Fermented Ginger Extract in Natural Deep Eutectic Solvent Enhances Cytotoxicity by Inhibiting NF-κB Mediated CXC Chemokine Receptor 4 Expression in Oxaliplatin-Resistant Human Colorectal Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. NaDES-Ultrasound-Assisted Extraction of Ginger Powder
2.3. Fermentation of NaDES-Ginger Extract with L. reuteri
2.4. High-Performance Liquid Chromatography (HPLC) Analysis
2.5. The DPPH and ABTS Antioxidant Assays
2.6. Total Phenolic Content (TPC) Analysis
2.7. Cell Culture
2.8. Cell Viability Assay
2.9. Trypan Blue Staining Assay
2.10. Live/Dead Assay
2.11. Real-Time Quantitative Polymerase Chain Reaction (PCR)
2.12. Western Blot Analysis
2.13. siRNA Transfection
2.14. NF-κB p65 Transcription Factor Activity Assay
2.15. Statistical Analysis
3. Results
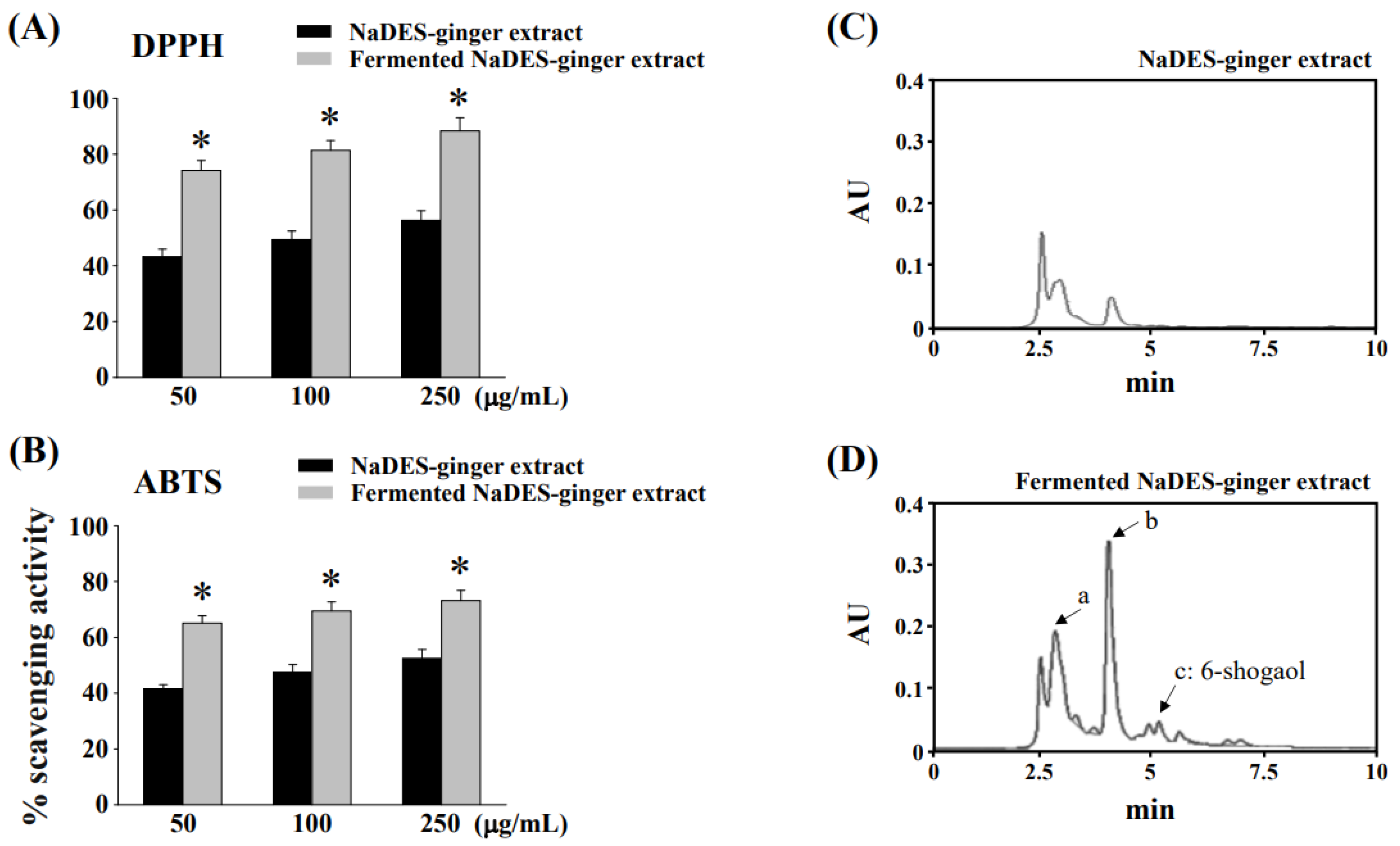
3.1. Fermentation Process to Changes of Chemical Compositions and Antioxidant Properties of Ginger Extract in NaDES
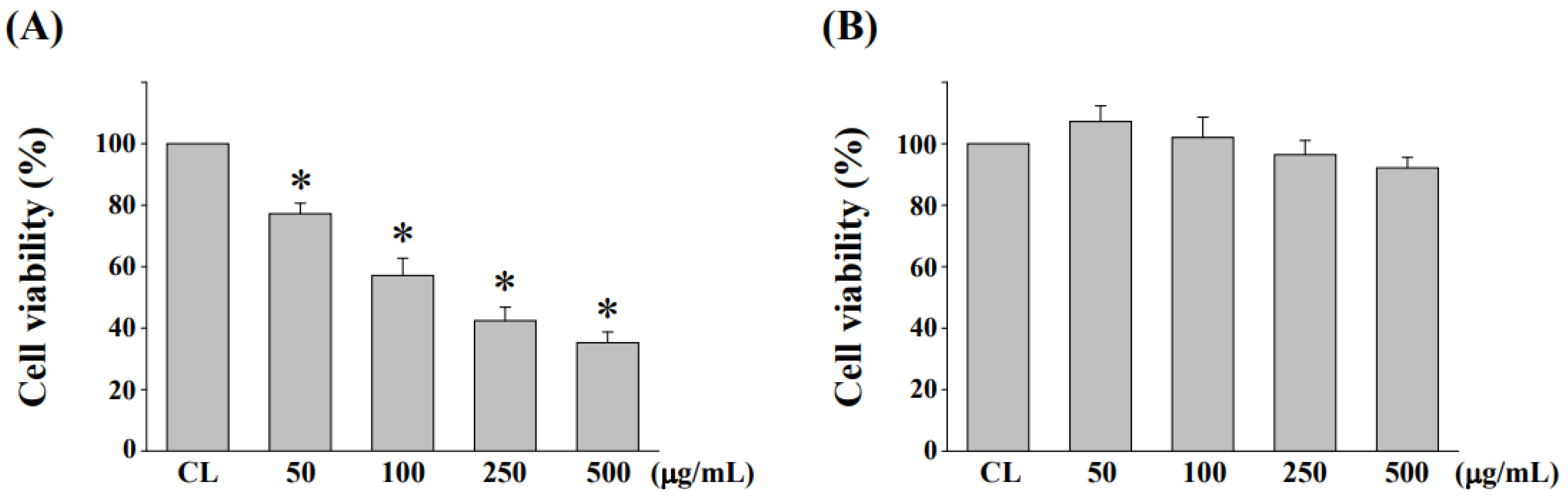
3.2. Cytotoxicity Evaluation of NaDES-Ginger Extract on Human Normal Colonic Mucosa Cell Line NCM460 after L. reuteri Fermentation
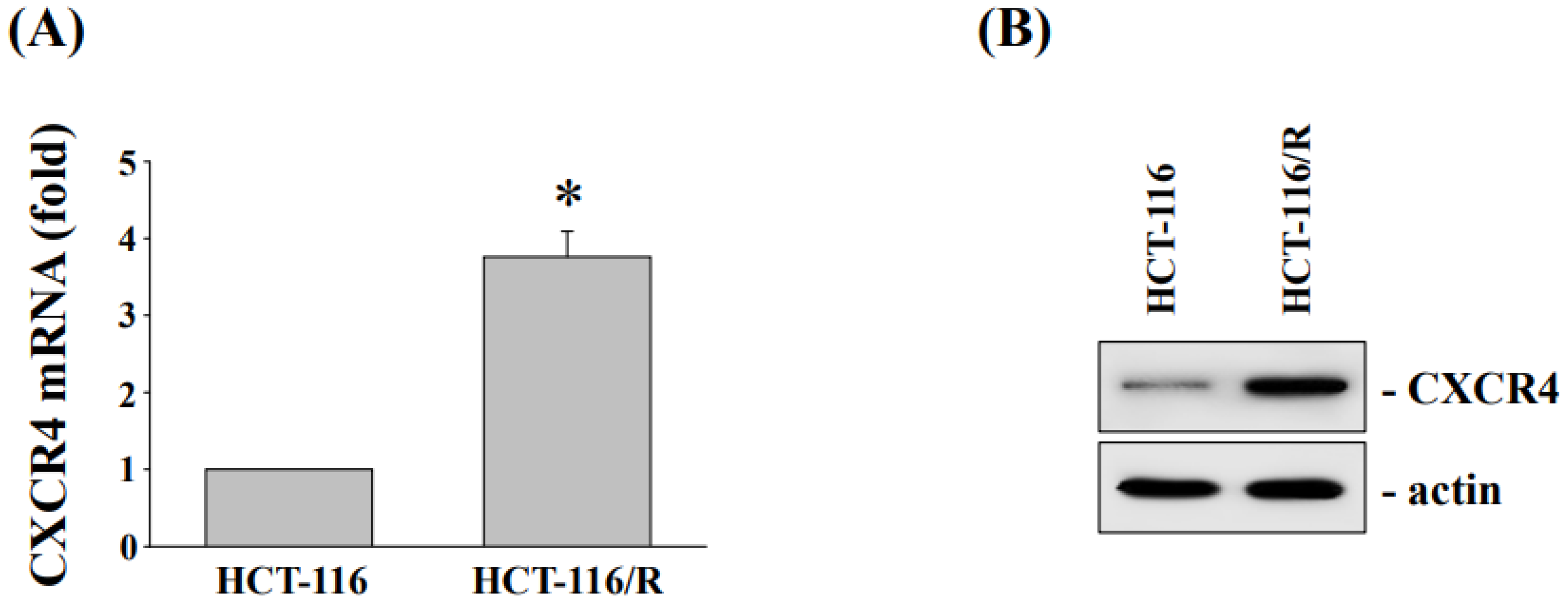
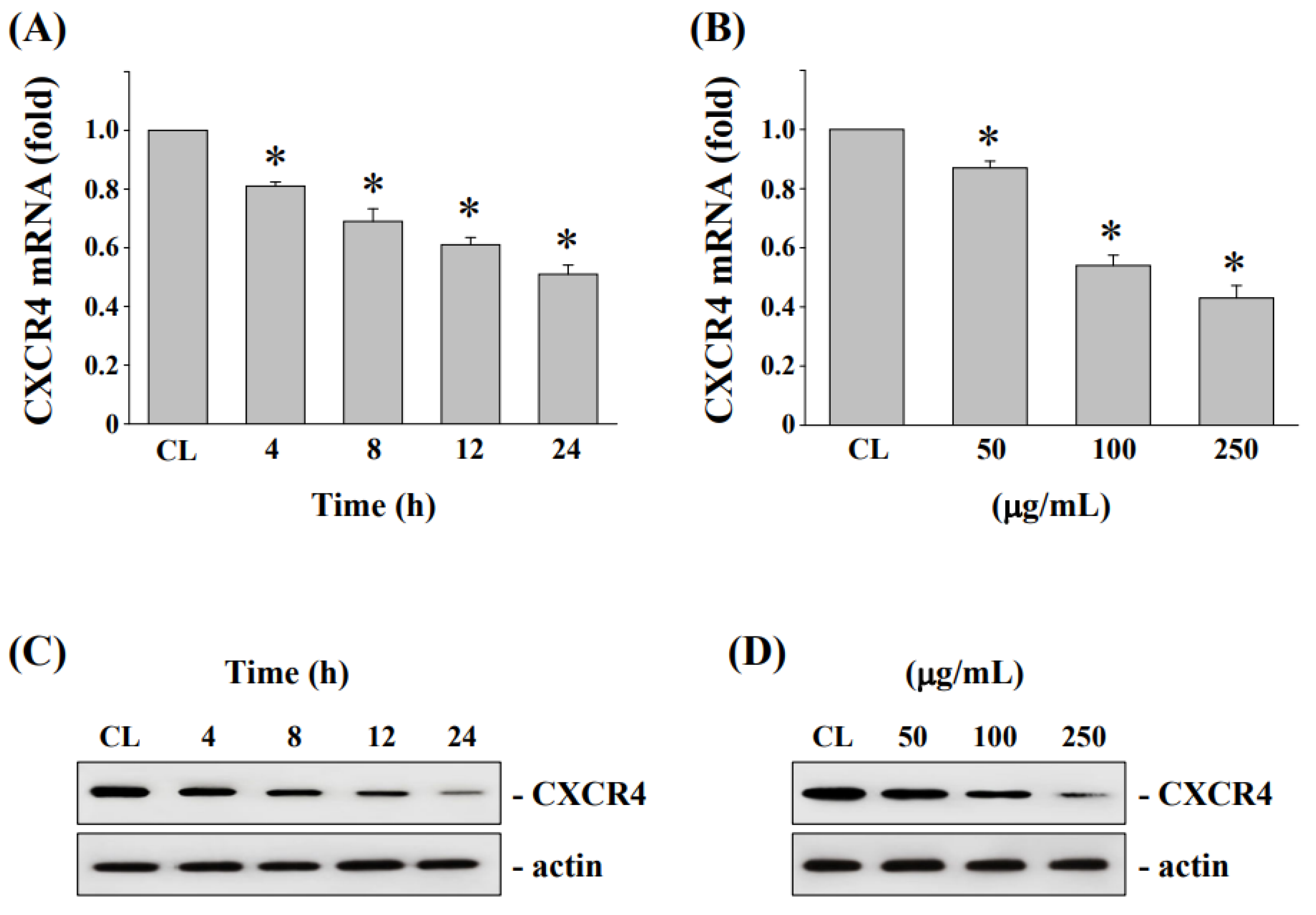
3.3. Cytotoxicity and CXC Chemokine Receptor 4 Expression of HCT-116 and Oxaliplatin-Resistant HCT116/R Cells
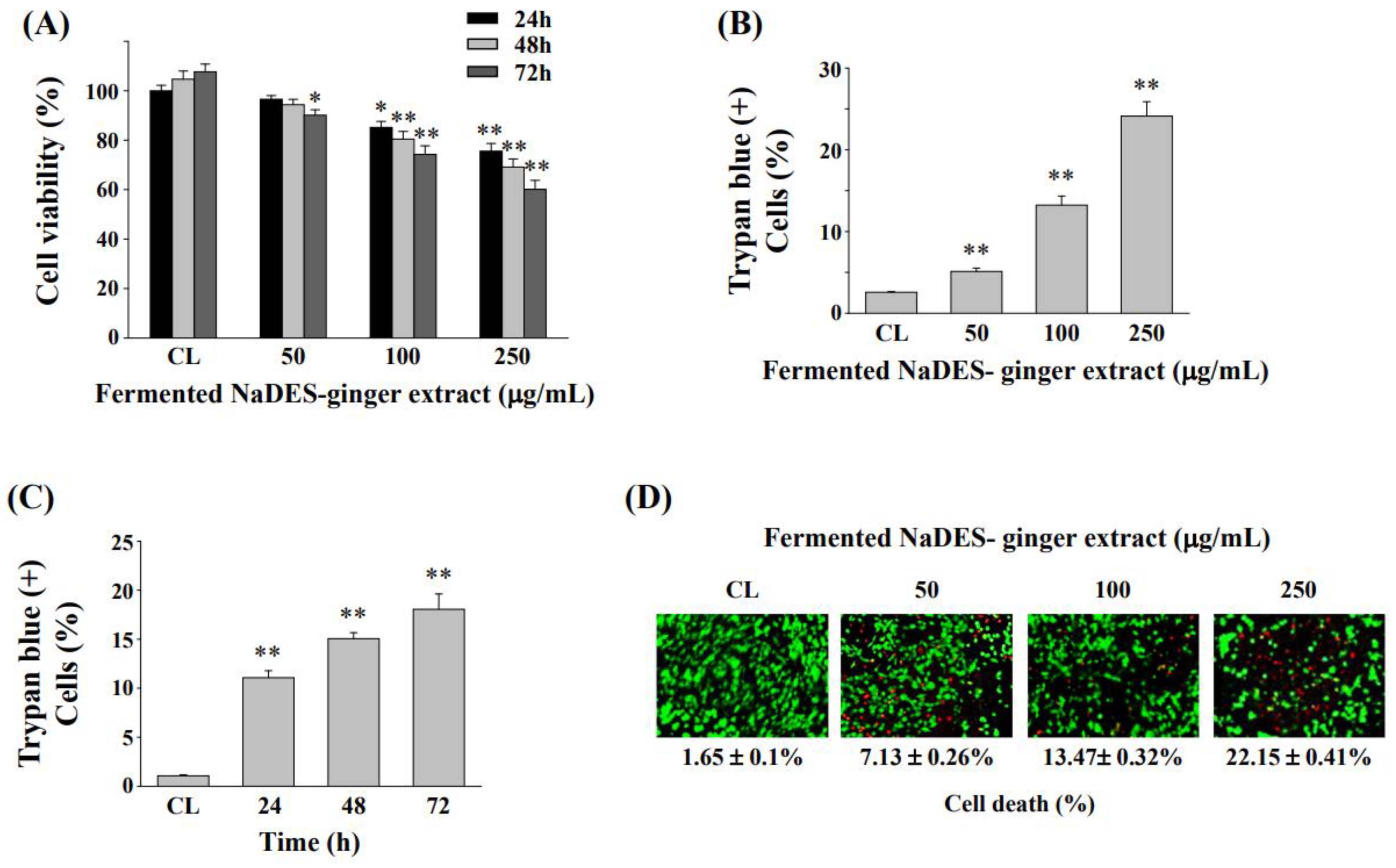
3.4. Fermented NaDES-Ginger Extract Decreased the Cell Viability of HCT-116/R Cells
3.5. Downregulation of CXCR4 mRNA and Protein Levels in HCT-116/R Cells after Treatment by Fermented NaDES-Ginger Extract
