Impact of N-Acetyl Cysteine (NAC) on Tuberculosis (TB) Patients—A Systematic Review
Abstract
:1. Introduction
1.1. What Is Already Known on This Topic?
1.2. What Does This Present Study Add?
2. Materials and Methods
Statistical Methods
3. Results
3.1. Data Charting
3.2. NAC Effect on Sputum Culture Conversion
3.3. Adverse Events of NAC in TB Subjects
3.4. Immunological Responses
3.5. GSH Expression Levels
3.6. Lung Function
4. Discussion
4.1. Mechanism of NAC
4.2. Effect of NAC on Microbial Activity
5. Limitations of the Review Process
6. Conclusions
7. Recommendation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Tuberculosis Report 2022. 2022. Available online: https://www.who.int/publications/i/item/9789240061729 (accessed on 13 August 2022).
- WHO. Global TB Report 2020; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Hnizdo, E.; Singh, T.; Churchyard, G. Chronic pulmonary function impairment caused by initial and recurrent pulmonary tuberculosis following treatment. Thorax 2000, 55, 32–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravimohan, S.; Kornfeld, H.; Weissman, D.; Bisson, G.P. Tuberculosis and lung damage: From epidemiology to pathophysiology. Eur. Respir. Rev. 2018, 27, 170077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, I.; MacNee, W. Oxidative Stress and Regulation of GSH in Lung Inflammation. Eur. Respir. J. 2000, 16, 534–554. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, G.; Hopkins, J. The response of Mycobacterium tuberculosis to reactive oxygen and nitrogen species. Front. Microbiol. 2011, 2, 105. [Google Scholar] [CrossRef] [Green Version]
- Hecker, L. Mechanisms and consequences of oxidative stress in lung disease: Therapeutic implications for an aging populace. Am. J. Physiol. Cell. Mol. Physiol. 2018, 314, L642–L653. [Google Scholar] [CrossRef]
- Silvagno, F.; Vernone, A.; Pescarmona, G.P. The Role of Glutathione in Protecting against the Severe Inflammatory Response Triggered by COVID-19. Antioxidants 2020, 9, 624. [Google Scholar] [CrossRef]
- Galano, A.; Alvarez-Idaboy, J.R. Glutathione: Mechanism and Kinetics of Its Non-Enzymatic Defense Action against Free Radicals. RSC Adv. 2011, 1, 1763–1771. [Google Scholar] [CrossRef]
- Ulrich, K.; Jakob, U. The role of thiols in antioxidant systems. Free Radic. Biol. Med. 2019, 140, 14–27. [Google Scholar] [CrossRef]
- Fagan, R.L.; Palfey, B.A. Flavin-Dependent Enzymes. In Comprehensive Natural Products II, 1st ed.; Mander, L., Liu, H.-W., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2010; pp. 37–113. [Google Scholar]
- Fanucchi, M.V. Pulmonary Developmental Responses to Toxicants. In Comprehensive Toxicology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 8, pp. 199–221. [Google Scholar]
- Venketaraman, V.; Dayaram, Y.K.; Amin, A.G.; Ngo, R.; Green, R.M.; Talaue, M.T.; Mann, J.; Connell, N.D. Role of Glutathione in Macrophage Control of Mycobacteria. Infect. Immun. 2003, 71, 1864–1871. [Google Scholar] [CrossRef] [Green Version]
