Circadian Clocks, Redox Homeostasis, and Exercise: Time to Connect the Dots?
Abstract
1. Introduction
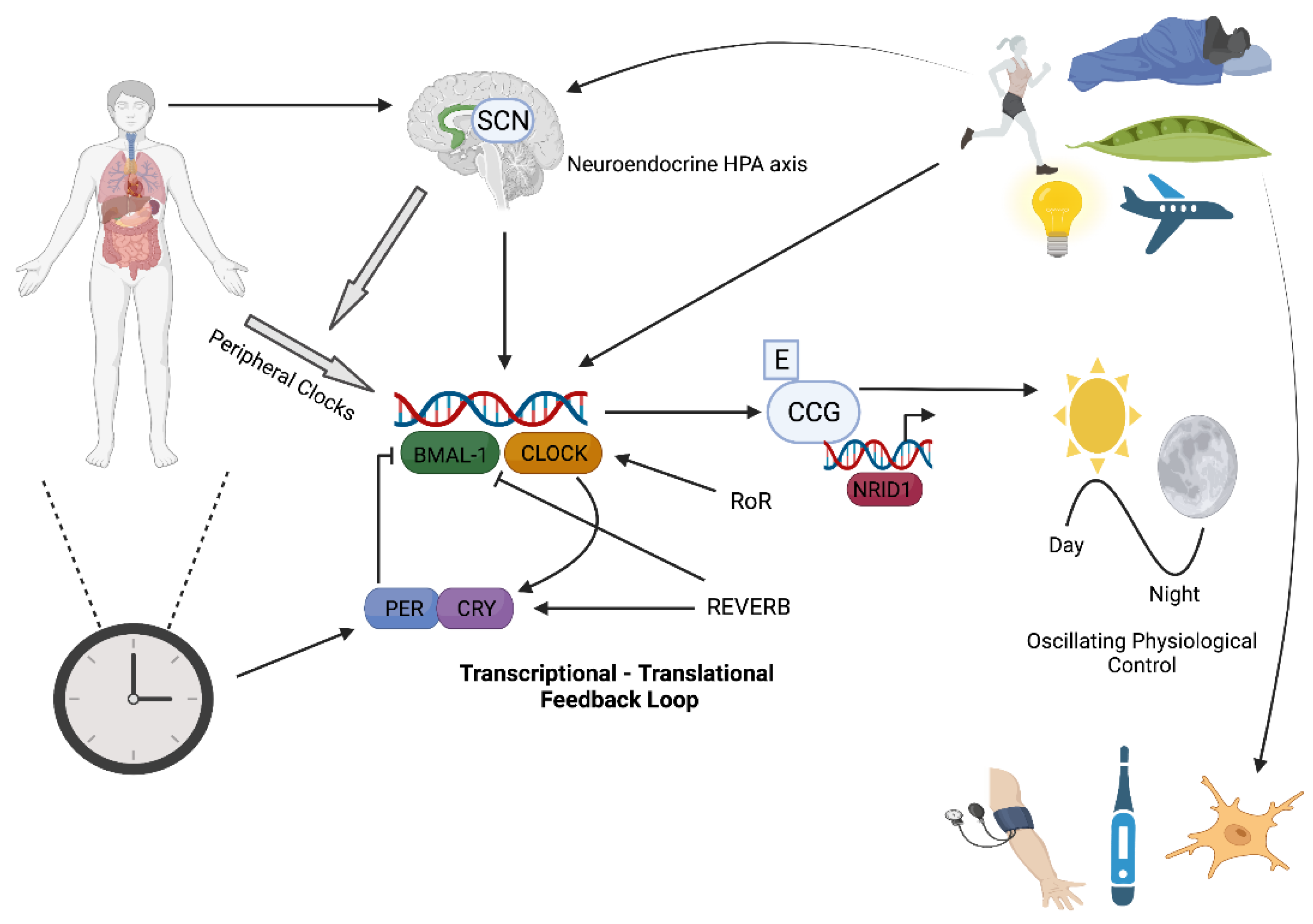
2. Circadian Rhythms and Molecular Clock Control
3. Oxidative Stress and Redox Homeostasis
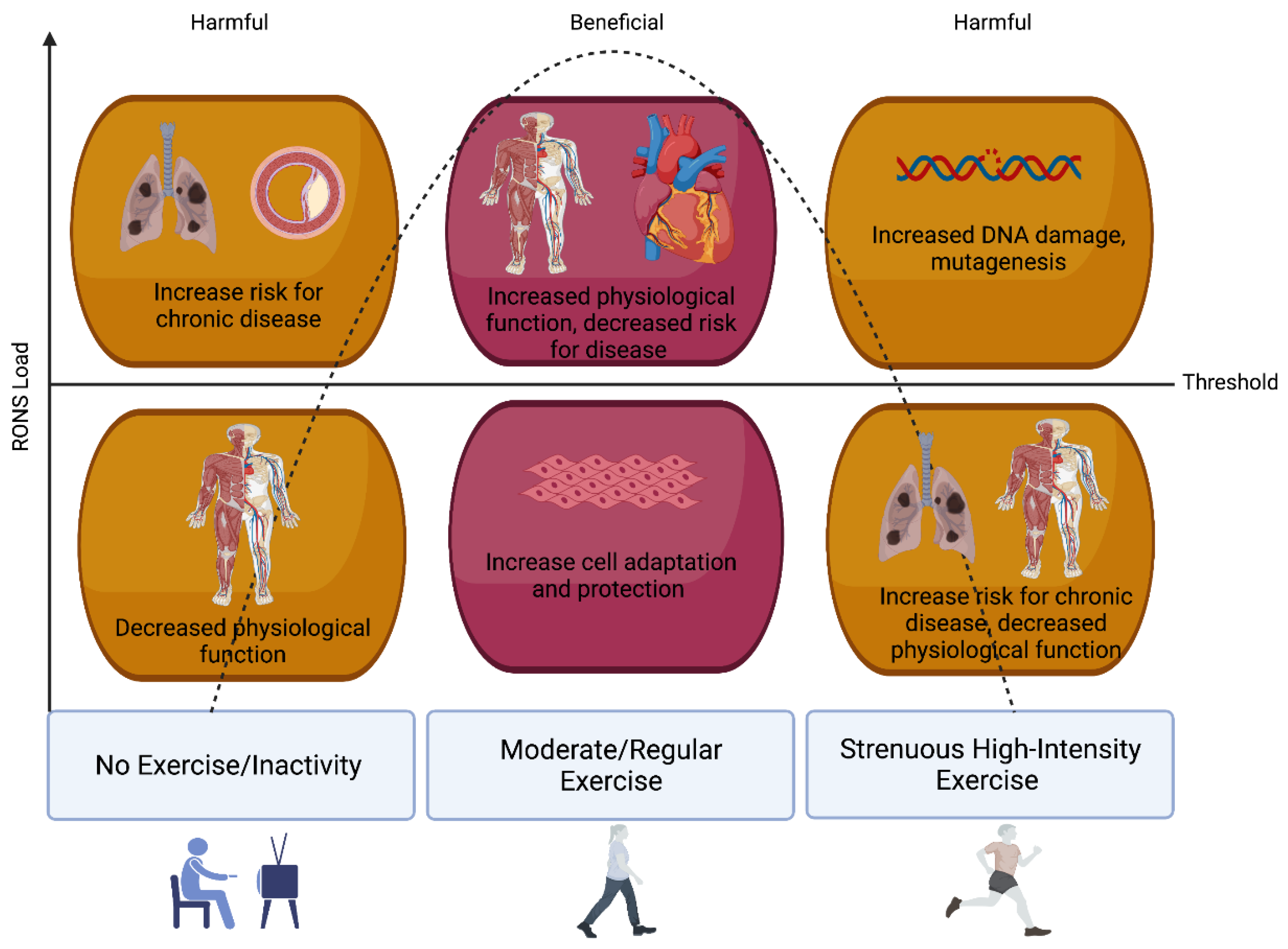
3.1. Oxidative Stress, Redox Homeostasis, and Exercise
3.2. Skeletal Muscle as a Biological Source of RONS
3.3. Antioxidants
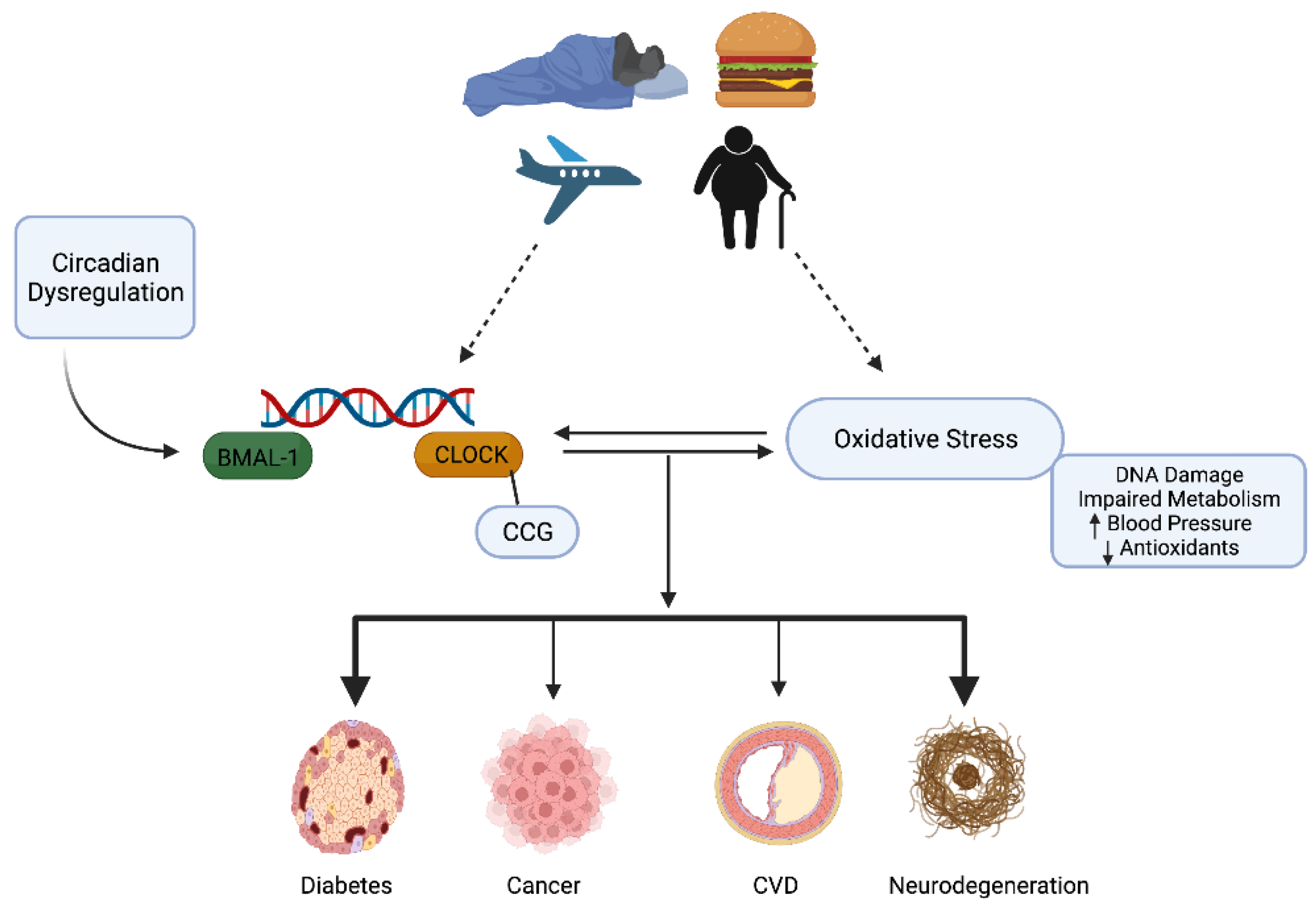
4. Circadian Rhythms, Oxidative Stress, and Redox Homeostasis
4.1. Antioxidant Regulation and Control
4.2. Circadian and Oxidative Influence on Cardiovascular Physiology and Disease
4.3. Shift Work: DNA Damage and Repair
4.4. Diabetes, Obesity, and Metabolic Control
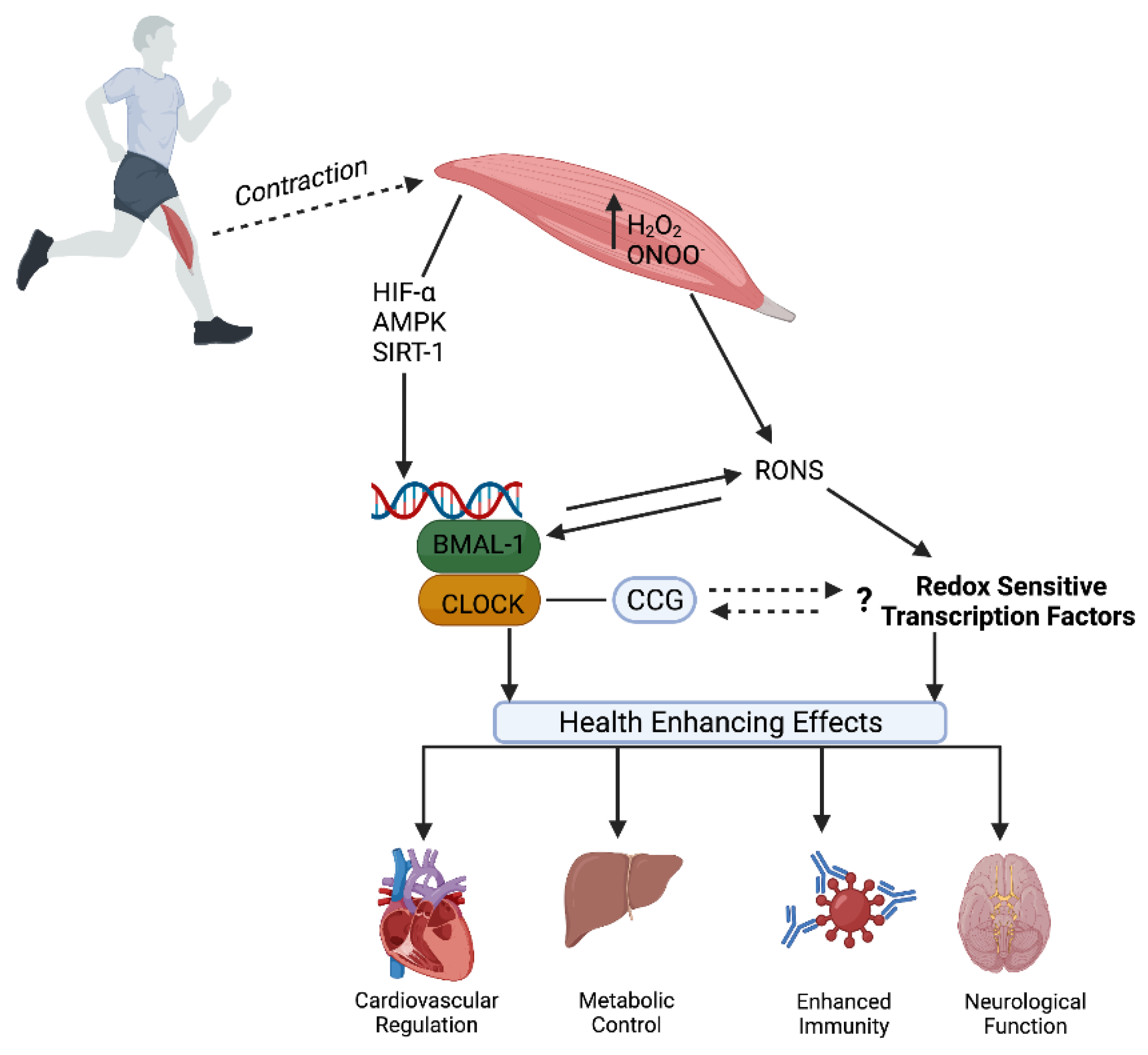
5. Exercise—Zeitgeber and Modulator of Redox Homeostasis
6. Future Directions
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Youngstedt, S.D.; Elliott, J.A.; Kripke, D.F. Human circadian phase-response curves for exercise. J. Physiol. 2019, 597, 2253–2268. [Google Scholar] [CrossRef]
- Booth, F.W.; Chakravarthy, M.V.; Spangenburg, E.E. Exercise and gene expression: Physiological regulation of the human genome through physical activity. J. Physiol. 2002, 543, 399–411. [Google Scholar] [CrossRef]
- Parr, E.B.; Heilbronn, L.K.; Hawley, J.A. A Time to Eat and a Time to Exercise. Exerc. Sport Sci. Rev. 2020, 48, 4–10. [Google Scholar] [CrossRef]
- Zimmet, P.; Thomas, C.R. Genotype, obesity and cardiovascular disease-has technical and social advancement outstripped evolution? J. Intern. Med. 2003, 254, 114–125. [Google Scholar] [CrossRef]
- Ceriello, A.; Motz, E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arter. Thromb. Vasc. Biol. 2004, 24, 816–823. [Google Scholar] [CrossRef]
- Wilking, M.; Ndiaye, M.; Mukhtar, H.; Ahmad, N. Circadian rhythm connections to oxidative stress: Implications for human health. Antioxid. Redox Signal. 2013, 19, 192–208. [Google Scholar] [CrossRef]
- Hower, I.M.; Harper, S.A.; Buford, T.W. Circadian Rhythms, Exercise, and Cardiovascular Health. J. Circadian Rhythm. 2018, 16, 7. [Google Scholar] [CrossRef]
- Ezagouri, S.; Zwighaft, Z.; Sobel, J.; Baillieul, S.; Doutreleau, S.; Ladeuix, B.; Golik, M.; Verges, S.; Asher, G. Physiological and Molecular Dissection of Daily Variance in Exercise Capacity. Cell Metab. 2019, 30, 78–91.e4. [Google Scholar] [CrossRef]
- Gabriel, B.M.; Zierath, J.R. Circadian rhythms and exercise—Re-setting the clock in metabolic disease. Nat. Rev. Endocrinol. 2019, 15, 197–206. [Google Scholar] [CrossRef]
- Tryfidou, D.V.; McClean, C.; Nikolaidis, M.G.; Davison, G.W. DNA Damage Following Acute Aerobic Exercise: A Systematic Review and Meta-analysis. Sports Med. 2020, 50, 103–127. [Google Scholar] [CrossRef]
- Bouviere, J.; Fortunato, R.S.; Dupuy, C.; Werneck-de-Castro, J.P.; Carvalho, D.P.; Louzada, R.A. Exercise-Stimulated ROS Sensitive Signaling Pathways in Skeletal Muscle. Antioxidants 2021, 10, 537. [Google Scholar] [CrossRef]
- Wolff, C.A.; Esser, K.A. Exercise Timing and Circadian Rhythms. Curr. Opin. Physiol. 2019, 10, 64–69. [Google Scholar] [CrossRef]
- Zhang, R.; Lahens, N.F.; Ballance, H.I.; Hughes, M.E.; Hogenesch, J.B. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. USA 2014, 111, 16219–16224. [Google Scholar] [CrossRef]
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935–941. [Google Scholar] [CrossRef]
- Hastings, M.H.; Maywood, E.S.; Brancaccio, M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 2018, 19, 453–469. [Google Scholar] [CrossRef]
- Lananna, B.V.; Musiek, E.S. The wrinkling of time: Aging, inflammation, oxidative stress, and the circadian clock in neurodegeneration. Neurobiol. Dis. 2020, 139, 104832. [Google Scholar] [CrossRef]
- Tahara, Y.; Shibata, S. Entrainment of the mouse circadian clock: Effects of stress, exercise, and nutrition. Free Radic. Biol. Med. 2018, 119, 129–138. [Google Scholar] [CrossRef]
- Bae, S.A.; Fang, M.Z.; Rustgi, V.; Zarbl, H.; Androulakis, I.P. At the Interface of Lifestyle, Behavior, and Circadian Rhythms: Metabolic Implications. Front. Nutr. 2019, 6, 132. [Google Scholar] [CrossRef]
- Roenneberg, T.; Merrow, M. The Circadian Clock and Human Health. Curr. Biol. 2016, 26, R432–R443. [Google Scholar] [CrossRef]
- Huang, R.C. The discoveries of molecular mechanisms for the circadian rhythm: The 2017 Nobel Prize in Physiology or Medicine. Biomed. J. 2018, 41, 5–8. [Google Scholar] [CrossRef]