3.6. Down-Regulation of CXCR4 Expression Enhanced Cell Cytotoxicity and Cell Death in HCT-116/R Cells
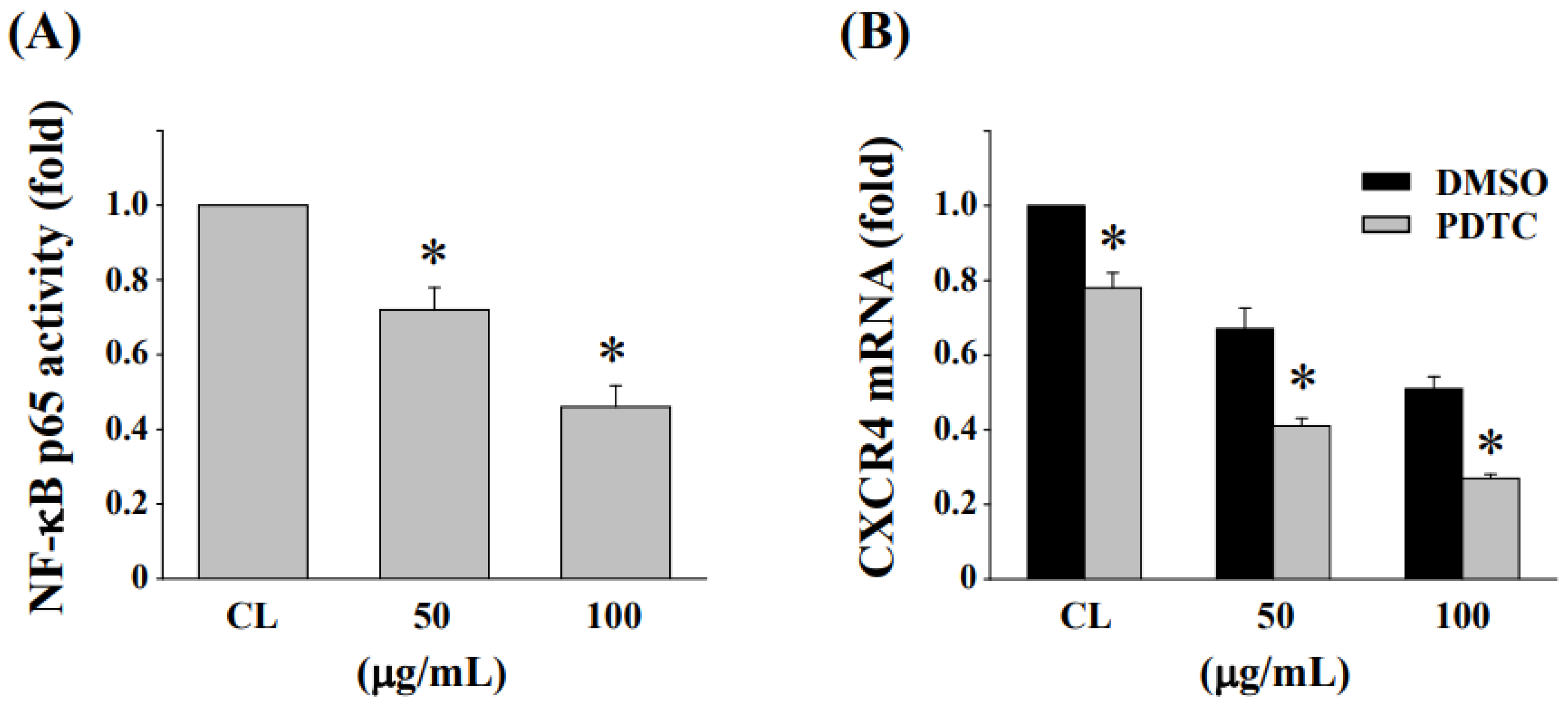
3.7. Inhibition of NF-κB Activation Enhances the Reduction in CXCR4 Expression in HCT-116/R Cells
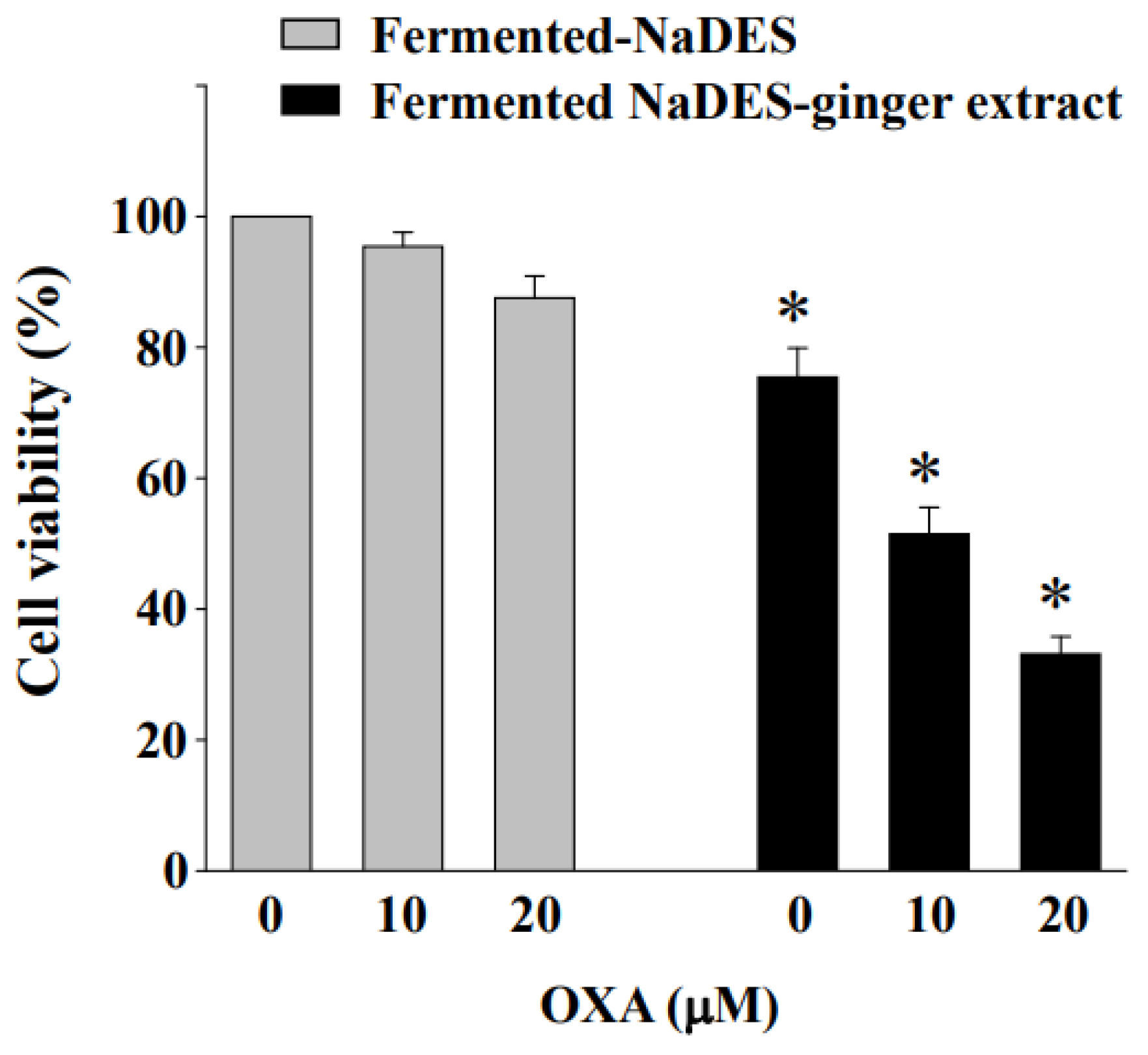
3.8. Fermented NaDES-Ginger Extract and Oxaliplatin Exert a Synergistic Cytotoxic Effect on HCT-116/R Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malki, A.; ElRuz, R.A.; Gupta, I.; Allouch, A.; Vranic, S.; Al Moustafa, A.E. Molecular Mechanisms of Colon Cancer Progression and Metastasis: Recent Insights and Advancements. Int. J. Mol. Sci. 2020, 22, 130. [Google Scholar] [CrossRef] [PubMed]
- Filip, S.; Vymetalkova, V.; Petera, J.; Vodickova, L.; Kubecek, O.; John, S.; Cecka, F.; Krupova, M.; Manethova, M.; Cervena, K.; et al. Distant Metastasis in Colorectal Cancer Patients-Do We Have New Predicting Clinicopathological and Molecular Biomarkers? A Comprehensive Review. Int. J. Mol. Sci. 2020, 21, 5255. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Kang, Y.; Chen, L.; Wang, H.; Liu, J.; Zeng, S.; Yu, L. The Drug-Resistance Mechanisms of Five Platinum-Based Antitumor Agents. Front. Pharmacol. 2020, 11, 343. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.K.U.; Hadinoto, K. Deep Eutectic Solvent as Green Solvent in Extraction of Biological Macromolecules: A Review. Int. J. Mol. Sci. 2022, 23, 3381. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H.B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Arcusa, R.; Villaño, D.; Marhuenda, J.; Cano, M.; Cerdà, B.; Zafrilla, P. Potential Role of Ginger (Zingiber officinale Roscoe) in the Prevention of Neurodegenerative Diseases. Front. Nutr. 2022, 9, 809621. [Google Scholar] [CrossRef]
