Oxidative Balance Scores (OBSs) Integrating Nutrient, Food and Lifestyle Dimensions: Development of the NutrientL-OBS and FoodL-OBS
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection: Dietary and Lifestyle Factors Assessment
2.3. Oxidative Balance Score (OBS) Components and Their Integration into Nutrient, Lifestyle and Food-Based OBSs
- -
- for PA, there were 3 categories established: sedentary/inactive = 1 point; moderately active = 3 points; active = 5 points;
- -
- for alcohol, 1 to 5 points were assigned according to sex-specific levels of its consumption (men who consumed: ≤10 g/day = 5 points, ≤20 g/day = 4 points, ≤50 g/d = 3 points, ≤75 g/d = 2 points, and >75 g/d = 1 point, and women who consumed: ≤5 g/d = 5 points, ≤15 g/d = 4 points, ≤25 g/d = 3 points, ≤50 g/d = 2 points, and >50 g/d = 1 point;
- -
- for overweight and obesity the assessment was made by classifying the subjects into 5 categories according to criteria established by the WHO and the Spanish Society for the Study of Obesity (SEEDO) [32,35]: <25 kg/m2 = 5 points), ≤ 26.9 kg/m2 = 4 points, ≤29.9 kg/m2 = 3 points, <35 kg/m2 = 2 points, and ≥ 35 kg/m2 = 1 point. Similarly, for abdominal obesity, following ATP III [33], the following points were assigned: men up to 102 cm of WC = 5 points, and 1 point otherwise, and women up to 88 cm = 5 points, while 1 point was given in the opposite case.
- -
- for smoking, participants were classified as non-smokers = 5 points, former smokers = 3 points, or current smokers = 1 point. for excessive energy intake, when the reported intake was close to estimated energy expenditure = 5 points, if the intake did not exceed 10% of the requirement = 4 points, if it exceeded up to 20% = 3 points, for 30% of excessive intake= 2 points, and if it was above this amount = 1 point. The estimated energy expenditure was calculated beforehand as the amount of energy needed to maintain essential body functions plus the amount required to support the daily PA [36].
2.4. Validation Study of the Oxidative Balance Scores: Relationship with Scores of Adherence to the Mediterranean Diet and with Biomarkers
2.5. Statistical Analyses
3. Results
3.1. Characteristics of the Study Sample by the NutrientL-OBSs and FoodL-OBS
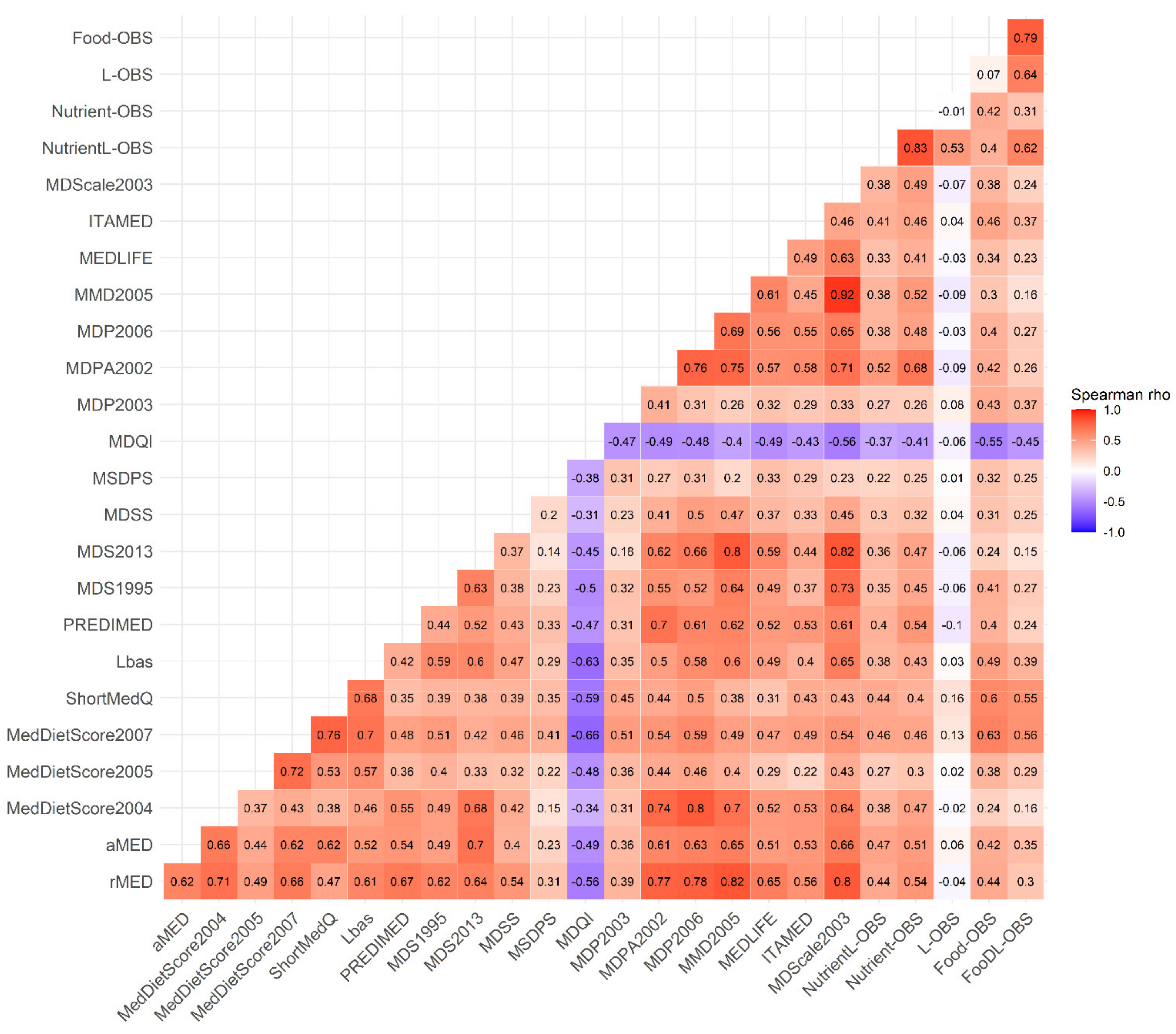
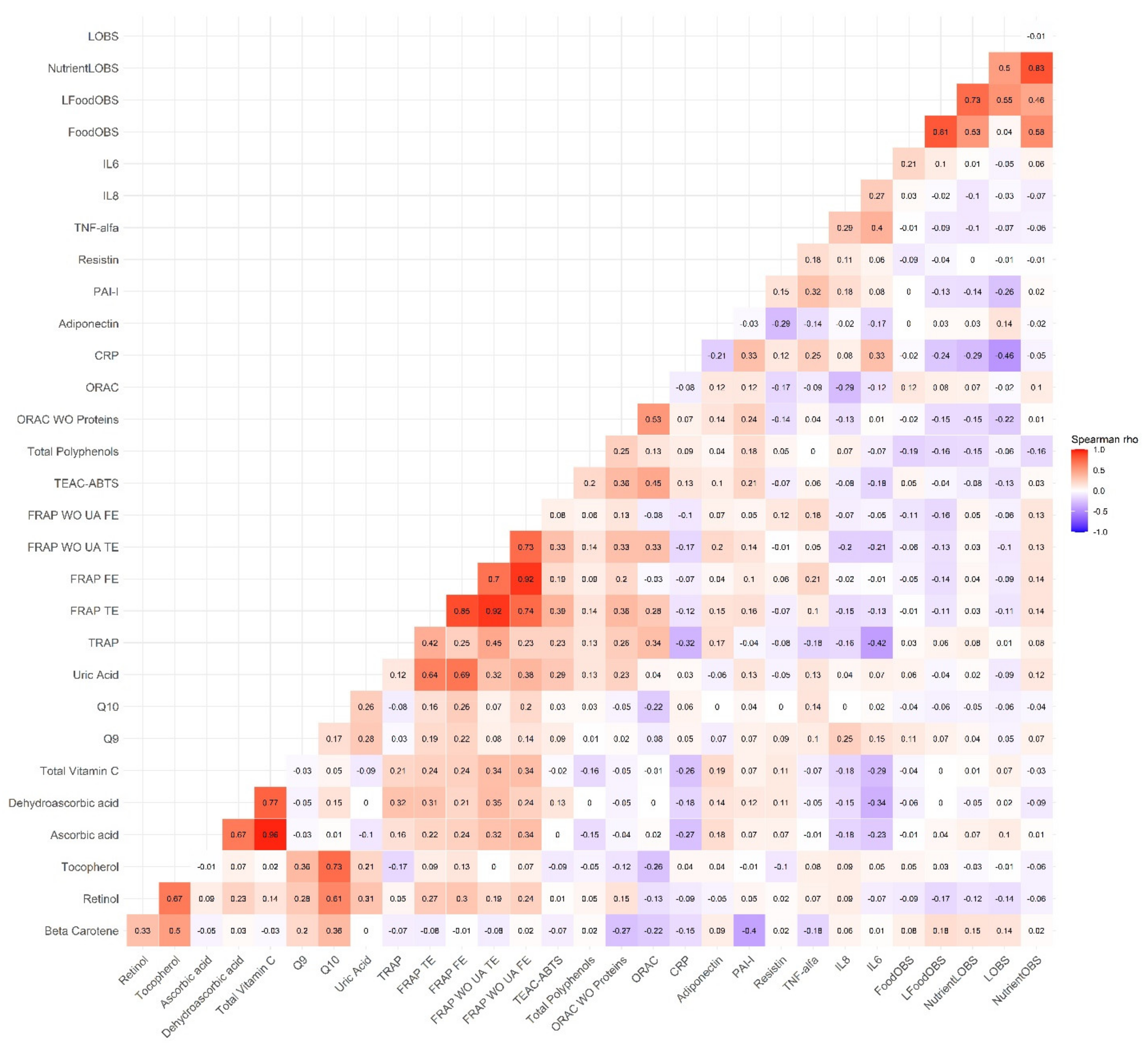
3.2. Correlations between the OBSs, the MD Adherence Scores and the Biomarkers
3.3. Linear Relationships between the OBSs, the MD Adherence Scores and the Biomarkers
3.4. Stratified and Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, D.P. Radical-free biology of oxidative stress. AJP Cell Physiol. 2008, 295, C849–C868. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M. The evolution of free radicals and oxidative stress. Am. J. Med. 2000, 108, 652–659. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Simone Reuter, B.B.A. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic Biol Med 2011, 49, 1603–1616. [Google Scholar] [CrossRef]
- Peng, C.; Wang, X.; Chen, J.; Jiao, R.; Wang, L.; Li, Y.M.; Zuo, Y.; Liu, Y.; Lei, L.; Ma, K.Y.; et al. Biology of ageing and role of dietary antioxidants. Biomed Res. Int. 2014, 2014, 831841. [Google Scholar] [CrossRef] [PubMed]
- Verdile, G.; Keane, K.N.; Cruzat, V.F.; Medic, S.; Sabale, M.; Rowles, J.; Wijesekara, N.; Martins, R.N.; Fraser, P.E.; Newsholme, P. Inflammation and Oxidative Stress: The Molecular Connectivity between Insulin Resistance, Obesity, and Alzheimer’s Disease. Mediators Inflamm. 2015, 105828, 1–17. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Phillips, D.H. Smoking-related DNA and protein adducts in human tissues. Carcinogenesis 2002, 23, 1979–2004. [Google Scholar] [CrossRef]
- Puntarulo, S. Iron, oxidative stress and human health. Mol. Aspects Med. 2005, 26, 299–312. [Google Scholar] [CrossRef]
- Boden, G.; Homko, C.; Barrero, C.A.; Stein, T.P.; Chen, X.; Cheung, P.; Fecchio, C.; Koller, S.; Merali, S. Excessive caloric intake acutely causes oxidative stress, GLUT4 carbonylation, and insulin resistance in healthy men. Sci. Transl. Med. 2015, 7, 304re7. [Google Scholar] [CrossRef]
- Kryston, T.B.; Georgiev, A.B.; Pissis, P.; Georgakilas, A.G. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2011, 711, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, C.V.; Sales, M.M.; Rosa, T.S.; Lewis, J.E.; de Andrade, R.V.; Simoes, H.G. The Antioxidant Effect of Exercise: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Zappe, K.; Pointner, A.; Switzeny, O.J.; Magnet, U.; Tomeva, E.; Heller, J.; Mare, G.; Wagner, K.H.; Knasmueller, S.; Haslberger, A.G. Counteraction of Oxidative Stress by Vitamin E Affects Epigenetic Regulation by Increasing Global Methylation and Gene Expression of MLH1 and DNMT1 Dose Dependently in Caco-2 Cells. Oxid. Med. Cell. Longev. 2018, 2018, 3734250. [Google Scholar] [CrossRef] [PubMed]
- Heber, D.; Lu, Q.-Y. Overview of mechanisms of action of lycopene. Exp. Biol. Med. 2002, 227, 920–923. [Google Scholar] [CrossRef]
- Wu, D.; Zhai, Q.; Shi, X. Alcohol-induced oxidative stress and cell responses. J. Gastroenterol. Hepatol. 2006, 21 (Suppl. 3), S26–S29. [Google Scholar] [CrossRef]
- Bjørklund, G.; Chirumbolo, S. Role of oxidative stress and antioxidants in daily nutrition and human health. Nutrition 2017, 33, 311–321. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Salvatore, S.; Del Rio, D.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of spices, dried fruits, nuts, pulses, cereals and sweets consumed in Italy assessed by three different in vitro assays. Mol. Nutr. Food Res. 2006, 50, 1030–1038. [Google Scholar] [CrossRef]