- Prescott, L.F.; Critchley, J.A.J.H.; Proudfoot, A.T.; Illingworth, R.N.; Stewart, M.J.; Adam, R.D. Intravenous N-acetylcysteine: The treatment of choice for paracetamol poisoning. Br. Med. J. 1979, 2, 1097–1100. [Google Scholar] [CrossRef]
- Li, X.; Wei, X.; Chen, C.; Zhang, Z.; Liu, D.; Hei, Z.; Yao, W. N-Acetylcysteine Inhalation Improves Pulmonary Function in Patients Received Liver Transplantation. Biosci. Rep. 2018, 38, BSR20180858. [Google Scholar] [CrossRef] [Green Version]
- Sadowska, A.M.; Verbraecken, J.; Darquennes, K.; de Backer, W.A. Role of N-acetylcysteine in the management of COPD. Ther. Clin. Risk Manag. 2006, 2, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Mahakalkar, S.; Nagrale, D.; Gaur, S.; Urade, C.; Murhar, B.; Turankar, A. N-acetylcysteine as an add-on to Directly Observed Therapy Short-I therapy in fresh pulmonary tuberculosis patients: A randomized, placebo-controlled, double-blinded study. Perspect. Clin. Res. 2017, 8, 132–136. [Google Scholar] [PubMed]
- Vilchèze, C.; Jacobs, W.R. The Promises and Limitations of N -Acetylcysteine as a Potentiator of First-Line and Second-Line Tuberculosis Drugs. Antimicrob. Agents Chemother. 2021, 65. [Google Scholar] [CrossRef] [PubMed]
- Safe, I.P.; Lacerda, M.V.G.; Printes, V.S.; Marins, A.F.P.; Rabelo, A.L.R.; Costa, A.A.; Tavares, M.A.; Jesus, J.S.; Souza, A.B.; Beraldi-Magalhães, F.; et al. Safety and efficacy of N-acetylcysteine in hospitalized patients with HIV-associated tuberculosis: An open-label, randomized, phase II trial (RIPENACTB Study). PLoS ONE 2020, 15, e0235381. [Google Scholar] [CrossRef]
- Amaral, E.P.; Conceição, E.L.; Costa, D.L.; Rocha, M.S.; Marinho, J.M.; Cordeiro-Santos, M.; D’Império-Lima, M.R.; Barbosa, T.; Sher, A.; Andrade, B.B. N-acetyl-cysteine exhibits potent anti-mycobacterial activity in addition to its known anti-oxidative functions. BMC Microbiol. 2016, 16, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Wei, M.; Song, F.; Xue, D.; Wang, Y. N-acetylcysteine (NAC) attenuating apoptosis and autophagy in RAW264.7 cells in response to incubation with mycolic acid from bovine mycobacterium tuberculosis complex. Pol. J. Microbiol. 2020, 69, 223–229. [Google Scholar] [CrossRef]
- Coleman, M.D.; Khan, N.; Welton, G.; Lambert, P.A.; Tims, K.J.; Rathbone, D.L. Effects of glutathione, N-acetyl-cysteine, α-lipoic acid and dihydrolipoic acid on the cytotoxicity of a 2-pyridylcarboxamidrazone antimycobacterial agent in human mononuclear leucocytes in vitro. Environ. Toxicol. Pharmacol. 2004, 17, 143–148. [Google Scholar] [CrossRef]
- Venketaraman, V.; Rodgers, T.; Linares, R.; Reilly, N.; Swaminathan, S.; Hom, D.; Millman, A.C.; Wallis, R.; Connell, N.D. Glutathione and growth inhibition of Mycobacterium tuberculosis in healthy and HIV infected subjects. AIDS Res. Ther. 2006, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Venketaraman, V.; Millman, A.; Salman, M.; Swaminathan, S.; Goetz, M.; Lardizabal, A.; Hom, D.; Connell, N.D. Glutathione levels and immune responses in tuberculosis patients. Microb. Pathog. 2008, 44, 255–261. [Google Scholar] [CrossRef]
