Zebrafish Model-Based Assessment of Indoxyl Sulfate-Induced Oxidative Stress and Its Impact on Renal and Cardiac Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Assessment of Cell Viability
2.3. Colony Formation Assay
2.4. Animals and IS Exposure
2.5. Histologic Studies
2.6. ROS Assessment
2.7. Analysis of the Effects of Treatment on Zebrafish Morphology and Survival
2.8. Renal Function Assay
2.9. Total RNA Extraction
2.10. Next-Generation Sequencing (NGS) Analysis
2.11. Real-Time Quantitative Polymerase Chain Reaction (RT–qPCR)
2.12. Statistical Analysis
3. Results
3.1. Assessment of the Effects of IS on the Survival Rate and Hatchability
3.2. Assessment of the Effect of IS on Cardiac Development
3.3. IS Treatment Led to Kidney Defects in Zebrafish
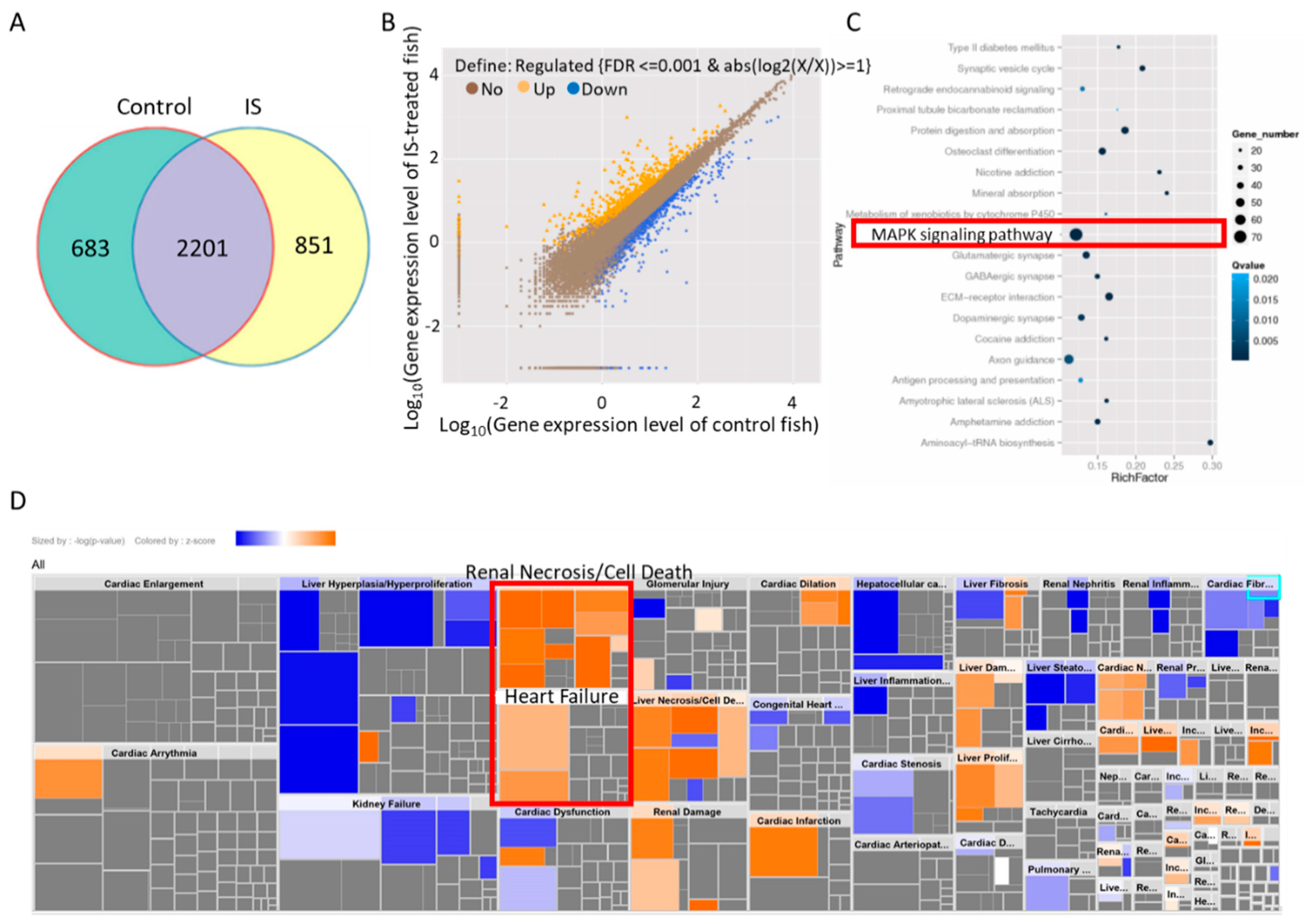
3.4. IS Induces MAPK Pathway-Associated Gene Expression
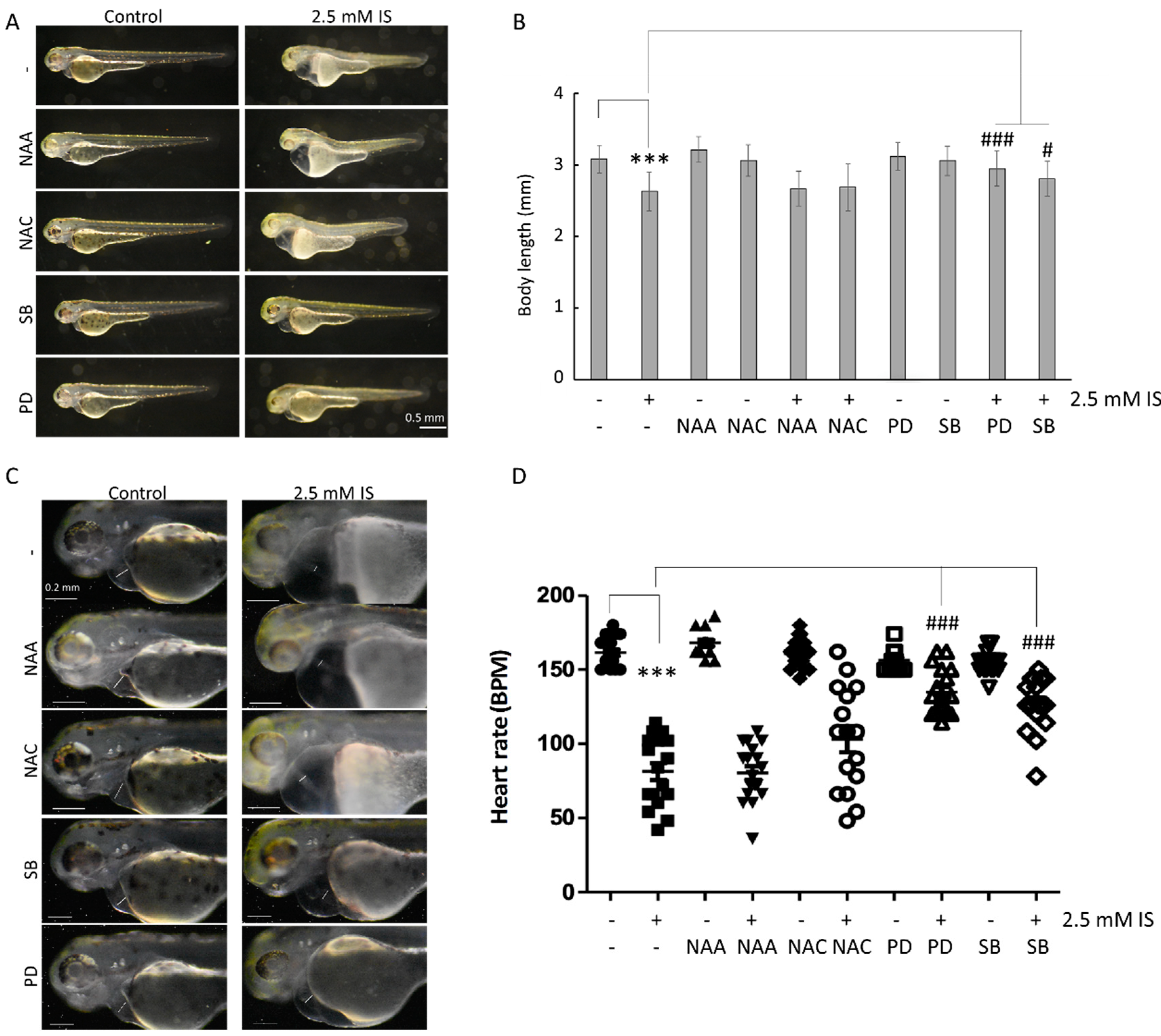
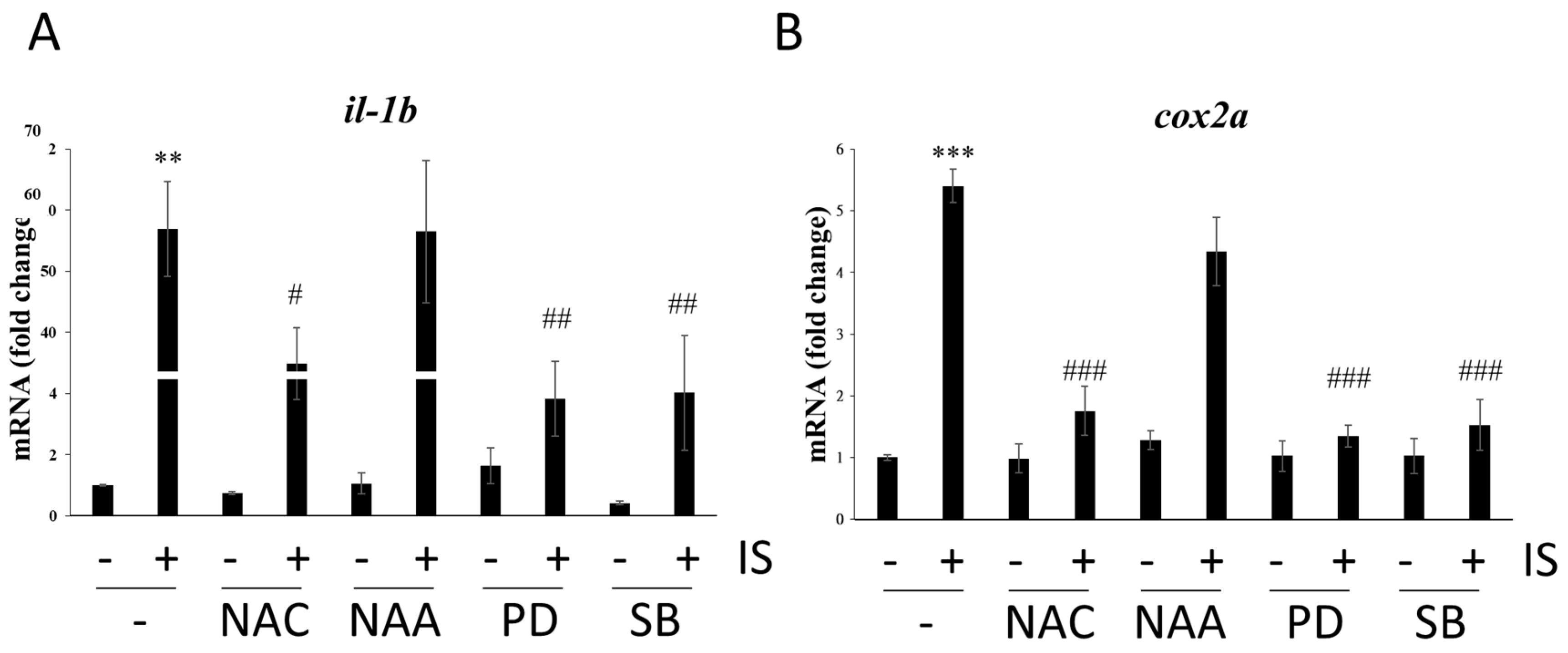
3.5. IS Affects Zebrafish Development via the MAPK Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tonelli, M.; Wiebe, N.; Culleton, B.; House, A.; Rabbat, C.; Fok, M.; McAlister, F.; Garg, A.X. Chronic kidney disease and mortality risk: A systematic review. J. Am. Soc. Nephrol. 2006, 17, 2034–2047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lano, G.; Burtey, S.; Sallee, M. Indoxyl Sulfate, a Uremic Endotheliotoxin. Toxins 2020, 12, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viaene, L.; Annaert, P.; de Loor, H.; Poesen, R.; Evenepoel, P.; Meijers, B. Albumin is the main plasma binding protein for indoxyl sulfate and p-cresyl sulfate. Biopharm. Drug Dispos. 2013, 34, 165–175. [Google Scholar] [CrossRef]
- Cheng, T.-H.; Ma, M.-C.; Liao, M.-T.; Zheng, C.-M.; Lu, K.-C.; Liao, C.-H.; Hou, Y.-C.; Liu, W.-C.; Lu, C.-L. Indoxyl Sulfate, a Tubular Toxin, Contributes to the Development of Chronic Kidney Disease. Toxins 2020, 12, 684. [Google Scholar] [CrossRef]
- Carmona, A.; Guerrero, F.; Buendia, P.; Obrero, T.; Aljama, P.; Carracedo, J. Microvesicles Derived from Indoxyl Sulfate Treated Endothelial Cells Induce Endothelial Progenitor Cells Dysfunction. Front. Physiol. 2017, 8, 666. [Google Scholar] [CrossRef] [PubMed]
