Antioxidant and Chemopreventive Activity of Protein Hydrolysates from Raw and Germinated Flour of Legumes with Commercial Interest in Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material and Germination Conditions
2.3. Preparation of Protein Hydrolysate
2.4. Characterization of Antioxidant Capacity
2.4.1. Quantification of Total Polyphenols
2.4.2. ABTS Radical Scavenging Assay
2.5. Cell Culture
2.6. In Vitro Antioxidant Capacity
2.7. Cell Viability Assay
2.8. Wound-Healing Assay
2.9. Detoxifying Enzyme Induction
2.9.1. Treatment and Purification of the Cytosolic Fraction
2.9.2. Glutathione S-Transferase (GST) Assay
2.9.3. NAD(P)H: Quinone Oxyidoreductase (QR) Assay
2.10. Statistical Analysis
3. Results
3.1. Analysis of Yield and Antioxidant Activity
3.2. Antioxidant Activity of Cultured Cells
3.3. Antiproliferative Activity
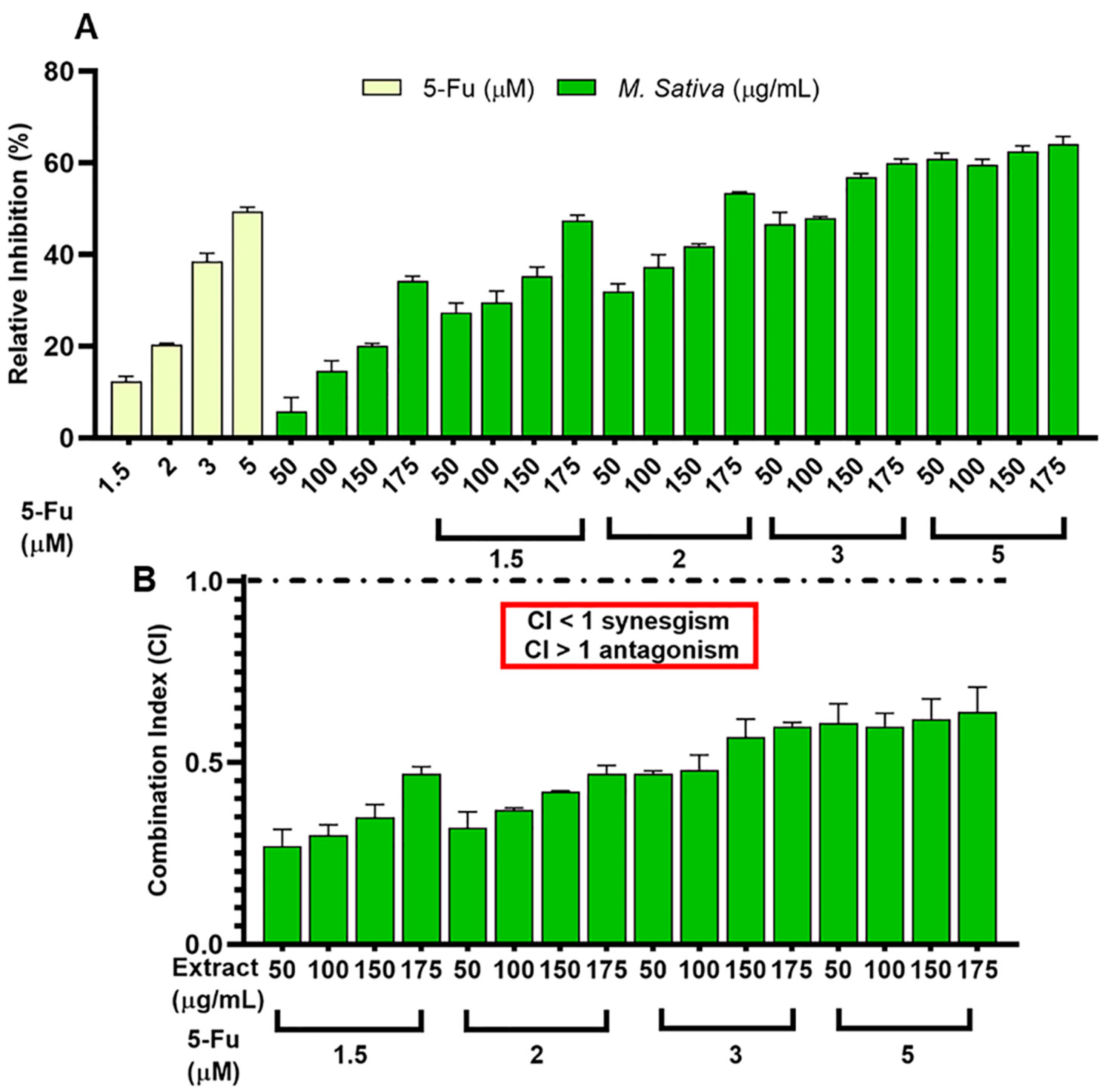
3.4. Synergistic Effect between 5-FU and Legume Extracts
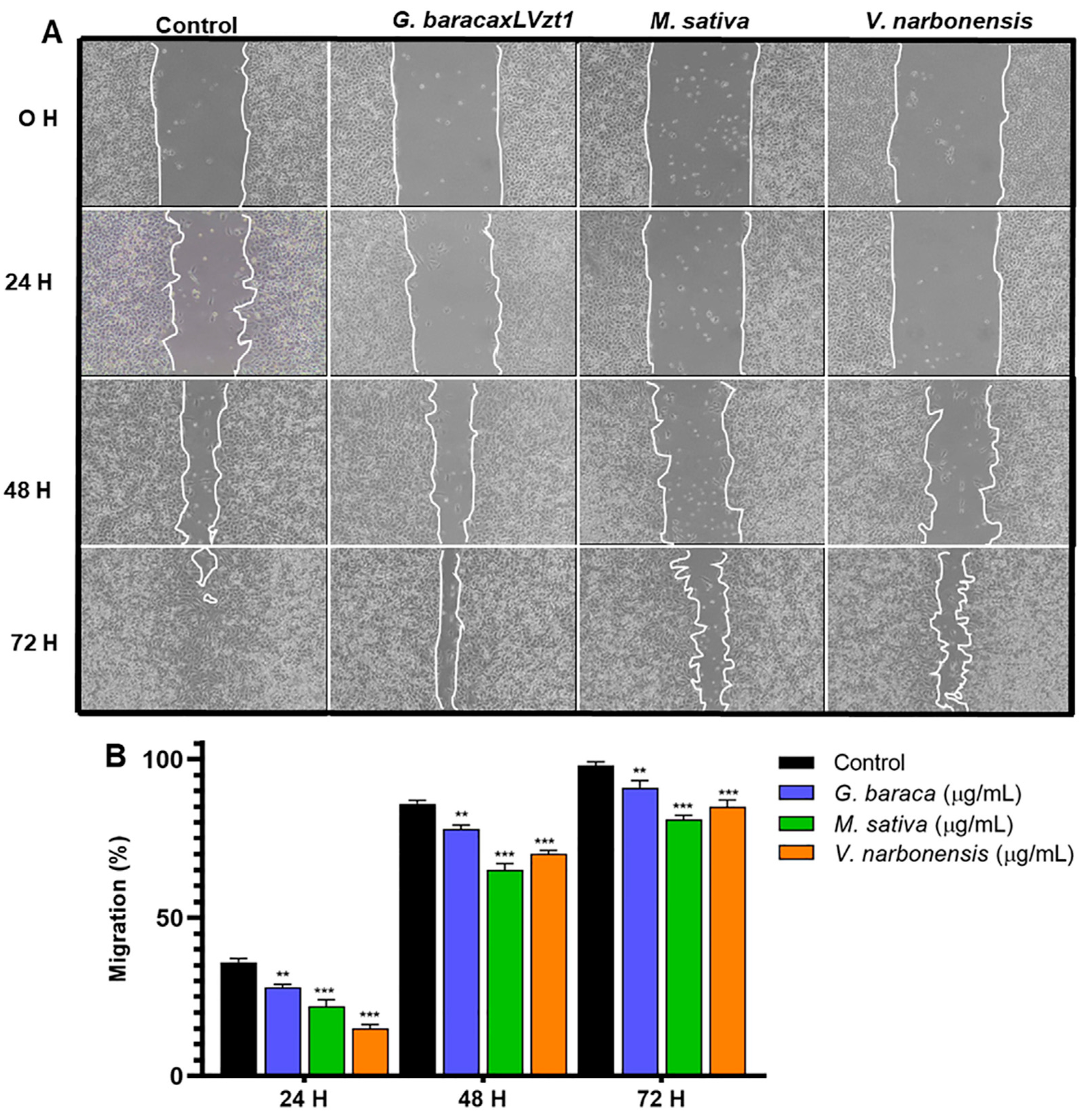
3.5. Cell Migration Analysis
3.6. Legume Extracts and Detoxifying Enzyme Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liu, X. Advances of Tumorigenesis, Diagnosis at Early Stage, and Cellular Immunotherapy in Gastrointestinal Malignancies. Front. Oncol. 2021, 11, 666340. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.S. Preclinical Drug Discovery in Colorectal Cancer: A Focus on Natural Compounds. Curr. Drug Targets 2021, 22, 977–997. [Google Scholar] [CrossRef]
- Khan, T.; Ali, M.; Khan, A.; Nisar, P.; Jan, S.A.; Afridi, S.; Shinwari, Z.K. Anticancer Plants: A Review of the Active Phytochemicals, Applications in Animal Models, and Regulatory Aspects. Biomolecules 2019, 10, E47. [Google Scholar] [CrossRef]
