Abstract
We here investigated the anti-inflammatory activity of a polymethoxylated flavone-containing fraction (PMFF) from Citrus sinensis and of a prenylflavonoid-containing one (PFF) from Humulus lupulus, either alone or in combination (MIX). To this end, an in vitro model of inflammatory bowel disease (IBD), consisting of differentiated, interleukin (IL)-1β-stimulated Caco-2 cells, was employed. We demonstrated that non-cytotoxic concentrations of either PMFF or PFF or MIX reduced nitric oxide (NO) production while PFF and MIX, but not PMFF, also inhibited prostaglandin E2 release. Coherently, MIX suppressed both inducible NO synthase and cyclooxygenase-2 over-expression besides NF-κB activation. Moreover, MIX increased nuclear factor erythroid 2–related factor 2 (Nrf2) activation, heme oxygenase-1 expression, restoring GSH and reactive oxygen and nitrogen species (RONs) levels. Remarkably, these effects with MIX were stronger than those produced by PMFF or PFF alone. Noteworthy, nobiletin (NOB) and xanthohumol (XTM), two of the most represented phytochemicals in PMFF and PFF, respectively, synergistically inhibited RONs production. Overall, our results demonstrate that MIX enhances the anti-inflammatory and anti-oxidative effects of the individual fractions in a model of IBD, via a mechanism involving modulation of NF-κB and Nrf2 signalling. Synergistic interactions between NOB and XTM emerge as a relevant aspect underlying this evidence.
1. Introduction
Inflammatory bowel disease (IBD) is a group of relapsing, idiopathic and chronic inflammatory conditions affecting the gastrointestinal tract [1,2]. It is globally prevalent, with increasing incidence in the newly industrialized countries and strictly related to mass consumption of refined sugars and ultra-processed food items, typical of the Westernized diets [3].
While the aetiology of IBD must be considered multifactorial and not clear yet, its development markedly relies on a vicious cycle between oxidative stress and inflammation that eventually impairs the gut barrier structure and function [2,4].
Indeed, alterations of the endocellular redox environment can generate a dysfunctional activation of specific, redox-dependent signal transduction pathways that generates constant and aberrant crosstalk between the immune cells and the intestinal epithelial ones [4,5,6]. These molecular events are orchestrated by an amazingly complex cellular machinery involving the activation of several redox-dependent transcription factors, with nuclear factor erythroid 2-related factor 2 (Nrf2) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) being key players [7,8]. The first one is responsible for maintaining the endocellular redox balance by activating the antioxidant response element (ARE)-dependent transcription of antioxidant defence enzymes such as heme oxygenase-1 (HO-1), superoxide dismutase-1 (SOD-1) and glutathione S-transferases (GSTs) [9]. On the other hand, NF-κB activation leads to the elaboration of a proinflammatory mediator system, including tumour necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, IL-8, prostaglandin E2 (PGE2) and to the over-expression of crucial, pro-inflammatory enzymes such as inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX-2) [10].
Amongst the pro-inflammatory mediators, IL-1β has repeatedly been reported to play a key role in the intestinal inflammatory response [11]. IL-1β is, indeed, a multifunctional cytokine released by several cell types, including monocytes, macrophages, neutrophils and endothelial cells. Increased levels of IL-1β have been found in the intestinal tissue of IBD patients and linked to higher disease severity [12]. Accordingly, a number of effects of IL-1β on intestinal epithelium have been reported both in vitro and in vivo. Indeed, Il-1β activates specific, redox-sensitive, signal transduction pathways that increase the expression of a wide range of pro-inflammatory genes such as those encoding for IL-8, IL-6, TNF-α and iNOS [13,14]. Moreover, a dysfunctional intestinal epithelium can also contribute to the pathogenesis of colitis-related cancer, non-alcoholic fatty liver disease, cardiovascular and kidney diseases and cognitive impairment [15]. All considered, targeting inflammation can be regarded, therefore, as a valid strategy to maintain and improve both intestinal and overall health.
Therapeutical control of IBD is unfortunately limited to a pharmacological approach (corticosteroids and immunomodulators) that is not free from adverse drug reactions [1].
A growing body of evidence strongly suggests that the administration of phytochemicals can support pharmacological therapies, especially for complex and multi-factorial pathological conditions such as inflammation [16]. Indeed, combining anti-inflammatory phytochemicals with standard drugs may reduce drug toxicity and realize a more effective treatment. Moreover, also the combined use of different phytochemicals, targeting different checkpoints of inflammatory diseases, could bring about an enhanced therapeutic outcome by producing synergistic interactions [17].
Over the last decades, citrus (Citrus sinensis) fruits and hop (Humulus lupulus) have been evaluated for their nutraceutical and health-promoting potential and reported to exert a plethora of beneficial effects in several pathological conditions, including IBD [18,19,20]. The anti-inflammatory effects of citrus and hop strongly depend on their biologically active polyphenols, able to interfere with crucial, redox-dependent signal transduction pathways [21,22].
Flavonoids in citrus juice mainly consist of flavanones and flavones, hesperetin, hesperidin, and naringin are the most abundant flavanones. Among the flavones, tangeretin (TGT) and nobiletin (NOB) are commonly found as polymethoxylated flavones (PMF) in citrus [23,24]. PMF are characterized by multiple methoxy (-OCH3) groups attached to the flavone structure [25]. The number and positions of methoxy groups in PMF significantly influence their bioactivity, with higher methoxy content enhancing hydrophobicity and biological effects [26]. PMF are well-known for their diverse therapeutic properties, including anti-cancer, anti-inflammatory, anti-dysmetabolic, neuroprotective, antimicrobial, and antioxidative activities [26,27,28,29].
Hops are a unique source of natural prenylated flavonoids (PF) [30]. These polyphenols are characterized by the presence of a prenyl group (-C5H8) attached to different positions of the aromatic ring of flavonoids, such as carbon 6 or carbon 8 [31]. The presence of the prenyl group in these compounds plays a significant role in their biological activities enhancing the hydrophobic nature of the molecules and modulating their interactions with target sites such as biomembranes and/or proteins [32]. PF are redox-active molecules able to modulate endocellular redox milieu and thus the redox-dependent signal transduction pathways sustaining the inflammatory response and the dysmetabolic conditions [33,34]. Accordingly, PF exhibits various biological effects including antioxidant, antiviral, antibacterial, antidiabetic, anti-inflammatory, and estrogenic activities [35,36,37,38,39,40,41,42,43].
In the light of the strict interconnections between IBD, oxidative stress and inflammation and considering the anti-oxidative and anti-inflammatory properties of PMF and PF, we here investigated whether these polyphenols subclasses, alone or in combination, display protective effects in an in vitro model of IBD. To this aim, we used selected fractions of PMF (PMFF) and PF (PFF) isolated from the extracts of Citrus sinensis and Humulus lupulus respectively, either alone or in combination (MIX) as elsewhere reported [44,45]. Moreover, differentiated Caco-2 cells, an established model of the human intestinal barrier, were exposed to IL-1β either in the absence or in the presence of PMFF or PFF or MIX. A number of inflammatory parameters, including pro-inflammatory mediators (NO, PGE2), inducible inflammatory enzymes (iNOS and COX-2) and redox-dependent transcription factors (NF-κB and Nrf2) were evaluated. Finally, considering that NOB is one of the major compounds in the PMFF and Xanthohumol (XTM) is one of the main abundant compounds identified in the PFF, we investigated the synergistic effects of NOB and XTM on reactive oxygen and nitrogen species (RONs) levels.
Overall, our results demonstrate for the first time that the association between PMFF and PFF enhances the anti-inflammatory and anti-oxidative effects of the individual fractions in an in vitro model of IBD. The synergistic interactions between NOB and XTM emerge as a relevant aspect underlying this evidence. A mechanistic basis for the observed effects, involving inhibition of NF-κB signalling and stimulation of the Nrf2/HO-1 pathway, is also suggested.
2. Materials and Methods
2.1. Reagents
Unless otherwise specified, all reagents and chemicals were purchased from Merck (Milan, Italy) and of the highest purity grade available.
2.2. PMFF and PFF Isolation
PMFF and PFF were isolated from red-orange fruits and hop flowers, respectively, according to Turdo et al. [35]. Briefly, Citrus sinensis fruits var. Tarocco, grown in Salerno, a city located in the Campania region, Italy, was collected in the year 2021, hand-squeezed, and the resulting juice clarified through centrifugation at 15,000× g for 15 min at 25 °C and lyophilized. The hop flowers purchased in a local herbalist’s shop were degreased thrice with hexane (1:25, w:v). The powdered samples of both preparations were subjected to three sequential extractions with methanol (MeOH) for a duration of 10 min each. This extraction process aimed to recover the polyphenolic compounds present in the samples. Finally, PMFF and PFF were purified by reversed-phase semi-preparative liquid chromatography as described by Turdo et al. [35].
