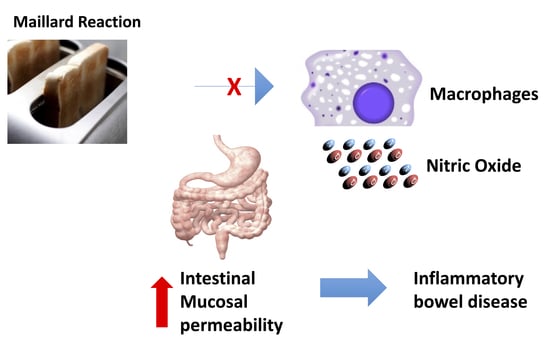
Antioxidant and Functional Activities of MRPs Derived from Different Sugar–Amino Acid Combinations and Reaction Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. MRP Sample Preparation
2.2.2. Sugar Analysis
2.2.3. Color Measurement in Soluble MRP Fraction
2.2.4. Free-Radical Scavenging Activity Measurements
DPPH Assay
TEAC Assay
ORAC Assay
2.2.5. Caco-2 Cell Culture Experiments
MTT Assay
MRP Effect on Nitric Oxide (NO) Production in Caco-2 Cells
Caco-2 Epithelial Monolayer Resistance (TEER) of Crude and Fractionated MRPs
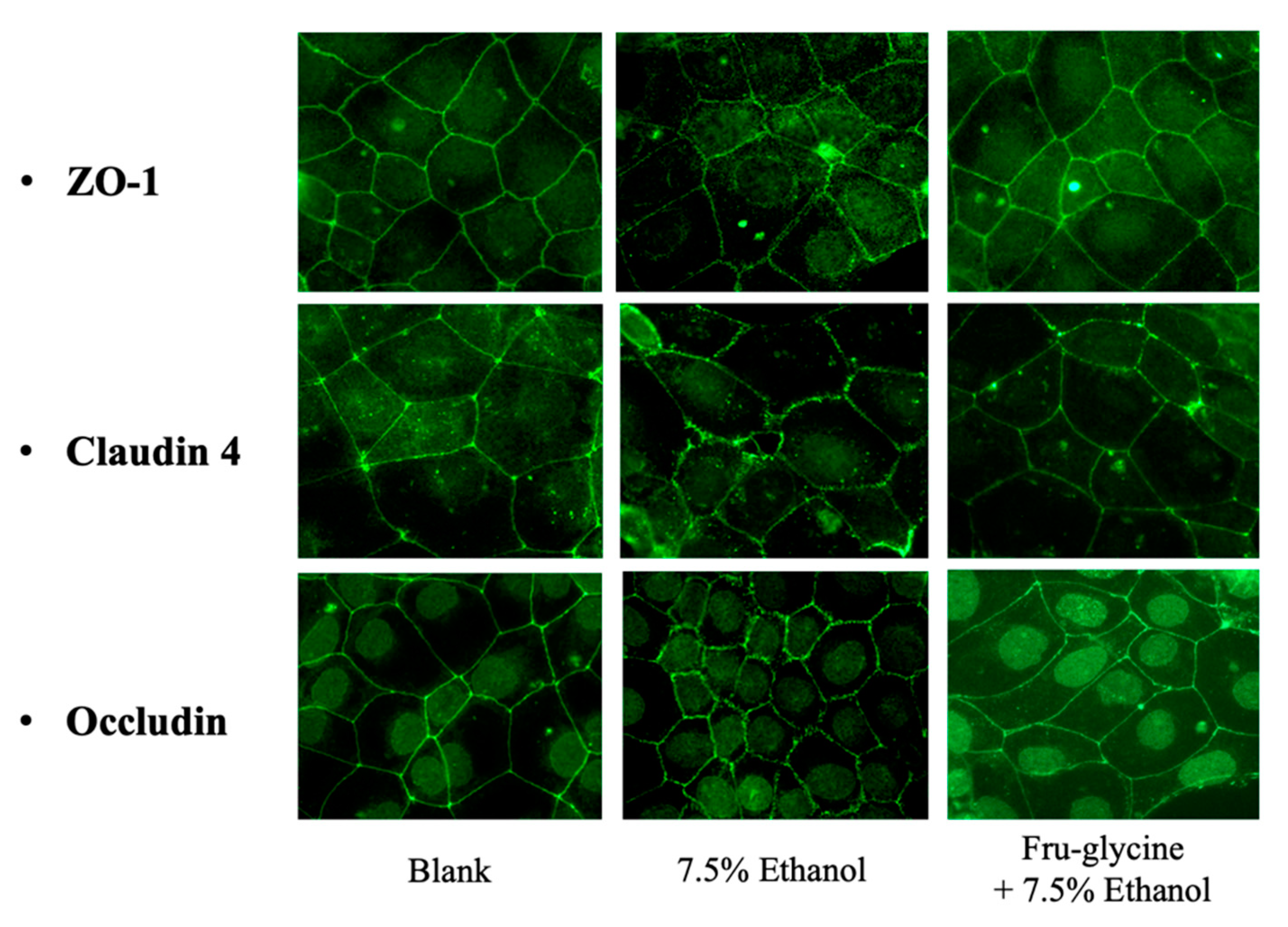
Visualization of Treated Tight-Junction Proteins
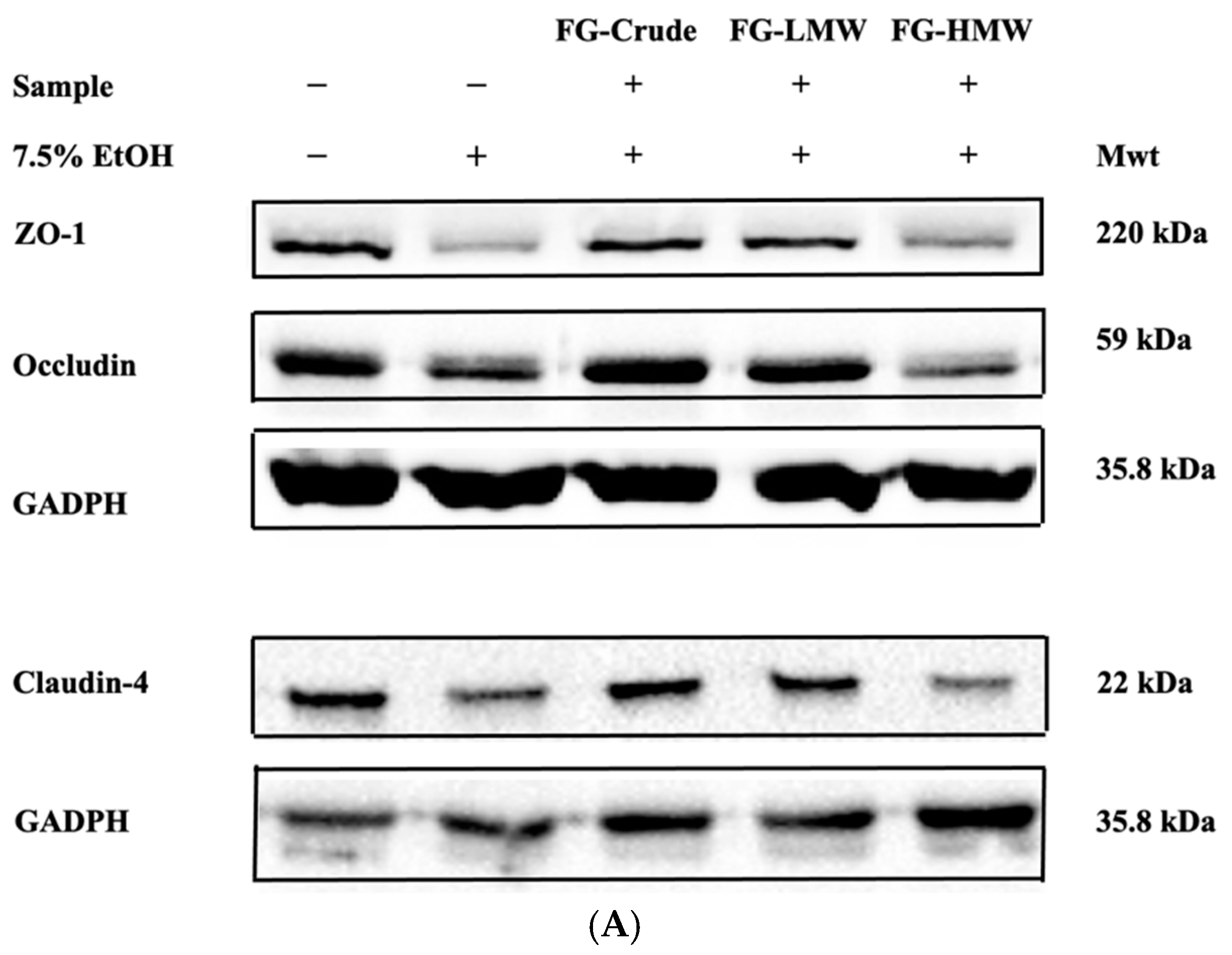
Western Blotting of Tight-Junction Proteins
2.3. Statistical Analysis
3. Results
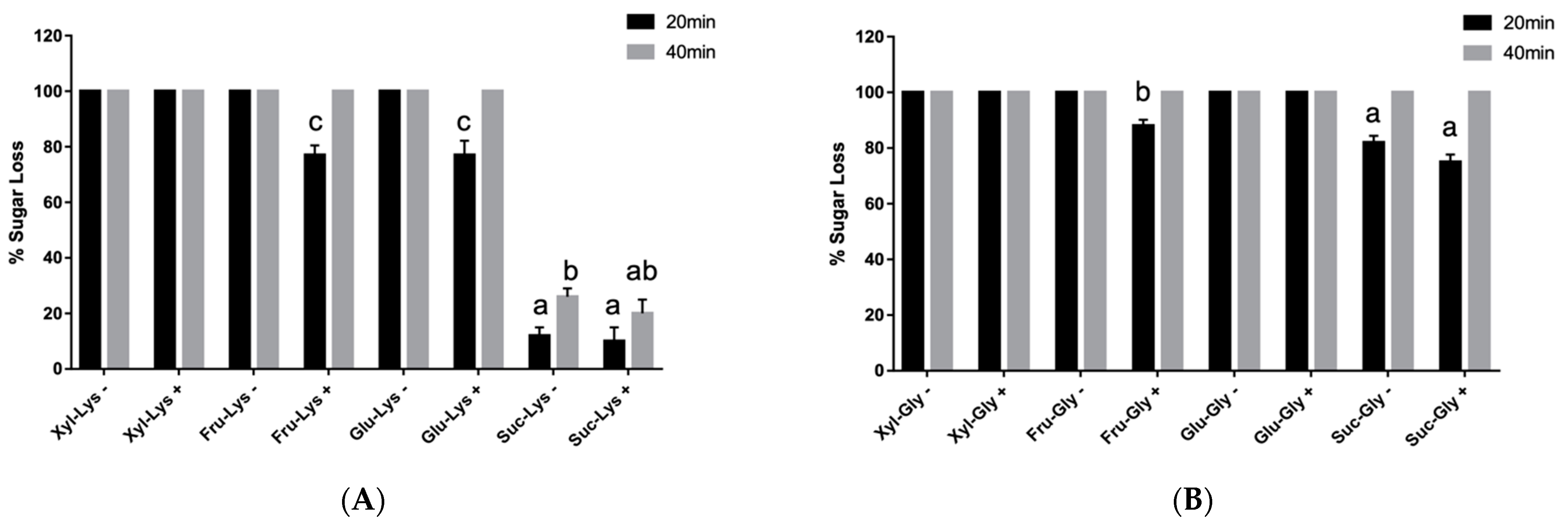
3.1. Effect of Alphacel on MRP Sugar Recovery and Color
3.2. MRP Chemical-Based Free-Radical Scavenging Activity
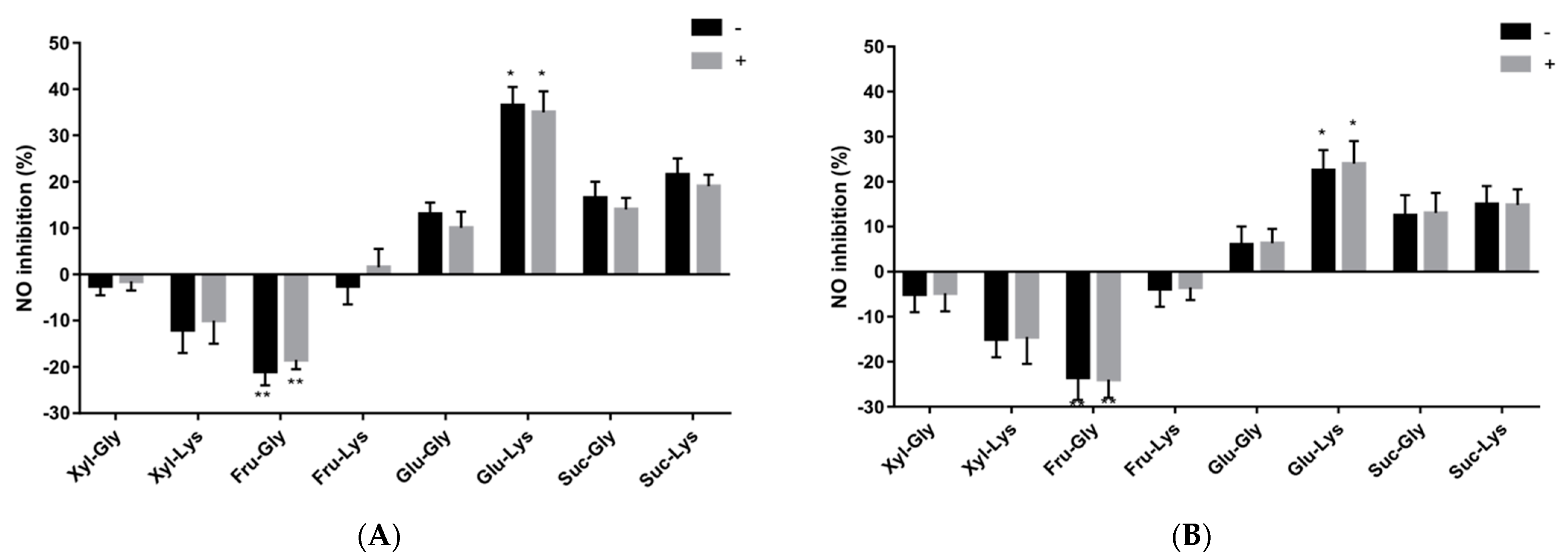
3.3. MRP and NO Production in Differentiated Caco-2 Cells
3.4. Effects of MRPs on Caco-2 Cell Viability and Paracellular Permeability
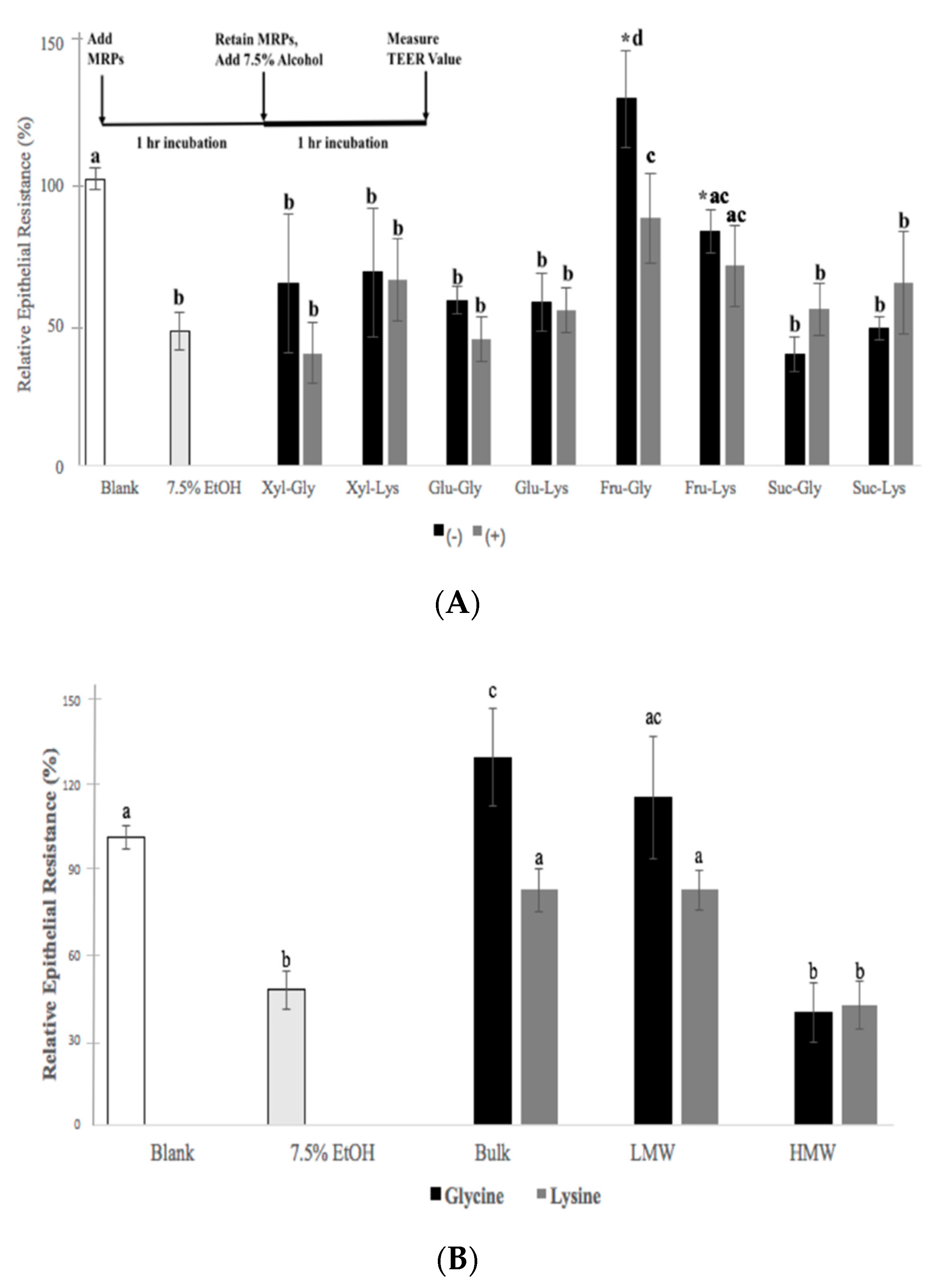
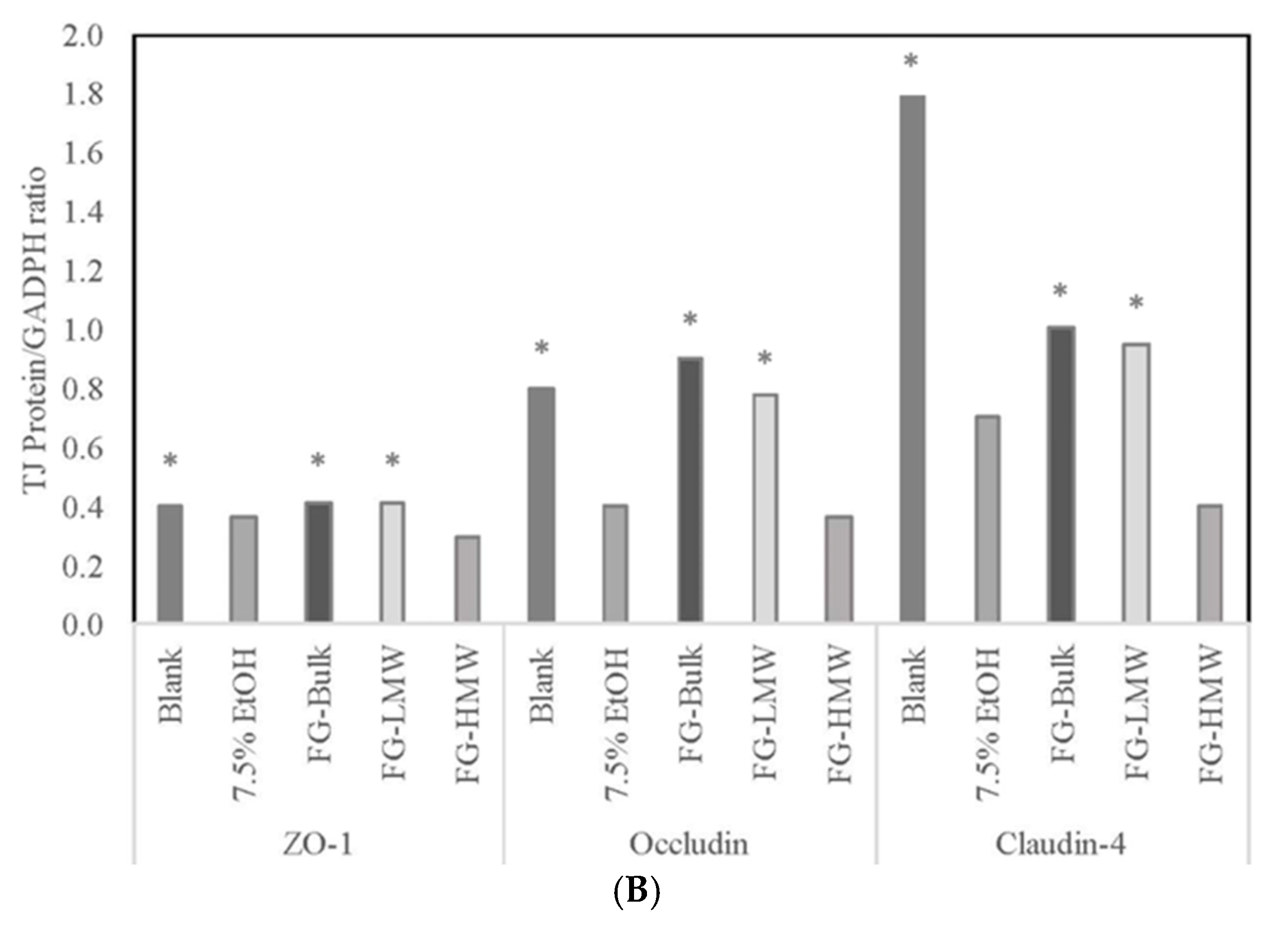
3.5. Effects of MRPs on Caco-2 Cell TEER Values and TJ Membrane Proteins
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A
| Sample | 20 min | 40 min | |||
|---|---|---|---|---|---|
| (+) | (−) | (+) | (−) | ||
| Glycine | Xylose | 49.48 ± 0.06 bx | 47.30 ± 0.02 bx | 36.23 ± 0.06 bx | 36.12 ± 0.02 bx |
| Fructose | 38.53 ± 0.05 ax | 33.53 ± 0.09 ay | 27.67 ± 0.0 ax | 22.57 ± 0.07ay | |
| Glucose | 65.73 ± 0.08 dx | 55.52 ± 0.05 cy | 45.81 ± 0.01 cx | 43.02 ± 0.04 cy | |
| Sucrose | 86.78 ± 0.08 cx | 82.05 ± 0.07 dy | 86.47 ± 0.08 dx | 80.87 ± 0.07 dy | |
| Lysine | Xylose | 41.89 ± 0.03 ax | 42.65 ± 0.05 ax | 33.64 ± 0.02 ax | 35.29 ± 0.12 ax |
| Fructose | 43.76 ± 0.03 ax | 40.44 ± 0.04 ax | 40.08 ± 0.02 ax | 37.30 ± 0.03 ay | |
| Glucose | 77.21 ± 0.18 bx | 70.64 ± 0.0 by | 73.97 ± 0.02 bx | 70.34 ± 0.02 by | |
| Sucrose | 98.63 ± 0.06 cx | 76.48 ± 0.02 by | 98.52 ± 0.06 cx | 76.25 ± 0.02 by | |
| Samples | Assays 2 | ||||||
|---|---|---|---|---|---|---|---|
| DPPH (% Inhibition) | TEAC (mg/mL Trolox Equivalent) | ORAC (µmol/Trolox Per g Samples) | |||||
| (−) 3 | (+) | (−) | (+) | (−) | (+) | ||
| Glycine | Xylose | 36.76 ± 7.41 bx | 35.41 ± 8.23 bx | 0.33 ± 0.03 bx | 0.32 ± 0.01 bx | 369 ± 23 bx | 273 ± 26 by |
| Fructose | 44.34 ± 5.23 cx | 44.21 ± 3.78 cx | 0.40 ± 0.02 bx | 0.33 ± 0.0 bx | 512 ± 28 cx | 434 ± 21 cy | |