- Wang, J.; Symul, L.; Yeung, J.; Gobet, C.; Sobel, J.; Luck, S.; Westermark, P.O.; Molina, N.; Naef, F. Circadian clock-dependent and -independent posttranscriptional regulation underlies temporal mRNA accumulation in mouse liver. Proc. Natl. Acad. Sci. USA 2018, 115, E1916–E1925. [Google Scholar] [CrossRef]
- Patke, A.; Young, M.W.; Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2020, 21, 67–84. [Google Scholar] [CrossRef]
- Koike, N.; Yoo, S.H.; Huang, H.C.; Kumar, V.; Lee, C.; Kim, T.K.; Takahashi, J.S. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science 2012, 338, 349–354. [Google Scholar] [CrossRef]
- Xie, Y.; Tang, Q.; Chen, G.; Xie, M.; Yu, S.; Zhao, J.; Chen, L. New Insights into the Circadian Rhythm and Its Related Diseases. Front. Physiol. 2019, 10, 682. [Google Scholar] [CrossRef]
- Khaper, N.; Bailey, C.D.C.; Ghugre, N.R.; Reitz, C.; Awosanmi, Z.; Waines, R.; Martino, T.A. Implications of disturbances in circadian rhythms for cardiovascular health: A new frontier in free radical biology. Free Radic. Biol. Med. 2018, 119, 85–92. [Google Scholar] [CrossRef]
- Ayyar, V.S.; Sukumaran, S. Circadian rhythms: Influence on physiology, pharmacology, and therapeutic interventions. J. Pharmacokinet. Pharmacodyn. 2021, 48, 321–338. [Google Scholar] [CrossRef]
- O’Connell, E.J.; Martinez, C.A.; Liang, Y.G.; Cistulli, P.A.; Cook, K.M. Out of breath, out of time: Interactions between HIF and circadian rhythms. Am. J. Physiol. Cell Physiol. 2020, 319, C533–C540. [Google Scholar] [CrossRef]
- Kondratov, R.V.; Gorbacheva, V.Y.; Antoch, M.P. The role of mammalian circadian proteins in normal physiology and genotoxic stress responses. Curr. Top. Dev. Biol. 2007, 78, 173–216. [Google Scholar]
- Cho, K. Chronic ‘jet lag’ produces temporal lobe atrophy and spatial cognitive deficits. Nat. Neurosci. 2001, 4, 567–568. [Google Scholar] [CrossRef]
- Roenneberg, T.; Allebrandt, K.V.; Merrow, M.; Vetter, C. Social jetlag and obesity. Curr. Biol. 2012, 22, 939–943. [Google Scholar] [CrossRef]
- James, S.M.; Honn, K.A.; Gaddameedhi, S.; Van Dongen, H.P.A. Shift Work: Disrupted Circadian Rhythms and Sleep-Implications for Health and Well-Being. Curr. Sleep Med. Rep. 2017, 3, 104–112. [Google Scholar] [CrossRef]
- Potter, G.D.M.; Wood, T.R. The Future of Shift Work: Circadian Biology Meets Personalised Medicine and Behavioural Science. Front. Nutr. 2020, 7, 116. [Google Scholar] [CrossRef]
- Chang, A.M.; Aeschbach, D.; Duffy, J.F.; Czeisler, C.A. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc. Natl. Acad. Sci. USA 2015, 112, 1232–1237. [Google Scholar] [CrossRef]
- Thomas, J.M.; Kern, P.A.; Bush, H.M.; McQuerry, K.J.; Black, W.S.; Clasey, J.L.; Pendergast, J.S. Circadian rhythm phase shifts caused by timed exercise vary with chronotype. JCI Insight 2020, 5, e134270. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Sen, C.K.; Packer, L. Thiol homeostasis and supplements in physical exercise. Am. J. Clin. Nutr. 2000, 72, 653S–669S. [Google Scholar] [CrossRef]
- Fukai, T.; Folz, R.J.; Landmesser, U.; Harrison, D.G. Extracellular superoxide dismutase and cardiovascular disease. Cardiovasc. Res. 2002, 55, 239–249. [Google Scholar] [CrossRef]
- McClean, C.M.; McLaughlin, J.; Burke, G.; Murphy, M.H.; Trinick, T.; Duly, E.; Davison, G.W. The effect of acute aerobic exercise on pulse wave velocity and oxidative stress following postprandial hypertriglyceridemia in healthy men. Eur. J. Appl. Physiol. 2007, 100, 225–234. [Google Scholar] [CrossRef]
- Jones, D.P. Redefining oxidative stress. Antioxid. Redox Signal. 2006, 8, 1865–1879. [Google Scholar] [CrossRef]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Louzada, R.A.; Bouviere, J.; Matta, L.P.; Werneck-de-Castro, J.P.; Dupuy, C.; Carvalho, D.P.; Fortunato, R.S. Redox Signaling in Widespread Health Benefits of Exercise. Antioxid. Redox Signal. 2020, 33, 745–760. [Google Scholar] [CrossRef]
- Stocker, R.; Keaney, J.F., Jr. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004, 84, 1381–1478. [Google Scholar] [CrossRef]
- Davison, G.W.; Hughes, C.M.; Bell, R.A. Exercise and mononuclear cell DNA damage: The effects of antioxidant supplementation. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 480–492. [Google Scholar] [CrossRef]
- Fogarty, M.C.; Devito, G.; Hughes, C.M.; Burke, G.; Brown, J.C.; McEneny, J.; Brown, D.; McClean, C.; Davison, G.W. Effects of alpha-lipoic acid on mtDNA damage after isolated muscle contractions. Med. Sci. Sports Exerc. 2013, 45, 1469–1477. [Google Scholar] [CrossRef]