2.3. Cell Culturing and Treatments
Human, colon adenocarcinoma-derived Caco-2 cells were purchased from Thermo Fisher Scientific (Milan, Italy) and used between passages 15 and 20. Cells were grown in 175 cm2 flasks in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% foetal bovine serum (FBS), 1% non-essential amino acids, 10 mM HEPES, 50 units/mL penicillin, 50 mg/mL streptomycin, and maintained at 37 °C in 5% CO2. To obtain fully differentiated cells, they were seeded at a density of 1.25 × 105 cells/mL in twenty-four-well plates in DMEM for 21 d. The culture medium was replaced thrice a week. Cell viability was routinely checked by the trypan blue exclusion method.
Before each treatment, differentiated Caco-2 cells were starved overnight in a serum-free medium and then placed in DMEM supplemented with 2% FBS. Differentiated Caco-2 cells were, then, either left untreated (control) or stimulated with IL-1β (25 ng/mL) for either 12, 24 or 48 h. When necessary, cells were preincubated for 1 h with either PMFF or PFF in a concentration range between 2.5 and 20 μg/mL or with a combination of both fractions (1:1, w:w, MIX) with each fraction at a concentration of 2.5 μg/mL.
2.4. Cell Viability
The cytotoxicity of PMFF or PFF against differentiated Caco-2 cells was determined by the MTT colorimetric assay as previously reported [46]. This assay is based on the reduction in 3-(4,5-dimethyl-2-thiazolyl)bromide-2,5-diphenyl-2-H tetrazolium to purple formazan by the mitochondrial dehydrogenases of living cells. Briefly, cells were seeded into 96-well plates (Corning Costar, Milan, Italy) at a density of 2.0 × 104 cells/cm2, incubated overnight and then treated either in the absence (control) or in the presence of the fractions for 24 or 48 h. Afterwards, the medium was carefully removed and 200 µL of 5 mg/mL MTT was added. The supernatant was discarded after 2 h of incubation at 37 °C and the formazan blue formed was dissolved in dimethyl sulfoxide. The absorbance at 565 nm that characterise the formazan product was measured using a microplate reader (LTek, INNO, Seongnam, Republic of Korea) and the value of control cells was taken as 100% of viability. Each experiment was repeated three times in triplicate to obtain the mean values.
2.5. Nitrite Assay
NO released by differentiated Caco-2 cells in the medium was evaluated as nitrite, by using the Griess reagent as previously reported [47].
2.6. Estimation of Combination Index
The Chou and Talalay method [48] was employed to investigate the combined effect of NOB and XTM. Differentiated Caco-2 cells were preincubated for 1 h with either NOB or XTM (in a concentration range between 2.5 and 10 μg/mL) or with MIX, (in a concentration range between 1 and 8 μg/mL). Cells were subsequently treated with IL-1β (25 ng/mL) for 24 h and NO released was determined.
The Combination Index (CI) was calculated using CompuSyn software, 2005 https://www.combosyn.com/register_process.php (accessed on 18 July 2023), (ComboSyn, Paramus, NJ, USA) to define the type of effect. CI is calculated for every fraction affected (fa) value, where fa is defined as percentage inhibition/100. CI > 1, CI < 1 and CI = 1 indicate antagonism, synergism and additive effects, respectively.
2.7. PGE2 Assay
PGE2 released by Caco-2 cells in the medium was measured by a PGE2 Enzyme Immunoassay Kit (Cayman Chemical Corporation, Milan, Italy) in accordance with the manufacturer’s instructions. Briefly, after treatment, cells were pelleted by centrifugation at 450× g for 5 min at 4 °C. Supernatants were diluted at 1:2.5 with assay buffer. A 100 µL sample, a 50 µL alkaline phosphatase PGE2 conjugate and a 50 µL monoclonal anti-PGE2 EIA antibody were, then, applied to a goat anti-mouse IgG-containing microtiter plate and incubated at room temperature for 2 h. After washing, 200 µL of p-nitrophenyl phosphate substrate solution was added and incubated at room temperature for 45 min. Finally, optical density at 405 nm was measured on a microplate reader (LTek, INNO, Seongnam, Republic of Korea). PGE2 concentrations in the samples were calculated from a PGE2 standard curve (25–2000 pg/mL) that was run in parallel.
2.8. Western Blot Analysis
Protein levels of COX-2, iNOS and HO-1 were evaluated as previously described [49] with some modifications. Briefly, cells were lysed on ice-cold buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 24 mM sodium deoxycholate, 0.01% SDS, 10 mM sodium pyrophosphate, 100 mM sodium fluoride, 10 mM sodium orthovanadate, 1.5 µM aprotinin, 1 mM phenylmethanesulfonylfluoride (PMSF) and 2.1 µM leupeptin. Lysates were centrifuged at 12,000× g at 4 °C for 10 min and the supernatants were used for protein determination [50]. Sample buffer (62.5 mM Tris-HCl, 10% glycerol, 2% SDS, 33.2 mM dithiothreitol (DTT) and 0.01% bromophenol blue, pH 6.8) was, then, added to the supernatants. Samples containing 50 µg protein were subjected to SDS-PAGE electrophoresis on 8% acrylamide gels and then electroblotted onto nitrocellulose membranes. Coloured protein markers were used to monitor the progress of protein electrophoresis and to assess the transfer efficiency and the molecular weight of blotted proteins (AmershamTM, ECLTM RainbowTM Marker-Full Range, VWR, Milan, Italy). Membranes were blocked for 2 h in 5% (w:v) skimmed, dry milk and subsequently incubated in the presence of the corresponding primary antibodies (Santa Cruz, Milan, Italy, 1:1000 dilution) overnight at 4 °C. After incubation for 90 min at room temperature in the presence of secondary, HRP-conjugated antibodies (Dako, Milan, Italy, 1:10,000 dilution), proteins were visualised by using an enhanced chemiluminescent substrate (1.1 mM luminol sodium salt, 2.0 mM 4-iodophenylboronic acid, 5.3 mM hydrogen peroxide and 0.1 M Tris–HCl, pH 8.6). Chemiluminescent bands were evaluated with a C-Digit Blot Scanner (LI-COR, Lincoln, NE, USA) and band intensities were analysed using LI-COR Image Studio 4.0.
To determine the protein levels of either cytosolic or nuclear p65 subunit and Nrf2, corresponding fractions were prepared according to Seubwai et al. [51]. Briefly, cells were lysed in hypotonic buffer (10 mM HEPES KOH at pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 1 mM EDTA, 1% NP-40, 0.5 mM DTT, 1 mM PMSF and 10 µg/mL aprotinin). After centrifugation at 2600× g for 3 min at 4 °C, the supernatant containing the cytosolic fraction was collected. The pellet was used as the nuclear fraction, lysed with nuclear lysis buffer (20 mM HEPES KOH at pH 7.9, 10% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 1 mM PMSF and 10 µg/mL aprotinin) and incubated on ice for 30 min. The nuclear fraction was obtained by centrifugation at 21,000× g for 10 min at 4 °C. Samples of the nuclear and cytosolic fractions containing 50 µg protein were used for analyses of p65 and Nrf2 levels as above described. All results were expressed as mean ± SD of the densitometric band analysis obtained from three replicates. All results were normalised to β-actin or laminin B. For each protein, a representative lane was selected to compose the figures.
2.9. Reactive Oxygen and Nitrogen Species (RONs)
Intracellular levels of RONs were measured by employing a fluorometric detection kit (abcam, Milan, Italy; catalogue number ab113851) according to the manufacturer’s instructions. The assay uses the cell-permeant reagent 2′,7′-dichlorofluorescin diacetate that diffuses through cell membranes and is then deacetylated by cellular esterases to a non-fluorescent compound. This latter is then oxidized by RONs into the highly fluorescent 2′,7′-dichlorofluorescein and detected by fluorescence spectroscopy with excitation/emission at 485 nm/535 nm.
2.10. GSH Measurements
Intracellular GSH/GSSG levels were measured by employing a glutathione colorimetric assay kit according to the manufacturer’s instruction (Invitrogen, Milan, Italy, catalogue number EIAGSHC).
2.11. Statistical Analysis
Results were reported as mean ± SD. Statistical analysis was performed either by an unpaired Student’s t-test or by ANOVA followed by Tukey’s post hoc test using Prism 8.0, GraphPad (San Diego, CA, USA). Results with a p-value < 0.05 were considered statistically significant.
3. Results
3.1. Evaluation of PMFF and PFF Chemical Composition
In this study, we employed a class-specific isolation approach using reverse-phase semi-preparative liquid chromatography to purify PMFF from citrus juice and PFF from hop flowers.
Fractionation techniques offer several advantages in terms of selectivity, cost-effectiveness, and higher yields over the isolation and purification of individual compounds in the large-scale production of functional ingredients. The isolation of single compounds often involves additional steps that are time-consuming and costly. In contrast, fractionation methods enable the production of larger quantities of bioactive fractions in a shorter time. This approach allows for the selective collection of bioactive compounds based on their structural and chemical similarities. This means that compounds with similar properties and potential benefits can be obtained together, enhancing the overall functionality of the isolated fraction.
In the present study, we have isolated two aliquots, PMFF or PFF from citrus juice and from hop flowers, respectively. The fractions were collected based on their elution times and thus hydrophobicity. Figure 1 shows the chemical characterization of both PMFF and PFF, including Total ion chromatogram, MS and MS/MS.