| Glucose | 27.45 ± 2.62 bx | 26.41 ± 2.91 bx | 0.39 ± 0.02 bx | 0.37 ± 0.03 bx | 555 ± 26 cx | 424 ± 28 cy | |
| Sucrose | 4.32 ± 0.81 ax | 4.27 ± 0.65 ax | 0.02 ± 0.00 ax | 0.01 ± 0.0 ax | 5.44 ± 0.9 ax | 4.83 ± 1.2 ax | |
| Lysine | Xylose | 27.53 ± 4.19 bx | 26.75 ± 3.68 bx | 0.23 ± 0.01 bx | 0.23 ± 0.01 by | 377 ± 27 bx | 355 ± 16 bx |
| Fructose | 39.36 ± 6.29 c | 38.74 ± 4.89 c | 0.40 ± 0.02 bx | 0.193 ± 0.0 by | 354 ± 27 bx | 296 ± 29 bx | |
| Glucose | 24.31 ± 3.67 bx | 23.73 ± 3.22 bx | 0.35 ± 0.01 bx | 0.31 ± 0.0 bx | 419 ± 34 bx | 355 ± 38 by | |
| Sucrose | 5.76 ± 0.42 ax | 5.31 ± 0.41 ax | 0.03 ± 0.0 ax | 0.02 ± 0.0 ax | 12.8 ± 3.3 ax | 14.0 ± 2.9 ax | |
References
- Wijewickreme, A.N.; Kitts, D.D.; Durance, T.D. Reaction conditions influence the elementary composition and metal chelating affinity of non-dialyzable model Maillard reaction products. J. Agric. Food Chem. 1997, 45, 4577–4583. [Google Scholar] [CrossRef]
- Shen, Y.; Tebben, L.; Chen, G.; Li, Y. Effect of amino acids on Maillard reaction product formation and total antioxidant capacity in white pan bread. Int. J. Food Sci. Technol. 2018, 54, 1372–1380. [Google Scholar] [CrossRef]
- Liu, S.C.; Yang, D.J.; Jin, S.Y.; Hsu, C.H.; Chen, S.L. Kinetics of color development, pH decreasing, and anti-oxidative activity reduction of Maillard reaction in galactose/glycine model systems. Food Chem. 2008, 108, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Sogut, E.; Filiz, B.E.; Seydim, A.C. A model system based on glucose-arginine to monitor the properties of Maillard reaction products. J. Food Sci. Technol. 2021, 58, 1005–1013. [Google Scholar] [CrossRef]
- Chen, X.M.; Kitts, D.D. Correlating changes that occur in chemical properties with the generation of antioxidant capacity in different sugar-amino acid Maillard reaction models. J. Food Sci. 2011, 76, C831–C837. [Google Scholar] [CrossRef]
- Hwang, S.H.; Wang, Z.; Suh, H.W.; Lim, S.S. Antioxidant activity and inhibitory effects of 2-hydroxy-3-methylcyclopent-2-enone isolated from ribose-histidine Maillard reaction products on aldose reductase and tyrosinase. Food Funct. 2018, 9, 1790–1799. [Google Scholar] [CrossRef]
- Wolfrom, M.L.; Kashimura, N.; Horton, D. Factors affecting the Maillard browning reaction between sugars and amino acids. Nonenzymic browning of dehydrated orange juice. J. Agric. Food Chem. 1974, 22, 796–800. [Google Scholar] [CrossRef]