- Serafini, M.; Del Rio, D. Understanding the association between dietary antioxidants, redox status and disease: Is the total antioxidant capacity the right tool? Redox Rep. 2004, 9, 145–152. [Google Scholar] [CrossRef]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef]
- Carrión-García, C.J.; Guerra-Hernández, E.J.; García-Villanova, B.; Serafini, M.; Sánchez, M.J.; Amiano, P.; Molina-Montes, E. Plasma non-enzymatic antioxidant capacity (NEAC) in relation to dietary NEAC, nutrient antioxidants and inflammation-related biomarkers. Antioxidants 2020, 9, 301. [Google Scholar] [CrossRef]
- Hernández-Ruiz, Á.; García-Villanova, B.; Guerra-Hernández, E.; Amiano, P.; Ruiz-Canela, M.; Molina-Montes, E. A Review of A Priori Defined Oxidative Balance Scores Relative to Their Components and Impact on Health Outcomes. Nutrients 2019, 11, 774. [Google Scholar] [CrossRef]
- Van Hoydonck, P.G.A.; Temme, E.H.M.; Schouten, E.G. A dietary oxidative balance score of vitamin C, beta-carotene and iron intakes and mortality risk in male smoking Belgians. J. Nutr. 2002, 132, 756–761. [Google Scholar] [CrossRef]
- Goodman, M.; Bostick, R.M.; Dash, C.; Flanders, W.D.; Mandel, J.S. Hypothesis: Oxidative Stress Score as a Combined Measure of Pro-oxidant and Antioxidant Exposures. Ann. Epidemiol. 2007, 17, 394–399. [Google Scholar] [CrossRef]
- González, C.A.; Navarro, C.; Martínez, C.; Quirós, J.R.; Dorronsoro, M.; Barricarte, A.; Tormo, M.J.; Agudo, A.; Chirlaque, M.D.; Amiano, P.; et al. El estudio prospectivo Europeo sobre cáncer y nutrición (EPIC). Rev. Esp. Salud Publica 2004, 78, 167–176. [Google Scholar] [CrossRef]
- Riboli, E.; Hunt, K.; Slimani, N.; Ferrari, P.; Norat, T.; Fahey, M.; Charrondière, U.; Hémon, B.; Casagrande, C.; Vignat, J.; et al. European Prospective Investigation into Cancer and Nutrition (EPIC): Study populations and data collection. Public Health Nutr. 2003, 5, 1113–1124. [Google Scholar] [CrossRef]
- van Liere, J.J.; Lucas, F.; Clavel, F.; Slimani, N.; Villeminot, S. Relative validity and reproducibility of a French dietary history questionnaire. Int. J. Epidemiol. 1997, 26 (Suppl. 1), S110–S117. [Google Scholar] [CrossRef]
- Slimani, N.; Deharveng, G.; Unwin, I.; Southgate, D.A.T.; Vignat, J.; Skeie, G.; Salvini, S.; Parpinel, M.; Møller, A.; Ireland, J.; et al. The EPIC nutrient database project (ENDB): A first attempt to standardize nutrient databases across the 10 European countries participating in the EPIC study. Eur. J. Clin. Nutr. 2007, 61, 1037–1056. [Google Scholar] [CrossRef]
- Hernández-Ruiz, A.; García-Villanova, B.; Guerra-Hernández, E.; Amiano, P.; Sánchez, M.-J.; Dorronsoro, M.; Molina-Montes, E. Comparison of the Dietary Antioxidant Profiles of 21 a priori Defined Mediterranean Diet Indexes. J. Acad. Nutr. Diet. 2018, 118, 2254–2268. [Google Scholar] [CrossRef]
- Pounis, G.; Di Castelnuovo, A.; Bonaccio, M.; Costanzo, S.; Persichillo, M.; Krogh, V.; Donati, M.B.; de Gaetano, G.; Iacoviello, L. Flavonoid and lignan intake in a Mediterranean population: Proposal for a holistic approach in polyphenol dietary analysis, the Moli-sani Study. Eur. J. Clin. Nutr. 2016, 70, 338–345. [Google Scholar] [CrossRef]
- Haftenberger, M.; Lahmann, P.H.; Panico, S.; Gonzalez, C.A.; Seidell, J.C.; Boeing, H.; Giurdanella, M.C.; Krogh, V.; Bueno-de-Mesquita, H.B.; Peeters, P.H.M.; et al. Overweight, obesity and fat distribution in 50- to 64-year-old participants in the European Prospective Investigation into Cancer and Nutrition (EPIC). Public Health Nutr. 2002, 5, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation (WHO). Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Grundy, S.M. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar]
- Wareham, N.J.; Jakes, R.W.; Rennie, K.L.; Schuit, J.; Mitchell, J.; Hennings, S.; Day, N.E. Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr. 2003, 6. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Rubio, M.A.; Barbany, M.; Moreno, B. SEEDO 2007 Consensus for the evaluation of overweight and obesity and the establishment of therapeutic intervention criteria. Med. Clin. 2007, 128, 184–196. [Google Scholar] [CrossRef]
- Schofield, W.N. Predicting basal metabolic rate, new standards and review of previous work. Hum. Nutr. Clin. Nutr. 1985, 39 (Suppl. S1), 5–41. [Google Scholar]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef]
- Mohammadzadeh Honarvar, N.; Saedisomeolia, A.; Abdolahi, M.; Shayeganrad, A.; Taheri Sangsari, G.; Hassanzadeh Rad, B.; Muench, G. Molecular Anti-inflammatory Mechanisms of Retinoids and Carotenoids in Alzheimer’s Disease: A Review of Current Evidence. J. Mol. Neurosci. 2017, 61, 289–304. [Google Scholar] [CrossRef]
- Hernández Ruiz, A.; García-Villanova, B.; Guerra Hernández, E.J.; Amiano, P.; Azpiri, M.; Molina Montes, E. Description of indexes based on the adherence to the mediterranean dietary pattern: A review. Nutr. Hosp. 2015, 32. [Google Scholar] [CrossRef]
- Ghiselli, A.; Serafini, M.; Maiani, G.; Azzini, E.; Ferro-Luzzi, A. A fluorescence method for measuring total plasma antioxidant capability. Free Rad. Biol. Med. 1995, 18, 29–36. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Activity, A.; An, A.; Abts, I.; Assay, C.D. Antioxidant Activity Applying An Improved Abts Radical. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for Hydrophilic and Lipophilic Antioxidant Capacity (oxygen radical absorbance capacity (ORACFL)) of Plasma and Other Biological and Food Samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Oxidants and Antioxidants Part A; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. ISBN 0076-6879. [Google Scholar]
- Desai, M.; Kubo, J.; Esserman, D.; Terry, M.B. The handling of missing data in molecular epidemiology studies. Cancer Epidemiol. Biomarkers Prev. 2011, 20, 1571–1579. [Google Scholar] [CrossRef][Green Version]