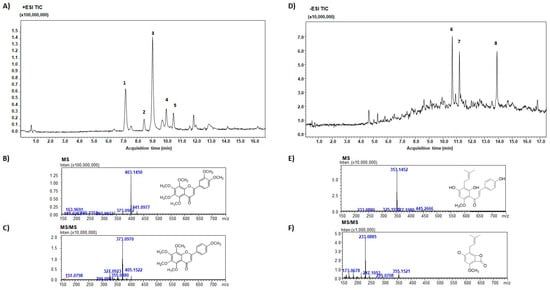
Figure 1.
TIC of PMFF isolated from Citrus sinensis (A); MS (B) and MS/MS (C) spectra of NOB. #1, SIN; #2, Hexamethoxyflavone; #3, NOB; #4, Heptamethoxyflavone; #5, TGT. TIC of PFF isolated from Humulus lupulus (D); MS (E) and MS/MS (F) spectra of XTM. #1, 5,7-Di-O-methyl-8-Prenylnaringenin; #2, Ox-XTM; #3, XTM.
LC-MS/MS analysis of PMFF revealed the presence of several biomolecules, including nobiletin (%peak area at 330 nm, 51.0% ± 0.2%), sinensetin (25.3% ± 0.1%), hexamethoxyflavone (3.8% ± 0.1%), heptamethoxyflavone (4.9% ± 0.1%), and tangeretin (5.1% ± 0.3%). MS/MS spectrum showed the characteristic fragment ions of PMFF, displaying a loss of a 31 Da group corresponding to CH3O.
On the other hand, the PFF was primarily composed of xanthohumol (% peak area at 370 nm, 27.5% ± 0.2%), ox-xanthohumol (21.8% ± 1.4%), and 5,7-di-O-methyl-8-prenylnaringenin (19.0% ± 1.2%). Figure 1E showed a typical fragmentation pattern of PFF, with the negative charge retained on the A-rings, resulting from retro-Diels-Alder fragmentation.
3.2. Evaluation of PMFF and PFF Cytotoxicity, Alone or in Combination, on Differentiated Caco-2 Cells
Before evaluating the anti-inflammatory potential of PMFF and PFF in our experimental system, their cytotoxicity on differentiated Caco-2 cells was assessed by MTT assay. To this aim, cells were either left untreated (control) or incubated with either PMFF or PFF or MIX in a concentration range between 2.5 and 20 µg/mL for 24 h.
As shown in Figure 2, when compared to control cells, neither treatment with PMFF, nor with PFF nor with MIX significantly affected cell viability in the concentration range tested and for the time period evaluated.
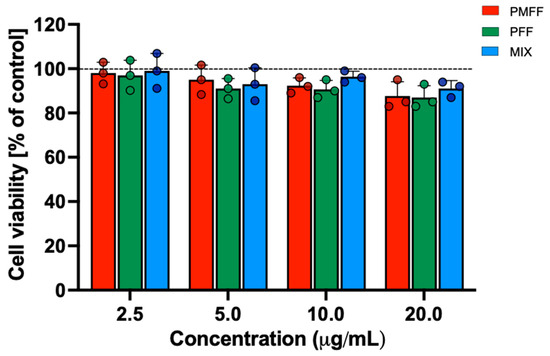
Figure 2.
Evaluation of cytotoxicity of PMFF, PFF or MIX on differentiated Caco-2 cells. Cell viability was assessed after a 24 h treatment. Values obtained are the mean ± SD of three separate experiments conducted in triplicates.
3.3. PMFF and PFF in Combination Inhibit NO and PGE2 Release in Differentiated, IL-1β-Stimulated Caco-2 Cells
NO and PGE2 are regarded as two of the most relevant mediators in gastrointestinal inflammatory diseases such as ulcerative colitis and Crohn’s disease [52,53].
The study then continued by assessing the effects of the two fractions on NO and PGE2 released by the activated, intestinal cells in our experimental system. To this end, differentiated Caco-2 cells were either left untreated (control) or stimulated with IL-1β (25 ng/mL) for 24 h. When necessary, cells were preincubated with either PMFF (2.5 μg/mL) or PFF (2.5 μg/mL) or MIX (2.5 μg/mL) for 1 h.
As shown in Figure 3A, with respect to control cells, IL-1β incubation resulted in a significant increase in NO released by Caco-2 cells (p < 0.001). Conversely, when Caco-2 cells were pre-incubated for 1 h with either PMFF or PFF at 2.5 μg/mL and then stimulated with IL-1β, a significant (p < 0.005) reduction in NO levels was observed with respect to treated cells. Relevantly, pre-incubation with MIX totally prevented IL-1β-induced NO release and brought its value back to control levels (Figure 3A).
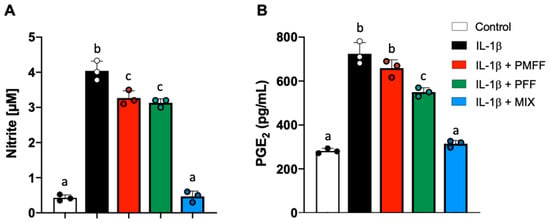
Figure 3.
Effect of PMFF, PFF or MIX on NO (A) and PGE2 (B) released from differentiated, IL-1β-activated Caco-2 cells. Values obtained are the mean ± SD of three separate experiments conducted in triplicates. Means with different letters are significantly different with p < 0.05 (one-way Anova with Tukey’s post hoc test).
On the other hand, as shown in Figure 3B, a baseline level of PGE2 was observed in control cells as a result of the constitutive cyclooxygenase 1 activity. Conversely, IL-1β stimulation significantly (p < 0.001) increased PGE2 release when compared to control. Interestingly, preincubation of Caco-2 cells with PMFF did not significantly affect PGE2 production with respect to IL-1β-treated cells. On the other hand, a significant (p < 0.02) reduction was observed in the presence of PFF vs. treated cells. Relevantly, pre-incubation with MIX totally prevented IL-1β-induced PGE2 release and brought its value back to.
3.4. PMFF and PFF in Combination Reduce iNOS and COX-2 Expression Levels in Differentiated, IL-1β-Activated Caco-2 Cells
Since MIX treatment strongly decreased NO and PGE2 release from differentiated IL-1β-activated Caco-2 cells, we then investigated whether PMFF or PFF treatment (either individually or in combination) affected iNOS and COX-2 protein levels. To this aim, differentiated Caco-2 cells were either left untreated (control) or stimulated with IL-1β (25 ng/mL) for 24 h. When necessary, cells were preincubated with either PMFF (2.5 μg/mL) or PFF (2.5 μg/mL) or MIX (2.5 μg/mL) for 1 h.
As shown in Figure 4, stimulation of cell monolayers with Il-1β induced a significant (p < 0.001) increase in both iNOS and COX-2 protein levels as compared to control. On the other hand, and coherently with the above-reported results on NO levels, co-incubation of cells with either PMFF or PFF significantly reduced the IL-1β-induced upregulation of iNOS (p < 0.005 and p < 0.001 respectively). Moreover, and in line with the observed effects on PGE2 release, PMFF did not induce any significant inhibition on COX-2 overexpression, while PFF treatment significantly reduced COX-2 protein levels (p < 0.001). Relevantly, co-incubation with MIX reduced iNOS and COX-2 overexpression either to control levels or below, respectively.
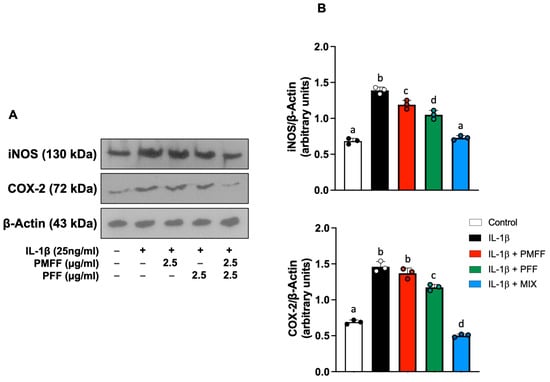
Figure 4.
Effect of PMFF, PFF or MIX on iNOS and COX-2 protein levels in differentiated, IL-1β activated Caco-2 cells. Representative images of Western blot analysis (A). Densitometric analysis of iNOS and COX-2 protein levels normalised for β actin levels (B). Values obtained are the mean ± SD of three separate experiments. Means with different letters are significantly different with p < 0.05 (one-way Anova with Tukey’s post hoc test).
3.5. PMFF and PFF in Combination Potentiate the Inhibition of IL-1β-Dependent Activation of NF-κB in Differentiated Caco-2 Cells
Our investigation next evaluated whether the reduction in IL-1β-induced COX-2 and iNOS over-expression by PMFF and PFF was associated with an inhibition of NF-κB activation. To this aim, differentiated Caco-2 cells were either left untreated (control) or stimulated with IL-1β (25 ng/mL) for 12 h. When necessary, cells were preincubated with either PMFF (2.5 μg/mL) or PFF (2.5 μg/mL) or MIX (2.5 μg/mL) for 1 h. The translocation of the p65 subunit to the nucleus was assessed as a marker of NF-κB activation.