- Karthika, C.; Hari, B.; Rahman, M.H.; Akter, R.; Najda, A.; Albadrani, G.M.; Sayed, A.A.; Akhtar, M.F.; Abdel-Daim, M.M. Multiple strategies with the synergistic approach for addressing colorectal cancer. Biomed. Pharmacother. 2021, 140, 111704. [Google Scholar] [CrossRef]
- Wang, K.; Liu, W.; Xu, Q.; Gu, C.; Hu, D. Tenacissoside G synergistically potentiates inhibitory effects of 5-fluorouracil to human colorectal cancer. Phytomed. Int. J. Phytother. Phytopharm. 2021, 86, 153553. [Google Scholar] [CrossRef]
- Conti, M.V.; Guzzetti, L.; Panzeri, D.; Giuseppe, R.D.; Coccetti, P.; Labra, M.; Cena, H. Bioactive compounds in legumes: Implications for sustainable nutrition and health in the elderly population. Trends Food Sci. Technol. 2021, 117, 139–147. [Google Scholar] [CrossRef]
- Mullins, A.P.; Arjmandi, B.H. Health Benefits of Plant-Based Nutrition: Focus on Beans in Cardiometabolic Diseases. Nutrients 2021, 13, 519. [Google Scholar] [CrossRef]
- Bouchenak, M.; Lamri-Senhadji, M. Nutritional quality of legumes, and their role in cardiometabolic risk prevention: A review. J. Med. Food 2013, 16, 185–198. [Google Scholar] [CrossRef]
- Afshin, A.; Micha, R.; Khatibzadeh, S.; Mozaffarian, D. Consumption of nuts and legumes and risk of incident ischemic heart disease, stroke, and diabetes: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2014, 100, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.Y.; Fabek, H.; Mollard, R.C.; Jones, P.J.H.; Tulbek, M.C.; Chibbar, R.N.; Gangola, M.P.; Ramadoss, B.R.; Sánchez-Hernández, D.; Anderson, G.H. Faba bean protein flours added to pasta reduce post-ingestion glycaemia, and increase satiety, protein content and quality. Food Funct. 2019, 10, 7476–7488. [Google Scholar] [CrossRef] [PubMed]
- Gregoriou, G.; Neophytou, C.M.; Vasincu, A.; Gregoriou, Y.; Hadjipakkou, H.; Pinakoulaki, E.; Christodoulou, M.C.; Ioannou, G.D.; Stavrou, I.J.; Christou, A.; et al. Anti-Cancer Activity and Phenolic Content of Extracts Derived from Cypriot Carob (Ceratonia siliqua L.) Pods Using Different Solvents. Mol. Basel Switz. 2021, 26, 5017. [Google Scholar] [CrossRef] [PubMed]