- Gökmen, V.; Şenyuva, H.Z. A simplified approach for the kinetic characterization of acrylamide formation in fructose-asparagine model system. Food Addit. Contam. 2006, 23, 348–354. [Google Scholar] [CrossRef]
- Kchaou, H.; Benbettaieb, N.; Jridi, M.; Nasri, M.; Debeaufort, F. Influence of Maillard reaction and temperature on functional, structure and bioactive properties of fish gelatin films. Food Hydrocoll. 2019, 97, 105196. [Google Scholar] [CrossRef]
- Cui, H.; Jia, C.; Hayat, K.; Yu, J.; Deng, S.; Karangwa, E.; Zhang, X. Controlled formation of flavor compounds by preparation and application of Maillard reaction intermediate (MRI) derived from xylose and phenylalanine. RSC Adv. 2017, 7, 45442–45451. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.J.; Sivasundaram, L.; Moreau, L.; Channell, G.A.; Hill, S.E. Effect of physical properties of food matrices on the Maillard Reaction. In Controlling Maillard Pathways to Generate Flavors; ACS Symposium Series: Chicago, IL, USA, 2010; Volume 1042, pp. 129–142. [Google Scholar]
- Jing, H.; Kitts, D.D. Comparison of the antioxidative and cytotoxic properties of glucose-lysine and fructose-lysine Maillard reaction products. Food Res. Int. 2000, 33, 509–516. [Google Scholar] [CrossRef]
- Morales, F.J.; Jiménez-Pérez, S. Free radical scavenging capacity of Maillard reaction products as related to colour and fluorescence. Food Chem. 2001, 72, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Jing, H.; Kitts, D.D. Chemical and biochemical properties of casein–sugar Maillard reaction products. Food Chem. Toxicol. 2002, 40, 1007–1015. [Google Scholar] [CrossRef]
- Gu, F.L.; Kim, J.M.; Abbas, S.; Zhang, X.M.; Xia, S.Q.; Chen, Z.X. Structure and antioxidant activity of high molecular weight Maillard reaction products from casein-glucose. Food Chem. 2010, 120, 505–511. [Google Scholar] [CrossRef]
- Park, C.K.; Kim, D.H. Relationship between fluorescence and antioxidant activity of ethanol extracts of a Maillard browning mixture. J. Am. Oil Chem. Soc. 1983, 60, 98–102. [Google Scholar] [CrossRef]
- Wijewickreme, A.N.; Kitts, D.D. Modulation of metal-induced genotoxicity by Maillard reaction products isolated from coffee. Food Chem. Toxicol. 1988, 36, 543–553. [Google Scholar] [CrossRef]
- Kitts, D.D.; Hu, C. Biological and chemical assessment of antioxidant activity of sugar-lysine model Maillard reaction products. Ann. N. Y. Acad. Sci. 2005, 1043, 501–512. [Google Scholar] [CrossRef]
- Chen, X.M.; Kitts, D.D. Antioxidant activity and chemical properties of crude and fractionated Maillard reaction products derived from four sugar–amino acid Maillard reaction model systems. Ann. N. Y. Acad. Sci. 2008, 1126, 220–224. [Google Scholar] [CrossRef]