- Madley-Dowd, P.; Hughes, R.; Tilling, K.; Heron, J. The proportion of missing data should not be used to guide decisions on multiple imputation. J. Clin. Epidemiol. 2019, 110, 63–73. [Google Scholar] [CrossRef]
- Little, R.J.A.; Rubin, D.B. Statistical analysis with missing data. J. Educ. Stat. 1987, 16, 150–155. [Google Scholar]
- Cook, R.D. Linear Regression. Regres. Methods Biostat. 1977, 19, 69–131. [Google Scholar] [CrossRef]
- Goodman, M.; Bostick, R.M.; Dash, C.; Terry, P.; Flanders, W.D.; Mandel, J. A summary measure of pro- and anti-oxidant exposures and risk of incident, sporadic, colorectal adenomas. Cancer Causes Control 2008, 19, 1051–1064. [Google Scholar] [CrossRef]
- Dash, C.; Goodman, M.; Dana Flanders, W.; Mink, P.J.; McCullough, M.L.; Bostick, R.M. Using pathway-specific comprehensive exposure scores in epidemiology: Application to oxidative balance in a pooled case-control study of incident, sporadic colorectal adenomas. Am. J. Epidemiol. 2013, 178, 610–624. [Google Scholar] [CrossRef]
- Dash, C.; Bostick, R.M.; Goodman, M.; Flanders, W.D.; Patel, R.; Shah, R.; Campbell, P.T.; McCullough, M.L. Oxidative balance scores and risk of incident colorectal cancer in a US prospective cohort study. Am. J. Epidemiol. 2015, 181, 584–594. [Google Scholar] [CrossRef]
- Amiano, P.; Molina-Montes, E.; Molinuevo, A.; Huerta, J.M.; Romaguera, D.; Gracia, E.; Martín, V.; Castaño-Vinyals, G.; Pérez-Gómez, B.; Moreno, V.; et al. Association study of dietary non-enzymatic antioxidant capacity (NEAC) and colorectal cancer risk in the Spanish Multicase–Control Cancer (MCC-Spain) study. Eur. J. Nutr. 2019, 58, 2229–2242. [Google Scholar] [CrossRef]
- Bastide, N.; Dartois, L.; Dyevre, V.; Dossus, L.; Fagherazzi, G.; Serafini, M.; Boutron-Ruault, M.C. Dietary antioxidant capacity and all-cause and cause-specific mortality in the E3N/EPIC cohort study. Eur. J. Nutr. 2016, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.; Jakszyn, P.; Luján-Barroso, L.; Agudo, A.; Bas Bueno-De-Mesquita, H.; Van Duijnhoven, F.J.B.; Jenab, M.; Navarro, C.; Palli, D.; Boeing, H.; et al. Dietary total antioxidant capacity and gastric cancer risk in the European prospective investigation into cancer and nutrition study. Int. J. Cancer 2012, 131, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Fedirko, V.; Trichopoulou, A.; González, C.A.; Bamia, C.; Trepo, E.; Nöthlings, U.; Duarte-Salles, T.; Serafini, M.; Bredsdorff, L.; et al. Dietary flavonoid, lignan and antioxidant capacity and risk of hepatocellular carcinoma in the European prospective investigation into cancer and nutrition study. Int. J. Cancer 2013, 133, 2429–2443. [Google Scholar] [CrossRef] [PubMed]
- Amiot, M.J.; Riva, C.; Vinet, A. Effects of dietary polyphenols on metabolic syndrome features in humans: A systematic review. Obes. Rev. 2016, 17, 573–586. [Google Scholar] [CrossRef]
- Glei, M.; Latunde-Dada, G.O.; Klinder, A.; Becker, T.W.; Hermann, U.; Voigt, K.; Pool-Zobel, B.L. Iron-overload induces oxidative DNA damage in the human colon carcinoma cell line HT29 clone 19A. Mutat. Res. Toxicol. Environ. Mutagen. 2002, 519, 151–161. [Google Scholar] [CrossRef]
- Ji, L.L.; Gomez-Cabrera, M.-C.; Vina, J. Exercise and hormesis: Activation of cellular antioxidant signaling pathway. Ann. N. Y. Acad. Sci. 2006, 1067, 425–435. [Google Scholar] [CrossRef]
- Das, S.K.; Vasudevan, D.M. Alcohol-induced oxidative stress. Life Sci. 2007, 81, 177–187. [Google Scholar] [CrossRef]
- van der Vaart, H. Acute effects of cigarette smoke on inflammation and oxidative stress: A review. Thorax 2004, 59, 713–721. [Google Scholar] [CrossRef]
- Van Cauwenberghe, C.; Vandendriessche, C.; Libert, C.; Vandenbroucke, R.E. Caloric restriction: Beneficial effects on brain aging and Alzheimer’s disease. Mamm. Genome 2016, 27, 300–319. [Google Scholar] [CrossRef]
- Speakman, J.R.; Mitchell, S.E. Caloric restriction. Mol. Aspects Med. 2011, 32, 159–221. [Google Scholar] [CrossRef]
- Golbidi, S.; Daiber, A.; Korac, B.; Li, H.; Essop, M.F.; Laher, I. Health Benefits of Fasting and Caloric Restriction. Curr. Diab. Rep. 2017, 17, 123. [Google Scholar] [CrossRef]
- Mao, Z.; Bostick, R.M. Associations of dietary, lifestyle, other participant characteristics, and oxidative balance scores with plasma F2-isoprostanes concentrations in a pooled cross-sectional study. Eur. J. Nutr. 2021. [Google Scholar] [CrossRef]
- Kong, S.Y.J.; Bostick, R.M.; Flanders, W.D.; McClellan, W.M.; Thyagarajan, B.; Gross, M.D.; Judd, S.; Goodman, M. Oxidative balance score, colorectal adenoma, and markers of oxidative stress and inflammation. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 545–554. [Google Scholar] [CrossRef]
- Lakkur, S.; Judd, S.; Bostick, R.M.; McClellan, W.; Flanders, W.D.; Stevens, V.L.; Goodman, M. Oxidative stress, inflammation, and markers of cardiovascular health. Atherosclerosis 2015, 243, 38–43. [Google Scholar] [CrossRef]
- Lee, H.; Park, T. Pathway-Driven Approaches of Interaction between Oxidative Balance and Genetic Polymorphism on Metabolic Syndrome. Oxidative Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef]
- Annor, F.B.; Goodman, M.; Okosun, I.S.; Wilmot, D.W.; Il’yasova, D.; Ndirangu, M.; Lakkur, S. Oxidative stress, oxidative balance score, and hypertension among a racially diverse population. J. Am. Soc. Hypertens. 2017, 9, 592–599. [Google Scholar] [CrossRef]
- Carrión-García, C.J.; Guerra-Hernández, E.J.; García-Villanova, B.; Molina-Montes, E. Non-enzymatic antioxidant capacity (NEAC) estimated by two different dietary assessment methods and its relationship with NEAC plasma levels. Eur. J. Nutr. 2016, 56, 1561–1576. [Google Scholar] [CrossRef]
- Ishizaka, Y.; Yamakado, M.; Toda, A.; Tani, M.; Ishizaka, N. Relationship between serum uric acid and serum oxidative stress markers in the Japanese general population. Nephron. Clin. Pract. 2014, 128, 49–56. [Google Scholar] [CrossRef]