- Heidari, S.; Mehri, S.; Hosseinzadeh, H. The genus Glycyrrhiza (Fabaceae family) and its active constituents as protective agents against natural or chemical toxicities. Phytother. Res. 2021, 35, 6552–6571. [Google Scholar] [CrossRef]
- Matemu, A.; Nakamura, S.; Katayama, S. Health Benefits of Antioxidative Peptides Derived from Legume Proteins with a High Amino Acid Score. Antioxidants 2021, 10, 316. [Google Scholar] [CrossRef]
- Urbano, G.; López-Jurado, M.; Frejnagel, S.; Gómez-Villalva, E.; Porres, J.M.; Frías, J.; Vidal-Valverde, C.; Aranda, P. Nutritional assessment of raw and germinated pea (Pisum sativum L.) protein and carbohydrate by in vitro and in vivo techniques. Nutr. Burbank Los Angel. Cty. Calif. 2005, 21, 230–239. [Google Scholar] [CrossRef]
- Kapravelou, G.; Martínez, R.; Nebot, E.; López-Jurado, M.; Aranda, P.; Arrebola, F.; Cantarero, S.; Galisteo, M.; Porres, J.M. The Combined Intervention with Germinated Vigna radiata and Aerobic Interval Training Protocol Is an Effective Strategy for the Treatment of Non-Alcoholic Fatty Liver Disease (NAFLD) and Other Alterations Related to the Metabolic Syndrome in Zucker Rats. Nutrients 2017, 9, 774. [Google Scholar] [CrossRef]
- Kapravelou, G.; Martínez, R.; Andrade, A.M.; Chaves, C.L.; López-Jurado, M.; Aranda, P.; Arrebola, F.; Cañizares, F.J.; Galisteo, M.; Porres, J.M. Improvement of the antioxidant and hypolipidaemic effects of cowpea flours (Vigna unguiculata) by fermentation: Results of in vitro and in vivo experiments. J. Sci. Food Agric. 2015, 95, 1207–1216. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. Lond. Engl. 1979 1993, 84, 407–412. [Google Scholar] [CrossRef]
