Therapeutic Potential of Perillaldehyde in Ameliorating Vulvovaginal Candidiasis by Reducing Vaginal Oxidative Stress and Apoptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Main Reagents
2.2. Candida Albicans Strain
2.3. Experimental Animals
2.4. Construction of the Animal Model of VVC
2.5. Measurement of Intracellular ROS in Vaginal Tissue
2.6. The Determination of Antioxidant Enzyme Activity in Vaginal Tissue
2.7. Western Blot Analysis
2.8. Immunofluorescence Staining
2.9. Apoptosis Detection
2.10. ELISA Measurement of IL-1β
2.11. Statistical Analyses
3. Results
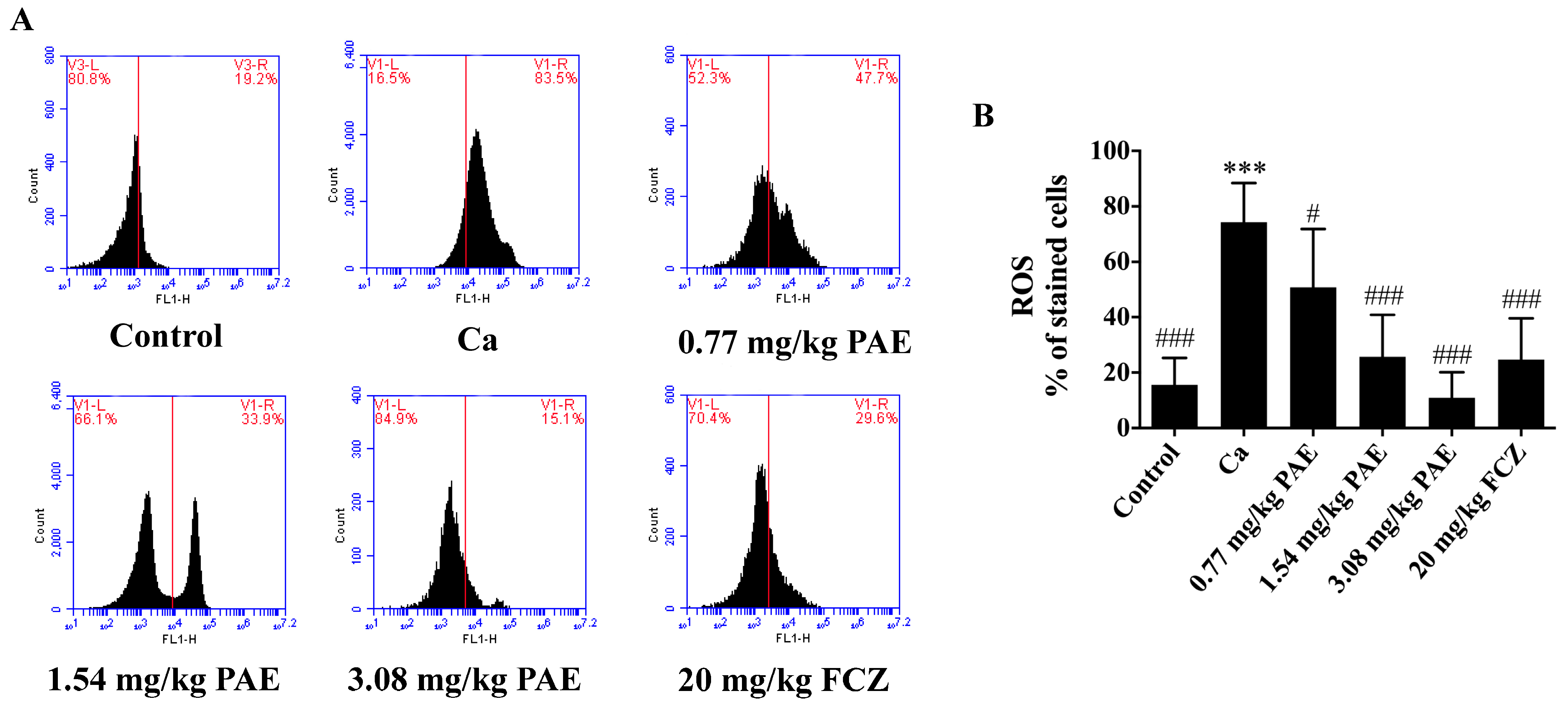
3.1. PAE Reduced Excessive ROS in VVC Mice
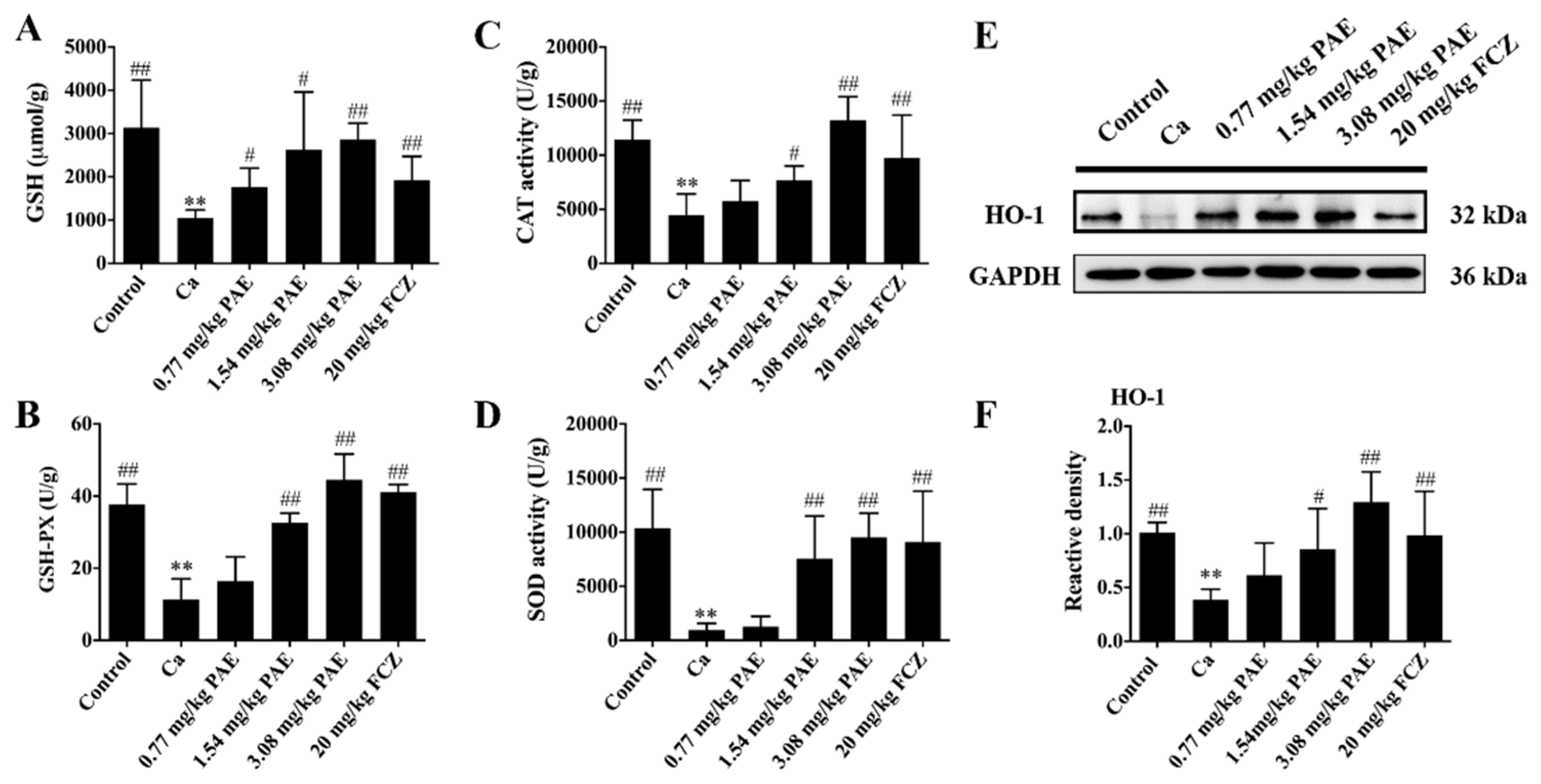
3.2. PAE Increased Vaginal Antioxidant Activities in VVC Mice
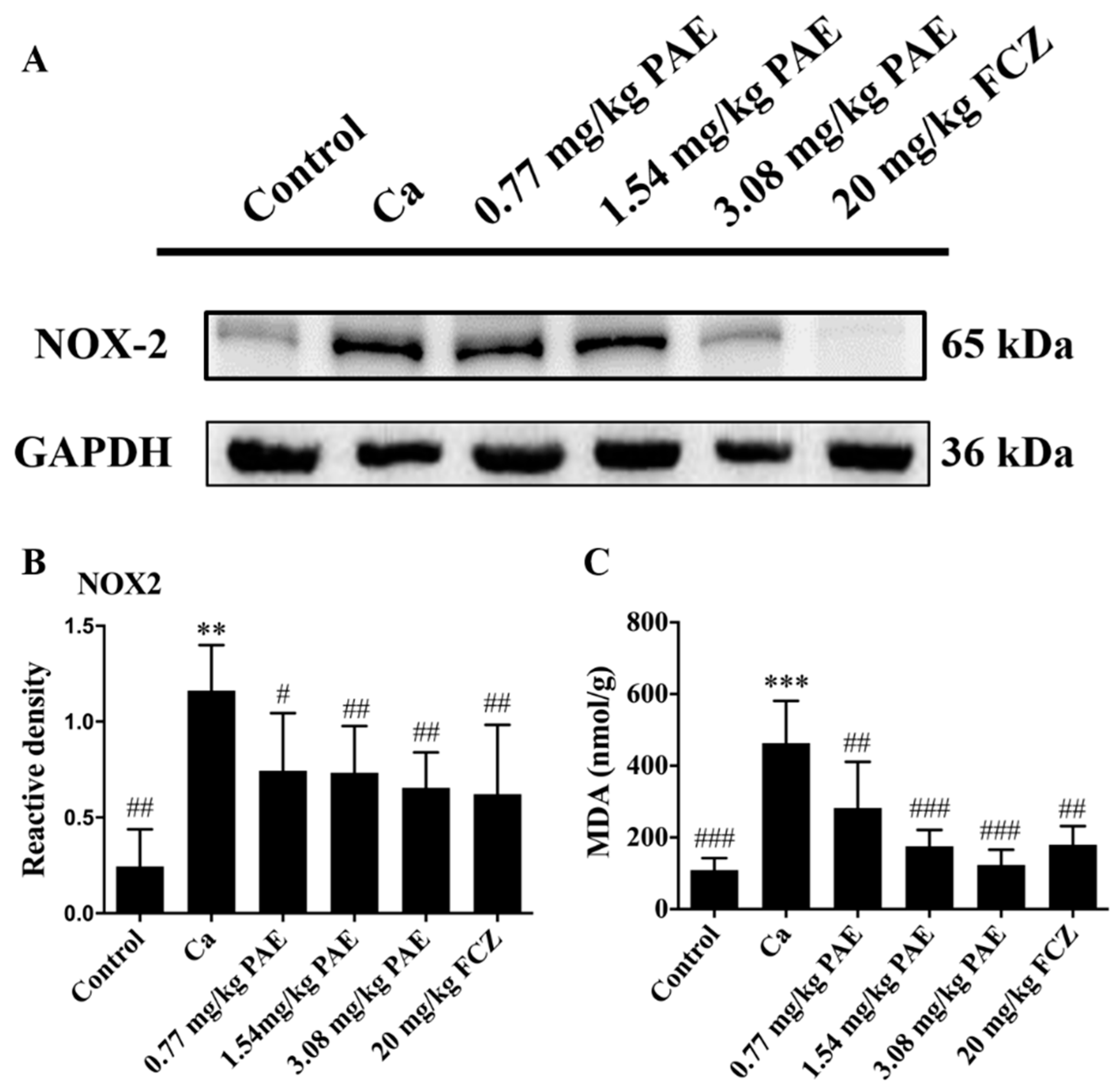
3.3. PAE Reduced Vaginal NOX2 Expression and Lipid Peroxide Content
3.4. PAE Enhanced Vaginal Nrf2 Activity in VVC Mice
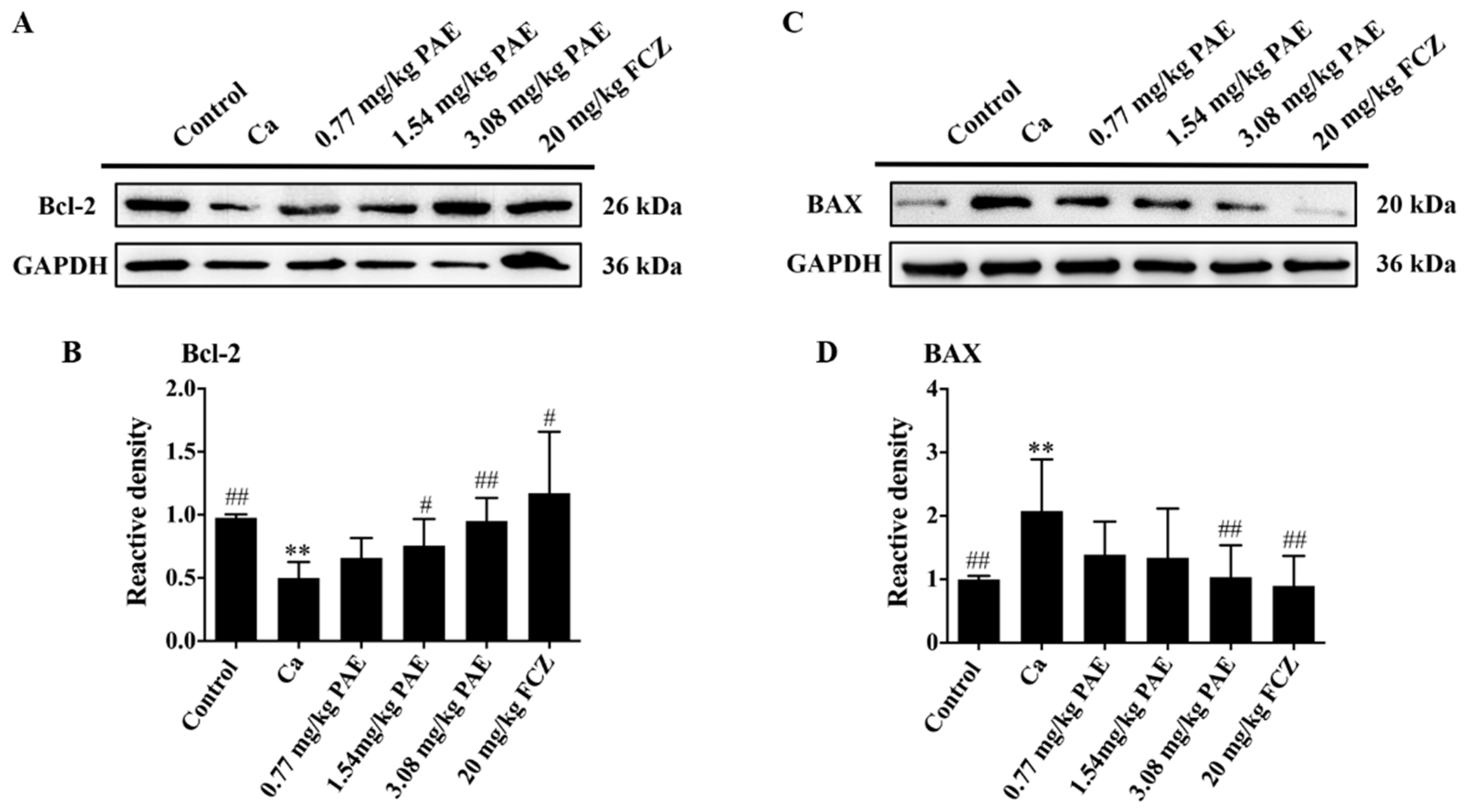
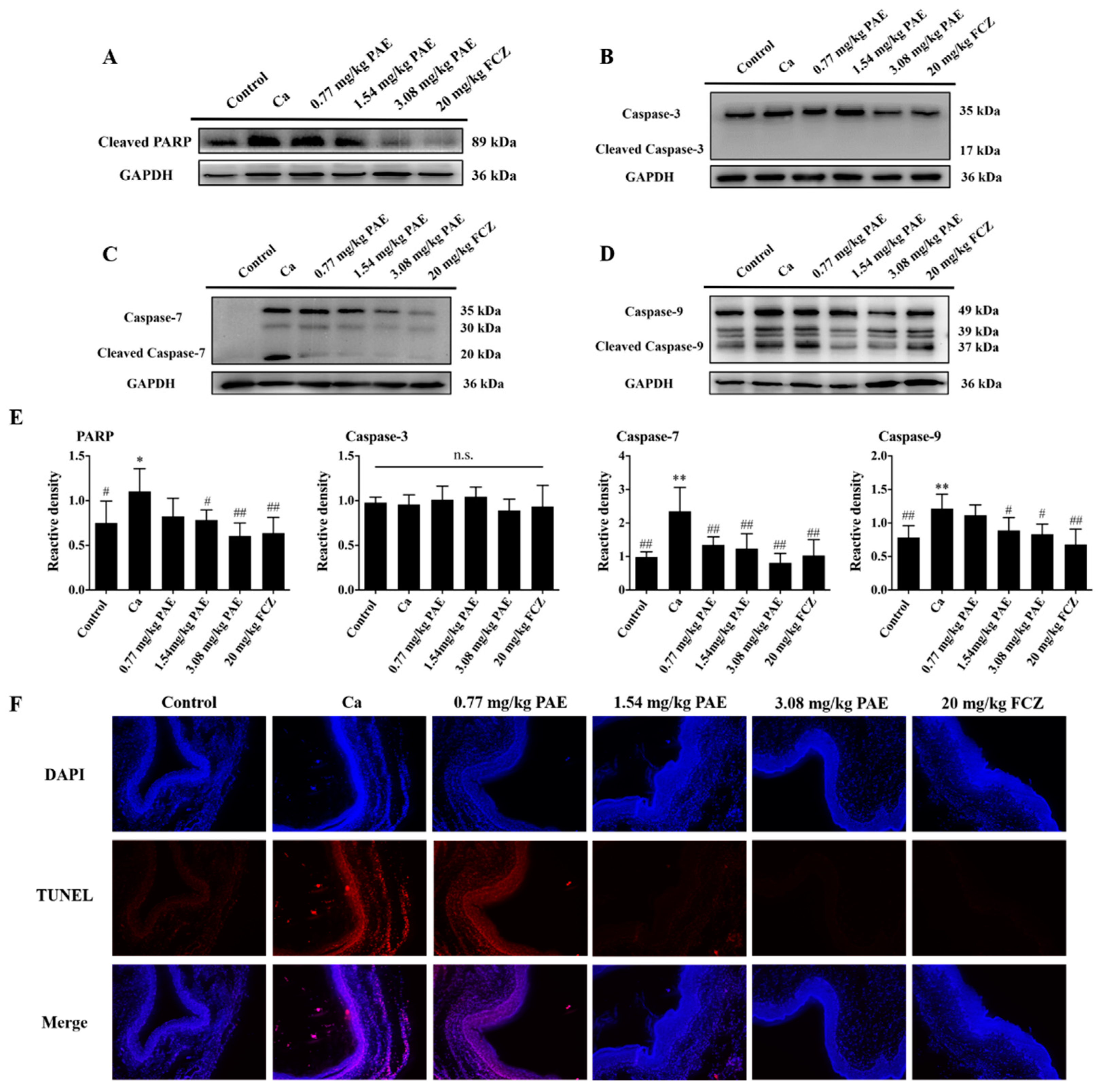
3.5. PAE Inhibited Vagina Apoptosis Induced by C. albicans Invasion
3.6. PAE Suppressed the IL-1β Release in VVC Mice
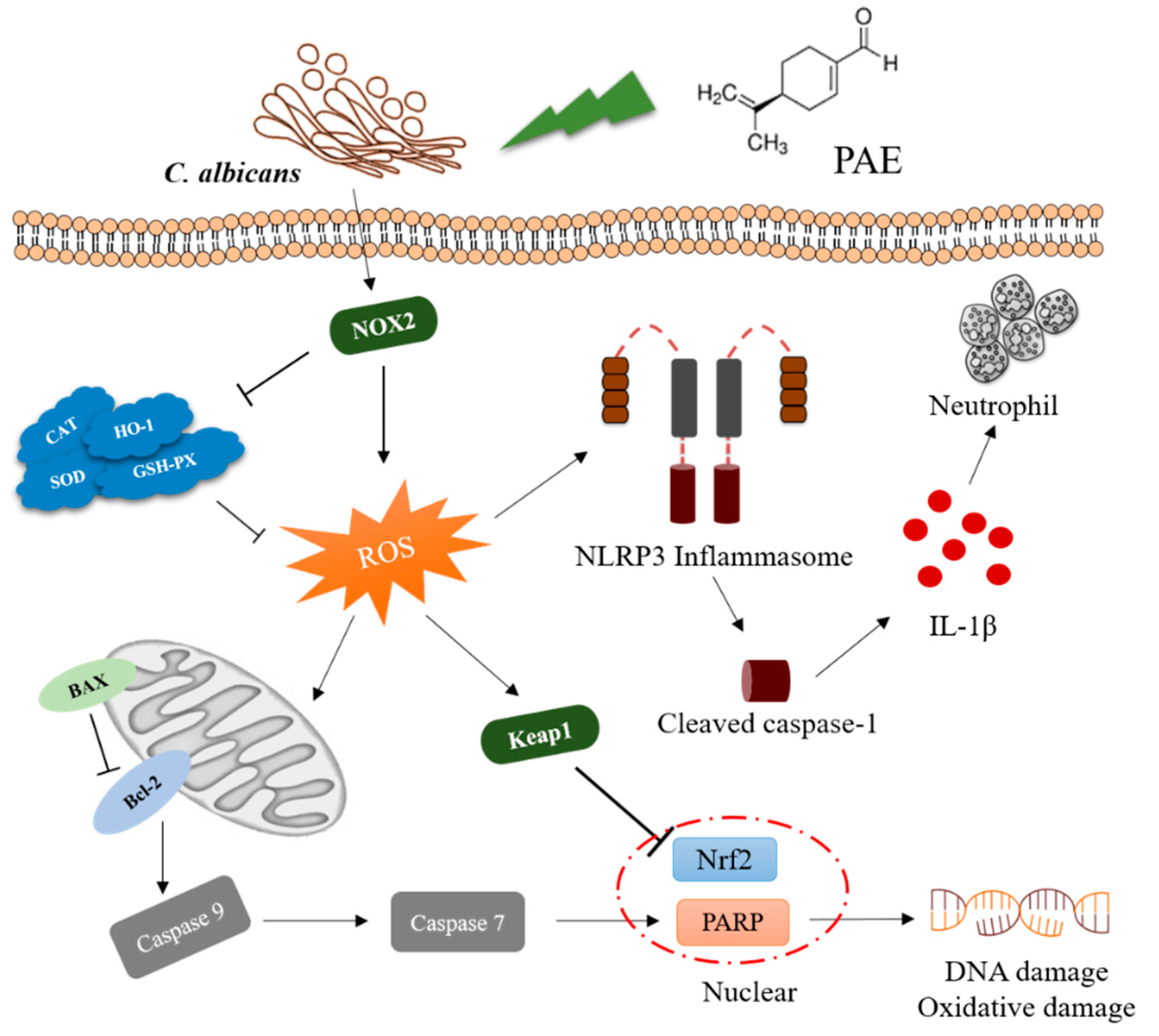
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peters, B.M.; Yano, J.; Noverr, M.C.; Fidel, P.L., Jr. Candida vaginitis: When opportunism knocks, the host responds. PLoS Pathog. 2014, 10, e1003965. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D. Vaginitis. N. Engl. J. Med. 1997, 337, 1896–1903. [Google Scholar] [CrossRef]
- Hong, E.; Dixit, S.; Fidel, P.L.; Bradford, J.; Fischer, G. Vulvovaginal candidiasis as a chronic disease: Diagnostic criteria and definition. J. Low. Genit. Tract. Dis. 2014, 18, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D.; Wiesenfeld, H.C.; Martens, M.; Danna, P.; Hooton, T.M.; Rompalo, A.; Sperling, M.; Livengood, C., 3rd; Horowitz, B.; Von Thron, J.; et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N. Engl. J. Med. 2004, 351, 876–883. [Google Scholar] [CrossRef]
