antioxidants, 11(2), 367. https://doi.org/10.3390/antiox11020367