- Chou, T.; Martin, N. CompuSyn for drug combinations. In PC Software and User’s Guide; ComboSyn: Paramus, NJ, USA, 2005. [Google Scholar]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research Techniques Made Simple: Analysis of Collective Cell Migration Using the Wound Healing Assay. J. Investig. Dermatol. 2017, 137, e11–e16. [Google Scholar] [CrossRef]
- Cerrito, M.G.; Grassilli, E. Identifying Novel Actionable Targets in Colon Cancer. Biomedicines 2021, 9, 579. [Google Scholar] [CrossRef]
- Malik, S.; Kaur, K.; Prasad, S.; Jha, N.K.; Kumar, V. A perspective review on medicinal plant resources for their antimutagenic potentials. Environ. Sci. Pollut. Res. Int. 2022, 29, 62014–62029. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wei, H.; Zhou, Y.; Szeto, C.-H.; Li, C.; Lin, Y.; Coker, O.O.; Lau, H.C.H.; Chan, A.W.H.; Sung, J.J.Y.; et al. High-Fat Diet Promotes Colorectal Tumorigenesis Through Modulating Gut Microbiota and Metabolites. Gastroenterology 2022, 162, 135–149.e2. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Augusti, P.R.; Quatrin, A.; Mello, R.; Bochi, V.C.; Rodrigues, E.; Prazeres, I.D.; Macedo, A.C.; Oliveira-Alves, S.C.; Emanuelli, T.; Bronze, M.R.; et al. Antiproliferative Effect of Colonic Fermented Phenolic Compounds from Jaboticaba (Myrciaria trunciflora) Fruit Peel in a 3D Cell Model of Colorectal Cancer. Mol. Basel Switz. 2021, 26, 4469. [Google Scholar] [CrossRef] [PubMed]
- Dabulici, C.M.; Sârbu, I.; Vamanu, E. The Bioactive Potential of Functional Products and Bioavailability of Phenolic Compounds. Foods 2020, 9, 953. [Google Scholar] [CrossRef]
- Grigalius, I.; Petrikaite, V. Relationship between Antioxidant and Anticancer Activity of Trihydroxyflavones. Molecules 2017, 22, 2169. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/es/#home (accessed on 26 May 2022).