- Brown, M.; McClean, C.M.; Davison, G.W.; Brown, J.C.W.; Murphy, M.H. Preceding exercise and postprandial hypertriglyceridemia: Effects on lymphocyte cell DNA damage and vascular inflammation. Lipids Health Dis. 2019, 18, 125. [Google Scholar] [CrossRef]
- McKeegan, K.; Mason, S.A.; Trewin, A.J.; Keske, M.A.; Wadley, G.D.; Della Gatta, P.A.; Nikolaidis, M.G.; Parker, L. Reactive oxygen species in exercise and insulin resistance: Working towards personalized antioxidant treatment. Redox Biol. 2021, 44, 102005. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.C.; Domenech, E.; Vina, J. Moderate exercise is an antioxidant: Upregulation of antioxidant genes by training. Free Radic. Biol. Med. 2008, 44, 126–131. [Google Scholar] [CrossRef]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Radak, Z.; Chung, H.Y.; Koltai, E.; Taylor, A.W.; Goto, S. Exercise, oxidative stress and hormesis. Ageing Res. Rev. 2008, 7, 34–42. [Google Scholar] [CrossRef]
- Powers, S.K.; Duarte, J.; Kavazis, A.N.; Talbert, E.E. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp. Physiol. 2010, 95, 1–9. [Google Scholar] [CrossRef]
- Irrcher, I.; Ljubicic, V.; Hood, D.A. Interactions between ROS and AMP kinase activity in the regulation of PGC-1alpha transcription in skeletal muscle cells. Am. J. Physiol.-Cell Physiol. 2009, 296, C116–C123. [Google Scholar] [CrossRef]
- Nemes, R.; Koltai, E.; Taylor, A.W.; Suzuki, K.; Gyori, F.; Radak, Z. Reactive Oxygen and Nitrogen Species Regulate Key Metabolic, Anabolic, and Catabolic Pathways in Skeletal Muscle. Antioxidants 2018, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Ristow, M.; Zarse, K.; Oberbach, A.; Kloting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Bluher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Chung, H.Y.; Goto, S. Exercise and hormesis: Oxidative stress-related adaptation for successful aging. Biogerontology 2005, 6, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, W.C.; Clarkson, P.M.; White, J.S.; Hsieh, S.S.; Frykman, P.N.; Maughan, R.J. Delayed onset muscle soreness following repeated bouts of downhill running. J. Appl. Physiol. 1985, 59, 710–715. [Google Scholar] [CrossRef]
- Radak, Z.; Taylor, A.W.; Ohno, H.; Goto, S. Adaptation to exercise-induced oxidative stress: From muscle to brain. Exerc. Immunol. Rev. 2001, 7, 90–107. [Google Scholar]
- Margaritelis, N.V.; Paschalis, V.; Theodorou, A.A.; Kyparos, A.; Nikolaidis, M.G. Redox basis of exercise physiology. Redox Biol. 2020, 35, 101499. [Google Scholar] [CrossRef]
- Radak, Z.; Ishihara, K.; Tekus, E.; Varga, C.; Posa, A.; Balogh, L.; Boldogh, I.; Koltai, E. Exercise, oxidants, and antioxidants change the shape of the bell-shaped hormesis curve. Redox Biol. 2017, 12, 285–290. [Google Scholar] [CrossRef]
- Lovlin, R.; Cottle, W.; Pyke, I.; Kavanagh, M.; Belcastro, A.N. Are indices of free radical damage related to exercise intensity. Eur. J. Appl. Physiol. Occup. Physiol. 1987, 56, 313–316. [Google Scholar] [CrossRef]
- Corbett, N.; Alda, M. On telomeres long and short. J. Psychiatry Neurosci. 2015, 40, 3–4. [Google Scholar] [CrossRef]
- Sousa, C.V.; Aguiar, S.S.; Santos, P.A.; Barbosa, L.P.; Knechtle, B.; Nikolaidis, P.T.; Deus, L.A.; Sales, M.M.; Rosa, E.C.C.C.; Rosa, T.S.; et al. Telomere length and redox balance in master endurance runners: The role of nitric oxide. Exp. Gerontol. 2019, 117, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Bogdanis, G.C.; Stavrinou, P.; Fatouros, I.G.; Philippou, A.; Chatzinikolaou, A.; Draganidis, D.; Ermidis, G.; Maridaki, M. Short-term high-intensity interval exercise training attenuates oxidative stress responses and improves antioxidant status in healthy humans. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 61, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Henriquez-Olguin, C.; Renani, L.B.; Arab-Ceschia, L.; Raun, S.H.; Bhatia, A.; Li, Z.; Knudsen, J.R.; Holmdahl, R.; Jensen, T.E. Adaptations to high-intensity interval training in skeletal muscle require NADPH oxidase 2. Redox Biol. 2019, 24, 101188. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Sies, H.; Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979, 59, 527–605. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.C.; Salvador-Pascual, A.; Cabo, H.; Ferrando, B.; Vina, J. Redox modulation of mitochondriogenesis in exercise. Does antioxidant supplementation blunt the benefits of exercise training? Free Radic. Biol. Med. 2015, 86, 37–46. [Google Scholar] [CrossRef]