As shown in Figure 5, when compared to control, stimulation with IL-1β resulted in a significant increase in NF-κB nuclear translocation, evident from the increased nuclear levels of p65 and the concomitant reduction in the cytosolic ones (p < 0.001). On the other hand, PMFF or PFF pre-treatment induced a significant reduction in p65 nuclear levels (p < 0.005 and p < 0.001, respectively). Interestingly, MIX preincubation completely inhibited IL-1β-induced p65 nuclear translocation, restoring nuclear and cytosolic protein levels to control ones.
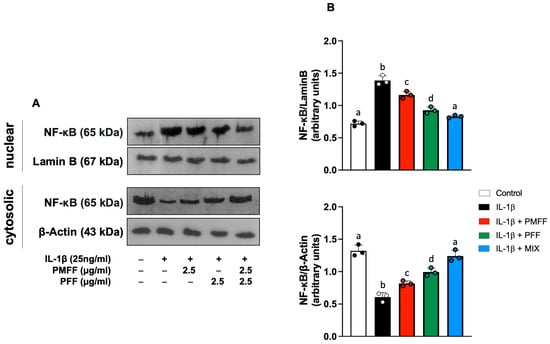
Figure 5.
Effect of PMFF, PFF or MIX on p65 nuclear translocation in differentiated, IL-1β-activated Caco-2 cells. Representative images of Western blot analysis (A). Densitometric analysis of p65 protein levels normalised for β actin or laminin b levels (B). Values obtained are the mean ± SD of three separate experiments. Means with different letters are significantly different with p < 0.05 (one-way Anova with Tukey’s post hoc test).
3.6. PMFF and PFF in Combination Enhances the Nuclear Translocation of Nrf2 and the Expression of HO-1 in Differentiated Caco-2 Cells
Taking into account the present results on the inhibition by PMFF and PFF of both NF-κB activation and the expression of its downstream proinflammatory products, we next investigated whether the two fractions, either alone or in combination, would also affect Nrf2 signalling. To this aim, differentiated Caco-2 cells were either left untreated (control) or stimulated with IL-1β (25 ng/mL) for 12 h. When necessary, cells were preincubated with either PMFF (2.5 μg/mL) or PFF (2.5 μg/mL) or MIX (2.5 μg/mL) for 1 h. The nuclear translocation of Nrf2 was assessed as a marker of its activation.
As shown in Figure 6, when compared to control cells, stimulation with IL-1β increases Nrf2 nuclear translocation (p < 0.01). Interestingly, both PMFF and PFF individually added, significantly increased Nrf2 activation (p < 0.05) with respect to IL-1β-treated cells. Remarkably, a significant further increase in its nuclear levels was evident when PMFF and PFF were added in combination (p < 0.001).
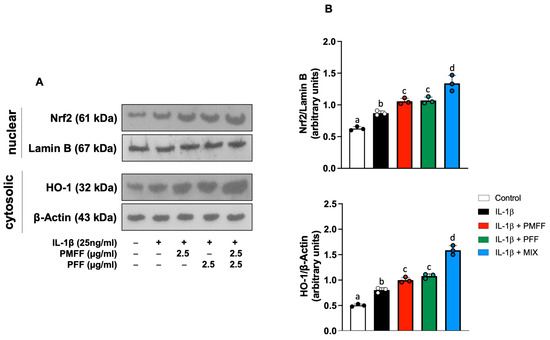
Figure 6.
Effect of PMFF, PFF or MIX on either Nrf2 nuclear translocation or cytosolic levels of HO-1 in differentiated, IL-1β-activated Caco-2 cells. Representative images of Western blot analysis (A). Densitometric analysis of either Nrf2 or HO-1 protein levels normalised for laminin b or β actin levels, respectively (B). Values obtained are the mean ± SD of three separate experiments. Means with different letters are significantly different with p < 0.05 (one-way Anova with Tukey’s post hoc test).
To further characterise the antioxidative response of the cells, induced by the fractions, the effect of PMFF and PFF on the protein expression of HO-1, an antioxidant enzyme representative of the transcriptional activity of Nrf2, was then evaluated. To this end, Caco-2 cells were treated as above described and stimulated with Il-1β for 24 h. As shown in Figure 6, with respect to control cells, stimulation with IL-1β increases HO-1 protein levels (p < 0.001). Moreover, preincubation with either PMFF or PFF induced a significant HO-1 protein over-expression with respect to IL-1β-treated cells (p < 0.01). Relevantly, MIX treatment generated a significantly higher increase in HO-1 protein levels with respect to cells incubated in the presence of the individual fractions (p < 0.001).
3.7. PMFF and PFF in Combination Enhances the Antioxidative Response in Differentiated, IL-1β-Activated Caco-2 Cells
By regulating the expression of key antioxidative enzymes, Nrf2 is a key modulator of cellular redox balance [54]. Finally, we measured both endocellular RONs and GSH levels in our experimental system. To this aim, differentiated Caco-2 cells were either left untreated (control) or stimulated with IL-1β (25 ng/mL) for 24 h. When necessary, cells were preincubated with either PMFF (2.5 μg/mL) or PFF (2.5 μg/mL) or MIX (2.5 μg/mL) for 1 h. As shown in Figure 7, with respect to control cells, treatment with IL-1β caused a significant increase in RONs levels and a decrease in the GSH/GSSG ratio, indicating a redox unbalance of cells towards a more oxidized state (p < 0.001). Interestingly, pre-treatment with either PMFF or PFF (2.5 μg/mL) significantly counteracted the IL-1β-induced RONs production and the reduction in GSH/GSSG ratio (p < 0.001 and p < 0.05, respectively). Remarkably, MIX pre-treatment totally prevented the IL-1β-induced redox unbalance, by restoring RONs levels and GSH/GSSG ratio back to control values.
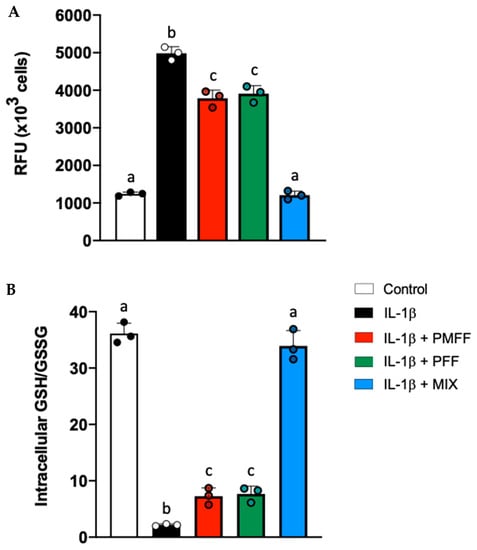
Figure 7.
Effect of PMFF, PFF or MIX on IL-1β-induced cellular redox unbalance in activated, differentiated Caco-2 cells. RONs, expressed as relative fluorescence units (RFU), (A) and GSH/GSSG (B). Values obtained are the mean ± SD of three separate experiments in triplicate. Means with different letters are significantly different with p < 0.05 (one-way Anova with Tukey’s post hoc test).
3.8. NOB and XTM in Combination Synergistically Counteract Endocellular Redox Unbalance in Differentiated, IL-1β-Activated Caco-2 Cells
As NOB and XTM were identified as the most abundant compounds of the PMFF and the PFF, respectively, we finally investigated whether the redox-modulating effects exerted by the two fractions were associated with the ability of these phytochemicals to synergistically reduce the IL-1β-induced RONs production in our experimental system.
To this aim, we followed the Chou–Talalay Combination Index (CI) model, based on the median effect equation. The CI values for the different inhibition fractions (fa) of RONs production and represented the data as a function of the measured effect (Figure 8B). According to Chou–Talalay, values of CI > 1, CI < 1 and CI = 1 indicate an antagonistic, synergistic or additive effect, respectively. In our study the CI values measured for the release of RONs by IL-1β-stimulated Caco-2 cells, were all far below 1, indicating a synergistic inhibitory effect of NOB and XTM in a wide range of concentrations.
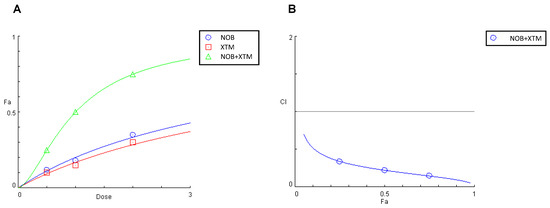
Figure 8.
Effect on RONs release in IL-1β-stimulated Caco-2 cells of NOB (0.5–2.0 μM), XTM (0.5–2.0 μM), and NOB combined with XTM (molar ratio of 1:1) (A) and plot of the combination index vs. fraction of the inhibitory effect (B). Cells were pre-treated individually or in combination with NOB and XTM for 1 h. The interactions on inhibitory effects were analysed using the median-effect analysis program where CI > 1, CI < 1 and CI =1 indicate an antagonistic, synergistic or additive effect, respectively.