- Kitts, D.D.; Chen, X.M.; Jing, H. Demonstration of antioxidant and anti-inflammatory bioactivities from sugar-amino acid Maillard reaction products. J. Agric. Food Chem. 2012, 60, 6718–6727. [Google Scholar] [CrossRef]
- Chen, X.M.; Liang, N.; Kitts, D.D. Chemical properties and reactive oxygen and nitrogen species quenching activities of dry sugar–amino acid maillard reaction mixtures exposed to baking temperatures. Food Res. Int. 2015, 76, 618–625. [Google Scholar] [CrossRef]
- Chen, X.M.; Kitts, D.D. Evidence for inhibition of nitric oxide and inducible nitric oxide synthase in Caco-2 and RAW 264.7 cells by a Maillard reaction product [5-(5,6-dihydro-4H-pyridin-3-ylidenemethyl) furan-2-yl]-methanol. Mol. Cell. Biochem. 2015, 406, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, M.G.; Palade, G.E. Junctional complexes in various epithelia. J. Cell Biol. 1963, 17, 375–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiba, H.; Osanai, M.; Murata, M.; Kojima, T.; Sawada, N. Transmembrane proteins of tight junctions. Biochim. Biophys. Acta (BBA) Biomembr. 2008, 1778, 588–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, T.Y.; Nguyen, D.; Bui, V.; Nguyen, H.; Hoa, N. Ethanol modulation of intestinal epithelial tight junction barrier. Am. J. Physiol. 1999, 276, G965–G974. [Google Scholar] [CrossRef]
- Chen, X.M.; Kitts, D.D. Flavonoid composition of orange peel extract ameliorates alcohol-induced tight junction dysfunction in Caco-2 monolayer. Food Chem. Toxicol. 2017, 105, 398–406. [Google Scholar] [CrossRef]
- Chen, M.; Liu, Y.; Xiong, S.; Wu, M.; Li, B.; Ruan, Z.; Hu, X. Dietary l-tryptophan alleviated LPS-induced intestinal barrier injury by regulating tight junctions in a Caco-2 cell monolayer model. Food Funct. 2019, 10, 2390–2398. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tong, J.; Chang, B.; Wang, B.; Zhang, D.; Wang, B. Effects of alcohol on intestinal epithelial barrier permeability and expression of tight junction associated proteins. Mol. Med. Rep. 2014, 9, 2352–2356. [Google Scholar] [CrossRef] [Green Version]
- Labuza, T.P.; Monnier, V.; Baynes, J.; O’Brien, J. (Eds.) Maillard Reactions in Chemistry, Food and Health; Woodhead Publishing Limited: Cambridge, UK, 1998; p. 435. [Google Scholar]