- Hsieh, Y.H.; Li, Y.; Pan, Z.; Chen, Z.; Lu, J.; Yuan, J.; Zhu, Z.; Zhang, J. Ultrasonication-assisted synthesis of alcohol-based deep eutectic solvents for extraction of active compounds from ginger. Ultrason. Sonochem. 2020, 63, 104915. [Google Scholar] [CrossRef]
- Tzani, A.; Kalafateli, S.; Tatsis, G.; Bairaktari, M.; Kostopoulou, I.; Pontillo, A.R.N.; Detsi, A. Natural Deep Eutectic Solvents (NaDESs) as Alternative Green Extraction Media for Ginger (Zingiber officinale Roscoe). Sustain. Chem. 2021, 2, 576–598. [Google Scholar] [CrossRef]
- Hayyan, M.; Mbous, Y.P.; Looi, C.Y.; Wong, W.F.; Hayyan, A.; Salleh, Z.; Mohd-Ali, O. Natural deep eutectic solvents: Cytotoxic profile. Springerplus 2016, 5, 913. [Google Scholar] [CrossRef]
- Lomba, L.; Ribate, M.P.; Sangüesa, E.; Concha, J.; Garralaga, M.P.; Errazquin, D.; García, C.B.; Giner, B. Deep Eutectic Solvents: Are They Safe? Appl. Sci. 2021, 11, 10061. [Google Scholar] [CrossRef]
- Lechner, J.F.; Stoner, G.D. Gingers and Their Purified Components as Cancer Chemopreventative Agents. Molecules 2019, 24, 2859. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, N.; Zhang, T.T.; Nakanishi, T. Involvement of CXCR4 in Normal and Abnormal Development. Cells 2019, 8, 185. [Google Scholar] [CrossRef] [PubMed]