- Mayer Labba, I.-C.; Frøkiær, H.; Sandberg, A.-S. Nutritional and antinutritional composition of fava bean (Vicia faba L., var. minor) cultivars. Food Res. Int. Ott. 2021, 140, 110038. [Google Scholar] [CrossRef]
- Turco, M.; Bedia, J.; Liberto, F.D.; Fiorucci, P.; von Hardenberg, J.; Koutsias, N.; Llasat, M.-C.; Xystrakis, F.; Provenzale, A. Decreasing Fires in Mediterranean Europe. PLoS ONE 2016, 11, e0150663. [Google Scholar] [CrossRef]
- Valente, M.; Oeding, K.; Brockmeyer, A.; Smith, S.; Kallogjeri, D. Differences in Word and Phoneme Recognition in Quiet, Sentence Recognition in Noise, and Subjective Outcomes between Manufacturer First-Fit and Hearing Aids Programmed to NAL-NL2 Using Real-Ear Measures. J. Am. Acad. Audiol. 2018, 29, 706–721. [Google Scholar] [CrossRef]
- Arese, P.; De Flora, A. Pathophysiology of hemolysis in glucose-6-phosphate dehydrogenase deficiency. Semin. Hematol. 1990, 27, 1–40. [Google Scholar] [PubMed]
- Du, G.; Xiao, M.; Chen, B.; Wang, A.; Zhu, Q.; Cai, W. Metabolic profiling reveals alterations in the erythrocyte response to fava bean ingestion in G6PD-deficient mice. J. Sci. Food Agric. 2021, 101, 1562–1571. [Google Scholar] [CrossRef]
- Valente, I.M.; Maia, M.R.G.; Malushi, N.; Oliveira, H.M.; Papa, L.; Rodrigues, J.A.; Fonseca, A.J.M.; Cabrita, A.R.J. Profiling of phenolic compounds and antioxidant properties of European varieties and cultivars of Vicia faba L. pods. Phytochemistry 2018, 152, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Oomah, B.D.; Luc, G.; Leprelle, C.; Drover, J.C.G.; Harrison, J.E.; Olson, M. Phenolics, phytic acid, and phytase in Canadian-grown low-tannin faba bean (Vicia faba L.) genotypes. J. Agric. Food Chem. 2011, 59, 3763–3771. [Google Scholar] [CrossRef]
- Bhadkaria, A.; Srivastava, N.; Bhagyawant, S.S. A prospective of underutilized legume moth bean (Vigna aconitifolia (Jacq.) Marechàl): Phytochemical profiling, bioactive compounds and in vitro pharmacological studies. Food Biosci. 2021, 42, 101088. [Google Scholar] [CrossRef]
- Samaei, S.P.; Ghorbani, M.; Tagliazucchi, D.; Martini, S.; Gotti, R.; Themelis, T.; Tesini, F.; Gianotti, A.; Gallina Toschi, T.; Babini, E. Functional, nutritional, antioxidant, sensory properties and comparative peptidomic profile of faba bean (Vicia faba, L.) seed protein hydrolysates and fortified apple juice. Food Chem. 2020, 330, 127120. [Google Scholar] [CrossRef] [PubMed]
- Taroncher, M.; Vila-Donat, P.; Tolosa, J.; Ruiz, M.J.; Rodríguez-Carrasco, Y. Biological activity and toxicity of plant nutraceuticals: An overview. Curr. Opin. Food Sci. 2021, 42, 113–118. [Google Scholar] [CrossRef]