4. Discussion
This work falls within the intense research on the advantage of combining different phytochemicals to improve the health benefits of single compounds. While the anti-inflammatory effects of PMFF and PFF are well established and both classes of compounds have been shown to exert significant protective effects in several in vitro and in vivo models of intestinal diseases, no study has yet evaluated their efficacy when added in combination with such experimental systems. Interestingly, we here demonstrate that a combination of PMFF and PFF exerts more effective anti-inflammatory effects in IL-1β-activated, intestinal cells with respect to a treatment with either PMFF or PFF alone. Inhibition of the redox-dependent NF-κB/Nrf2 activation and of their downstream signalling axis appears as key mechanisms underlying the PMFF- and PFF-mediated anti-inflammatory effects.
NO is a pleiotropic free radical messenger molecule, responsible for several physiological functions of the gastrointestinal mucosa such as the maintenance of perfusion, the regulation of microvascular epithelial permeability and the modulation of the immune response [52]. Increased output production of NO by intestinal cells, however, has been correlated to intestinal injury, mucosal inflammation, and enterocyte apoptosis through mechanisms correlated to the increased nitrosative stress and DNA damage [52,55,56]. Along these lines, the ability of PMFF and PFF to inhibit NO release in our in vitro model of IBD appears remarkable. Moreover, our data are in agreement with published evidence demonstrating the effectiveness of NOB and XTM to reduce NO production in an in vivo model of colitis, and the efficacy of SIN and TGT in a number of in vitro models of immunological dysfunctions in several cell types [19,57,58,59,60,61].
PGE2 is one of the most relevant downstream products of COX-2 enzymatic activity. Together with NO, it modulates several physiological intestinal functions such as stimulation of mucus, bicarbonate secretion, mucosal blood flow, leucocyte recruitment, fever and pain [62,63]. At the same time, multiple lines of evidence demonstrated that PGE2 is a key mediator of IBD, exerting significant pro-inflammatory and tumor-promoting effects [53]. Indeed, PGE2 overproduction by intestinal cells has been reported to exacerbate the inflammatory process, inducing the disruption of the intestinal epithelial barrier function and promoting proinflammatory Th17 cell signalling [64,65]. Interestingly, our results demonstrated that PFF, but not PMFF, is able to significantly reduce the release of PGE2 in our experimental system. Relevantly, when PMFF acts in combination with PFF the phytochemicals exert a much more evident anti-inflammatory response, reducing PGE2 levels to control. This evidence further reinforces the concept that the association between the two fractions can be envisaged as a rational and effective anti-inflammatory strategy for the treatment of IBD.
The present effects of PMFF and PFF on NO and PGE2 levels mirror their effects on both iNOS and COX-2 expression, observed in our experimental system. Indeed, and coherently with the ability of both fractions to inhibit the release of NO, either PMFF or PFF counteract IL-1β-induced iNOS over-expression. Conversely, and in line with the incapacity of PMFF to inhibit PGE2 release, this fraction is not able to reduce COX-2 levels. Remarkably, only the contemporaneous presence of both PMFF and PFF results in the inhibition of both enzymes, reducing iNOS or COX-2 expression to control levels or even below, respectively. These effects, obtained only when the fractions were combined, appear of particular interest as both these pro-inflammatory enzymes are dramatically upregulated in IBD patients. Indeed, intestinal chronic inflammation can orchestrate the development of a tumour-supporting microenvironment that promotes tumour initiation, progression and metastasis. Remarkably, COX-2 over-expression plays a key role in IBD-associated colon rectal cancer (CRC) being strongly associated with worse survival among CRC patients [66,67]. Therefore, the simultaneous inhibition of iNOS and COX-2 is crucial to achieve maximal protection as demonstrated in chemically-induced colitis [68], colitis-related carcinogenesis and CRC development in patients with IBD [69,70,71]. Within this scenario, these results relevantly underlie how important is the combination of the PMFF and PFF, which appears necessary to achieve the contemporary inhibition of both iNOS and COX-2.
The IL-1β-induced rapid transcription of iNOS, COX-2 and several other crucial genes involved in the inflammatory response, strongly involves the activation of the redox-sensitive transcription factor NF-κB [72]. A growing number of evidence has revealed how critical is the role of NF-κB activation on both the onset and the progression of IBD [73,74]. Upon activation, the protein translocates from the cytoplasm to the nucleus, where binds to specific DNA sequences, initiating the transcription of its downstream target genes [75]. Interestingly, our results indicate that both PMFF and PFF are able to inhibit NF-κB activation and, more importantly, that only when are added in combination do they bring back the nuclear levels of the transcription factor to control values. These effects can be related to previously published evidence showing that XTM inhibits NF-κB nuclear translocation by counteracting IκBα phosphorylation both in vitro and in vivo [20] and to the ability of NOB, SIN and TGT to inhibit the NF-κB pathway [18,76,77,78,79,80].
Nrf2 is a key transcription factor controlling many aspects of cell homoeostasis in response to oxidative and toxic insults. Indeed, Nrf2 modulates the transcription of several cytoprotective, detoxification and antioxidant genes such as HO-1 [54,81]. NOB, SIN, TGT and XTM have been previously demonstrated to positively modulate Nrf2 activation and HO-1 protein levels in other experimental systems [82,83,84,85,86,87,88,89]. Accordingly, our results demonstrate that either PMFF or PFF can further increase the IL-1β-induced Nrf2 activation and HO-1 over-expression in an in vitro model of IBD. Remarkably, present data also show, for the first time, that the combination of both fractions results in an enhanced antioxidative response (in terms of Nrf2 activation and HO-1 overexpression) with respect to the one induced by the treatment with the single fractions, in our experimental system.
Moreover, a growing number of evidence demonstrates that the Nrf2 function is strictly integrated and interconnected with NF-κB signalling [90]. Indeed, Nrf2 can negatively regulate the NF-κB activation pathway, counteracting NF-κB-related pro-inflammatory response and oxidative injury [91,92]. Along these lines, it is tempting to speculate that the inhibition of NF-κB observed in this work may be related to the activation of Nrf2 and the subsequent overexpression of HO-1 by PMFF and PFF treatment.
GSH is a ubiquitous intracellular peptide with diverse functions that helps to scavenge RONs, reduces peroxides and reacts with electrophilic compounds providing cells with multiple defences against RONs and their by-products [93]. Consistent with the activation of the Nrf2/HO-1 axis by PMFF and PFF, our results also showed for the first time, in an in vitro model of IBD, a significant amelioration of the endocellular oxidative stress, in terms of RONs reduction and GSH levels increase. These results are in line with the antioxidative effects exerted by the phytochemicals of both fractions in other IBD-unrelated experimental models either in vitro or in vivo [82,84,86,87,88,89,94]. Relevantly, these effects were significantly more evident when both fractions were combined and might well be related to the Nrf2-dependent enhancement of the endogenous antioxidant defence systems by the phytochemicals, especially when present in combination.
Finally, our current results relevantly demonstrate for the first time the ability of NOB and XTM, the most abundant components of PMFF and PFF, respectively, to synergistically reduce the IL-1β-induced RONs production in differentiated and activated Caco-2 cells. While suggesting that the redox-modulating effects exerted by the two fractions could be associated with the synergistic interactions between these phytochemicals, this evidence could also foster further research aimed to explore other relevant synergistic interactions between the phytochemicals of PMFF and PFF.
5. Conclusions
As a whole, our data support the concept that phytocomplexes can potentiate the health effects of their single components. Indeed, we here demonstrate for the first time that PMFF and PFF exert more effective anti-inflammatory effects in an in vitro model of IBD when added in combination. Modulation of the redox-dependent NF-κB/Nrf2 activation and of their downstream signalling axis appears key mechanisms underlying the PMFF- and PFF-mediated anti-inflammatory effects. Synergistic interactions between NOB and XTM emerge as a relevant aspect underlying this evidence.
Author Contributions
Conceptualization, M.A., L.T. and G.P.; methodology, M.A., L.T., A.D. and M.G.B.; validation, L.T., M.A., I.R. and A.D.; formal analysis, A.A. and F.P.; investigation, M.A., I.R., M.G.B., I.C.G., A.M., C.P., D.C. and E.S.; resources, P.D.; writing—original draft preparation, M.A. and L.T.; writing—review and editing, M.A., L.T. and A.D.; visualization, S.C., B.P. and I.R.; supervision, M.A.; project administration, C.O., P.C. and F.P.; funding acquisition, P.D. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by a 2014–2020 PON Ricerca e Innovazione grant from the Italian Ministry of Education, University and Research, European Union, entitled “PROGEMA—Processi Green per l’Estrazione di Principi Attivi e la Depurazione di Matrici di Scarto e Non” (ARS01_00432) to P.C.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting reported results can be requested to the corresponding authors.