- Denning, D.W.; Kneale, M.; Sobel, J.D.; Rautemaa-Richardson, R. Global burden of recurrent vulvovaginal candidiasis: A systematic review. Lancet Infect. Dis. 2018, 18, e339–e347. [Google Scholar] [CrossRef]
- Fidel, P.L., Jr. History and update on host defense against vaginal candidiasis. Am. J. Reprod. Immunol. 2007, 57, 2–12. [Google Scholar] [CrossRef]
- Yano, J.; Noverr, M.C.; Fidel, P.L., Jr. Cytokines in the host response to Candida vaginitis: Identifying a role for non-classical immune mediators, S100 alarmins. Cytokine 2012, 58, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Warnatsch, A.; Tsourouktsoglou, T.D.; Branzk, N.; Wang, Q.; Reincke, S.; Herbst, S.; Gutierrez, M.; Papayannopoulos, V. Reactive oxygen species localization programs inflammation to clear microbes of different size. Immunity 2017, 46, 421–432. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.; Liu, L.; Qu, S.; Mao, Y.; Peng, X.; Li, Y.X.; Tian, J. Cinnamaldehyde inhibits Candida albicans growth by causing apoptosis and its treatment on vulvovaginal candidiasis and oropharyngeal candidiasis. Appl. Microbiol. Biotechnol. 2019, 103, 9037–9055. [Google Scholar] [CrossRef]
- Qu, S.; Yang, K.; Chen, L.; Liu, M.; Geng, Q.; He, X.; Li, Y.; Liu, Y.; Tian, J. Cinnamaldehyde, a promising natural preservative against Aspergillus flavus. Front. Microbiol. 2019, 10, 2895. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zeng, X.B.; Zhang, S.; Wang, Y.Z.; Zhang, P.; Lu, A.J.; Peng, X. Regional variation in components and antioxidant and antifungal activities of Perilla frutescens essential oils in China. Ind. Crop Prod. 2014, 59, 69–79. [Google Scholar] [CrossRef]