- Omar, A.; Kalra, R.S.; Putri, J.; Elwakeel, A.; Kaul, S.C.; Wadhwa, R. Soyasapogenol-A targets CARF and results in suppression of tumor growth and metastasis in p53 compromised cancer cells. Sci. Rep. 2020, 10, 6323. [Google Scholar] [CrossRef]
- Liu, L.-F.; Li, W.-H.; Li, M.-Y.; Wu, X.-Z.; Yang, F.; Xu, J.-N.; Yuan, C.-S. Chemical constituents from common vetch (Vicia sativa L.) and their antioxidant and cytotoxic activities. Nat. Prod. Res. 2020, 34, 3205–3211. [Google Scholar] [CrossRef]
- Kobbi, S.; Nedjar, N.; Chihib, N.; Balti, R.; Chevalier, M.; Silvain, A.; Chaabouni, S.; Dhulster, P.; Bougatef, A. Synthesis and antibacterial activity of new peptides from Alfalfa RuBisCO protein hydrolysates and mode of action via a membrane damage mechanism against Listeria innocua. Microb. Pathog. 2018, 115, 41–49. [Google Scholar] [CrossRef]
- Pandurangi, R.S.; Karwa, A.; Sagaram, U.S.; Shah, D. Medicago Sativa Defensin 1 (MsDef1), A Natural Tumor Targeted Sensitizer for Improving Chemotherapy: Translation from Anti-Fungal Agent to Potential Anti-Cancer Agent. bioRxiv 2021. [Google Scholar] [CrossRef]
- Salehi, B.; Abu-Reidah, I.M.; Sharopov, F.; Karazhan, N.; Sharifi-Rad, J.; Akram, M.; Daniyal, M.; Khan, F.S.; Abbaass, W.; Zainab, R.; et al. Vicia plants-A comprehensive review on chemical composition and phytopharmacology. Phytother. Res. PTR 2021, 35, 790–809. [Google Scholar] [CrossRef] [PubMed]
- Kubina, R.; Iriti, M.; Kabała-Dzik, A. Anticancer Potential of Selected Flavonols: Fisetin, Kaempferol, and Quercetin on Head and Neck Cancers. Nutrients 2021, 13, 845. [Google Scholar] [CrossRef] [PubMed]
- Vamanu, E.; Gatea, F. Correlations between Microbiota Bioactivity and Bioavailability of Functional Compounds: A Mini-Review. Biomedicines 2020, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Eagles, S.K.; Gross, A.S.; McLachlan, A.J. The Effects of Cruciferous Vegetable-Enriched Diets on Drug Metabolism: A Systematic Review and Meta-Analysis of Dietary Intervention Trials in Humans. Clin. Pharmacol. Ther. 2020, 108, 212–227. [Google Scholar] [CrossRef]
- Hodges, R.E.; Minich, D.M. Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components: A Scientific Review with Clinical Application. J. Nutr. Metab. 2015, 2015, 760689. [Google Scholar] [CrossRef]
| Concentration | Lines/Varieties | |
|---|---|---|
| High Tannins/High V-C | Chipen | Raw |
| Germinated | ||
| Low Tannins/High V-C | Aldaba | Raw |
| Germinated | ||
| Cana | Raw Germinated | |