- Nengroo, M.A.; Maheshwari, S.; Singh, A.; Verma, A.; Arya, R.K.; Chaturvedi, P.; Saini, K.K.; Singh, A.K.; Sinha, A.; Meena, S.; et al. CXCR4 intracellular protein promotes drug resistance and tumorigenic potential by inversely regulating the expression of Death Receptor 5. Cell Death Dis. 2021, 12, 464. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.S.; Hsieh, M.C.; Huang, C.Y.; Kuo, Y.H.; Tung, S.Y.; Shen, C.H.; Hsieh, Y.Y.; Teng, C.C.; Lee, K.F.; Chen, T.C.; et al. The Association of CXC Receptor 4 Mediated Signaling Pathway with Oxaliplatin-Resistant Human Colorectal Cancer Cells. PLoS ONE 2016, 11, e0159927. [Google Scholar] [CrossRef] [PubMed]
- Goïta, A.A.; Guenot, D. Colorectal Cancer: The Contribution of CXCL12 and Its Receptors CXCR4 and CXCR7. Cancers 2022, 14, 1810. [Google Scholar] [CrossRef]
- Lee, K.C.; Wu, K.L.; Yen, C.K.; Chang, S.F.; Chen, C.N.; Lu, Y.C. Inhibition of NLRP3 by Fermented Quercetin Decreases Resistin-Induced Chemoresistance to 5-Fluorouracil in Human Colorectal Cancer Cells. Pharmaceuticals 2022, 15, 798. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteau reagent. Method. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Lee, K.C.; Wu, K.L.; Yen, C.K.; Chen, C.N.; Chang, S.F.; Huang, W.S. 6-Shogaol Antagonizes the Adipocyte-Conditioned Medium-Initiated 5-Fluorouracil Resistance in Human Colorectal Cancer Cells through Controlling the SREBP-1 Level. Life 2021, 11, 1067. [Google Scholar] [CrossRef]
- Huang, W.S.; Chen, C.N.; Sze, C.I.; Teng, C.C. Visfatin induces stromal cell-derived factor-1 expression by β1 integrin signaling in colorectal cancer cells. J. Cell. Physiol. 2013, 228, 1017–1024. [Google Scholar] [CrossRef]