- Tian, H.; Qu, S.; Wang, Y.; Lu, Z.; Zhang, M.; Gan, Y.; Zhang, P.; Tian, J. Calcium and oxidative stress mediate perillaldehyde-induced apoptosis in Candida albicans. Appl. Microbiol. Biotechnol. 2017, 101, 3335–3345. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Chen, L.; Tian, H.; Wang, Z.; Wang, F.; Wang, L.; Li, J.; Ji, H.; Xi, L.; Feng, Z.; et al. Effect of perillaldehyde on prophylaxis and treatment of vaginal candidiasis in a murine model. Front. Microbiol. 2019, 10, 1466. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qu, S.; Yang, K.; Liu, M.; Li, Y.X.; Keller, N.P.; Zeng, X.; Tian, J. Perillaldehyde: A promising antifungal agent to treat oropharyngeal candidiasis. Biochem. Pharmacol. 2020, 180, 114201. [Google Scholar] [CrossRef]
- Erhunmwunsee, F.; Pan, C.; Yang, K.; Li, Y.; Liu, M.; Tian, J. Recent development in biological activities and safety concerns of perillaldehyde from perilla plants: A review. Crit. Rev. Food Sci. Nutr. 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Krist, S.; Sato, K.; Glasl, S.; Hoeferl, M.; Saukel, J. Antimicrobial effect of vapours of terpineol, (R)-(-)-linalool, carvacrol, (S)-(-)-perillaldehyde and 1,8-cineole on airborne microbes using a room diffuser. Flavour Frag. J. 2008, 23, 353–356. [Google Scholar] [CrossRef]
- Tian, J.; Wang, Y.; Lu, Z.; Sun, C.; Zhang, M.; Zhu, A.; Peng, X. Perillaldehyde, a promising antifungal agent used in food preservation, triggers apoptosis through a metacaspase-dependent pathway in Aspergillus flavus. J. Agric. Food Chem. 2016, 64, 7404–7413. [Google Scholar] [CrossRef]
- Tian, J.; Wang, Y.; Zeng, H.; Li, Z.; Zhang, P.; Tessema, A.; Peng, X. Efficacy and possible mechanisms of perillaldehyde in control of Aspergillus niger causing grape decay. Int. J. Food Microbiol. 2015, 202, 27–34. [Google Scholar] [CrossRef]