- Adesso, S.; Magnus, T.; Cuzzocrea, S.; Campolo, M.; Rissiek, B.; Paciello, O.; Pinto, A.; Marzocco, S. Indoxyl sulfate affects glial function increasing oxidative stress and neuroinflammation in chronic kidney disease: Interaction between astrocytes and microglia. Front. Pharmacol. 2017, 8, 370. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Schepers, E.; Pletinck, A.; Nagler, E.V.; Glorieux, G. The Uremic Toxicity of Indoxyl Sulfate and p-Cresyl Sulfate: A Systematic Review. J. Am. Soc. Nephrol. 2014, 25, 1897–1907. [Google Scholar] [CrossRef]
- Parng, C.; Seng, W.L.; Semino, C.; McGrath, P. Zebrafish: A preclinical model for drug screening. Assay Drug Dev. Technol. 2002, 1, 41–48. [Google Scholar] [CrossRef]
- Lessman, C.A. The developing zebrafish (Danio rerio): A vertebrate model for high-throughput screening of chemical libraries. Birth Defects Res. Part C Embryo Today Rev. 2011, 93, 268–280. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498. [Google Scholar] [CrossRef] [Green Version]
- Langheinrich, U. Zebrafish: A new model on the pharmaceutical catwalk. Bioessays 2003, 25, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Horzmann, K.A.; Freeman, J.L. Making Waves: New Developments in Toxicology with the Zebrafish. Toxicol. Sci. 2018, 163, 5–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dooley, K.; Zon, L.I. Zebrafish: A model system for the study of human disease. Curr. Opin. Genet. Dev. 2000, 10, 252–256. [Google Scholar] [CrossRef]
- Bradford, Y.; Conlin, T.; Dunn, N.; Fashena, D.; Frazer, K.; Howe, D.G.; Knight, J.; Mani, P.; Martin, R.; Moxon, S.A. ZFIN: Enhancements and updates to the Zebrafish Model Organism Database. Nucleic Acids Res. 2010, 39, D822–D829. [Google Scholar] [CrossRef]
- Truong, L.; Bugel, S.M.; Chlebowski, A.; Usenko, C.Y.; Simonich, M.T.; Simonich, S.L.M.; Tanguay, R.L. Optimizing multi-dimensional high throughput screening using zebrafish. Reprod. Toxicol. 2016, 65, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Sipes, N.S.; Padilla, S.; Knudsen, T.B. Zebrafish—As an integrative model for twenty-first century toxicity testing. Birth Defects Res. Part C Embryo Today Rev. 2011, 93, 256–267. [Google Scholar] [CrossRef]