- Ok, E.J.; Kim, K.; Park, S.B. Association between Serum Uric Acid and Oxidative Stress in Korean Adults. Korean J. Fam. Med. 2018, 39, 295–299. [Google Scholar] [CrossRef]
- Hayden, M.R.; Tyagi, S.C. Uric acid: A new look at an old risk marker for cardiovascular disease, metabolic syndrome, and type 2 diabetes mellitus: The urate redox shuttle. Nutr. Metab. 2004, 1, 10. [Google Scholar] [CrossRef]
- Lacey, B.; Herrington, W.G.; Preiss, D.; Lewington, S.; Armitage, J. The Role of Emerging Risk Factors in Cardiovascular Outcomes. Curr. Atheroscler. Rep. 2017, 19, 28. [Google Scholar] [CrossRef]
- Nosalski, R.; Guzik, T.J. Perivascular adipose tissue inflammation in vascular disease. Br. J. Pharmacol. 2017, 174, 3496–3513. [Google Scholar] [CrossRef]
- Yang, X.D.; Jiang, S.; Wang, G.; Zhang, R.; Zhang, J.; Zhu, J.S. Link of obesity and gastrointestinal cancer: Crossroad of inflammation and oxidative stress. J. Biol. Regul. Homeost. Agents 2015, 29, 755–760. [Google Scholar]
- Lim, S.; Quon, M.J.; Koh, K.K. Modulation of adiponectin as a potential therapeutic strategy. Atherosclerosis 2014, 233, 721–728. [Google Scholar] [CrossRef]
- Selthofer-Relatic, K.; Radic, R.; Stupin, A.; Sisljagic, V.; Bosnjak, I.; Bulj, N.; Selthofer, R.; Delic Brkljacic, D. Leptin/adiponectin ratio in overweight patients—Gender differences. Diabetes Vasc. Dis. Res. 2018, 15, 260–262. [Google Scholar] [CrossRef]
| Men | Women | Granada | Gipuzkoa | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | p-Value a | N | % | N | % | p-Value a | |
| Eight Nutrient components of NutrientL-OBS (in quintiles, Q) | ||||||||||
| Vitamin C, mg/d γ | 0.007 | <0.001 | ||||||||
| Q1 (1 point) | 1095 | 19.8% | 1857 | 20.1% | 1093 | 16.5% | 1859 | 22.9% | ||
| Q2 (2 points) | 1044 | 18.9% | 1907 | 20.6% | 1462 | 22.1% | 1489 | 18.3% | ||
| Q3 (3 points) | 1077 | 19.5% | 1874 | 20.3% | 1441 | 21.8% | 1510 | 18.6% | ||
| Q4 (4 points) | 1172 | 21.2% | 1779 | 19.3% | 1345 | 20.3% | 1606 | 19.8% | ||
| Q5 (5 points) | 1129 | 20.5% | 1822 | 19.7% | 1284 | 19.4% | 1667 | 20.5% | ||
| β-carotene, µg/d γ | <0.001 | <0.001 | ||||||||
| Q1 (1 point) | 998 | 18.1% | 1954 | 21.1% | 1460 | 22.0% | 1492 | 18.3% | ||
| Q2 (2 points) | 1079 | 19.6% | 1872 | 20.3% | 1456 | 22.0% | 1495 | 18.4% | ||
| Q3 (3 points) | 1114 | 20.2% | 1837 | 19.9% | 1363 | 20.6% | 1588 | 19.5% | ||
| Q4 (4 points) | 1139 | 20.6% | 1812 | 19.6% | 1214 | 18.3% | 1737 | 21.4% | ||
| Q5 (5 points) | 1187 | 21.5% | 1764 | 19.1% | 1132 | 17.1% | 1819 | 22.4% | ||
| α-Tocopherol, mg/d γ | <0.001 | <0.001 | ||||||||
| Q1 (1 point) | 523 | 9.48% | 2429 | 26.3% | 2028 | 30.6% | 924 | 11.4% | ||
| Q2 (2 points) | 795 | 14.4% | 2156 | 23.3% | 1688 | 25.5% | 1263 | 15.5% | ||
| Q3 (3 points) | 1066 | 19.3% | 1885 | 20.4% | 1348 | 20.3% | 1603 | 19.7% | ||
| Q4 (4 points) | 1375 | 24.9% | 1576 | 17.1% | 1014 | 15.3% | 1937 | 23.8% | ||
| Q5 (5 points) | 1758 | 31.9% | 1193 | 12.9% | 547 | 8.26% | 2404 | 29.6% | ||
| TRAP, µmol trolox γ | <0.001 | <0.001 | ||||||||
| Q1 (1 point) | 460 | 8.34% | 2492 | 27.0% | 1836 | 27.7% | 1116 | 13.7% | ||
| Q2 (2 points) | 631 | 11.4% | 2320 | 25.1% | 1669 | 25.2% | 1282 | 15.8% | ||
| Q3 (3 points) | 878 | 15.9% | 2073 | 22.4% | 1399 | 21.1% | 1552 | 19.1% | ||
| Q4 (4 points) | 1331 | 24.1% | 1620 | 17.5% | 1128 | 17.0% | 1823 | 22.4% | ||
| Q5 (5 points) | 2217 | 40.2% | 734 | 7.94% | 593 | 8.95% | 2358 | 29.0% | ||
| FRAP, µmol iron/d γ | <0.001 | <0.001 | ||||||||
| Q1 (1 point) | 407 | 7.38% | 2545 | 27.5% | 1772 | 26.7% | 1180 | 14.5% | ||
| Q2 (2 points) | 620 | 11.2% | 2331 | 25.2% | 1615 | 24.4% | 1336 | 16.4% | ||
| Q3 (3 points) | 924 | 16.7% | 2027 | 21.9% | 1439 | 21.7% | 1512 | 18.6% | ||
| Q4 (4 points) | 1382 | 25.0% | 1569 | 17.0% | 1140 | 17.2% | 1811 | 22.3% | ||
| Q5 (5 points) | 2184 | 39.6% | 767 | 8.30% | 659 | 9.95% | 2292 | 28.2% | ||
| PAC score, -28-28 γ | <0.001 | <0.001 | ||||||||
| Q1 (1 point) | 485 | 8.79% | 2609 | 28.2% | 1760 | 26.6% | 1334 | 16.4% | ||
| Q2 (2 points) | 719 | 13.0% | 2396 | 25.9% | 1660 | 25.1% | 1455 | 17.9% | ||
| Q3 (3 points) | 993 | 18.0% | 2007 | 21.7% | 1402 | 21.2% | 1598 | 19.7% | ||
| Q4 (4 points) | 1339 | 24.3% | 1504 | 16.3% | 1078 | 16.3% | 1765 | 21.7% | ||
| Q5 (5 points) | 1981 | 35.9% | 723 | 7.83% | 725 | 10.9% | 1979 | 24.3% | ||
| PUFA, g/d ≠ | <0.001 | <0.001 | ||||||||
| Q1 (5 points) | 1836 | 33.3% | 1115 | 12.1% | 467 | 7.05% | 2484 | 30.5% | ||
| Q2 (4 points) | 1406 | 25.5% | 1545 | 16.7% | 1029 | 15.5% | 1922 | 23.6% | ||
| Q3 (3 points) | 1176 | 21.3% | 1775 | 19.2% | 1396 | 21.1% | 1555 | 19.1% | ||
| Q4 (2 points) | 747 | 13.5% | 2204 | 23.9% | 1732 | 26.1% | 1219 | 15.0% | ||
| Q5 (1 point) | 352 | 6.38% | 2600 | 28.1% | 2001 | 30.2% | 951 | 11.7% | ||
| Heme-iron, mg/d ≠ | <0.001 | <0.001 | ||||||||
| Q1 (5 points) | 2114 | 38.3% | 837 | 9.06% | 521 | 7.86% | 2430 | 29.9% | ||
| Q2 (4 points) | 1425 | 25.8% | 1526 | 16.5% | 804 | 12.1% | 2147 | 26.4% | ||
| Q3 (3 points) | 981 | 17.8% | 1970 | 21.3% | 1274 | 19.2% | 1677 | 20.6% | ||
| Q4 (2 points) | 643 | 11.7% | 2308 | 25.0% | 1752 | 26.4% | 1199 | 14.7% | ||
| Q5 (1 point) | 354 | 6.42% | 2598 | 28.1% | 2274 | 34.3% | 678 | 8.34% | ||
| Six Lifestyle factors components of NutrientL-OBS (in categories) | ||||||||||
| PA, METs/d γ | <0.001 | <0.001 | ||||||||
| Inactive (1 points) | 1132 | 20.5% | 4604 | 49.8% | 3486 | 52.6% | 2250 | 27.7% | ||
| Moderate (3 points) | 3174 | 57.5% | 4715 | 46.0% | 2705 | 40.8% | 4715 | 58.0% | ||
| Active (5 points) | 1211 | 22.0% | 389 | 4.21% | 434 | 6.55% | 1166 | 14.3% | ||
| Alcohol, g/d ≠ | <0.001 | <0.001 | ||||||||