- Rodriguez, A.; Lema, P.; Bessio, I.M.; Moyna, G.; Olivaro, C.; Ferreira, F.; Panizzolo, L.A. Characterization of melanoidins and color development in Dulce de Leche, a confectionary dairy product with high sucrose content: Evaluation of pH effect, and essential manufacturing process parameter. Front. Nutr. 2021, 8, 864. [Google Scholar] [CrossRef]
- Lund, M.N.; Ray, C.A. Control of Maillard reactions in foods: Strategies and chemical mechanisms. J. Agric. Food Chem. 2017, 65, 4537–4552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedinghaus, A.J.; Ockerman, H.W. Antioxidative Maillard reaction products from reducing sugars and free amino acids in cooked ground pork patties. J. Food Sci. 1995, 60, 992–995. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Zeng, X.A.; Brennan, C.S.; Ma, H.; Aadil, R.M. Preparation and characterization of novelty food preservatives by Maillard reaction between ε-polylysine and reducing sugars. Int. J. Food Sci. Technol. 2019, 54, 1824–1835. [Google Scholar] [CrossRef]
- Jing, H.; Kitts, D.D. Antioxidant activity of sugar-lysine Maillard reaction products in cell free and cell culture systems. Arch. Biochem. Biophys. 2004, 429, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Morales, F.J.; Jiménez-Pérez, S. Peroxyl radical scavenging activity of melanoidins in aqueous systems. Eur. Food Res. Technol. 2004, 218, 515–520. [Google Scholar] [CrossRef] [Green Version]
- Somoza, V.; Wenzel, E.; Lindenmeier, M.; Grothe, D.; Erbersdobler, H.F.; Hofmann, T. Influence of feeding malt, bread crust, and a pronylated protein on the activity of chemopreventive enzymes and antioxidative defense parameters in vivo. J. Agric. Food Chem. 2005, 53, 8176–8182. [Google Scholar] [CrossRef] [PubMed]
- Seiquer, I.; Ruiz-Roca, B.; Mesías, M.; Muñoz-Hoyos, A.; Galdó, G.; Ochoa, J.J.; Navarro, M.P. The antioxidant effect of a diet rich in Maillard reaction products is attenuated after consumption by healthy male adolescents. In vitro and in vivo comparative study. J. Sci. Food Agric. 2008, 88, 1245–1252. [Google Scholar] [CrossRef]
- Hwang, I.G.; Kim, H.Y.; Woo, K.S.; Lee, J.; Jeong, H.S. Biological activities of Maillard reaction products (MRPs) in a sugar–amino acid model system. Food Chem. 2011, 126, 221–227. [Google Scholar] [CrossRef]
- ALjahdali, N.; Carbonero, F. Impact of Maillard reaction products on nutrition and health: Current knowledge and need to understand their fate in the human digestive system. Crit. Rev. Food Sci. Nutr. 2019, 59, 474–487. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R. Antioxidant activity of water-soluble Maillard reaction products. Food Chem. 2005, 93, 273–278. [Google Scholar] [CrossRef]