- Henriquez-Olguin, C.; Meneses-Valdes, R.; Jensen, T.E. Compartmentalized muscle redox signals controlling exercise metabolism—Current state, future challenges. Redox Biol. 2020, 35, 101473. [Google Scholar] [CrossRef]
- Ito, N.; Ruegg, U.T.; Kudo, A.; Miyagoe-Suzuki, Y.; Takeda, S. Activation of calcium signaling through Trpv1 by nNOS and peroxynitrite as a key trigger of skeletal muscle hypertrophy. Nat. Med. 2013, 19, 101–106. [Google Scholar] [CrossRef]
- Ito, N.; Ruegg, U.T.; Kudo, A.; Miyagoe-Suzuki, Y.; Takeda, S. Capsaicin mimics mechanical load-induced intracellular signaling events: Involvement of TRPV1-mediated calcium signaling in induction of skeletal muscle hypertrophy. Channels 2013, 7, 221–224. [Google Scholar] [CrossRef]
- Ito, N.; Ruegg, U.T.; Takeda, S. ATP-Induced Increase in Intracellular Calcium Levels and Subsequent Activation of mTOR as Regulators of Skeletal Muscle Hypertrophy. Int. J. Mol. Sci. 2018, 19, 2804. [Google Scholar] [CrossRef]
- McClean, C.M.; McNeilly, A.M.; Trinick, T.R.; Murphy, M.H.; Duly, E.; McLaughlin, J.; McEneny, J.; Burke, G.; Davison, G.W. Acute exercise and impaired glucose tolerance in obese humans. J. Clin. Lipidol. 2009, 3, 262–268. [Google Scholar] [CrossRef]
- McConell, G.K.; Rattigan, S.; Lee-Young, R.S.; Wadley, G.D.; Merry, T.L. Skeletal muscle nitric oxide signaling and exercise: A focus on glucose metabolism. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E301–E307. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.W.; Prosser, B.L.; Lederer, W.J. Mechanical stretch-induced activation of ROS/RNS signaling in striated muscle. Antioxid. Redox Signal. 2014, 20, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.F.; Laitano, O. Regulation of NADPH oxidases in skeletal muscle. Free Radic. Biol. Med. 2016, 98, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Nordberg, J.; Arner, E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Balsera, M.; Buchanan, B.B. Evolution of the thioredoxin system as a step enabling adaptation to oxidative stress. Free Radic. Biol. Med. 2019, 140, 28–35. [Google Scholar] [CrossRef]
- Perkins, A.; Nelson, K.J.; Parsonage, D.; Poole, L.B.; Karplus, P.A. Peroxiredoxins: Guardians against oxidative stress and modulators of peroxide signaling. Trends Biochem. Sci. 2015, 40, 435–445. [Google Scholar] [CrossRef]
- Cobley, J.N.; Husi, H. Immunological Techniques to Assess Protein Thiol Redox State: Opportunities, Challenges and Solutions. Antioxidants 2020, 9, 315. [Google Scholar] [CrossRef]
- Wadley, A.J.; Aldred, S.; Coles, S.J. An unexplored role for Peroxiredoxin in exercise-induced redox signalling? Redox Biol. 2016, 8, 51–58. [Google Scholar] [CrossRef]
- Fanjul-Moles, M.L.; Lopez-Riquelme, G.O. Relationship between Oxidative Stress, Circadian Rhythms, and AMD. Oxidative Med. Cell. Longev. 2016, 2016, 7420637. [Google Scholar] [CrossRef]
- Yang, Z.; Kim, H.; Ali, A.; Zheng, Z.; Zhang, K. Interaction between stress responses and circadian metabolism in metabolic disease. Liver Res. 2017, 1, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Timmons, G.A.; O’Siorain, J.R.; Kennedy, O.D.; Curtis, A.M.; Early, J.O. Innate Rhythms: Clocks at the Center of Monocyte and Macrophage Function. Front. Immunol. 2020, 11, 1743. [Google Scholar] [CrossRef] [PubMed]
- Early, J.O.; Menon, D.; Wyse, C.A.; Cervantes-Silva, M.P.; Zaslona, Z.; Carroll, R.G.; Palsson-McDermott, E.M.; Angiari, S.; Ryan, D.G.; Corcoran, S.E.; et al. Circadian clock protein BMAL1 regulates IL-1beta in macrophages via NRF2. Proc. Natl. Acad. Sci. USA 2018, 115, E8460–E8468. [Google Scholar] [CrossRef] [PubMed]
- Rutter, J.; Reick, M.; Wu, L.C.; McKnight, S.L. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science 2001, 293, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Wible, R.S.; Ramanathan, C.; Sutter, C.H.; Olesen, K.M.; Kensler, T.W.; Liu, A.C.; Sutter, T.R. NRF2 regulates core and stabilizing circadian clock loops, coupling redox and timekeeping in Mus musculus. Elife 2018, 7, e31656. [Google Scholar] [CrossRef]
- O’Neill, J.S.; Reddy, A.B. Circadian clocks in human red blood cells. Nature 2011, 469, 498–503. [Google Scholar] [CrossRef]