- Teskey, G.; Cao, R.; Islamoglu, H.; Medina, A.; Prasad, C.; Prasad, R.; Sathananthan, A.; Fraix, M.; Subbian, S.; Zhong, L.; et al. The Synergistic Effects of the Glutathione Precursor, NAC and First-Line Antibiotics in the Granulomatous Response Against Mycobacterium tuberculosis. Front. Immunol. 2018, 9, 2069. [Google Scholar] [CrossRef] [Green Version]
- Khameneh, B.; Bazzaz, B.S.F.; Amani, A.; Rostami, J.; Vahdati-Mashhadian, N. Combination of anti-tuberculosis drugs with vitamin C or NAC against different Staphylococcus aureus and Mycobacterium tuberculosis strains. Microb. Pathog. 2016, 93, 83–87. [Google Scholar] [CrossRef]
- Guerra, C.; Johal, K.; Morris, D.; Moreno, S.; Alvarado, O.; Gray, D.; Tanzil, M.; Pearce, D.; Venketaraman, V. Control of Mycobacterium tuberculosis growth by activated natural killer cells. Clin. Exp. Immunol. 2011, 168, 142–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palanisamy, G.S.; Kirk, N.M.; Ackart, D.F.; Shanley, C.A.; Orme, I.M.; Basaraba, R.J. Evidence for Oxidative Stress and Defective Antioxidant Response in Guinea Pigs with Tuberculosis. PLoS ONE 2011, 6, e26254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baniasadi, S.; Eftekhari, P.; Tabarsi, P.; Fahimi, F.; Raoufy, M.R.; Masjedi, M.; Velayati, A.A. Protective effect of N-acetylcysteine on antituberculosis drug-induced hepatotoxicity. Eur. J. Gastroenterol. Hepatol. 2010, 22, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- Moosa, M.S.; Maartens, G.; Gunter, H.; Allie, S.; Chughlay, M.F.; Setshedi, M.; Wasserman, S.; Stead, D.F.; Hickman, N.; Stewart, A.; et al. A Randomized Controlled Trial of Intravenous N-Acetylcysteine in the Management of Anti-tuberculosis Drug–Induced Liver Injury. Clin. Infect. Dis. 2020, 73, e3377–e3383. [Google Scholar] [CrossRef] [PubMed]
- Safe, I.P.; Amaral, E.P.; Araújo-Pereira, M.; Lacerda, M.V.G.; Printes, V.S.; Souza, A.B.; Beraldi-Magalhães, F.; Monteiro, W.M.; Sampaio, V.S.; Barreto-Duarte, B.; et al. Adjunct N-acetylcysteine treatment in hospitalized patients with HIV-associated tuberculosis dampens the oxidative stress in peripheral blood: Results from the RIPENACTB Study trial. Front. Immunol. 2021, 11, 602589. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.N.; Nation, B.E.; Autry, M.T.; Johnson, P.N. Possible role for acetylcysteine as a treatment for acute liver failure secondary to antitubercular medication use. Am. J. Health Pharm. 2020, 77, 1482–1487. [Google Scholar] [CrossRef]
- Atkuri, K.R.; Mantovani, J.J.; Herzenberg, L.A. N-Acetylcysteine—A safe antidote for cysteine/glutathione deficiency. Curr. Opin. Pharmacol. 2007, 7, 355–359. [Google Scholar] [CrossRef] [Green Version]
- Kranzer, K.; Elamin, W.F.; Cox, H.; Seddon, J.A.; Ford, N.; Drobniewski, F. A systematic review and meta-analysis of the efficacy and safety ofN-acetylcysteine in preventing aminoglycoside-induced ototoxicity: Implications for the treatment of multidrug-resistant TB. Thorax 2015, 70, 1070–1077. [Google Scholar] [CrossRef]