| >75, M->50, W (1 point) | 338 | 6.13% | 29 | 0.31% | 27 | 0.41% | 340 | 4.18% | ||
| ≤75, M-≤50, W (2 points) | 641 | 11.6% | 325 | 3.52% | 101 | 1.52% | 865 | 10.6% | ||
| ≤50, M-≤25, W (3 points) | 1855 | 33.6% | 486 | 5.26% | 516 | 7.79% | 1825 | 22.4% | ||
| ≤20, M-≤15, W (4 points) | 838 | 15.2% | 1208 | 13.1% | 681 | 10.3% | 1365 | 16.8% | ||
| ≤10, M-≤5, W (5 points) | 1845 | 33.4% | 7191 | 77.8% | 5300 | 80.0% | 3736 | 45.9% | ||
| BMI, kg/m2 ≠ | <0.001 | <0.001 | ||||||||
| ≥35 (1 point) | 180 | 3.26% | 852 | 9.22% | 688 | 10.4% | 344 | 4.23% | ||
| <35 (2 points) | 1306 | 23.7% | 2112 | 22.9% | 1923 | 29.0% | 1495 | 18.4% | ||
| ≤29.9 (3 points) | 2006 | 36.4% | 2135 | 23.1% | 1793 | 27.1% | 2348 | 28.9% | ||
| ≤26.9 (4 points) | 1194 | 21.6% | 1576 | 17.1% | 1004 | 15.2% | 1766 | 21.7% | ||
| <25 (5 points) | 831 | 15.1% | 2564 | 27.8% | 1217 | 18.4% | 2178 | 26.8% | ||
| WC, cm ≠ | <0.001 | <0.001 | ||||||||
| >102, M; >88, W (1 point) | 1814 | 32.9% | 3927 | 42.5% | 3229 | 48.7% | 2512 | 30.9% | ||
| <102. M; <88, W (5 points) | 3703 | 67.1% | 5312 | 57.5% | 3396 | 51.3% | 5619 | 69.1% | ||
| Smoking status ≠ | <0.001 | <0.001 | ||||||||
| Never (5 points) | 1674 | 30.3% | 6835 | 74.0% | 4423 | 66.8% | 4086 | 50.3% | ||
| Former (3 points) | 1679 | 30.4% | 942 | 10.2% | 996 | 15.0% | 1625 | 20.0% | ||
| Current (1 points) | 2164 | 39.2% | 1462 | 15.8% | 1206 | 18.2% | 2420 | 29.8% | ||
| Excess energy intake, kcal ≠ | <0.001 | <0.001 | ||||||||
| Excess energy >30% (1 points) | 296 | 5.37% | 322 | 3.49% | 170 | 2.57% | 448 | 5.51% | ||
| Excess energy <30% (2 points) | 317 | 5.75% | 292 | 3.16% | 155 | 2.34% | 454 | 5.58% | ||
| Excess energy <20% (3 points) | 485 | 8.79% | 468 | 5.07% | 270 | 4.08% | 683 | 8.40% | ||
| Excess energy <10% (4 points) | 735 | 13.3% | 769 | 8.32% | 439 | 6.63% | 1065 | 13.1% | ||
| Similar intake (5 points) | 3684 | 66.8% | 7388 | 80.0% | 5591 | 84.4% | 5481 | 67.4% | ||
| Distribution of the NutrientL-OBS | ||||||||||
| NutrientL-OBS (by tertiles) | <0.001 | <0.001 | ||||||||
| T1 | 1117 | 20.2% | 3874 | 41.9% | 2891 | 43.6% | 2100 | 25.8% | ||
| T2 | 2178 | 39.5% | 3077 | 33.3% | 2154 | 32.5% | 3101 | 38.1% | ||
| T3 | 2222 | 40.3% | 2288 | 24.8% | 1580 | 23.8% | 2930 | 36.0% | ||
| Tertile 1 (T1); N = 4917 | Tertile 2 (T2); N = 4900 | Tertile 3 (T3); N = 4939 | |||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | p-Value a | |
| Eleven Food components of FoodL-OBS (in quintiles, Q) | |||||||
| Vegetable, g/d γ | <0.001 | ||||||
| Q1 (1 point) | 1644 | 33.4% | 935 | 19.1% | 373 | 7.55% | |
| Q2 (2 points) | 1176 | 23.9% | 1100 | 22.4% | 675 | 13.7% | |
| Q3 (3 points) | 928 | 19.0% | 1042 | 21.3% | 971 | 19.7% | |
| Q4 (4 points) | 712 | 14.5% | 988 | 20.2% | 1251 | 25.3% | |
| Q5 (5 points) | 447 | 9.09% | 835 | 17.0% | 1669 | 33.8% | |
| Fruits and juices, g/d γ | <0.001 | ||||||
| Q1 (1 point) | 1636 | 33.3% | 898 | 18.3% | 418 | 8.46% | |
| Q2 (2 points) | 1203 | 24.5% | 1075 | 21.9% | 727 | 14.7% | |
| Q3 (3 points) | 901 | 18.3% | 1018 | 20.8% | 978 | 19.8% | |
| Q4 (4 points) | 699 | 14.2% | 1020 | 20.8% | 1232 | 24.9% | |
| Q5 (5 points) | 478 | 9.72% | 889 | 18.1% | 1584 | 32.1% | |
| Legumes, g/d γ. | <0.001 | ||||||
| Q1 (0.5 points) | 1085 | 22.1% | 955 | 19.5% | 913 | 18.5% | |
| Q2 (1 point) | 1021 | 20.8% | 1010 | 20.6% | 937 | 19.0% | |
| Q3 (1.5 points) | 944 | 19.2% | 1032 | 21.1% | 1024 | 20.7% | |
| Q4 (2 points) | 868 | 17.7% | 965 | 19.7% | 1058 | 21.4% | |
| Q5 (2.5 points) | 999 | 20.3% | 938 | 19.1% | 1007 | 20.4% | |
| Olive oil, g/d γ. | <0.001 | ||||||
| Q1 (0.5 points) | 1830 | 37.2% | 786 | 16.0% | 336 | 6.80% | |
| Q2 (1 point) | 1175 | 23.9% | 1095 | 22.3% | 681 | 13.8% | |
| Q3 (1.5 points) | 796 | 16.2% | 1095 | 22.3% | 1060 | 21.5% | |
| Q4 (2 points) | 597 | 12.1% | 995 | 20.3% | 1359 | 27.5% | |
| Q5 (2.5 points) | 519 | 10.6% | 929 | 19.0% | 1503 | 30.4% | |
| Fatty fish, g/d γ | <0.001 | ||||||
| Q1 (0.5 points) | 1212 | 24.6% | 945 | 19.3% | 850 | 17.2% | |
| Q2 (1 point) | 993 | 20.2% | 1044 | 21.3% | 831 | 16.8% | |
| Q3 (1.5 points) | 1056 | 21.5% | 924 | 18.9% | 998 | 20.2% | |
| Q4 (2 points) | 845 | 17.2% | 1118 | 22.8% | 988 | 20.0% | |
| Q5 (2.5 points) | 811 | 16.5% | 869 | 17.7% | 1272 | 25.8% | |
| Coffee and tea, g/d γ | <0.001 | ||||||
| Q1 (1 point) | 1252 | 25.5% | 1018 | 20.8% | 682 | 13.8% | |
| Q2 (2 points) | 1168 | 23.8% | 977 | 19.9% | 810 | 16.4% | |
| Q3 (3 points) | 1123 | 22.8% | 1138 | 23.2% | 1003 | 20.3% | |
| Q4 (4 points) | 733 | 14.9% | 850 | 17.3% | 1055 | 21.4% | |
| Q5 (5 points) | 641 | 13.0% | 917 | 18.7% | 1389 | 28.1% | |
| Meat and meat products, g/d ≠ | <0.001 | ||||||
| Q5 (1 point) | 1490 | 28.0% | 869 | 18.4% | 592 | 12.6% | |
| Q4 (2 points) | 1331 | 25.0% | 920 | 19.5% | 700 | 14.8% | |
| Q3 (3 points) | 1052 | 19.8% | 1001 | 21.2% | 898 | 19.1% | |
| Q2 (4 points) | 793 | 14.9% | 935 | 19.8% | 1223 | 25.9% | |
| Q1 (5 points) | 656 | 12.3% | 996 | 21.1% | 1300 | 27.6% | |
| Cookies and pastries, g/d ≠ | <0.001 | ||||||
| Q5 (0.5 points) | 1073 | 21.8% | 836 | 17.1% | 628 | 12.7% | |
| Q4 (1 point) | 939 | 19.1% | 856 | 17.5% | 723 | 14.6% | |
| Q3 (1.5 points) | 876 | 17.8% | 878 | 17.9% | 805 | 16.3% | |
| Q2 (2 points) | 719 | 14.6% | 859 | 17.5% | 960 | 19.4% | |
| Q1 (2.5 points) | 1310 | 26.6% | 1471 | 30.0% | 1823 | 36.9% | |
| Fats and oils, g/d ≠ | <0.001 | ||||||
| Q5 (1 point) | 1596 | 30.0% | 631 | 13.4% | 218 | 4.63% | |
| Q4 (2 points) | 1354 | 25.4% | 741 | 15.7% | 349 | 7.41% | |
| Q3 (3 points) | 852 | 16.0% | 875 | 18.5% | 717 | 15.2% | |
| Q2 (4 points) | 570 | 10.7% | 832 | 17.6% | 1043 | 22.1% | |
| Q1 (5 points) | 950 | 17.8% | 1642 | 34.8% | 2386 | 50.6% | |
| Snacks and sauces, g/d ≠ | <0.001 | ||||||