| Low Tannins/Low V-C | Alameda × LVzt2 | Raw |
| Baraca × LVzt1 | Germinated | |
| Varieties of V. faba | Yield (mg/g Flour) | Total Polyphenols (µg GAE/mg PH) | ABTS (µg GAE/mg PH) | |
|---|---|---|---|---|
| Chipen | Raw | 384.5 ± 1.89 A | 34.9 ± 0.07 A | 5.26 ± 0.02 A |
| Germinated | 478.9 ± 0.61 B | 23.5 ± 0.14 B | 3.62 ± 0.04 B | |
| Aldaba | Raw | 427.3 ± 2.95 C | 28.9 ± 0.33 C | 3.76 ± 0.07 C |
| Germinated | 477.5 ± 0.87 B | 22.5 ± 0.11 D | 2.61 ± 0.03 D | |
| Cana | Raw | 413.3 ± 0.59 C | 18.1 ± 0.02 E | 1.02 ± 0.03 E |
| Germinated | 510.3 ± 1.40 D | 20.8 ± 0.03 F | 1.87 ± 0.03 F | |
| Alameda × LVzt2 | Raw | 351.9 ± 2.72 E | 18.7 ± 0.17 E | 0.61 ± 0.01 G |
| Baraca × LVzt1 | Germinated | 455.2 ± 0.90 F | 31.6 ± 0.02 G | 0.91 ± 0.01 H |
| Legumes | Yield (mg/g Flour) | Total Polyphenols (µg GAE/mg PH) | ABTS (µg GAE/mg PH) |
|---|---|---|---|
| L. luteus | 553.6 ± 1.72 A | 13.8 ± 0.12 A | 2.90 ± 0.01 A |
| M. sativa | 384.4 ± 0.64 B | 91.8 ± 0.82 B | 12.18 ± 0.02 B |
| V. ervilia | 385.6 ± 0.80 B | 27.6 ± 0.11 C | 2.51 ± 0.02 C |
| V. narbonensis | 262.1 ± 4.03 C | 24.1 ± 0.02 D | 5.21 ± 0.06 D |
| V. sativa | 365.8 ± 1.07 D | 26.4 ± 0.06 C | 3.57 ± 0.01 E |
| Varieties of V. faba | In Vitro Antioxidant Activity (mUAA/mg) | |
|---|---|---|
| Paraquat | ||
| Chipen | Raw | 302.9 ± 1.77 |
| Germinated | 219.3 ± 1.16 | |
| Aldaba | Raw | 143.0 ± 1.76 |
| Germinated | 130.3 ± 2.18 | |
| Cana | Raw | 267.7 ± 1.73 |
| Germinated | 309.1 ± 1.89 | |
| Alameda × LVzt2 | Raw | 283.8 ± 0.63 |
| Baraca × LVzt1 | Germinated | 368.9 ± 1.91 |
| Legumes | In Vitro Antioxidant Activity (mUAA/mg) |
|---|---|
| Paraquat | |
| L. luteus | 273.2 ± 1.42 A |
| M. sativa | 616.2 ± 1.58 B |
| V. ervilia | 236.7 ± 1.98 C |
| V. narbonensis | 301.4 ± 1.51 D |
| V. sativa | 437.2 ± 1.61 E |
| IC50 (µg/mL) | ||||
|---|---|---|---|---|
| T84 | HCT-15 | SW480 | CCD18 | |
| V. faba var. Chipen | ||||
| raw | - | - | - | - |
| germinated | - | - | - | - |
| V. faba var. Aldaba | ||||
| raw | - | - | - | - |
| germinated | - | - | - | - |
| V. fabavar. Alameda × LVzt2 | ||||
| raw | - | - | - | - |
| V. faba var. Cana | ||||
| raw | - | - | - | - |
| germinated | - | - | - | - |
| V. fabavar. Baraca × LVzt1 | ||||
| germinated | 360.8 ± 2.35 | - | 397.3 ± 1.06 | - |
| L. luteus | - | - | - | - |
| raw | ||||
| V. narbonensis | 328.8 ± 3.46 | - | 405.5 ± 1.03 | 879.5 ± 1.20 |
| raw | ||||
| M. sativa | 269.2 ± 0.32 | 452.8 ± 5.49 | 328.2 ± 1.68 | 710.4 ± 5.90 |
| raw | ||||
| V. ervilia | 954.9 ± 0.58 | - | - | - |
| raw | ||||