- Sato, T.; Greco, C.M. Expanding the link between circadian rhythms and redox metabolism of epigenetic control. Free Radic. Biol. Med. 2021, 170, 50–58. [Google Scholar] [CrossRef]
- Krishnan, N.; Kretzschmar, D.; Rakshit, K.; Chow, E.; Giebultowicz, J.M. The circadian clock gene period extends healthspan in aging Drosophila melanogaster. Aging 2009, 1, 937–948. [Google Scholar] [CrossRef]
- Patel, S.A.; Velingkaar, N.S.; Kondratov, R.V. Transcriptional control of antioxidant defense by the circadian clock. Antioxid. Redox Signal. 2014, 20, 2997–3006. [Google Scholar] [CrossRef]
- Ashok Kumar, P.V.; Dakup, P.P.; Sarkar, S.; Modasia, J.B.; Motzner, M.S.; Gaddameedhi, S. It’s About Time: Advances in Understanding the Circadian Regulation of DNA Damage and Repair in Carcinogenesis and Cancer Treatment Outcomes. Yale J. Biol. Med. 2019, 92, 305–316. [Google Scholar]
- Lapenna, D.; De Gioia, S.; Mezzetti, A.; Porreca, E.; Ciofani, G.; Marzio, L.; Capani, F.; Di Ilio, C.; Cuccurullo, F. Circadian variations in antioxidant defences and lipid peroxidation in the rat heart. Free Radic. Res. Commun. 1992, 17, 187–194. [Google Scholar] [CrossRef]
- Tomas-Zapico, C.; Coto-Montes, A.; Martinez-Fraga, J.; Rodriguez-Colunga, M.J.; Tolivia, D. Effects of continuous light exposure on antioxidant enzymes, porphyric enzymes and cellular damage in the Harderian gland of the Syrian hamster. J. Pineal Res. 2003, 34, 60–68. [Google Scholar] [CrossRef]
- Diaz-Munoz, M.; Hernandez-Munoz, R.; Suarez, J.; Chagoya de Sanchez, V. Day-night cycle of lipid peroxidation in rat cerebral cortex and their relationship to the glutathione cycle and superoxide dismutase activity. Neuroscience 1985, 16, 859–863. [Google Scholar] [CrossRef]
- Kondratov, R.V.; Vykhovanets, O.; Kondratova, A.A.; Antoch, M.P. Antioxidant N-acetyl-L-cysteine ameliorates symptoms of premature aging associated with the deficiency of the circadian protein BMAL1. Aging 2009, 1, 979–987. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Willich, S.N.; Goldberg, R.J.; Maclure, M.; Perriello, L.; Muller, J.E. Increased onset of sudden cardiac death in the first three hours after awakening. Am. J. Cardiol. 1992, 70, 65–68. [Google Scholar] [CrossRef]
- Takeda, N.; Maemura, K. Circadian clock and the onset of cardiovascular events. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2016, 39, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, R.; Fabbian, F.; Cappadona, R.; De Giorgi, A.; Bravi, F.; Carradori, T.; Flacco, M.E.; Manzoli, L. Daylight Saving Time and Acute Myocardial Infarction: A Meta-Analysis. J. Clin. Med. 2019, 8, 404. [Google Scholar] [CrossRef] [PubMed]
- Gewaltig, M.T.; Kojda, G. Vasoprotection by nitric oxide: Mechanisms and therapeutic potential. Cardiovasc. Res. 2002, 55, 250–260. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Man, A.W.C.; Li, H.; Xia, N. Circadian Rhythm: Potential Therapeutic Target for Atherosclerosis and Thrombosis. Int. J. Mol. Sci. 2021, 22, 676. [Google Scholar] [CrossRef] [PubMed]
- Thosar, S.S.; Berman, A.M.; Herzig, M.X.; McHill, A.W.; Bowles, N.P.; Swanson, C.M.; Clemons, N.A.; Butler, M.P.; Clemons, A.A.; Emens, J.S.; et al. Circadian Rhythm of Vascular Function in Midlife Adults. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1203–1211. [Google Scholar] [CrossRef]
- Teixeira, K.R.C.; Dos Santos, C.P.; de Medeiros, L.A.; Mendes, J.A.; Cunha, T.M.; De Angelis, K.; Penha-Silva, N.; de Oliveira, E.P.; Crispim, C.A. Night workers have lower levels of antioxidant defenses and higher levels of oxidative stress damage when compared to day workers. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tynes, T.; Hannevik, M.; Andersen, A.; Vistnes, A.I.; Haldorsen, T. Incidence of breast cancer in Norwegian female radio and telegraph operators. Cancer Causes Control 1996, 7, 197–204. [Google Scholar] [CrossRef]
- Schernhammer, E.S.; Laden, F.; Speizer, F.E.; Willett, W.C.; Hunter, D.J.; Kawachi, I.; Fuchs, C.S.; Colditz, G.A. Night-shift work and risk of colorectal cancer in the nurses’ health study. J. Natl. Cancer Inst. 2003, 95, 825–828. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, P.; Mirick, D.K.; Randolph, T.W.; Gong, J.; Buchanan, D.T.; Zhang, J.J.; Davis, S. Oxidative DNA damage during sleep periods among nightshift workers. Occup. Environ. Med. 2016, 73, 537–544. [Google Scholar] [CrossRef]