| Q5 (0.5 points) | 1554 | 31.6% | 914 | 18.7% | 483 | 9.78% | |
| Q4 (1 point) | 1122 | 22.8% | 990 | 20.2% | 839 | 17.0% | |
| Q3 (1.5 points) | 883 | 18.0% | 1016 | 20.7% | 1024 | 20.7% | |
| Q2 (2 points) | 740 | 15.0% | 961 | 19.6% | 1276 | 25.8% | |
| Q1 (2.5 points) | 618 | 12.6% | 1019 | 20.8% | 1317 | 26.7% | |
| Cereals and refined products, g/d ≠ | <0.001 | ||||||
| Q5 (0.5 points) | 1543 | 31.4% | 917 | 18.7% | 491 | 9.94% | |
| Q4 (1 point) | 1161 | 23.6% | 1019 | 20.8% | 770 | 15.6% | |
| Q3 (1.5 points) | 944 | 19.2% | 981 | 20.0% | 942 | 19.1% | |
| Q2 (2 points) | 764 | 15.5% | 1047 | 21.4% | 1224 | 24.8% | |
| Q1 (2.5 points) | 505 | 10.3% | 936 | 19.1% | 1512 | 30.6% | |
| Six Lifestyle factors components of FoodL-OBS (in categories) | |||||||
| PA, METs/d γ | <0.001 | ||||||
| Inactive (1 point) | 2239 | 45.5% | 2023 | 41.3% | 1474 | 29.8% | |
| Moderate (3 points) | 2289 | 46.6% | 2388 | 48.7% | 2743 | 55.5% | |
| Active (5 points) | 389 | 7.91% | 489 | 9.98% | 722 | 14.6% | |
| Alcohol consumption, g/d ≠ | <0.001 | ||||||
| >75, M->50, W (1 point) | 300 | 6.10% | 55 | 1.12% | 12 | 0.24% | |
| ≤75, M-≤50, W (2 points) | 600 | 12.2% | 265 | 5.41% | 101 | 2.04% | |
| ≤50, M-≤25, W (3 points) | 1101 | 22.4% | 763 | 15.6% | 477 | 9.66% | |
| ≤20, M-≤15, W (4 points) | 639 | 13.0% | 713 | 14.6% | 694 | 14.1% | |
| ≤10, M-≤5, W (5 points) | 2277 | 46.3% | 3104 | 63.3% | 3655 | 74.0% | |
| BMI, kg/m2 ≠ | <0.001 | ||||||
| ≥35 (1 point) | 596 | 12.1% | 321 | 6.55% | 115 | 2.33% | |
| <35 (2 points) | 1663 | 33.8% | 1172 | 23.9% | 583 | 11.8% | |
| ≤29.9 (3 points) | 1401 | 28.5% | 1408 | 28.7% | 1332 | 27.0% | |
| ≤26.9 (4 points) | 616 | 12.5% | 875 | 17.9% | 1279 | 25.9% | |
| <25 (5 points) | 641 | 13.0% | 1124 | 22.9% | 1630 | 33.0% | |
| WC, cm ≠ | <0.001 | ||||||
| >102, M; >88, W (1 point) | 2988 | 60.8% | 1937 | 39.5% | 816 | 16.5% | |
| <102, M; <88, W (5 points) | 1929 | 39.2% | 2963 | 60.5% | 4123 | 83.5% | |
| Smoking status ≠ | <0.001 | ||||||
| Current (1 point) | 1860 | 37.8% | 1092 | 22.3% | 674 | 13.6% | |
| Former (3 points) | 847 | 17.2% | 893 | 18.2% | 881 | 17.8% | |
| Never (5 points) | 2210 | 44.9% | 2915 | 59.5% | 3384 | 68.5% | |
| Excess energy intake according to total energy expenditure component, kcal (categories) | <0.001 | ||||||
| Excess energy intake >30% (1 point) | 372 | 7.57% | 178 | 3.63% | 68 | 1.38% | |
| Excess energy intake <30% (2 points) | 304 | 6.18% | 189 | 3.86% | 116 | 2.35% | |
| Excess energy intake <20% (3 points) | 434 | 8.83% | 293 | 5.98% | 226 | 4.58% | |
| Excess energy intake <10% (4 points) | 575 | 11.7% | 486 | 9.92% | 443 | 8.97% | |
| Similar energy intake (5 points) | 3232 | 65.7% | 3754 | 76.6% | 4086 | 82.7% | |
| NutrientL-OBS | FoodL-OBS | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||||||||||||||
| β | 95% CI | p-Value | R2 | β | 95% CI | p-Value | R2 | β | 95% CI | p-Value | R2 | β | 95% CI | p-Value | R2 | |||||
| rMED | 0.023 | 0.022 | 0.023 | <0.001 | 0.243 | 0.021 | 0.021 | 0.022 | <0.001 | 0.288 | 0.023 | 0.022 | 0.024 | <0.001 | 0.221 | 0.028 | 0.028 | 0.029 | <0.001 | 0.342 |
| aMED | 0.062 | 0.058 | 0.065 | <0.001 | 0.076 | 0.06 | 0.057 | 0.064 | <0.001 | 0.079 | 0.064 | 0.06 | 0.068 | <0.001 | 0.067 | 0.072 | 0.068 | 0.076 | <0.001 | 0.087 |
| MedDietScore2004 | 0.008 | 0.007 | 0.008 | <0.001 | 0.259 | 0.007 | 0.007 | 0.007 | <0.001 | 0.384 | 0.006 | 0.006 | 0.006 | <0.001 | 0.194 | 0.009 | 0.008 | 0.009 | <0.001 | 0.405 |
| MedDietScore2005 | 0.006 | 0.005 | 0.006 | <0.001 | 0.105 | 0.006 | 0.005 | 0.006 | <0.001 | 0.113 | 0.007 | 0.007 | 0.008 | <0.001 | 0.129 | 0.008 | 0.008 | 0.009 | <0.001 | 0.169 |
| MedDietScore2007 | 0.009 | 0.009 | 0.009 | <0.001 | 0.251 | 0.009 | 0.009 | 0.01 | <0.001 | 0.252 | 0.012 | 0.012 | 0.012 | <0.001 | 0.344 | 0.013 | 0.013 | 0.013 | <0.001 | 0.371 |
| ShortMedQ | 0.024 | 0.023 | 0.024 | <0.001 | 0.172 | 0.024 | 0.023 | 0.024 | <0.001 | 0.172 | 0.031 | 0.03 | 0.032 | <0.001 | 0.226 | 0.033 | 0.032 | 0.034 | <0.001 | 0.248 |
| Lbas | 0.014 | 0.013 | 0.014 | <0.001 | 0.143 | 0.013 | 0.013 | 0.014 | <0.001 | 0.151 | 0.016 | 0.015 | 0.016 | <0.001 | 0.148 | 0.018 | 0.017 | 0.018 | <0.001 | 0.197 |
| PREDIMED | 0.009 | 0.009 | 0.01 | <0.001 | 0.241 | 0.009 | 0.008 | 0.009 | <0.001 | 0.287 | 0.009 | 0.008 | 0.009 | <0.001 | 0.205 | 0.011 | 0.011 | 0.011 | <0.001 | 0.319 |
| MDS1995 | 0.03 | 0.028 | 0.032 | <0.001 | 0.058 | 0.029 | 0.027 | 0.031 | <0.001 | 0.068 | 0.026 | 0.024 | 0.028 | <0.001 | 0.035 | 0.032 | 0.03 | 0.034 | <0.001 | 0.065 |
| MDS2013 | 0.029 | 0.027 | 0.031 | <0.001 | 0.084 | 0.027 | 0.026 | 0.029 | <0.001 | 0.097 | 0.021 | 0.019 | 0.023 | <0.001 | 0.054 | 0.027 | 0.025 | 0.029 | <0.001 | 0.086 |
| MDSS | 0.012 | 0.011 | 0.012 | <0.001 | 0.085 | 0.011 | 0.01 | 0.012 | <0.001 | 0.107 | 0.012 | 0.012 | 0.013 | <0.001 | 0.082 | 0.015 | 0.015 | 0.016 | <0.001 | 0.143 |
| MSDPS | 0.006 | 0.006 | 0.007 | <0.001 | 0.057 | 0.006 | 0.006 | 0.007 | <0.001 | 0.057 | 0.008 | 0.008 | 0.009 | <0.001 | 0.073 | 0.009 | 0.008 | 0.009 | <0.001 | 0.08 |
| MDQI | −0.023 | −0.023 | −0.02 | <0.001 | 0.176 | −0.02 | −0.02 | −0.02 | <0.001 | 0.185 | −0.028 | −0.029 | −0.027 | <0.001 | 0.214 | −0.03 | −0.03 | −0.03 | <0.001 | 0.217 |
| MDP2003 | 0.008 | 0.007 | 0.008 | <0.001 | 0.096 | 0.008 | 0.008 | 0.008 | <0.001 | 0.113 | 0.011 | 0.011 | 0.012 | <0.001 | 0.165 | 0.011 | 0.011 | 0.012 | <0.001 | 0.165 |
| MDPA2002 | 0.014 | 0.014 | 0.015 | <0.001 | 0.412 | 0.013 | 0.013 | 0.014 | <0.001 | 0.515 | 0.012 | 0.011 | 0.012 | <0.001 | 0.311 | 0.015 | 0.015 | 0.016 | <0.001 | 0.529 |
| MDP2006 | 0.008 | 0.008 | 0.008 | <0.001 | 0.22 | 0.007 | 0.007 | 0.007 | <0.001 | 0.362 | 0.008 | 0.008 | 0.008 | <0.001 | 0.202 | 0.011 | 0.011 | 0.011 | <0.001 | 0.458 |