- Andrés-Delgado, L.; Mercader, N. Interplay between cardiac function and heart development. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 1707–1716. [Google Scholar] [CrossRef] [Green Version]
- Elmonem, M.A.; Berlingerio, S.P.; Van den Heuvel, L.P.; De Witte, P.A.; Lowe, M.; Levtchenko, E.N. Genetic renal diseases: The emerging role of zebrafish models. Cells 2018, 7, 130. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.T.; Wu, C.Y.; Lu, M.J.; Chuang, Y.J.; Tsai, E.M.; Leu, S.; Lin, I.L.; Ko, C.J.; Chiu, C.C.; Chang, W.T. The Phenoxyphenol Compound 4-HPPP Selectively Induces Antiproliferation Effects and Apoptosis in Human Lung Cancer Cells through Aneupolyploidization and ATR DNA Repair Signaling. Oxid. Med. Cell. Longev. 2020, 2020, 5167292. [Google Scholar] [CrossRef]
- Perner, B.; Englert, C.; Bollig, F. The Wilms tumor genes wt1a and wt1b control different steps during formation of the zebrafish pronephros. Dev. Biol. 2007, 309, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, C.-K.; Liu, W.; Hong, Y.-J.; Dahms, H.-U.; Chiu, C.-H.; Chang, W.-T.; Chien, C.-M.; Yen, C.-H.; Cheng, Y.-B.; Chiu, C.-C. Ethyl Acetate Extract of Scindapsus cf. hederaceus Exerts the Inhibitory Bioactivity on Human Non-Small Cell Lung Cancer Cells through Modulating ER Stress. Int. J. Mol. Sci. 2018, 19, 1832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hentschel, D.M.; Park, K.M.; Cilenti, L.; Zervos, A.S.; Drummond, I.; Bonventre, J.V. Acute renal failure in zebrafish: A novel system to study a complex disease. Am. J. Physiol.-Ren. Physiol. 2005, 288, F923–F929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, W.T.; Bow, Y.D.; Chen, Y.C.; Li, C.Y.; Chen, J.Y.; Chu, Y.C.; Teng, Y.N.; Li, R.N.; Chiu, C.C. The Phenoxyphenol Compound diTFPP Mediates Exogenous C2-Ceramide Metabolism, Inducing Cell Apoptosis Accompanied by ROS Formation and Autophagy in Hepatocellular Carcinoma Cells. Antioxidants 2021, 10, 394. [Google Scholar] [CrossRef] [PubMed]