- Koritala, B.S.C.; Porter, K.I.; Arshad, O.A.; Gajula, R.P.; Mitchell, H.D.; Arman, T.; Manjanatha, M.G.; Teeguarden, J.; Van Dongen, H.P.A.; McDermott, J.E.; et al. Night shift schedule causes circadian dysregulation of DNA repair genes and elevated DNA damage in humans. J. Pineal Res. 2021, 70, e12726. [Google Scholar] [CrossRef]
- Bhatti, P.; Mirick, D.K.; Randolph, T.W.; Gong, J.; Buchanan, D.T.; Zhang, J.J.; Davis, S. Oxidative DNA damage during night shift work. Occup. Environ. Med. 2017, 74, 680–683. [Google Scholar] [CrossRef]
- Hudec, M.; Dankova, P.; Solc, R.; Bettazova, N.; Cerna, M. Epigenetic Regulation of Circadian Rhythm and Its Possible Role in Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 3005. [Google Scholar] [CrossRef]
- Pan, A.; Schernhammer, E.S.; Sun, Q.; Hu, F.B. Rotating night shift work and risk of type 2 diabetes: Two prospective cohort studies in women. PLoS Med. 2011, 8, e1001141. [Google Scholar] [CrossRef]
- Lee, J.; Ma, K.; Moulik, M.; Yechoor, V. Untimely oxidative stress in beta-cells leads to diabetes—Role of circadian clock in beta-cell function. Free Radic. Biol. Med. 2018, 119, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Bell, G.I.; Polonsky, K.S. Diabetes mellitus and genetically programmed defects in beta-cell function. Nature 2001, 414, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.J.; Bonadonna, R.C. Role of reduced beta-cell mass versus impaired beta-cell function in the pathogenesis of type 2 diabetes. Diabetes Care 2013, 36 (Suppl. 2), S113–S119. [Google Scholar] [CrossRef]
- Burrack, A.L.; Martinov, T.; Fife, B.T. T Cell-Mediated Beta Cell Destruction: Autoimmunity and Alloimmunity in the Context of Type 1 Diabetes. Front. Endocrinol. 2017, 8, 343. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, N.; Vaziri, N.D.; Dafoe, D.C.; Ichii, H. The Role of Oxidative Stress in Pancreatic beta Cell Dysfunction in Diabetes. Int. J. Mol. Sci. 2021, 22, 1509. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Moulik, M.; Fang, Z.; Saha, P.; Zou, F.; Xu, Y.; Nelson, D.L.; Ma, K.; Moore, D.D.; Yechoor, V.K. Bmal1 and beta-cell clock are required for adaptation to circadian disruption, and their loss of function leads to oxidative stress-induced beta-cell failure in mice. Mol. Cell. Biol. 2013, 33, 2327–2338. [Google Scholar] [CrossRef]
- Patti, M.E.; Corvera, S. The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr. Rev. 2010, 31, 364–395. [Google Scholar] [CrossRef]
- Man, A.W.C.; Xia, N.; Li, H. Circadian Rhythm in Adipose Tissue: Novel Antioxidant Target for Metabolic and Cardiovascular Diseases. Antioxidants 2020, 9, 968. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Froy, O.; Garaulet, M. The Circadian Clock in White and Brown Adipose Tissue: Mechanistic, Endocrine, and Clinical Aspects. Endocr. Rev. 2018, 39, 261–273. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, L.; Wu, S.; Li, W.; Sun, M.; Feng, W.; Ding, D.; Yeung-Shan Wong, S.; Zhu, P.; Evans, G.J.; et al. Night shift work and abnormal liver function: Is non-alcohol fatty liver a necessary mediator? Occup. Environ. Med. 2019, 76, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, T.; Leonardi, S.; Cuppari, C.; Manti, S.; Lanzafame, A.; D’Angelo, G.; Gitto, E.; Marseglia, L.; Salpietro, C. Role of the diet as a link between oxidative stress and liver diseases. World J. Gastroenterol. 2015, 21, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Meng, F.; Francis, H.; Wu, N.; Chen, L.; Kennedy, L.; Zhou, T.; Franchitto, A.; Onori, P.; Gaudio, E.; et al. Melatonin and circadian rhythms in liver diseases: Functional roles and potential therapies. J. Pineal Res. 2020, 68, e12639. [Google Scholar] [CrossRef] [PubMed]
- Stangherlin, A.; Reddy, A.B. Regulation of circadian clocks by redox homeostasis. J. Biol. Chem. 2013, 288, 26505–26511. [Google Scholar] [CrossRef] [PubMed]
- Haskell, W.L.; Lee, I.M.; Pate, R.R.; Powell, K.E.; Blair, S.N.; Franklin, B.A.; Macera, C.A.; Heath, G.W.; Thompson, P.D.; Bauman, A. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 2007, 116, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Academy of Medical Royal Colleges. Exercise: The Miracle Cure and the Role of the Doctor in Promoting It. 2015. Available online: https://www.aomrc.org.uk/reports-guidance/exercise-the-miracle-cure-0215/ (accessed on 26 November 2021).
- Zilberter, T.; Paoli, A. Editorial: Metabolic Shifting: Nutrition, Exercise, and Timing. Front. Nutr. 2020, 7, 592863. [Google Scholar] [CrossRef]