- Mokhtari, V.; Afsharian, P.; Shahhoseini, M.; Kalantar, M.; Moini, A. A Review on Various Uses of N-Acetyl Cysteine. Cell J. 2017, 19, 11–17. [Google Scholar] [PubMed]
- Ejigu, D.A.; Abay, S.M. N-Acetyl Cysteine as an Adjunct in the Treatment of Tuberculosis. Tuberc. Res. Treat. 2020, 2020, 5907839. [Google Scholar] [CrossRef]
- Young, C.; Walzl, G.; Du Plessis, N. Therapeutic host-directed strategies to improve outcome in tuberculosis. Mucosal Immunol. 2020, 13, 190–204. [Google Scholar] [CrossRef] [Green Version]
- Schwalfenberg, G.K. N-Acetylcysteine: A Review of Clinical Usefulness (an Old Drug with New Tricks). J. Nutr. Metab. 2021, 2021, 9949453. [Google Scholar] [CrossRef]
- Tenório, M.C.d.S.; Graciliano, N.G.; Moura, F.; de Oliveira, A.C.M.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef]
- Conrad, C.; Lymp, J.; Thompson, V.; Dunn, C.; Davies, Z.; Chatfield, B.; Nichols, D.; Clancy, J.; Vender, R.; Egan, M.E.; et al. Long-term treatment with oral N-acetylcysteine: Affects lung function but not sputum inflammation in cystic fibrosis subjects. A phase II randomized placebo-controlled trial. J. Cyst. Fibros. 2015, 14, 219–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.O.; Byun, Y.J.; Cho, K.O.; Kim, S.Y.; Lee, S.B.; Kim, H.S.; Kwon, O.J.; Jeong, S.W. GS28 Protects Neuronal Cell Death Induced by Hydrogen Peroxide under Glutathione-Depleted Condition. Korean J. Physiol. Pharmacol. 2011, 15, 149–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dringen, R.; Pfeiffer, B.; Hamprecht, B. Synthesis of the Antioxidant Glutathione in Neurons: Supply by Astrocytes of CysGly as Precursor for Neuronal Glutathione. J. Neurosci. 1999, 19, 562–569. [Google Scholar] [CrossRef] [Green Version]
- Franco, R.; Schoneveld, O.J.; Pappa, A.; Panayiotidis, M.I. The Central Role of Glutathione in the Pathophysiology of Human Diseases. Arch. Physiol. Biochem. 2007, 113, 234–258. [Google Scholar] [CrossRef]
- Palmen, N.G.M.; Evelo, C.T.A. Glutathione depletion in human erythrocytes as an indicator for microsomal activation of cyclophosphamide and 3-hydroxyacetanilide. Toxicology 1993, 84, 157–170. [Google Scholar] [CrossRef]
- Rahman, I.; MacNee, W. Role of oxidants/antioxidants in smoking-induced lung diseases. Free Radic. Biol. Med. 1996, 21, 669–681. [Google Scholar] [CrossRef]
- Oxidants, Antioxidants and the Pathogenesis of Emphysema—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/2995106/ (accessed on 13 August 2022).
- Metinko, A.P.; Kunkel, S.L.; Standiford, T.J.; Strieter, R.M. Anoxia-hyperoxia induces monocyte-derived interleukin-8. J. Clin. Investig. 1992, 90, 791–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dekhuijzen, P.N.R.; Van Beurden, W.J.C. The role for N-acetylcysteine in the management of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2006, 1, 99–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 4117–4129. [Google Scholar] [CrossRef] [PubMed]