- Tian, J.; Pan, C.; Zhang, M.; Gan, Y.Y.; Pan, S.Y.; Liu, M.; Li, Y.X.; Zeng, X.B. Induced cell death in Ceratocystis fimbriata by pro-apoptotic activity of a natural organic compound, perillaldehyde, through Ca2+ overload and accumulation of reactive oxygen species. Plant Pathol. 2019, 68, 344–357. [Google Scholar] [CrossRef]
- Keesen, T.S.L.; da Silva, L.V.; da Camara Rocha, J.; Andrade, L.N.; Lima, T.C.; de Sousa, D.P. Anti-Leishmania and cytotoxic activities of perillaldehyde epoxide synthetic positional isomers. Nat. Prod. Res. 2019, 33, 2536–2540. [Google Scholar] [CrossRef]
- Hobbs, C.A.; Taylor, S.V.; Beevers, C.; Lloyd, M.; Bowen, R.; Lillford, L.; Maronpot, R.; Hayashi, S.M. Genotoxicity assessment of the flavouring agent, perillaldehyde. Food Chem. Toxicol. 2016, 97, 232–242. [Google Scholar] [CrossRef]
- Song, Y.; Sun, R.; Ji, Z.; Li, X.; Fu, Q.; Ma, S. Perilla aldehyde attenuates CUMS-induced depressive-like behaviors via regulating TXNIP/TRX/NLRP3 pathway in rats. Life Sci. 2018, 206, 117–124. [Google Scholar] [CrossRef]
- Uemura, T.; Yashiro, T.; Oda, R.; Shioya, N.; Nakajima, T.; Hachisu, M.; Kobayashi, S.; Nishiyama, C.; Arimura, G.I. Intestinal anti-inflammatory activity of perillaldehyde. J. Agric. Food Chem. 2018, 66, 3443–3448. [Google Scholar] [CrossRef] [PubMed]
- Fuyuno, Y.; Uchi, H.; Yasumatsu, M.; Morino-Koga, S.; Tanaka, Y.; Mitoma, C.; Furue, M. Perillaldehyde inhibits AHR signaling and activates NRF2 antioxidant pathway in human keratinocytes. Oxid. Med. Cell Longev. 2018, 2018, 9524657. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Kumar, A. Multiple drug targeting potential of novel ligands against virulent proteins of Candida albicans. Int. J. Pept. Res. Ther. 2020, 26, 921–942. [Google Scholar] [CrossRef]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef]