- Finaud, J.; Lac, G.; Filaire, E. Oxidative stress: Relationship with exercise and training. Sports Med. 2006, 36, 327–358. [Google Scholar] [CrossRef]
- Warburton, D.E.; Nicol, C.W.; Bredin, S.S. Prescribing exercise as preventive therapy. CMAJ Can. Med. Assoc. J. 2006, 174, 961–974. [Google Scholar] [CrossRef]
- Hawley, J.A.; Hargreaves, M.; Joyner, M.J.; Zierath, J.R. Integrative biology of exercise. Cell 2014, 159, 738–749. [Google Scholar] [CrossRef]
- Song, Y.; Choi, G.; Laeguen, J.; Kim, S.-W.; Jung, K.-H.; Park, H. Circadian rhythm gene expression and daily melatonin levels vary in athletes and sedentary males. Biol. Rhythm Res. 2018, 49, 237–245. [Google Scholar] [CrossRef]
- Harfmann, B.D.; Schroder, E.A.; Kachman, M.T.; Hodge, B.A.; Zhang, X.; Esser, K.A. Muscle-specific loss of Bmal1 leads to disrupted tissue glucose metabolism and systemic glucose homeostasis. Skelet. Muscle 2016, 6, 12. [Google Scholar] [CrossRef]
- Erickson, M.L.; Zhang, H.; Mey, J.T.; Kirwan, J.P. Exercise Training Impacts Skeletal Muscle Clock Machinery in Prediabetes. Med. Sci. Sports Exerc. 2020, 52, 2078–2085. [Google Scholar] [CrossRef] [PubMed]
- Van Moorsel, D.; Hansen, J.; Havekes, B.; Scheer, F.A.J.L.; Jorgensen, J.A.; Hoeks, J.; Schrauwen-Hinderling, V.B.; Duez, H.; Lefebvre, P.; Schaper, N.C.; et al. Demonstration of a day-night rhythm in human skeletal muscle oxidative capacity. Mol. Metab. 2016, 5, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Basti, A.; Yalcin, M.; Herms, D.; Hesse, J.; Aboumanify, O.; Li, Y.; Aretz, Z.; Garmshausen, J.; El-Athman, R.; Hastermann, M.; et al. Diurnal variations in the expression of core-clock genes correlate with resting muscle properties and predict fluctuations in exercise performance across the day. BMJ Open Sport Exerc. Med. 2021, 7, e000876. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, J.; Drust, B.; Weinert, D.; Edwards, B.; Gregson, W.; Atkinson, G.; Kao, S.; Aizawa, S.; Reilly, T. The circadian rhythm of core temperature: Origin and some implications for exercise performance. Chronobiol. Int. 2005, 22, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, G.; Reilly, T. Circadian variation in sports performance. Sports Med. 1996, 21, 292–312. [Google Scholar] [CrossRef]
- Savikj, M.; Gabriel, B.M.; Alm, P.S.; Smith, J.; Caidahl, K.; Bjornholm, M.; Fritz, T.; Krook, A.; Zierath, J.R.; Wallberg-Henriksson, H. Afternoon exercise is more efficacious than morning exercise at improving blood glucose levels in individuals with type 2 diabetes: A randomised crossover trial. Diabetologia 2019, 62, 233–237. [Google Scholar] [CrossRef]
- Teo, S.Y.M.; Kanaley, J.A.; Guelfi, K.J.; Marston, K.J.; Fairchild, T.J. The Effect of Exercise Timing on Glycemic Control: A Randomized Clinical Trial. Med. Sci. Sports Exerc. 2020, 52, 323–334. [Google Scholar] [CrossRef]
- Morris, J.N.; Heady, J.A.; Raffle, P.A.; Roberts, C.G.; Parks, J.W. Coronary heart-disease and physical activity of work. Lancet 1953, 265, 1053–1057. [Google Scholar] [CrossRef]
- Morris, J.N.; Heady, J.A.; Raffle, P.A.; Roberts, C.G.; Parks, J.W. Coronary heart-disease and physical activity of work. Lancet 1953, 265, 1111–1120. [Google Scholar] [CrossRef]
- Thompson, P.D.; Buchner, D.; Pina, I.L.; Balady, G.J.; Williams, M.A.; Marcus, B.H.; Berra, K.; Blair, S.N.; Costa, F.; Franklin, B.; et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: A statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation 2003, 107, 3109–3116. [Google Scholar] [PubMed]
- Safar, M.E.; Czernichow, S.; Blacher, J. Obesity, arterial stiffness, and cardiovascular risk. J. Am. Soc. Nephrol. 2006, 17, S109–S111. [Google Scholar] [CrossRef] [PubMed]
- Van Gaal, L.F.; Mertens, I.L.; De Block, C.E. Mechanisms linking obesity with cardiovascular disease. Nature 2006, 444, 875–880. [Google Scholar] [CrossRef]
- MacDonald, J.R.; Hogben, C.D.; Tarnopolsky, M.A.; MacDougall, J.D. Post exercise hypotension is sustained during subsequent bouts of mild exercise and simulated activities of daily living. J. Hum. Hypertens. 2001, 15, 567–571. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jones, H.; George, K.; Edwards, B.; Atkinson, G. Is the magnitude of acute post-exercise hypotension mediated by exercise intensity or total work done? Eur. J. Appl. Physiol. 2007, 102, 33–40. [Google Scholar] [CrossRef]