- Van Boekel, M.A.J.S. Kinetic aspects of the Maillard reaction: A critical review. Food/Nahrung 2001, 45, 150–159. [Google Scholar] [CrossRef]
- Rongsirikul, N.; Hongsprabhas, P. Brown pigment formation in heated sugar–protein mixed suspensions containing unmodified and peptically modified whey protein concentrates. J. Food Sci. Technol. 2016, 53, 800–807. [Google Scholar] [CrossRef] [Green Version]
- Ameur, L.A.; Mathieu, O.; Lalanne, V.; Trystram, G.; Birlouez-Aragon, I. Comparison of the effects of sucrose and hexose on furfural formation and browning in cookies baked at different temperatures. Food Chem. 2007, 101, 1407–1416. [Google Scholar] [CrossRef]
- Yeboah, F.K.; Alli, I.; Yaylayan, V.A. Reactivities of D-glucose and D-fructose during glycation of bovine serum albumin. J. Agric. Food Chem. 1999, 47, 3164–3172. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kitts, D.D. Confirmation that the Maillard reaction is the principle contributor to the antioxidant capacity of coffee brews. Food Res. Int. 2011, 44, 2418–2424. [Google Scholar] [CrossRef]
- Benjakul, S.; Lertittikul, W.; Bauer, F. Antioxidant activity of Maillard reaction products from a porcine plasma protein-sugar model system. Food Chem. 2005, 93, 189–196. [Google Scholar] [CrossRef]
- Chen, X.M.; Kitts, D.D. Identification and quantification of α-dicarbonyl compounds produced in different sugar-amino acid Maillard reaction model systems. Food Res. Int. 2011, 44, 2775–2782. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Iijima, T.; Watanabe, T.; Nakazawa, H. Antioxidative effect of Maillard reaction products using glucose-glycine model system. J. Agric. Food Chem. 1997, 45, 4106–4109. [Google Scholar] [CrossRef]
- Morales, F.J. Assessing the non-specific hydroxyl radical scavenging properties of melanoidins in a Fenton-type reaction system. Anal. Chim. Acta 2005, 534, 171–176. [Google Scholar] [CrossRef]
- Wijewickreme, A.N.; Krejpcio, Z.; Kitts, D.D. Hydroxyl scavenging activity of glucose, fructose, and ribose-lysine model Maillard products. J. Food Sci. 1999, 64, 457–461. [Google Scholar] [CrossRef]
- Vhangani, L.N.; Van Wyk, J. Antioxidant activity of Maillard reaction products (MRPs) derived from fructose reaction ribose-lysine model systems. Food Chem. 2013, 137, 92–98. [Google Scholar] [CrossRef]
- Cao, C.; Xie, J.; Hou, L.; Zhao, J.; Chen, F.; Xiao, Q.; Zhao, M.; Fan, M. Effect of glycine on reaction of cysteine-xylose: Insights on initial Maillard stage intermediates to develop meat flavor. Food Res. Int. 2017, 99, 444–453. [Google Scholar] [CrossRef]