- Munoz, J.E.; Rossi, D.C.P.; Ishida, K.; Spadari, C.C.; Melhem, M.S.C.; Garcia, D.M.; Caires, A.C.F.; Taborda, C.P.; Rodrigues, E.G. Antifungal activity of the biphosphinic cyclopalladate C7a against Candida albicans yeast forms in vitro and in vivo. Front. Microbiol. 2017, 8, 771. [Google Scholar] [CrossRef]
- Kaur, G.; Mehta, S.K. Developments of polysorbate (tween) based microemulsions: Preclinical drug delivery, toxicity and antimicrobial applications. Int. J. Pharm. 2017, 529, 134–160. [Google Scholar] [CrossRef] [PubMed]
- Kogan, A.; Garti, N. Microemulsions as transdermal drug delivery vehicles. Adv. Colloid. Interface Sci. 2006, 123-126, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, T.; Uchima, F.D.; Ostrander, P.L.; Bern, H.A. Growth of normal mouse vaginal epithelial cells in and on collagen gels. Proc. Natl. Acad. Sci. USA 1983, 80, 3743–3747. [Google Scholar] [CrossRef]
- Indran, I.R.; Tufo, G.; Pervaiz, S.; Brenner, C. Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. Biochim. Biophys. Acta 2011, 1807, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Kurata, M.; Suzuki, M.; Agar, N.S. Antioxidant systems and erythrocyte life-span in mammals. Comp. Biochem. Physiol. B 1993, 106, 477–487. [Google Scholar] [CrossRef]
- Ando, T.; Mimura, K.; Johansson, C.C.; Hanson, M.G.; Mougiakakos, D.; Larsson, C.; Martins da Palma, T.; Sakurai, D.; Norell, H.; Li, M.; et al. Transduction with the antioxidant enzyme catalase protects human T cells against oxidative stress. J. Immunol. 2008, 181, 8382–8390. [Google Scholar] [CrossRef]
- Kirkman, H.N.; Gaetani, G.F. Mammalian catalase: A venerable enzyme with new mysteries. Trends Biochem. Sci. 2007, 32, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Somwar, R.; Erdjument-Bromage, H.; Larsson, E.; Shum, D.; Lockwood, W.W.; Yang, G.; Sander, C.; Ouerfelli, O.; Tempst, P.J.; Djaballah, H.; et al. Superoxide dismutase 1 (SOD1) is a target for a small molecule identified in a screen for inhibitors of the growth of lung adenocarcinoma cell lines. Proc. Natl. Acad. Sci. USA 2011, 108, 16375–16380. [Google Scholar] [CrossRef] [PubMed]