- Moldéus, P.; Cotgreave, I.A.; Berggren, M. Lung Protection by a Thiol-Containing Antioxidant: N-Acetylcysteine. Respiration 1986, 50, 31–42. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, P.; Martinez, L.; Yang, H.; Lu, W.; Ding, X.; Zhu, L. Factors Affecting Time to Sputum Culture Conversion and Treatment Outcome of Patients with Multidrug-Resistant Tuberculosis in China. BMC Infect. Dis. 2018, 18, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Tekalegn, Y.; Woldeyohannes, D.; Assefa, T.; Aman, R.; Sahiledengle, B. Predictors of Time to Sputum Culture Conversion among Drug-Resistant Tuberculosis Patients in Oromia Region Hospitals, Ethiopia. Infect. Drug Resist. 2020, 13, 2547–2556. [Google Scholar] [CrossRef]
- Salindri, A.D.; Kipiani, M.; Kempker, R.R.; Gandhi, N.R.; Darchia, L.; Tukvadze, N.; Blumberg, H.M.; Magee, M.J. Diabetes Reduces the Rate of Sputum Culture Conversion in Patients with Newly Diagnosed Multidrug-Resistant Tuberculosis. Open Forum Infect. Dis. 2016, 3, 2009–2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yihunie Akalu, T.; Fentahun Muchie, K.; Alemu Gelaye, K. Time to Sputum Culture Conversion and Its Determinants among Multi-Drug Resistant Tuberculosis Patients at Public Hospitals of the Amhara Regional State: A Multicenter Retrospective Follow up Study. PLoS ONE 2018, 13, 1–14. [Google Scholar] [CrossRef]
- Reliene, R.; Schiestl, R.H. Glutathione Depletion by Buthionine Sulfoximine Induces DNA Deletions in Mice. Carcinogenesis 2006, 27, 240–244. [Google Scholar] [CrossRef]
- Griffith, O.W.; Meister, A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J. Biol. Chem. 1979, 254, 7558–7560. [Google Scholar] [CrossRef]
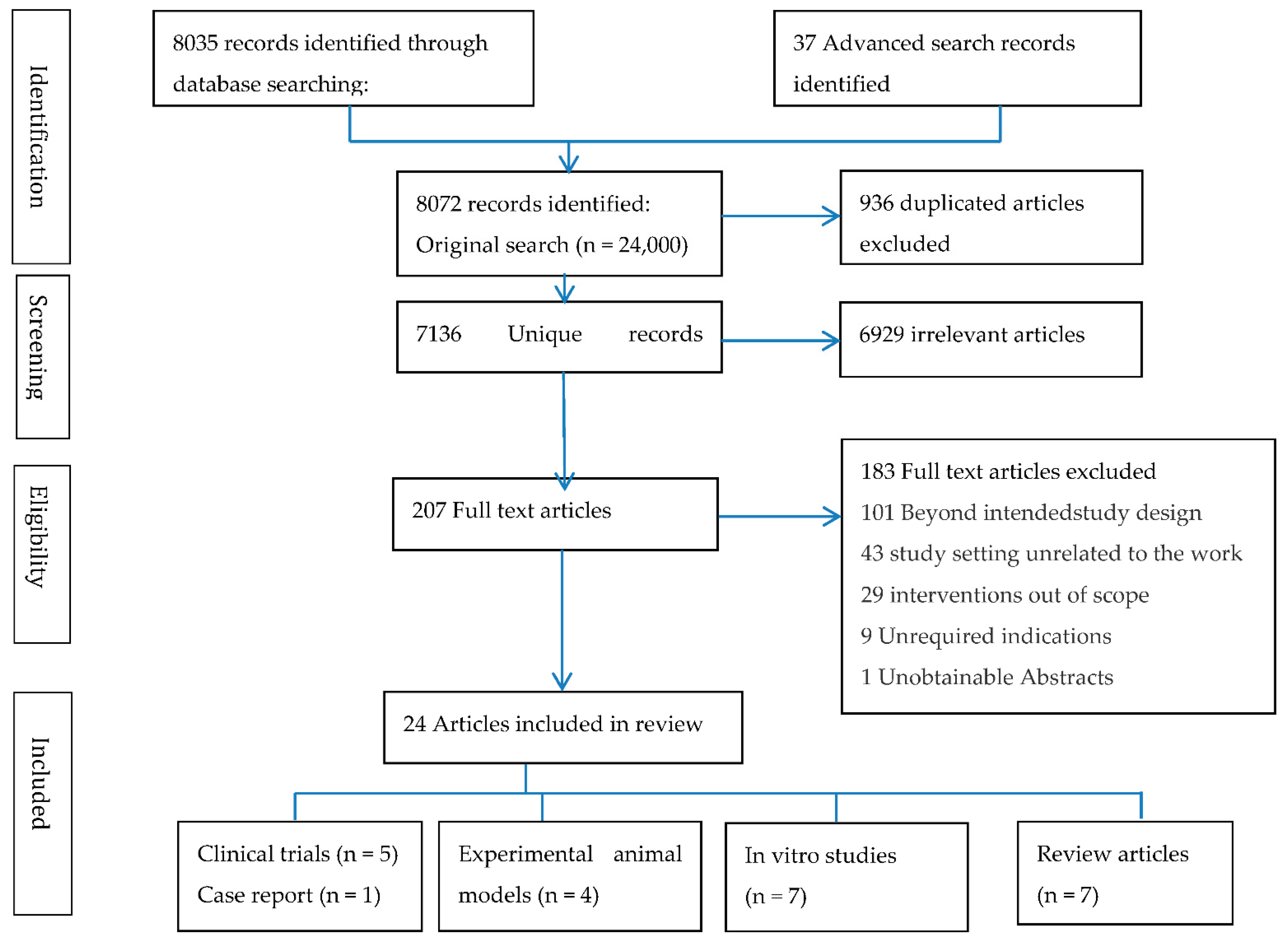
| Author and Time of Publication | In Vitro Experiment | Objectives | Intervention (Methods Overview) | Results/Outcome | Reference |
|---|---|---|---|---|---|
| Lin (2020) | Cell culture experiment, derived from Experimental animal model | RAW264.7 macrophages were used to explore the immunological response and cell damage of host cells after incubation with Mycolic Acid. | NAC conc. of 600 mg/mL for 2 h was used to treat incubated cells. | NAC inhibited the expression of the TNF-α and caspase-9 genes and reduced the translation of apoptotic proteins. | [21] |
| Amaral (2016) | 70 (32) Plasma from 30 TB, subjects 20 LTBI, and 20 Healthy control | NAC directly impairs the growth of several species of M.tb in vitro independent of its inhibitory effects on the host NADPH oxidase System. | 10 mM NAC was used to treat M.tb infected Macrophages from 70 subjects. | NAC significantly decreased ROS accumulation, lipid peroxidation, and DNA oxidation, while restoring cell viability. | [20] |
| Coleman (2004) | Monocyte cells derived from blood collected from 24 Healthy volunteers (21–24) | Use of GSH, NAC, Lipoic acid (LA), dihydrolipoic acid (DHLA; to modulate the toxicity of (N1-4-N,N-dimethylamino-1-naphthylidene) pyridine-2-carboxamidrazone (Compound 1), and Isoniazid (INH), which has demonstrated both Mononuclear leukocyte toxicity and anti-mycobacterial action. | Isoniazid (INH) and Compound 1-treated cells were incubated with either NAC, GSH, DHLA, or LA in 1 mM; final concentration. | GSH and NAC showed abolition of Isoniazid (INH) toxicity to the mononuclear leukocyte cells | [22] |