- De Brito, L.C.; Ely, M.R.; Sieck, D.C.; Mangum, J.E.; Larson, E.A.; Minson, C.T.; Forjaz, C.L.M.; Halliwill, J.R. Effect of Time of Day on Sustained Postexercise Vasodilation Following Small Muscle-Mass Exercise in Humans. Front. Physiol. 2019, 10, 762. [Google Scholar] [CrossRef]
- Roberts, C.K.; Vaziri, N.D.; Barnard, R.J. Effect of diet and exercise intervention on blood pressure, insulin, oxidative stress, and nitric oxide availability. Circulation 2002, 106, 2530–2532. [Google Scholar] [CrossRef]
- Portaluppi, F.; Hermida, R.C. Circadian rhythms in cardiac arrhythmias and opportunities for their chronotherapy. Adv. Drug Deliv. Rev. 2007, 59, 940–951. [Google Scholar] [CrossRef]
- Jones, H.; Pritchard, C.; George, K.; Edwards, B.; Atkinson, G. The acute post-exercise response of blood pressure varies with time of day. Eur. J. Appl. Physiol. 2008, 104, 481–489. [Google Scholar] [CrossRef]
- De Brito, L.C.; Pecanha, T.; Fecchio, R.Y.; Rezende, R.A.; Sousa, P.; DASilva-Junior, N.; Abreu, A.; Silva, G.; Mion-Junior, D.; Halliwill, J.R.; et al. Morning versus Evening Aerobic Training Effects on Blood Pressure in Treated Hypertension. Med. Sci. Sports Exerc. 2019, 51, 653–662. [Google Scholar] [CrossRef]
- De Brito, L.C.; Rezende, R.A.; da Silva Junior, N.D.; Tinucci, T.; Casarini, D.E.; Cipolla-Neto, J.; Forjaz, C.L. Post-Exercise Hypotension and Its Mechanisms Differ after Morning and Evening Exercise: A Randomized Crossover Study. PLoS ONE 2015, 10, e0132458. [Google Scholar] [CrossRef] [PubMed]
- Walther, C.; Gielen, S.; Hambrecht, R. The effect of exercise training on endothelial function in cardiovascular disease in humans. Exerc. Sport Sci. Rev. 2004, 32, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J. Exercise training as vascular medicine: Direct impacts on the vasculature in humans. Exerc. Sport Sci. Rev. 2009, 37, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, D.H.; Black, M.A.; Pyke, K.E.; Padilla, J.; Atkinson, G.; Harris, R.A.; Parker, B.; Widlansky, M.E.; Tschakovsky, M.E.; Green, D.J. Assessment of flow-mediated dilation in humans: A methodological and physiological guideline. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H2–H12. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.; Green, D.J.; George, K.; Atkinson, G. Intermittent exercise abolishes the diurnal variation in endothelial-dependent flow-mediated dilation in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R427–R432. [Google Scholar] [CrossRef]
- Ballard, K.D.; Timsina, R.; Timmerman, K.L. Influence of time of day and intermittent aerobic exercise on vascular endothelial function and plasma endothelin-1 in healthy adults. Chronobiol. Int. 2021, 38, 1064–1071. [Google Scholar] [CrossRef]
- Hood, D.A. Invited Review: Contractile activity-induced mitochondrial biogenesis in skeletal muscle. J. Appl. Physiol. 2001, 90, 1137–1157. [Google Scholar] [CrossRef]
- Andrews, J.L.; Zhang, X.; McCarthy, J.J.; McDearmon, E.L.; Hornberger, T.A.; Russell, B.; Campbell, K.S.; Arbogast, S.; Reid, M.B.; Walker, J.R.; et al. CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proc. Natl. Acad. Sci. USA 2010, 107, 19090–19095. [Google Scholar] [CrossRef]
- Wu, R.; Dang, F.; Li, P.; Wang, P.; Xu, Q.; Liu, Z.; Li, Y.; Wu, Y.; Chen, Y.; Liu, Y. The Circadian Protein Period2 Suppresses mTORC1 Activity via Recruiting Tsc1 to mTORC1 Complex. Cell Metab. 2019, 29, 653–667.e6. [Google Scholar] [CrossRef]
- Reid, K.J. Assessment of Circadian Rhythms. Neurol. Clin. 2019, 37, 505–526. [Google Scholar] [CrossRef]
- Huang, C.J.; McAllister, M.J.; Slusher, A.L.; Webb, H.E.; Mock, J.T.; Acevedo, E.O. Obesity-Related Oxidative Stress: The Impact of Physical Activity and Diet Manipulation. Sports Med. Open 2015, 1, 32. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McClean, C.; Davison, G.W. Circadian Clocks, Redox Homeostasis, and Exercise: Time to Connect the Dots? Antioxidants 2022, 11, 256. https://doi.org/10.3390/antiox11020256
McClean C, Davison GW. Circadian Clocks, Redox Homeostasis, and Exercise: Time to Connect the Dots? Antioxidants. 2022; 11(2):256. https://doi.org/10.3390/antiox11020256
Chicago/Turabian StyleMcClean, Conor, and Gareth W. Davison. 2022. "Circadian Clocks, Redox Homeostasis, and Exercise: Time to Connect the Dots?" Antioxidants 11, no. 2: 256. https://doi.org/10.3390/antiox11020256
APA StyleMcClean, C., & Davison, G. W. (2022). Circadian Clocks, Redox Homeostasis, and Exercise: Time to Connect the Dots? Antioxidants, 11(2), 256. https://doi.org/10.3390/antiox11020256