- Jadhao, M.; Tsai, E.-M.; Yang, H.-C.; Chen, Y.-F.; Liang, S.-S.; Wang, T.-N.; Teng, Y.-N.; Huang, H.-W.; Wang, L.-F.; Chiu, C.-C. The Long-Term DEHP Exposure Confers Multidrug Resistance of Triple-Negative Breast Cancer Cells through ABC Transporters and Intracellular ROS. Antioxidants 2021, 10, 949. [Google Scholar] [CrossRef]
- Tang, Q.; Moore, J.C.; Ignatius, M.S.; Tenente, I.M.; Hayes, M.N.; Garcia, E.G.; Yordan, N.T.; Bourque, C.; He, S.N.; Blackburn, J.S.; et al. Imaging tumour cell heterogeneity following cell transplantation into optically clear immune-deficient zebrafish. Nat. Commun. 2016, 7, 10358. [Google Scholar] [CrossRef]
- Liu, J.D.; Stainier, D.Y.R. Zebrafish in the Study of Early Cardiac Development. Circ. Res. 2012, 110, 870–874. [Google Scholar] [CrossRef]
- Dou, L.; Jourde-Chiche, N.; Faure, V.; Cerini, C.; Berland, Y.; DIGNAT-GEORGE, F.; Brunet, P. The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J. Thromb. Haemost. 2007, 5, 1302–1308. [Google Scholar] [CrossRef]
- Bollig, F.; Perner, B.; Besenbeck, B.; Kothe, S.; Ebert, C.; Taudien, S.; Englert, C. A highly conserved retinoic acid responsive element controls wt1a expression in the zebrafish pronephros. Development 2009, 136, 2883–2892. [Google Scholar] [CrossRef] [Green Version]
- Rapa, S.F.; Prisco, F.; Popolo, A.; Iovane, V.; Autore, G.; Di Iorio, B.R.; Dal Piaz, F.; Paciello, O.; Nishijima, F.; Marzocco, S. Pro-Inflammatory Effects of Indoxyl Sulfate in Mice: Impairment of Intestinal Homeostasis and Immune Response. Int. J. Mol. Sci. 2021, 22, 1135. [Google Scholar] [CrossRef]
- USRDS. 2017 USRDS Annual Data Report: Executive Summary. Am. J. Kidney Dis 2018, 71, S1–S8. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic kidney disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Drey, N.; Roderick, P.; Mullee, M.; Rogerson, M. A population-based study of the incidence and outcomes of diagnosed chronic kidney disease. Am. J. Kidney Dis. 2003, 42, 677–684. [Google Scholar] [CrossRef]