- Abraham, N.G.; Kappas, A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 2008, 60, 79–127. [Google Scholar] [CrossRef]
- Forte, M.; Nocella, C.; De Falco, E.; Palmerio, S.; Schirone, L.; Valenti, V.; Frati, G.; Carnevale, R.; Sciarretta, S. The pathophysiological role of NOX2 in hypertension and organ damage. High Blood Press Cardiovasc. Prev. 2016, 23, 355–364. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Amoutzias, G.D.; Bornberg-Bauer, E.; Oliver, S.G.; Robertson, D.L. Reduction/oxidation-phosphorylation control of DNA binding in the bZIP dimerization network. BMC Genom. 2006, 7, 107. [Google Scholar] [CrossRef]
- Cullinan, S.B.; Gordan, J.D.; Jin, J.; Harper, J.W.; Diehl, J.A. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: Oxidative stress sensing by a Cul3-Keap1 ligase. Mol. Cell Biol. 2004, 24, 8477–8486. [Google Scholar] [CrossRef]
- Bhola, P.D.; Letai, A. Mitochondria-judges and executioners of cell death sentences. Mol. Cell 2016, 61, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Burke, P.J. Mitochondria, bioenergetics and apoptosis in cancer. Trends Cancer 2017, 3, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.C.; Zong, W.X.; Cheng, E.H.; Lindsten, T.; Panoutsakopoulou, V.; Ross, A.J.; Roth, K.A.; MacGregor, G.R.; Thompson, C.B.; Korsmeyer, S.J. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science 2001, 292, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.M.; Ranganathan, V.; Farnsworth, M.L.; Kavallaris, M.; Lock, R.B. Bcl-2 inhibits Bax translocation from cytosol to mitochondria during drug-induced apoptosis of human tumor cells. Cell Death Differ. 2000, 7, 102–111. [Google Scholar] [CrossRef]