- Ali, A.M.A.; El-Nour, M.E.M.; Yagi, S.M. Total phenolic and flavonoid contents and antioxidant activity of ginger (Zingiber officinale Rosc.) rhizome, callus and callus treated with some elicitors. J. Genet. Eng. Biotechnol. 2018, 16, 677–682. [Google Scholar] [CrossRef]
- Van der Jeught, K.; Xu, H.C.; Li, Y.J.; Lu, X.B.; Ji, G. Drug resistance and new therapies in colorectal cancer. World J. Gastroenterol. 2018, 24, 3834–3848. [Google Scholar] [CrossRef] [PubMed]
- Hikmawanti, N.P.E.; Ramadon, D.; Jantan, I.; Mun’im, A. Natural Deep Eutectic Solvents (NADES): Phytochemical Extraction Performance Enhancer for Pharmaceutical and Nutraceutical Product Development. Plants 2021, 10, 2091. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Benlebna, M.; Ruesgas-Ramón, M.; Bonafos, B.; Fouret, G.; Casas, F.; Coudray, C.; Durand, E.; Cruz Figueroa-Espinoza, M.; Feillet-Coudray, C. Toxicity of Natural Deep Eutectic Solvent Betaine:Glycerol in Rats. J. Agric. Food Chem. 2018, 66, 6205–6212. [Google Scholar] [CrossRef] [PubMed]
- Klaschka, U. Naturally toxic: Natural substances used in personal care products. Environ. Sci. Eur. 2015, 27, 1. [Google Scholar] [CrossRef]
- Son, C.G.; Lee, S.K.; Choi, I.K.; Jang, E.S.; Bang, K.J. Herbal Transformation by Fermentation. J. Acupunct. Meridian Stud. 2020, 13, 167–168. [Google Scholar] [CrossRef]