- Himmelfarb, J.; Ikizler, T.A. Hemodialysis. N. Engl. J. Med. 2010, 363, 1833–1845. [Google Scholar] [CrossRef]
- Karkar, A. Modalities of hemodialysis: Quality improvement. Saudi J. Kidney Dis. Transplant. 2012, 23, 1145. [Google Scholar]
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaziri, N.D.; Zhao, Y.-Y.; Pahl, M.V. Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: The nature, mechanisms, consequences and potential treatment. Nephrol. Dial. Transplant. 2015, 31, 737–746. [Google Scholar] [CrossRef] [Green Version]
- Niwa, T. Indoxyl sulfate is a nephro-vascular toxin. J. Ren. Nutr. 2010, 20, S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, A.; Takeda, M.; Tojo, A.; Sekine, T.; Cha, S.H.; Khamdang, S.; Takayama, F.; Aoyama, I.; Nakamura, S.; Endou, H. Role of organic anion transporters in the tubular transport of indoxyl sulfate and the induction of its nephrotoxicity. J. Am. Soc. Nephrol. 2002, 13, 1711–1720. [Google Scholar] [CrossRef] [Green Version]
- Jansen, J.; Fedecostante, M.; Wilmer, M.; Peters, J.; Kreuser, U.; van den Broek, P.; Mensink, R.; Boltje, T.; Stamatialis, D.; Wetzels, J. Bioengineered kidney tubules efficiently excrete uremic toxins. Sci. Rep. 2016, 6, 26715. [Google Scholar] [CrossRef]
- Villain, C.; Metzger, M.; Combe, C.; Fouque, D.; Frimat, L.; Jacquelinet, C.; Laville, M.; Briançon, S.; Klein, J.; Schanstra, J.P. Prevalence of atheromatous and non-atheromatous cardiovascular disease by age in chronic kidney disease. Nephrol. Dial. Transplant. 2020, 35, 827–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutz, J.; Menke, J.; Sollinger, D.; Schinzel, H.; Thürmel, K. Haemostasis in chronic kidney disease. Nephrol. Dial. Transplant. 2014, 29, 29–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathew, R.O.; Bangalore, S.; Lavelle, M.P.; Pellikka, P.A.; Sidhu, M.S.; Boden, W.E.; Asif, A. Diagnosis and management of atherosclerotic cardiovascular disease in chronic kidney disease: A review. Kidney Int. 2017, 91, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Garimella, P.S.; Hart, P.D.; O’Hare, A.; DeLoach, S.; Herzog, C.A.; Hirsch, A.T. Peripheral artery disease and CKD: A focus on peripheral artery disease as a critical component of CKD care. Am. J. Kidney Dis. 2012, 60, 641–654. [Google Scholar] [CrossRef]