- Tewari, M.; Quan, L.T.; O’Rourke, K.; Desnoyers, S.; Zeng, Z.; Beidler, D.R.; Poirier, G.G.; Salvesen, G.S.; Dixit, V.M. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell 1995, 81, 801–809. [Google Scholar] [CrossRef]
- Nicholson, D.W.; Ali, A.; Thornberry, N.A.; Vaillancourt, J.P.; Ding, C.K.; Gallant, M.; Gareau, Y.; Griffin, P.R.; Labelle, M.; Lazebnik, Y.A.; et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 1995, 376, 37–43. [Google Scholar] [CrossRef]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Shadel, G.S.; Horvath, T.L. Mitochondrial ROS signaling in organismal homeostasis. Cell 2015, 163, 560–569. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S.; Okamoto, K.; Yodoi, J.; Hiai, H. Persistent oxidative stress in cancer. FEBS Lett. 1995, 358, 1–3. [Google Scholar] [CrossRef]
- Johnson, L.A.; June, C.H. Driving gene-engineered T cell immunotherapy of cancer. Cell Res. 2017, 27, 38–58. [Google Scholar] [CrossRef] [PubMed]
- Vila, T.V.; Chaturvedi, A.K.; Rozental, S.; Lopez-Ribot, J.L. In vitro activity of miltefosine against Candida albicans under planktonic and biofilm growth conditions and in vivo efficacy in a murine model of oral candidiasis. Antimicrob. Agents Chemother. 2015, 59, 7611–7620. [Google Scholar] [CrossRef]
- Franchina, D.G.; He, F.; Brenner, D. Survival of the fittest: Cancer challenges T cell metabolism. Cancer Lett. 2018, 412, 216–223. [Google Scholar] [CrossRef]
- Storz, P. Reactive oxygen species-mediated mitochondria-to-nucleus signaling: A key to aging and radical-caused diseases. Sci. STKE 2006, 2006, re3. [Google Scholar] [CrossRef] [PubMed]
- Parkes, T.L.; Elia, A.J.; Dickinson, D.; Hilliker, A.J.; Phillips, J.P.; Boulianne, G.L. Extension of drosophila lifespan by overexpression of human SOD1 in motorneurons. Nat. Genet. 1998, 19, 171–174. [Google Scholar] [CrossRef]
- Singel, K.L.; Segal, B.H. NOX2-dependent regulation of inflammation. Clin. Sci. 2016, 130, 479–490. [Google Scholar] [CrossRef]