- Chen, X.M.; Kitts, D.D. Characterization of antioxidant and anti-inflammatory activities of bioactive fractions recovered from a glucose-lysine Maillard reaction model system. Mol. Cell Biochem. 2012, 364, 147–157. [Google Scholar] [CrossRef]
- Wu, C.H.; Huang, C.M.; Lin, C.H.; Ho, Y.S.; Chen, C.M.; Lee, H.M. Advanced glycosylation end products induce NF-kB-dependent iNOS expression in RAW 264.7 cells. Mol. Cell Endocrinol. 2002, 194, 9–17. [Google Scholar] [CrossRef]
- Ruiz-Roca, B.; Delgado-Andrade, C.; Navarro, M.P.; Seiquer, I. Effects of Maillard reaction products from glucose-lysine model systems on oxidative stress markers and against oxidative induction by hydrogen peroxide in Caco-2 cells. J. Food Nutr. Res. 2011, 50, 237–248. [Google Scholar] [CrossRef]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardi, S.; Del Bo’, C.; Marino, M.; Gargari, G.; Cherubini, A.; Andrés-Lacueva, C.; Riso, P. Polyphenols and intestinal permeability: Rationale and future perspectives. J. Agric. Food Chem. 2019, 68, 1816–1829. [Google Scholar] [CrossRef]
- Mohebali, N.; Ekat, K.; Kreikemeyer, B.; Breitrück, A. Barrier Protection and Recovery Effects of Gut Commensal Bacteria on Differentiated Intestinal Epithelial Cells in vitro. Nutrients 2020, 12, 2251. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.G.; Kim, H.Y.; Lee, S.H.; Woo, K.S.; Ban, J.O.; Hong, J.T.; Jeong, H.S. Isolation and identification of an antiproliferative substance from fructose-tyrosine Maillard reaction products. Food Chem. 2012, 130, 547–551. [Google Scholar] [CrossRef]
- Lee, S.H.; Jeong, S.J.; Jang, G.Y.; Kim, M.Y.; Hwang, I.G.; Kim, H.Y.; Jeong, H.S. Isolation and identification of an antiproliferative compound from fructosese-tyrosine Maillard reaction products. J. Agric. Food Chem. 2016, 64, 3041–3047. [Google Scholar] [CrossRef]
- Chen, X.M.; Chen, G.; Chen, H.; Zhang, Y.; Kitts, D.D. Elucidation of the chemical structure and determination of the production conditions for a bioactive Maillard reaction product, [5-(5,6-dihydro-4H-pyridin-3-ylidenemethyl)furan-2-yl] methanol, isolated from a glucose-lysine heated mixture. J. Agric. Food Chem. 2015, 63, 1739–1746. [Google Scholar] [CrossRef]
| Sample | 20 min | 40 min | |||
|---|---|---|---|---|---|
| (−) | (+) | (−) | (+) | ||
| Glycine | Xylose | 81.27 ± 0.87 ex | 76.69 ± 0.41 dy | 80.44 ± 0.21 cx | 80.29 ± 0.07 cx |
| Fructose | 79.87 ± 0.11 dx | 73.23 ± 0.01 dy | 80.71 ± 0.43 cx | 81.64 ± 0.33 cx | |