- Kuo, H.C.; Kwong, H.K.; Chen, H.Y.; Hsu, H.Y.; Yu, S.H.; Hsieh, C.W.; Lin, H.W.; Chu, Y.L.; Cheng, K.C. Enhanced antioxidant activity of Chenopodium formosanum Koidz. by lactic acid bacteria: Optimization of fermentation conditions. PLoS ONE 2021, 16, e0249250. [Google Scholar] [CrossRef]
- Kwon, J.E.; Lee, J.W.; Park, Y.; Sohn, E.H.; Choung, E.S.; Jang, S.A.; Kim, I.; Lee, D.E.; Koo, H.J.; Bak, J.P.; et al. Biotransformation of Pueraria lobata Extract with Lactobacillus rhamnosus vitaP1 Enhances Anti-Melanogenic Activity. J. Microbiol. Biotechnol. 2018, 28, 22–31. [Google Scholar] [CrossRef]
- Hati, S.; Vij, S.; Singh, B.P.; Mandal, S. β-Glucosidase activity and bioconversion of isoflavones during fermentation of soymilk. J. Sci. Food Agric. 2015, 95, 216–220. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Eweys, A.S.; Zhang, J.Y.; Zhu, Y.; Bai, J.; Darwesh, O.M.; Zhang, H.B.; Xiao, X. Fermentation Affects the Antioxidant Activity of Plant-Based Food Material through the Release and Production of Bioactive Components. Antioxidants 2021, 10, 2004. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation transforms the phenolic profiles and bioactivities of plant-based foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.Y.; Kang, H.R.; Cho, S.K. Changes Over the Fermentation Period in Phenolic Compounds and Antioxidant and Anticancer Activities of Blueberries Fermented by Lactobacillus plantarum. J. Food Sci. 2019, 84, 2347–2356. [Google Scholar] [CrossRef] [PubMed]
- de Lima, R.M.T.; Dos Reis, A.C.; de Menezes, A.P.M.; Santos, J.V.O.; Filho, J.W.G.O.; Ferreira, J.R.O.; de Alencar, M.V.O.B.; da Mata, A.M.O.F.; Khan, I.N.; Islam, A.; et al. Protective and therapeutic potential of ginger (Zingiber officinale) extract and [6]-gingerol in cancer: A comprehensive review. Phytother. Res. 2018, 32, 1885–1907. [Google Scholar] [CrossRef] [PubMed]