| MMD2005 | 0.028 | 0.026 | 0.03 | <0.001 | 0.131 | 0.026 | 0.024 | 0.027 | <0.001 | 0.171 | 0.024 | 0.022 | 0.026 | <0.001 | 0.104 | 0.032 | 0.03 | 0.034 | <0.001 | 0.181 |
| MEDLIFE | 0.009 | 0.009 | 0.01 | <0.001 | 0.141 | 0.009 | 0.009 | 0.009 | <0.001 | 0.147 | 0.009 | 0.009 | 0.01 | <0.001 | 0.128 | 0.011 | 0.01 | 0.011 | <0.001 | 0.161 |
| ITAMED | 0.028 | 0.026 | 0.029 | <0.001 | 0.074 | 0.027 | 0.026 | 0.029 | <0.001 | 0.075 | 0.033 | 0.031 | 0.035 | <0.001 | 0.085 | 0.037 | 0.035 | 0.039 | <0.001 | 0.099 |
| MDScale2003 | 0.03 | 0.028 | 0.031 | <0.001 | 0.101 | 0.029 | 0.027 | 0.03 | <0.001 | 0.106 | 0.029 | 0.027 | 0.031 | <0.001 | 0.089 | 0.034 | 0.032 | 0.036 | <0.001 | 0.112 |
| NutrientL-OBS | FoodL-OBS | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||||||||||||||
| β | 95% CI | p-Value | R2 | β | 95% CI | p-Value | R2 | β | 95% CI | p-Value | R2 | β | 95% CI | p-Value | R2 | |||||
| β-carotene | 0.02 | 0.004 | 0.035 | 0.012 | 0.078 | 0.02 | 0.005 | 0.036 | 0.010 | 0.086 | 0.012 | −0.004 | 0.029 | 0.143 | 0.06 | 0.014 | −0.002 | 0.031 | 0.096 | 0.069 |
| Retinol | −0.004 | −0.01 | 0.001 | 0.116 | 0.086 | −0.004 | −0.01 | 0.001 | 0.112 | 0.087 | −0.005 | −0.011 | 0.001 | 0.078 | 0.089 | −0.005 | −0.011 | 0.000 | 0.068 | 0.090 |
| Tocopherol | −0.001 | −0.008 | 0.007 | 0.876 | 0.01 | −0.001 | −0.009 | 0.007 | 0.827 | 0.015 | 0.002 | −0.006 | 0.011 | 0.587 | 0.011 | 0.002 | −0.007 | 0.01 | 0.691 | 0.015 |
| Ascorbic acid | 0.012 | 0.002 | 0.023 | 0.025 | 0.273 | 0.012 | 0.002 | 0.023 | 0.022 | 0.275 | 0.014 | 0.003 | 0.026 | 0.013 | 0.277 | 0.015 | 0.004 | 0.027 | 0.008 | 0.282 |
| Dehydroascorbic acid | −0.017 | −0.104 | 0.07 | 0.705 | 0.339 | −0.015 | −0.103 | 0.072 | 0.728 | 0.339 | 0.013 | −0.08 | 0.105 | 0.787 | 0.338 | 0.017 | −0.077 | 0.111 | 0.727 | 0.339 |
| Total Vitamin C | 0.011 | −0.001 | 0.023 | 0.077 | 0.308 | 0.011 | −0.001 | 0.023 | 0.065 | 0.313 | 0.013 | 0 | 0.026 | 0.049 | 0.311 | 0.014 | 0.001 | 0.027 | 0.030 | 0.318 |
| Q9 | 0.004 | −0.011 | 0.019 | 0.593 | 0.029 | 0.003 | −0.012 | 0.018 | 0.667 | 0.042 | 0.007 | −0.009 | 0.023 | 0.408 | 0.03 | 0.005 | −0.011 | 0.021 | 0.564 | 0.042 |
| Q10 | −0.004 | −0.012 | 0.005 | 0.410 | 0.019 | −0.004 | −0.012 | 0.005 | 0.383 | 0.022 | −0.003 | −0.012 | 0.006 | 0.557 | 0.018 | −0.003 | −0.012 | 0.006 | 0.480 | 0.021 |
| Uric Acid | −0.005 | −0.01 | 0.00 | 0.052 | 0.26 | −0.005 | −0.01 | 0.000 | 0.045 | 0.264 | −0.001 | −0.007 | 0.004 | 0.619 | 0.247 | −0.002 | −0.007 | 0.004 | 0.520 | 0.251 |
| TRAP | 0.00 | −0.002 | 0.003 | 0.825 | 0.187 | 0.000 | −0.002 | 0.003 | 0.845 | 0.188 | 0.000 | −0.002 | 0.003 | 0.776 | 0.187 | 0.00 | −0.002 | 0.003 | 0.822 | 0.188 |
| FRAP TE | −0.002 | −0.005 | 0.001 | 0.133 | 0.319 | −0.002 | −0.005 | 0.001 | 0.133 | 0.319 | −0.001 | −0.004 | 0.002 | 0.477 | 0.313 | −0.001 | −0.004 | 0.002 | 0.473 | 0.313 |
| FRAP FE | −0.002 | −0.005 | 0.001 | 0.130 | 0.237 | −0.002 | −0.005 | 0.001 | 0.140 | 0.238 | −0.002 | −0.004 | 0.001 | 0.268 | 0.233 | −0.002 | −0.004 | 0.001 | 0.304 | 0.233 |
| FRAP WO UA TE | −0.002 | −0.005 | 0.001 | 0.287 | 0.322 | −0.002 | −0.005 | 0.002 | 0.305 | 0.323 | −0.001 | −0.005 | 0.002 | 0.452 | 0.32 | −0.001 | −0.005 | 0.002 | 0.507 | 0.321 |
| FRAP WO UA FE | −0.002 | −0.004 | 0.001 | 0.316 | 0.172 | −0.001 | −0.004 | 0.002 | 0.354 | 0.178 | −0.002 | −0.005 | 0.001 | 0.216 | 0.174 | −0.002 | −0.005 | 0.001 | 0.291 | 0.179 |
| TEAC-ABTS | −0.001 | −0.006 | 0.004 | 0.597 | 0.019 | −0.001 | −0.006 | 0.004 | 0.589 | 0.019 | −0.002 | −0.007 | 0.004 | 0.550 | 0.019 | −0.002 | −0.007 | 0.004 | 0.528 | 0.019 |
| Total Polyphenols | −0.002 | −0.003 | 0.00 | 0.063 | 0.022 | −0.002 | −0.003 | 0.000 | 0.042 | 0.051 | −0.002 | −0.004 | 0.000 | 0.055 | 0.023 | −0.002 | −0.004 | 0.000 | 0.020 | 0.057 |
| ORAC WO Proteins | −0.005 | −0.01 | 0.00 | 0.049 | 0.144 | −0.005 | −0.01 | 0.000 | 0.050 | 0.144 | −0.005 | −0.011 | 0.00 | 0.072 | 0.142 | −0.005 | −0.011 | 0.000 | 0.072 | 0.142 |
| ORAC | 0.001 | −0.003 | 0.005 | 0.513 | 0.117 | 0.001 | −0.003 | 0.005 | 0.496 | 0.118 | 0.000 | −0.004 | 0.004 | 0.922 | 0.116 | 0.00 | −0.004 | 0.004 | 0.871 | 0.116 |
| CRP | −0.019 | −0.036 | −0.002 | 0.032 | 0.152 | −0.02 | −0.037 | −0.003 | 0.022 | 0.172 | −0.018 | −0.036 | 0.00 | 0.051 | 0.149 | −0.021 | −0.039 | −0.003 | 0.021 | 0.172 |
| Adiponectin | 0.012 | −0.004 | 0.029 | 0.143 | 0.056 | 0.014 | −0.003 | 0.03 | 0.105 | 0.081 | 0.01 | −0.007 | 0.028 | 0.255 | 0.052 | 0.014 | −0.004 | 0.032 | 0.131 | 0.079 |
| PAI-I | −0.007 | −0.016 | 0.002 | 0.132 | 0.126 | −0.007 | −0.016 | 0.002 | 0.129 | 0.126 | −0.007 | −0.017 | 0.002 | 0.141 | 0.125 | −0.008 | −0.017 | 0.002 | 0.131 | 0.126 |
| Resistin | −0.001 | −0.007 | 0.006 | 0.870 | 0.082 | −0.001 | −0.008 | 0.006 | 0.784 | 0.096 | −0.005 | −0.013 | 0.002 | 0.147 | 0.092 | −0.007 | −0.014 | 0.001 | 0.081 | 0.109 |
| TNF-alfa | −0.005 | −0.014 | 0.003 | 0.237 | 0.082 | −0.005 | −0.014 | 0.004 | 0.260 | 0.085 | −0.005 | −0.014 | 0.004 | 0.264 | 0.082 | −0.005 | −0.014 | 0.005 | 0.320 | 0.084 |
| IL8 | −0.008 | −0.024 | 0.008 | 0.328 | 0.103 | −0.008 | −0.024 | 0.008 | 0.319 | 0.104 | −0.006 | −0.023 | 0.011 | 0.498 | 0.101 | −0.006 | −0.024 | 0.011 | 0.465 | 0.102 |
| IL6 | 0.011 | −0.016 | 0.037 | 0.441 | 0.19 | 0.01 | −0.017 | 0.037 | 0.455 | 0.19 | 0.012 | −0.017 | 0.041 | 0.408 | 0.19 | 0.012 | −0.018 | 0.041 | 0.437 | 0.19 |
| Nutrient-OBS | Food-OBS | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 2 | Model 3 | Model 2 | Model 3 | |||||||||||||||||
| β | 95% CI | p-Value | R2 | β | 95% CI | p-Value | R2 | β | 95% CI | p-Value | R2 | β | 95% CI | p-Value | R2 | |||||
| β-carotene | 0.018 | 0.00 | 0.037 | 0.052 | 0.073 | 0.016 | −0.003 | 0.034 | 0.100 | 0.13 | 0.008 | −0.012 | 0.028 | 0.424 | 0.059 | 0.002 | −0.019 | 0.022 | 0.873 | 0.118 |