- Shan, Q.; Zhuang, J.; Zheng, G.; Zhang, Z.; Zhang, Y.; Lu, J.; Zheng, Y. Troxerutin reduces kidney damage against BDE-47-induced apoptosis via inhibiting NOX2 activity and increasing Nrf2 activity. Oxid. Med. Cell Longev. 2017, 2017, 6034692. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, Q.; Mu, X.; Zhu, H.; Liu, B.; Yao, B.; Liu, X.; Xue, W.; Wang, B.; Liu, S. SREBP1 promotes 5-FU resistance in colorectal cancer cells by inhibiting the expression of caspase7. Int. J. Clin. Exp. Pathol. 2019, 12, 1095–1100. [Google Scholar] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Wang, F.; Qu, S.; He, X.; Zhu, Y.; Zhou, Y.; Yang, K.; Li, Y.-X.; Liu, M.; Peng, X.; et al. Therapeutic Potential of Perillaldehyde in Ameliorating Vulvovaginal Candidiasis by Reducing Vaginal Oxidative Stress and Apoptosis. Antioxidants 2022, 11, 178. https://doi.org/10.3390/antiox11020178
Chen L, Wang F, Qu S, He X, Zhu Y, Zhou Y, Yang K, Li Y-X, Liu M, Peng X, et al. Therapeutic Potential of Perillaldehyde in Ameliorating Vulvovaginal Candidiasis by Reducing Vaginal Oxidative Stress and Apoptosis. Antioxidants. 2022; 11(2):178. https://doi.org/10.3390/antiox11020178
Chicago/Turabian StyleChen, Lei, Fei Wang, Su Qu, Xiaona He, Yongxin Zhu, Yi Zhou, Kunlong Yang, Yong-Xin Li, Man Liu, Xue Peng, and et al. 2022. "Therapeutic Potential of Perillaldehyde in Ameliorating Vulvovaginal Candidiasis by Reducing Vaginal Oxidative Stress and Apoptosis" Antioxidants 11, no. 2: 178. https://doi.org/10.3390/antiox11020178
APA StyleChen, L., Wang, F., Qu, S., He, X., Zhu, Y., Zhou, Y., Yang, K., Li, Y.-X., Liu, M., Peng, X., & Tian, J. (2022). Therapeutic Potential of Perillaldehyde in Ameliorating Vulvovaginal Candidiasis by Reducing Vaginal Oxidative Stress and Apoptosis. Antioxidants, 11(2), 178. https://doi.org/10.3390/antiox11020178