| V. sativa | - | - | - | - |
| raw | ||||
| GST | QR | ||||||
|---|---|---|---|---|---|---|---|
| Concentration of PH | U/mL | U/mg | Induction Rate (Treated/Control) | U/mL | U/mg | Induction Rate (Treated/Control) | |
| Control | 72.9 ± 2.66 | 20.6 ± 0.76 | 1.00 ± 0.00 | 229.4 ± 1.72 | 591.8 ± 0.24 | 1.00 ± 0.00 | |
| M. sativa | 25 µg/mL | 220.9 ± 4.18 | 51.9 ± 1.29 | 2.53 ± 0.05 *** | 6684.4 ± 3.91 | 1768.4 ± 0.51 | 2.99 ± 0.02 *** |
| V. faba line Baraca × LVzt1 germinated | 25 µg/mL | 179.1 ± 1.80 | 37.9 ± 0.38 | 1.84 ± 0.02 *** | 6383.4 ± 2.61 | 1305.4 ± 0.34 | 2.21 ± 0.01 *** |
| V. narbonensis | 25 µg/mL | 197.9 ± 3.43 | 37.9 ± 0.66 | 1.84 ± 0.03 *** | 5661.7 ± 4.19 | 1139.2 ± 0.64 | 1.92 ± 0.03 *** |
| Sulforaphane | 5 µM | 66.3 ± 1.49 | 22.3 ± 0.50 | 1.08 ± 0.02 * | 5852.4 ± 1.20 | 1336.1 ± 0.14 | 2.28 ± 0.01 *** |
| Sulforaphane | 10 µM | 116.2 ± 3.87 | 26.5 ± 0.88 | 1.29 ± 0.04 *** | 4947.9 ± 2.64 | 1660.4 ± 0.12 | 2.81 ± 0.01 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuel, M.; Mesas, C.; Martínez, R.; Ortiz, R.; Quiñonero, F.; Bermúdez, F.; Gutiérrez, N.; Torres, A.M.; Kapravelou, G.; Lozano, A.; et al. Antioxidant and Chemopreventive Activity of Protein Hydrolysates from Raw and Germinated Flour of Legumes with Commercial Interest in Colorectal Cancer. Antioxidants 2022, 11, 2421. https://doi.org/10.3390/antiox11122421
Fuel M, Mesas C, Martínez R, Ortiz R, Quiñonero F, Bermúdez F, Gutiérrez N, Torres AM, Kapravelou G, Lozano A, et al. Antioxidant and Chemopreventive Activity of Protein Hydrolysates from Raw and Germinated Flour of Legumes with Commercial Interest in Colorectal Cancer. Antioxidants. 2022; 11(12):2421. https://doi.org/10.3390/antiox11122421
Chicago/Turabian StyleFuel, Marco, Cristina Mesas, Rosario Martínez, Raúl Ortiz, Francisco Quiñonero, Francisco Bermúdez, Natalia Gutiérrez, Ana M. Torres, Garyfallia Kapravelou, Aída Lozano, and et al. 2022. "Antioxidant and Chemopreventive Activity of Protein Hydrolysates from Raw and Germinated Flour of Legumes with Commercial Interest in Colorectal Cancer" Antioxidants 11, no. 12: 2421. https://doi.org/10.3390/antiox11122421
APA StyleFuel, M., Mesas, C., Martínez, R., Ortiz, R., Quiñonero, F., Bermúdez, F., Gutiérrez, N., Torres, A. M., Kapravelou, G., Lozano, A., Perazzoli, G., Prados, J., Porres, J. M., & Melguizo, C. (2022). Antioxidant and Chemopreventive Activity of Protein Hydrolysates from Raw and Germinated Flour of Legumes with Commercial Interest in Colorectal Cancer. Antioxidants, 11(12), 2421. https://doi.org/10.3390/antiox11122421