- Aprile, A.; Negro, C.; Sabella, E.; Luvisi, A.; Nicolì, F.; Nutricati, E.; Vergine, M.; Miceli, A.; Blando, F.; De Bellis, L. Antioxidant Activity and Anthocyanin Contents in Olives (cv Cellina di Nardò) during Ripening and after Fermentation. Antioxidants 2019, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, H.F.; Algonaiman, R.; Barakat, H. Ameliorative and Antioxidative Potential of Lactobacillus plantarum-Fermented Oat (Avena sativa) and Fermented Oat Supplemented with Sidr Honey against Streptozotocin-Induced Type 2 Diabetes in Rats. Antioxidants 2022, 11, 1122. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K. Ginger and its constituents: Role in prevention and treatment of gastrointestinal cancer. Gastroenterol. Res. Pract. 2015, 2015, 142979. [Google Scholar] [CrossRef]
- Liu, T.; Wei, R.; Zhang, Y.; Chen, W.; Liu, H. Association between NF-κB expression and drug resistance of liver cancer. Oncol. Lett. 2019, 17, 1030–1034. [Google Scholar] [CrossRef]
- Zhi, Y.; Lu, H.; Duan, Y.; Sun, W.; Guan, G.; Dong, Q.; Yang, C. Involvement of the nuclear factor-κB signaling pathway in the regulation of CXC chemokine receptor-4 expression in neuroblastoma cells induced by tumor necrosis factor-α. Int. J. Mol. Med. 2015, 35, 349–357. [Google Scholar] [CrossRef]