Acknowledgments
F.P. was supported by Fondazione Umberto Veronesi.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ko, J.; Auyeung, K. Inflammatory Bowel Disease: Etiology, Pathogenesis and Current Therapy. Curr. Pharm. Des. 2014, 20, 1082–1096. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Yang, M.F.; Liang, Y.J.; Xu, J.; Xu, H.M.; Nie, Y.Q.; Wang, L.S.; Yao, J.; Li, D.F. Immunology of Inflammatory Bowel Disease: Molecular Mechanisms and Therapeutics. J. Inflamm. Res. 2022, 15, 1825–1844. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, F.; Spisni, E.; Giovanardi, E.; Imbesi, V.; Salice, M.; Alvisi, P.; Valerii, M.C.; Gionchetti, P. Implications of the Westernized Diet in the Onset and Progression of IBD. Nutrients 2019, 11, 1033. [Google Scholar] [CrossRef] [PubMed]
- Moura, F.A.; Goulart, M.O.F.; Campos, S.B.G.; da Paz Martins, A.S. The Close Interplay of Nitro-Oxidative Stress, Advanced Glycation End Products and Inflammation in Inflammatory Bowel Diseases. Curr. Med. Chem. 2018, 27, 2059–2076. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, Y.R. Oxidative Stress and Redox Signaling Mechanisms of Inflammatory Bowel Disease: Updated Experimental and Clinical Evidence. Exp. Biol. Med. 2012, 237, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Dziąbowska-Grabias, K.; Sztanke, M.; Zając, P.; Celejewski, M.; Kurek, K.; Szkutnicki, S.; Korga, P.; Bulikowski, W.; Sztanke, K. Antioxidant Therapy in Inflammatory Bowel Diseases. Antioxidants 2021, 10, 412. [Google Scholar] [CrossRef]
- Piotrowska, M.; Swierczynski, M.; Fichna, J.; Piechota-Polanczyk, A. The Nrf2 in the Pathophysiology of the Intestine: Molecular Mechanisms and Therapeutic Implications for Inflammatory Bowel Diseases. Pharmacol. Res. 2021, 163, 105243. [Google Scholar] [CrossRef]
- Atreya, I.; Atreya, R.; Neurath, M.F. NF-KappaB in Inflammatory Bowel Disease. J. Intern. Med. 2008, 263, 591–596. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of Reactive Oxygen Species and NF-ΚB Signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Ligumsky, M. Role of Interleukin 1 in Inflammatory Bowel Disease-Enhanced Production during Active Disease. Gut 1990, 31, 686–689. [Google Scholar] [CrossRef]
- Reinecker, H.C.; Steffen, M.; Witthoeft, T.; Pflueger, I.; Schreiber, S.; MacDermott, R.P.; Raedler, A. Enhanced Secretion of Tumour Necrosis Factor-Alpha, IL-6, and IL-1β by Isolated Lamina Propria Mononuclear Cells from Patients with Ulcerative Colitis and Crohn’s Disease. Clin. Exp. Immunol. 1993, 94, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Van De Walle, J.; Hendrickx, A.; Romier, B.; Larondelle, Y.; Schneider, Y.J. Inflammatory Parameters in Caco-2 Cells: Effect of Stimuli Nature, Concentration, Combination and Cell Differentiation. Toxicol. In Vitro 2010, 24, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Schuerer-Maly, C.C.; Eckmann, L.; Kagnoff, M.F.; Falco, M.T.; Maly, F.E. Colonic Epithelial Cell Lines as a Source of Interleukin-8: Stimulation by Inflammatory Cytokines and Bacterial Lipopolysaccharide. Immunology 1994, 81, 85–91. [Google Scholar]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.J. Human Intestinal Barrier Function in Health and Disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. Anti-Inflammatory Effects of Phytochemicals from Fruits, Vegetables, and Food Legumes: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1260–1270. [Google Scholar] [CrossRef]
- Zhang, L.; Virgous, C.; Si, H. Synergistic Anti-Inflammatory Effects and Mechanisms of Combined Phytochemicals. J. Nutr. Biochem. 2019, 69, 19–30. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, D.; Yu, C.; Lv, B.; Peng, J.; Wang, J.; Lin, Y. Citrus Nobiletin Ameliorates Experimental Colitis by Reducing Inflammation and Restoring Impaired Intestinal Barrier Function. Mol. Nutr. Food Res. 2015, 59, 829–842. [Google Scholar] [CrossRef]
- Hagenlocher, Y.; Gommeringer, S.; Held, A.; Feilhauer, K.; Köninger, J.; Bischoff, S.C.; Lorentz, A. Nobiletin Acts Anti-Inflammatory on Murine IL-10−/− Colitis and Human Intestinal Fibroblasts. Eur. J. Nutr. 2019, 58, 1391–1401. [Google Scholar] [CrossRef]
- Cho, J.M.; Yun, S.M.; Choi, Y.H.; Heo, J.; Kim, N.J.; Kim, S.H.; Kim, E.H. Xanthohumol Prevents Dextran Sulfate Sodium-Induced Colitis via Inhibition of IKKβ/NF-ΚB Signaling in Mice. Oncotarget 2018, 9, 866–880. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, H.; Guo, L.; Zhao, H.; Ho, C.T. Chemistry and Bioactivity of Nobiletin and Its Metabolites. J. Funct. Foods 2014, 6, 2–10. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.E.; Wang, G.; Qiu, L.; Dong, J.; Yin, H.; Qian, Z.; Yang, M.; Miao, J. Pharmacological Profile of Xanthohumol, a Prenylated Flavonoid from Hops (Humulus lupulus). Molecules 2015, 20, 754–779. [Google Scholar] [CrossRef] [PubMed]
- Whitman, S.C.; Kurowska, E.M.; Manthey, J.A.; Daugherty, A. Nobiletin, a Citrus Flavonoid Isolated from Tangerines, Selectively Inhibits Class A Scavenger Receptor-Mediated Metabolism of Acetylated LDL by Mouse Macrophages. Atherosclerosis 2005, 178, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Raciti, G.A.; Fiory, F.; Campitelli, M.; Desiderio, A.; Spinelli, R.; Longo, M.; Nigro, C.; Pepe, G.; Sommella, E.; Campiglia, P.; et al. Citrus aurantium L. Dry Extracts Promote C/ Ebpβ Expression and Improve Adipocyte Differentiation in 3T3-L1 Cells. PLoS ONE 2018, 13, e0193704. [Google Scholar] [CrossRef]
- Gao, Z.; Gao, W.; Zeng, S.L.; Li, P.; Liu, E.H. Chemical Structures, Bioactivities and Molecular Mechanisms of Citrus Polymethoxyflavones. J. Funct. Foods 2018, 40, 498–509. [Google Scholar] [CrossRef]
- Walle, T. Methoxylated Flavones, a Superior Cancer Chemopreventive Flavonoid Subclass? Semin. Cancer Biol. 2007, 17, 354–362. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, S.; Ho, C.T.; Huang, Q. Citrus Polymethoxyflavones as Regulators of Metabolic Homoeostasis: Recent Advances for Possible Mechanisms. Trends Food Sci. Technol. 2021, 110, 743–753. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zhang, H.; Chen, J.; Cao, J.; Chen, Q.; Li, X.; Sun, C. Polymethoxyflavones from Citrus Inhibited Gastric Cancer Cell Proliferation through Inducing Apoptosis by Upregulating RARβ, Both in Vitro and in Vivo. Food Chem. Toxicol. 2020, 146, 111811. [Google Scholar] [CrossRef]
- Ke, Z.; Zhao, Y.; Tan, S.; Chen, H.; Li, Y.; Zhou, Z.; Huang, C. Citrus Reticulata Blanco Peel Extract Ameliorates Hepatic Steatosis, Oxidative Stress and Inflammation in HF and MCD Diet-Induced NASH C57BL/6 J Mice. J. Nutr. Biochem. 2020, 83, 108426. [Google Scholar] [CrossRef]
- Ambrož, M.; Lněničková, K.; Matoušková, P.; Skálová, L.; Boušová, I. Antiproliferative Effects of Hop-Derived Prenylflavonoids and Their Influence on the Efficacy of Oxaliplatine, 5-Fluorouracil and Irinotecan in Human ColorectalC Cells. Nutrients 2019, 11, 879. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, Y.; Yang, J.; He, J.; Sun, J.; Chen, F.; Zhang, M.; Yang, B. Prenylated Flavonoids, Promising Nutraceuticals with Impressive Biological Activities. Trends Food Sci. Technol. 2015, 44, 93–104. [Google Scholar] [CrossRef]