| Retinol | −0.003 | −0.009 | 0.004 | 0.396 | 0.079 | −0.003 | −0.01 | 0.003 | 0.294 | 0.133 | −0.004 | −0.011 | 0.003 | 0.288 | 0.081 | −0.005 | −0.012 | 0.002 | 0.132 | 0.138 |
| Tocopherol | −0.001 | −0.011 | 0.008 | 0.773 | 0.015 | −0.002 | −0.011 | 0.008 | 0.697 | 0.06 | 0.002 | −0.008 | 0.012 | 0.658 | 0.016 | 0 | −0.01 | 0.011 | 0.932 | 0.059 |
| Ascorbic acid (AA) | 0.012 | 0.00 | 0.025 | 0.054 | 0.27 | 0.013 | 0.00 | 0.026 | 0.052 | 0.297 | 0.016 | 0.003 | 0.03 | 0.021 | 0.276 | 0.017 | 0.003 | 0.031 | 0.018 | 0.304 |
| Dehydro AA | −0.019 | −0.122 | 0.084 | 0.722 | 0.339 | −0.003 | −0.109 | 0.103 | 0.950 | 0.358 | 0.027 | −0.085 | 0.14 | 0.632 | 0.34 | 0.05 | −0.065 | 0.165 | 0.393 | 0.361 |
| Total Vitamin C | 0.012 | −0.002 | 0.026 | 0.105 | 0.311 | 0.013 | −0.002 | 0.027 | 0.091 | 0.34 | 0.016 | 0.00 | 0.031 | 0.047 | 0.315 | 0.017 | 0.001 | 0.033 | 0.038 | 0.345 |
| Q9 | 0.008 | −0.01 | 0.026 | 0.372 | 0.045 | 0.008 | −0.01 | 0.027 | 0.382 | 0.066 | 0.011 | −0.008 | 0.031 | 0.267 | 0.047 | 0.009 | −0.011 | 0.029 | 0.402 | 0.066 |
| Q10 | −0.003 | −0.013 | 0.007 | 0.551 | 0.02 | −0.003 | −0.013 | 0.007 | 0.543 | 0.089 | −0.002 | −0.013 | 0.009 | 0.708 | 0.019 | −0.004 | −0.015 | 0.006 | 0.425 | 0.091 |
| Uric Acid | −0.003 | −0.009 | 0.003 | 0.274 | 0.254 | −0.002 | −0.008 | 0.004 | 0.557 | 0.344 | 0.002 | −0.005 | 0.009 | 0.542 | 0.251 | 0.002 | −0.004 | 0.008 | 0.531 | 0.344 |
| TRAP | 0.001 | −0.002 | 0.003 | 0.574 | 0.189 | 0.001 | −0.002 | 0.004 | 0.457 | 0.207 | 0.001 | −0.002 | 0.004 | 0.528 | 0.189 | 0.001 | −0.002 | 0.004 | 0.625 | 0.206 |
| FRAP TE | −0.001 | −0.004 | 0.003 | 0.700 | 0.312 | 0.00 | −0.004 | 0.003 | 0.938 | 0.362 | 0.001 | −0.002 | 0.005 | 0.514 | 0.313 | 0.001 | −0.003 | 0.004 | 0.714 | 0.363 |
| FRAP FE | −0.001 | −0.004 | 0.002 | 0.443 | 0.232 | −0.001 | −0.004 | 0.002 | 0.661 | 0.281 | 0.00 | −0.004 | 0.003 | 0.866 | 0.23 | −0.001 | −0.004 | 0.003 | 0.644 | 0.281 |
| FRAP WO UA TE | 0.00 | −0.004 | 0.004 | 0.921 | 0.319 | 0.00 | −0.004 | 0.004 | 0.953 | 0.343 | 0.001 | −0.003 | 0.005 | 0.677 | 0.32 | 0 | −0.004 | 0.005 | 0.893 | 0.343 |
| FRAP WO UA FE | −0.001 | −0.004 | 0.003 | 0.587 | 0.176 | −0.001 | −0.004 | 0.003 | 0.727 | 0.209 | −0.001 | −0.005 | 0.003 | 0.508 | 0.176 | −0.002 | −0.006 | 0.002 | 0.329 | 0.212 |
| TEAC-ABTS | 0.001 | −0.005 | 0.007 | 0.745 | 0.018 | 0.001 | −0.005 | 0.007 | 0.757 | 0.057 | 0.001 | −0.006 | 0.007 | 0.768 | 0.018 | 0.001 | −0.005 | 0.008 | 0.727 | 0.057 |
| Total Polyphenols | −0.002 | −0.004 | 0 | 0.053 | 0.049 | −0.002 | −0.004 | 0 | 0.046 | 0.15 | −0.003 | −0.005 | 0 | 0.022 | 0.056 | −0.003 | −0.005 | −0.001 | 0.005 | 0.167 |
| ORAC WO Proteins | −0.004 | −0.01 | 0.003 | 0.249 | 0.134 | −0.004 | −0.01 | 0.003 | 0.263 | 0.169 | −0.003 | −0.01 | 0.004 | 0.374 | 0.131 | −0.003 | −0.009 | 0.004 | 0.447 | 0.166 |
| ORAC | 0.002 | −0.002 | 0.007 | 0.290 | 0.121 | 0.003 | −0.002 | 0.008 | 0.221 | 0.148 | 0.001 | −0.004 | 0.006 | 0.639 | 0.117 | 0.001 | −0.004 | 0.006 | 0.659 | 0.142 |
| CRP | −0.003 | −0.023 | 0.017 | 0.773 | 0.151 | −0.003 | −0.022 | 0.016 | 0.759 | 0.283 | −0.001 | −0.023 | 0.021 | 0.906 | 0.15 | −0.004 | −0.025 | 0.017 | 0.680 | 0.283 |
| Adiponectin | 0.01 | −0.01 | 0.029 | 0.336 | 0.073 | 0.009 | −0.011 | 0.029 | 0.377 | 0.101 | 0.008 | −0.013 | 0.03 | 0.444 | 0.072 | 0.011 | −0.011 | 0.032 | 0.346 | 0.102 |
| PAI-I | 0 | −0.011 | 0.011 | 0.953 | 0.116 | 0.003 | −0.008 | 0.014 | 0.638 | 0.182 | 0.001 | −0.011 | 0.013 | 0.834 | 0.116 | 0.002 | −0.01 | 0.014 | 0.788 | 0.181 |
| Resistin | 0.001 | −0.007 | 0.009 | 0.861 | 0.095 | 0.001 | −0.008 | 0.009 | 0.890 | 0.107 | −0.007 | −0.016 | 0.002 | 0.123 | 0.106 | −0.008 | −0.017 | 0.002 | 0.107 | 0.119 |
| TNF-alfa | −0.004 | −0.014 | 0.006 | 0.462 | 0.082 | −0.004 | −0.015 | 0.006 | 0.417 | 0.127 | −0.003 | −0.014 | 0.008 | 0.589 | 0.081 | −0.005 | −0.016 | 0.007 | 0.432 | 0.127 |
| IL8 | −0.009 | −0.028 | 0.01 | 0.337 | 0.103 | −0.011 | −0.03 | 0.009 | 0.278 | 0.13 | −0.007 | −0.028 | 0.014 | 0.523 | 0.101 | −0.008 | −0.03 | 0.013 | 0.446 | 0.127 |
| IL6 | 0.02 | −0.012 | 0.052 | 0.218 | 0.194 | 0.022 | −0.01 | 0.054 | 0.175 | 0.259 | 0.023 | −0.011 | 0.058 | 0.190 | 0.195 | 0.024 | −0.011 | 0.059 | 0.174 | 0.259 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Ruiz, Á.; García-Villanova, B.; Guerra-Hernández, E.J.; Carrión-García, C.J.; Amiano, P.; Sánchez, M.-J.; Molina-Montes, E. Oxidative Balance Scores (OBSs) Integrating Nutrient, Food and Lifestyle Dimensions: Development of the NutrientL-OBS and FoodL-OBS. Antioxidants 2022, 11, 300. https://doi.org/10.3390/antiox11020300
Hernández-Ruiz Á, García-Villanova B, Guerra-Hernández EJ, Carrión-García CJ, Amiano P, Sánchez M-J, Molina-Montes E. Oxidative Balance Scores (OBSs) Integrating Nutrient, Food and Lifestyle Dimensions: Development of the NutrientL-OBS and FoodL-OBS. Antioxidants. 2022; 11(2):300. https://doi.org/10.3390/antiox11020300
Chicago/Turabian StyleHernández-Ruiz, Ángela, Belén García-Villanova, Eduardo J. Guerra-Hernández, Cayetano Javier Carrión-García, Pilar Amiano, María-José Sánchez, and Esther Molina-Montes. 2022. "Oxidative Balance Scores (OBSs) Integrating Nutrient, Food and Lifestyle Dimensions: Development of the NutrientL-OBS and FoodL-OBS" Antioxidants 11, no. 2: 300. https://doi.org/10.3390/antiox11020300
APA StyleHernández-Ruiz, Á., García-Villanova, B., Guerra-Hernández, E. J., Carrión-García, C. J., Amiano, P., Sánchez, M.-J., & Molina-Montes, E. (2022). Oxidative Balance Scores (OBSs) Integrating Nutrient, Food and Lifestyle Dimensions: Development of the NutrientL-OBS and FoodL-OBS. Antioxidants, 11(2), 300. https://doi.org/10.3390/antiox11020300