- Singh, A.; Srivastava, N.; Yadav, A.; Ateeq, B. Targeting AGTR1/NF-κB/CXCR4 axis by miR-155 attenuates oncogenesis in glioblastoma. Neoplasia 2020, 22, 497–510. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-C.; Wu, K.-L.; Chang, S.-F.; Chang, H.-I.; Chen, C.-N.; Chen, Y.-Y. Fermented Ginger Extract in Natural Deep Eutectic Solvent Enhances Cytotoxicity by Inhibiting NF-κB Mediated CXC Chemokine Receptor 4 Expression in Oxaliplatin-Resistant Human Colorectal Cancer Cells. Antioxidants 2022, 11, 2057. https://doi.org/10.3390/antiox11102057
Lee K-C, Wu K-L, Chang S-F, Chang H-I, Chen C-N, Chen Y-Y. Fermented Ginger Extract in Natural Deep Eutectic Solvent Enhances Cytotoxicity by Inhibiting NF-κB Mediated CXC Chemokine Receptor 4 Expression in Oxaliplatin-Resistant Human Colorectal Cancer Cells. Antioxidants. 2022; 11(10):2057. https://doi.org/10.3390/antiox11102057
Chicago/Turabian StyleLee, Ko-Chao, Kuen-Lin Wu, Shun-Fu Chang, Hsin-I Chang, Cheng-Nan Chen, and Yih-Yuan Chen. 2022. "Fermented Ginger Extract in Natural Deep Eutectic Solvent Enhances Cytotoxicity by Inhibiting NF-κB Mediated CXC Chemokine Receptor 4 Expression in Oxaliplatin-Resistant Human Colorectal Cancer Cells" Antioxidants 11, no. 10: 2057. https://doi.org/10.3390/antiox11102057
APA StyleLee, K.-C., Wu, K.-L., Chang, S.-F., Chang, H.-I., Chen, C.-N., & Chen, Y.-Y. (2022). Fermented Ginger Extract in Natural Deep Eutectic Solvent Enhances Cytotoxicity by Inhibiting NF-κB Mediated CXC Chemokine Receptor 4 Expression in Oxaliplatin-Resistant Human Colorectal Cancer Cells. Antioxidants, 11(10), 2057. https://doi.org/10.3390/antiox11102057