- Mukai, R. Prenylation Enhances the Biological Activity of Dietary Flavonoids by Altering Their Bioavailability. Biosci. Biotechnol. Biochem. 2018, 82, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.L.; Stevens, J.F.; Ivanov, V.; Mccall, M.; Frei, B.; Deinzer, M.L.; Buhler, D.R. Antioxidant and Prooxidant Actions of Prenylated and Nonprenylated Chalcones and Flavanones in Vitro. J. Agric. Food Chem. 2000, 48, 3876–3884. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.M.M.; Silva, A.M.S. The Antioxidant Activity of Prenylflavonoids. Molecules 2020, 25, 696. [Google Scholar] [CrossRef] [PubMed]
- Dorn, C.; Massinger, S.; Wuzik, A.; Heilmann, J.; Hellerbrand, C. Xanthohumol Suppresses Inflammatory Response to Warm Ischemia–Reperfusion Induced Liver Injury. Exp. Mol. Pathol. 2013, 94, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Legette, L.L.; Moreno Luna, A.Y.; Reed, R.L.; Miranda, C.L.; Bobe, G.; Proteau, R.R.; Stevens, J.F. Xanthohumol Lowers Body Weight and Fasting Plasma Glucose in Obese Male Zucker Fa/Fa Rats. Phytochemistry 2013, 91, 236–241. [Google Scholar] [CrossRef]
- Gerhäuser, C. Broad Spectrum Antiinfective Potential of Xanthohumol from Hop (Humulus lupulus L.) in Comparison with Activities of Other Hop Constituents and Xanthohumol Metabolites. Mol. Nutr. Food Res. 2005, 49, 827–831. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, C.; Zhang, B.; Huang, Q. The Inhibitory Effects of Flavonoids on α-Amylase and α-Glucosidase. Crit. Rev. Food Sci. Nutr. 2020, 60, 695–708. [Google Scholar] [CrossRef]
- Tronina, T.; Popłonski, J.; Bartmanska, A. Flavonoids as Phytoestrogenic Components of Hops and Beer. Molecules 2020, 25, 4201. [Google Scholar] [CrossRef]
- Lv, H.W.; Wang, Q.L.; Luo, M.; Zhu, M.D.; Liang, H.M.; Li, W.J.; Cai, H.; Zhou, Z.B.; Wang, H.; Tong, S.Q.; et al. Phytochemistry and Pharmacology of Natural Prenylated Flavonoids. Arch. Pharm. Res. 2023, 46, 207–272. [Google Scholar] [CrossRef]
- Shi, S.; Li, J.; Zhao, X.; Liu, Q.; Song, S.J. A Comprehensive Review: Biological Activity, Modification and Synthetic Methodologies of Prenylated Flavonoids. Phytochemistry 2021, 191, 112895. [Google Scholar] [CrossRef] [PubMed]
- Sychrová, A.; Škovranová, G.; Čulenová, M.; Bittner Fialová, S. Prenylated Flavonoids in Topical Infections and Wound Healing. Molecules 2022, 27, 4491. [Google Scholar] [CrossRef] [PubMed]
- Carbone, K.; Gervasi, F. An Updated Review of the Genus Humulus: A Valuable Source of Bioactive Compounds for Health and Disease Prevention. Plants 2022, 11, 3434. [Google Scholar] [CrossRef]
- Turdo, A.; Glaviano, A.; Pepe, G.; Calapà, F.; Raimondo, S.; Fiori, M.E.; Carbone, D.; Basilicata, M.G.; Di Sarno, V.; Ostacolo, C.; et al. Nobiletin and Xanthohumol Sensitize Colorectal Cancer Stem Cells to Standard Chemotherapy. Cancers 2021, 13, 3927. [Google Scholar] [CrossRef] [PubMed]
- Corrado, C.; Barreca, M.M.; Raimondo, S.; Diana, P.; Pepe, G.; Basilicata, M.G.; Conigliaro, A.; Alessandro, R. Nobiletin and Xanthohumol Counteract the TNFα-Mediated Activation of Endothelial Cells through the Inhibition of the NF-ΚB Signaling Pathway. Cell Biol. Int. 2023, 47, 634–647. [Google Scholar] [CrossRef]
- Allegra, M.; de Cicco, P.; Ercolano, G.; Attanzio, A.; Busà, R.; Cirino, G.; Tesoriere, L.; Livrea, M.A.; Ianaro, A. Indicaxanthin from Opuntia Ficus Indica (L. Mill) Impairs Melanoma Cell Proliferation, Invasiveness, and Tumor Progression. Phytomedicine 2018, 50, 19–24. [Google Scholar] [CrossRef]
- Grisham, M.B.; Johnson, G.G.; Lancaster, J.R. Quantitation of Nitrate and Nitrite in Extracellular Fluids. Methods Enzymol. 1996, 268, 237–246. [Google Scholar]
- Chou, T.C. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Allegra, M.; D’Acquisto, F.; Tesoriere, L.; Attanzio, A.; Livrea, M.A. Pro-Oxidant Activity of Indicaxanthin from Opuntia Ficus Indica Modulates Arachidonate Metabolism and Prostaglandin Synthesis through Lipid Peroxide Production in LPS-Stimulated RAW 264.7 Macrophages. Redox Biol. 2014, 2, 892–900. [Google Scholar] [CrossRef][Green Version]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Seubwai, W.; Wongkham, C.; Puapairoj, A.; Khuntikeo, N.; Pugkhem, A.; Hahnvajanawong, C.; Chaiyagool, J.; Umezawa, K.; Okada, S.; Wongkham, S. Aberrant Expression of NF-ΚB in Liver Fluke Associated Cholangiocarcinoma: Implications for Targeted Therapy. PLoS ONE 2014, 9, e106056. [Google Scholar] [CrossRef] [PubMed]
- Kolios, G.; Valatas, V.; Ward, S.G. Nitric Oxide in Inflammatory Bowel Disease: A Universal Messenger in an Unsolved Puzzle. Immunology 2004, 113, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Sheibanie, A.F.; Yen, J.-H.; Khayrullina, T.; Emig, F.; Zhang, M.; Tuma, R.; Ganea, D. The Proinflammatory Effect of Prostaglandin E2 in Experimental Inflammatory Bowel Disease Is Mediated through the IL-23→IL-17 Axis. J. Immunol. 2007, 178, 8138–8147. [Google Scholar] [CrossRef]
- Dodson, M.; Redmann, M.; Rajasekaran, N.S.; Darley-Usmar, V.; Zhang, J. KEAP1-NRF2 Signalling and Autophagy in Protection against Oxidative and Reductive Proteotoxicity. Biochem. J. 2015, 469, 347–355. [Google Scholar] [CrossRef]
- Rana, T. Influence and Implications of the Molecular Paradigm of Nitric Oxide Underlying Inflammatory Reactions of the Gastrointestinal Tract of Dog: A Major Hallmark of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2022, 28, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Grishin, A.; Bowling, J.; Bell, B.; Wang, J.; Ford, H.R. Roles of Nitric Oxide and Intestinal Microbiota in the Pathogenesis of Necrotizing Enterocolitis. J. Pediatr. Surg. 2016, 51, 13–17. [Google Scholar] [CrossRef]
- Choi, S.Y.; Ko, H.C.; Ko, S.Y.; Hwang, J.H.; Park, J.G.; Kang, S.H.; Han, S.H.; Yun, S.H.; Kim, S.J. Correlation between Flavonoid Content and the NO Production Inhibitory Activity of Peel Extracts from Various Citrus Fruits. Biol. Pharm. Bull. 2007, 30, 772–778. [Google Scholar] [CrossRef]
- Choi, S.Y.; Hwang, J.H.; Ko, H.C.; Park, J.G.; Kim, S.J. Nobiletin from Citrus Fruit Peel Inhibits the DNA-Binding Activity of NF-ΚB and ROS Production in LPS-Activated RAW 264.7 Cells. J. Ethnopharmacol. 2007, 113, 149–155. [Google Scholar] [CrossRef]
- Rong, X.; Xu, J.; Jiang, Y.; Li, F.; Chen, Y.; Dou, Q.P.; Li, D. Citrus Peel Flavonoid Nobiletin Alleviates Lipopolysaccharide-Induced Inflammation by Activating IL-6/STAT3/FOXO3a-Mediated Autophagy. Food Funct. 2021, 12, 1305–1317. [Google Scholar] [CrossRef]
- Funaro, A.; Wu, X.; Song, M.; Zheng, J.; Guo, S.; Rakariyatham, K.; Rodriguez-Estrada, M.T.; Xiao, H. Enhanced Anti-Inflammatory Activities by the Combination of Luteolin and Tangeretin. J. Food Sci. 2016, 81, H1320–H1327. [Google Scholar] [CrossRef]
- Laavola, M.; Nieminen, R.; Yam, M.; Sadikun, A.; Asmawi, M.; Basir, R.; Welling, J.; Vapaatalo, H.; Korhonen, R.; Moilanen, E. Flavonoids Eupatorin and Sinensetin Present in Orthosiphon Stamineus Leaves Inhibit Inflammatory Gene Expression and STAT1 Activation. Planta Med. 2012, 78, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, T.; Tohnai, N. Cyclooxygenase Isozymes and Their Gene Structures and Expression. Prostaglandins Other Lipid. Mediat. 2002, 68–69, 95–114. [Google Scholar] [CrossRef]
- Martin, G.R.; Wallace, J.L. Gastrointestinal Inflammation: A Central Component of Mucosal Defense and Repair. Exp. Biol. Med. 2006, 231, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lagunas, M.J.; Martín-Venegas, R.; Moreno, J.J.; Ferrer, R. PGE2 Promotes Ca2+-Mediated Epithelial Barrier Disruption through EP1 and EP4 Receptors in Caco-2 Cell Monolayers. Am. J. Physiol. Cell Physiol. 2010, 299, C324–C334. [Google Scholar] [CrossRef]