- Arinze, N.V.; Yin, W.; Lotfollahzadeh, S.; Napoleon, M.A.; Richards, S.; Walker, J.A.; Belghasem, M.; Ravid, J.D.; Hassan Kamel, M.; Whelan, S.A.; et al. Tryptophan metabolites suppress the Wnt pathway and promote adverse limb events in chronic kidney disease. J. Clin. Investig. 2022, 132, e142260. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Li, J.-R.; Wang, Y.-Y.; Lin, S.-Y.; Ou, Y.-C.; Lin, C.-J.; Wang, J.-D.; Liao, S.-L.; Chen, C.-J. Indoxyl sulfate caused behavioral abnormality and neurodegeneration in mice with unilateral nephrectomy. Aging 2021, 13, 6681–6701. [Google Scholar] [CrossRef]
- Small, D.M.; Coombes, J.S.; Bennett, N.; Johnson, D.W.; Gobe, G.C. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology 2012, 17, 311–321. [Google Scholar] [CrossRef]
- Yang, K.; Du, C.; Wang, X.; Li, F.; Xu, Y.; Wang, S.; Chen, S.; Chen, F.; Shen, M.; Chen, M.; et al. Indoxyl sulfate induces platelet hyperactivity and contributes to chronic kidney disease–associated thrombosis in mice. Blood 2017, 129, 2667–2679. [Google Scholar] [CrossRef] [Green Version]
- Can, H.; Chanumolu, S.K.; Gonzalez-Munoz, E.; Prukudom, S.; Otu, H.H.; Cibelli, J.B. Comparative analysis of single-cell transcriptomics in human and Zebrafish oocytes. BMC Genom. 2020, 21, 471. [Google Scholar] [CrossRef]
- Dou, L.; Sallee, M.; Cerini, C.; Poitevin, S.; Gondouin, B.; Jourde-Chiche, N.; Fallague, K.; Brunet, P.; Calaf, R.; Dussol, B.; et al. The cardiovascular effect of the uremic solute indole-3 acetic acid. J. Am. Soc. Nephrol. 2015, 26, 876–887. [Google Scholar] [CrossRef]
- Gondouin, B.; Cerini, C.; Dou, L.; Sallee, M.; Duval-Sabatier, A.; Pletinck, A.; Calaf, R.; Lacroix, R.; Jourde-Chiche, N.; Poitevin, S.; et al. Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int. 2013, 84, 733–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Addi, T.; Poitevin, S.; McKay, N.; El Mecherfi, K.E.; Kheroua, O.; Jourde-Chiche, N.; de Macedo, A.; Gondouin, B.; Cerini, C.; Brunet, P.; et al. Mechanisms of tissue factor induction by the uremic toxin indole-3 acetic acid through aryl hydrocarbon receptor/nuclear factor-kappa B signaling pathway in human endothelial cells. Arch. Toxicol. 2019, 93, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Muslin, A.J. MAPK signalling in cardiovascular health and disease: Molecular mechanisms and therapeutic targets. Clin. Sci. 2008, 115, 203–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denise Martin, E.; De Nicola, G.F.; Marber, M.S. New therapeutic targets in cardiology: p38 alpha mitogen-activated protein kinase for ischemic heart disease. Circulation 2012, 126, 357–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, P.W.-H.; Wu, P.-H.; Lin, Y.-T.; Chiu, C.-H.; Cheng, T.-L.; Guan, W.-H.; Lin, H.Y.-H.; Lee, K.-T.; Chen, Y.-H.; Chiu, C.-C.; et al. Zebrafish Model-Based Assessment of Indoxyl Sulfate-Induced Oxidative Stress and Its Impact on Renal and Cardiac Development. Antioxidants 2022, 11, 400. https://doi.org/10.3390/antiox11020400
Tang PW-H, Wu P-H, Lin Y-T, Chiu C-H, Cheng T-L, Guan W-H, Lin HY-H, Lee K-T, Chen Y-H, Chiu C-C, et al. Zebrafish Model-Based Assessment of Indoxyl Sulfate-Induced Oxidative Stress and Its Impact on Renal and Cardiac Development. Antioxidants. 2022; 11(2):400. https://doi.org/10.3390/antiox11020400
Chicago/Turabian StyleTang, Paul Wei-Hua, Ping-Hsun Wu, Yi-Ting Lin, Chen-Hao Chiu, Tien-Li Cheng, Wen-Hui Guan, Hugo You-Hsien Lin, Kun-Tai Lee, Yau-Hung Chen, Chien-Chih Chiu, and et al. 2022. "Zebrafish Model-Based Assessment of Indoxyl Sulfate-Induced Oxidative Stress and Its Impact on Renal and Cardiac Development" Antioxidants 11, no. 2: 400. https://doi.org/10.3390/antiox11020400
APA StyleTang, P. W.-H., Wu, P.-H., Lin, Y.-T., Chiu, C.-H., Cheng, T.-L., Guan, W.-H., Lin, H. Y.-H., Lee, K.-T., Chen, Y.-H., Chiu, C.-C., & Liu, W. (2022). Zebrafish Model-Based Assessment of Indoxyl Sulfate-Induced Oxidative Stress and Its Impact on Renal and Cardiac Development. Antioxidants, 11(2), 400. https://doi.org/10.3390/antiox11020400