| Glucose | 83.23 ± 0.50 ex | 78.17 ± 0.78 ey | 75.15 ± 1.48 cx | 81.07 ± 1.61 cx | |
| Sucrose | 27.89 ± 0.33 ax | 18.54 ± 0.35 ay | 74.03 ± 0.81 cx | 66.85 ± 0.98 cx | |
| Lysine | Xylose | 75.00 ± 0.49 dx | 71.76 ± 1.36 dy | 73.36 ± 0.34 cx | 74.98 ± 1.56 cx |
| Fructose | 55.39 ± 0.30 cx | 46.36 ± 0.18 cy | 74.55 ± 1.10 cx | 69.24 ± 2.77 cx | |
| Glucose | 74.04 ± 1.16 dx | 58.39 ± 1.01 cy | 66.45 ± 1.21 bx | 65.34 ± 0.29 bx | |
| Sucrose | 42.96 ± 2.52 bx | 23.26 ± 0.37 by | 47.47 ± 0.12 ax | 46.14 ± 0.30 ax | |
| Samples | Assays 2 | ||||||
|---|---|---|---|---|---|---|---|
| DPPH (% Inhibition) | TEAC (mmol TE Per g Sample) | ORAC (µmol TE Per g Sample) | |||||
| (−) 3 | (+) | (−) | (+) | (−) | (+) | ||
| Glycine | Xylose | 43.20 ± 1.80 cx | 34.61 ± 2.22 by | 0.39 ± 0.02 bx | 0.27 ± 0.03 by | 304 ± 28 bx | 275 ± 29 by |
| Fructose | 47.89 ± 2.64 cx | 38.13 ± 2.18 cy | 0.35 ± 0.02 bx | 0.27 ± 0.01 by | 510 ± 27 cx | 412 ± 20 cy | |
| Glucose | 57.76 ± 3.32 dx | 43.12 ± 2.71 dy | 0.48 ± 0.02 cx | 0.36 ± 0.03 cy | 562 ± 29 cx | 439 ± 28 cy | |
| Sucrose | 4.83 ± 0.31 ax | 4.96 ± 0.84 ax | 0.01 ± 0.00 ax | 0.01 ± 0.00 ax | 4.60 ± 0.98 ax | 4.5 ± 1.2 ax | |
| Lysine | Xylose | 48.90 ± 3.39 cx | 36.60 ± 2.03 cy | 0.37 ± 0.01 bx | 0.23 ± 0.01 by | 382 ± 26 bx | 309 ± 18 by |
| Fructose | 38.68 ± 1.19 bx | 27.12 ± 1.11 by | 0.28 ± 0.02 bx | 0.19 ± 0.01 by | 365 ± 23 bx | 277 ± 23 by | |
| Glucose | 50.56 ± 3.07 dx | 38.31 ± 2.62 cy | 0.39 ± 0.01 bx | 0.30 ± 0.01 bx | 416 ± 37 cx | 335 ± 42 by | |
| Sucrose | 5.83 ± 0.52 ax | 5.72 ± 0.39 ax | 0.02 ± 0.00 ax | 0.02 ± 0.00 ax | 13.8 ± 3.2 ax | 14.9 ± 2.7 ax | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitts, D.D. Antioxidant and Functional Activities of MRPs Derived from Different Sugar–Amino Acid Combinations and Reaction Conditions. Antioxidants 2021, 10, 1840. https://doi.org/10.3390/antiox10111840
Kitts DD. Antioxidant and Functional Activities of MRPs Derived from Different Sugar–Amino Acid Combinations and Reaction Conditions. Antioxidants. 2021; 10(11):1840. https://doi.org/10.3390/antiox10111840
Chicago/Turabian StyleKitts, David D. 2021. "Antioxidant and Functional Activities of MRPs Derived from Different Sugar–Amino Acid Combinations and Reaction Conditions" Antioxidants 10, no. 11: 1840. https://doi.org/10.3390/antiox10111840
APA StyleKitts, D. D. (2021). Antioxidant and Functional Activities of MRPs Derived from Different Sugar–Amino Acid Combinations and Reaction Conditions. Antioxidants, 10(11), 1840. https://doi.org/10.3390/antiox10111840