- Stenson, W.F. The Universe of Arachidonic Acid Metabolites in Inflammatory Bowel Disease: Can We Tell the Good from the Bad? Curr. Opin. Gastroenterol. 2014, 30, 347–351. [Google Scholar] [CrossRef]
- Yang, L.; Wu, G.; Wu, Q.; Peng, L.; Yuan, L. METTL3 Overexpression Aggravates LPS-Induced Cellular Inflammation in Mouse Intestinal Epithelial Cells and DSS-Induced IBD in Mice. Cell Death. Discov. 2022, 8, 62. [Google Scholar] [CrossRef]
- Wang, D.; Dubois, R.N. The Role of COX-2 in Intestinal Inflammation and Colorectal Cancer. Oncogene 2010, 29, 781–788. [Google Scholar] [CrossRef]
- Dudhgaonkar, S.P.; Tandan, S.K.; Kumar, D.; Raviprakash, V.; Kataria, M. Influence of Simultaneous Inhibition of Cyclooxygenase-2 and Inducible Nitric Oxide Synthase in Experimental Colitis in Rats. Inflammopharmacology 2007, 15, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Kohno, H.; Suzuki, R.; Sugie, S.; Tanaka, T. Suppression of Colitis-Related Mouse Colon Carcinogenesis by a COX-2 Inhibitor and PPAR Ligands. BMC Cancer 2005, 5, 46. [Google Scholar] [CrossRef]
- Eaden, J. Review Article: The Data Supporting a Role for Aminosalicylates in the Chemoprevention of Colorectal Cancer in Patients with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2003, 18, 15–21. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Eaden, J.; Steinhart, A.H.; Munkholm, P.; Gordon, P.H. Cancer Prevention in Inflammatory Bowel Disease and the Chemoprophylactic Potential of 5-Aminosalicylic Acid. Inflamm. Bowel Dis. 2002, 8, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J.; Chun, K.S.; Cha, H.H.; Han, S.S.; Keum, Y.S.; Park, K.K.; Lee, S.S. Molecular Mechanisms Underlying Chemopreventive Activities of Anti-Inflammatory Phytochemicals: Down-Regulation of COX-2 and INOS through Suppression of NF-ΚB Activation. Mutat. Res. 2001, 480–481, 243–268. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Freitas, V.; Almeida, L.; Laranjinha, J. Red Wine Extract Preserves Tight Junctions in Intestinal Epithelial Cells under Inflammatory Conditions: Implications for Intestinal Inflammation. Food Funct. 2019, 10, 1364–1374. [Google Scholar] [CrossRef]
- Nunes, C.; Almeida, L.; Barbosa, R.M.; Laranjinha, J. Luteolin Suppresses the JAK/STAT Pathway in a Cellular Model of Intestinal Inflammation. Food Funct. 2017, 8, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Bernotti, S.; Seidman, E.; Sinnett, D.; Brunet, S.; Dionne, S.; Delvin, E.; Levy, E. Inflammatory Reaction without Endogenous Antioxidant Response in Caco-2 Cells Exposed to Iron/Ascorbate-Mediated Lipid Peroxidation. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G898–G906. [Google Scholar] [CrossRef][Green Version]
- Zhi, Z.; Tang, X.; Wang, Y.; Chen, R.; Ji, H. Sinensetin Attenuates Amyloid Beta25-35-Induced Oxidative Stress, Inflammation, and Apoptosis in SH-SY5Y Cells Through the TLR4/NF-ΚB Signaling Pathway. Neurochem. Res. 2021, 46, 3012–3024. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, R.; Wang, R.; Dai, J.; Chen, H.; Wang, J.; Li, X. Sinensetin Attenuates IL-1β-Induced Cartilage Damage and Ameliorates Osteoarthritis by Regulating SERPINA3. Food Funct. 2022, 13, 9973–9987. [Google Scholar] [CrossRef]
- Hagenlocher, Y.; Feilhauer, K.; Schäffer, M.; Bischoff, S.C.; Lorentz, A. Citrus Peel Polymethoxyflavones Nobiletin and Tangeretin Suppress LPS- and IgE-Mediated Activation of Human Intestinal Mast Cells. Eur. J. Nutr. 2017, 56, 1609–1620. [Google Scholar] [CrossRef]
- Chang, S.N.; Dey, D.K.; Oh, S.T.; Kong, W.H.; Cho, K.H.; Al-Olayan, E.M.; Hwang, B.S.; Kang, S.C.; Park, J.G. Phorbol 12-Myristate 13-Acetate Induced Toxicity Study and the Role of Tangeretin in Abrogating Hif-1α-Nf-Κb Crosstalk in Vitro and in Vivo. Int. J. Mol. Sci. 2020, 21, 9261. [Google Scholar] [CrossRef]
- Eun, S.H.; Woo, J.T.; Kim, D.H. Tangeretin Inhibits IL-12 Expression and NF-ΚB Activation in Dendritic Cells and Attenuates Colitis in Mice. Planta Med. 2017, 83, 527–533. [Google Scholar] [CrossRef]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/Small Maf Heterodimer Mediates the Induction of Phase II Detoxifying Enzyme Genes through Antioxidant Response Elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, X.; Zhang, C.; Bai, X.; Zhang, J.; Zhao, X.; Chen, L.; Wang, L.; Zhu, C.; Cui, L.; et al. Nobiletin Promotes Antioxidant and Anti-Inflammatory Responses and Elicits Protection against Ischemic Stroke in Vivo. Brain Res. 2016, 1636, 130–141. [Google Scholar] [CrossRef]
- He, Z.; Li, X.; Chen, H.; He, K.; Liu, Y.; Gong, J.; Gong, J. Nobiletin Attenuates Lipopolysaccharide/D-Galactosamine-Induced Liver Injury in Mice by Activating the Nrf2 Antioxidant Pathway and Subsequently Inhibiting NF-ΚB-Mediated Cytokine Production. Mol. Med. Rep. 2016, 14, 5595–5600. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yao, Y.; Huang, H.; Hao, H.; Ying, M. Xanthohumol Attenuates Cisplatin-Induced Nephrotoxicity through Inhibiting NF-ΚB and Activating Nrf2 Signaling Pathways. Int. Immunopharmacol. 2018, 61, 277–282. [Google Scholar] [CrossRef]
- Rahman, S.U.; Ali, T.; Hao, Q.; He, K.; Li, W.; Ullah, N.; Zhang, Z.; Jiang, Y.; Li, S. Xanthohumol Attenuates Lipopolysaccharide-Induced Depressive Like Behavior in Mice: Involvement of NF-ΚB/Nrf2 Signaling Pathways. Neurochem. Res. 2021, 46, 3135–3148. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Liu, Q.; Wen, Z.; Feng, H.; Deng, X.; Ci, X. Xanthohumol Ameliorates Lipopolysaccharide (LPS)-Induced Acute Lung Injury via Induction of AMPK/GSK3β-Nrf2 Signal Axis. Redox Biol. 2017, 12, 311–324. [Google Scholar] [CrossRef]
- Lv, C.; Li, Y.; Liang, R.; Huang, W.; Xiao, Y.; Ma, X.; Wang, Y.; Zou, H.; Qin, F.; Sun, C.; et al. Characterization of Tangeretin as an Activator of Nuclear Factor Erythroid 2-Related Factor 2/Antioxidant Response Element Pathway in HEK293T Cells. Curr. Res. Food Sci. 2023, 6, 100459. [Google Scholar] [CrossRef]
- Liang, F.; Fang, Y.; Cao, W.; Zhang, Z.; Pan, S.; Xu, X. Attenuation of Tert-Butyl Hydroperoxide (t-BHP)-Induced Oxidative Damage in HepG2 Cells by Tangeretin: Relevance of the Nrf2-ARE and MAPK Signaling Pathways. J. Agric. Food Chem. 2018, 66, 6317–6325. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, J.; Li, S.; Wu, Y.; Yu, C.; Ni, L.B.; Xiao, J.; Shao, Z.; Zhu, H.; Wang, J.; et al. Tangeretin Suppresses Osteoarthritis Progression via the Nrf2/NF-ΚB and MAPK/NF-ΚB Signaling Pathways. Phytomedicine 2022, 98, 153928. [Google Scholar] [CrossRef]
- Chiou, Y.S.; Huang, Q.; Ho, C.T.; Wang, Y.J.; Pan, M.H. Directly Interact with Keap1 and LPS Is Involved in the Anti-Inflammatory Mechanisms of (-)-Epicatechin-3-Gallate in LPS-Induced Macrophages and Endotoxemia. Free Radic. Biol. Med. 2016, 94, 1–16. [Google Scholar] [CrossRef]
- Keleku-Lukwete, N.; Suzuki, M.; Yamamoto, M. An Overview of the Advantages of KEAP1-NRF2 System Activation during Inflammatory Disease Treatment. Antioxid. Redox Signal. 2018, 29, 1746–1755. [Google Scholar] [CrossRef] [PubMed]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting Molecular Cross-Talk between Nrf2 and NF-ΚB Response Pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Maher, P. The Effects of Stress and Aging on Glutathione Metabolism. Ageing Res. Rev. 2005, 4, 288–314. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Yang, J.J.; Ni, W. Immunomodulatory Effects of Sinensetin on Macrophage and Cyclophosphamide-Induced Immunosuppression in Mice. Pharmazie 2022, 77, 147–151. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).