| Venketaraman (2006) | 20 Healthy and TB subjects | Study examined the role of GSH in immunity against TB infection in samples derived from healthy and HIV-human subjects. | 10 mM NAC conc. was used in cell culture experiment to find the relationship between GSH levels and the ability to kill intracellular M. tb. | NAC resulted in more efficient control of intracellular M. tb infection in blood cultures derived from healthy subjects compared to TB Patients; NAC treatment caused down-regulation of the synthesis of IL-1, IL-6, and TNF-α. | [23] |
| Venketaraman (2008) | 12 Healthy and TB subjects | To determine the extent to which GSH levels are decreased in patients with active TB and examine the relationship between GSH and the ability to kill intracellular M.tb and other host immune functions, such as cytokine production. | The effect of 10 mM NAC/BSO in altering the intracellular survival of H37Rv M.tb strain. | NAC decreased the levels of IL-10, IL-6, TNF-a, and IL-1 in blood cultures derived from TB patients and also showed efficient control of intracellular M. tb infection in blood cultures derived from healthy subjects compared to TB patients | [24] |
| Teskey (2018) | 13 subjects (20–65) | Examined the effect of NAC on M. tb infection. | NAC 10 mM + antibiotic in altering the survival of M. tb, Elevated GSH levels. | NAC results in significant reduction of M. tb burden in both healthy and diabetic individuals. | [25] |
| Khameneh (2016) | NA | Investigation of the antibacterial activity of vitamin C and NAC individually and in combination with RIF and INH against different strains of S. aureus and M.tb. | MIC on cell cultures NAC (final conc. 40.0 mg/L) or vitamin C (final conc. 40.0 mg/L). | Combination of vitamin C and NAC was able to reduce the hepatotoxicity of the anti-tb drugs and enhanced antimicrobial activity. | [26] |
| Guerra (2012) | 23 subjects | The study demonstrated the treatment of NK cells with IL-2 + IL-12 + NAC resulted in inhibition in the growth of H37Rv M.tb strain. | 20 mM NAC was used in treatment with IL-2 + IL-12. | Results unveil an important pathway by which cytokines in conjunction with GSH, enhanced the functions of NK cells to control M. tb infection. | [27] |
| Author And Publication Time | Animal Species | Study Design | Objectives | NAC Dose | Results/Outcome | Reference |
|---|---|---|---|---|---|---|
| Amaral (2016) | C57BL/6 Micen = 20 | NAC directly impairs the growth of several species of mycobacteria in vitro independent of its inhibitory effects on the host NADPH oxidase system. This anti-mycobacterial effect was also observed in an experimental model in vivo. | NAC exerts anti mycobacterial activity in vivo. | 400 mg/Kg daily for 6 days. | Lung burden of M. tb-infected mice decreased by 0.5 log10 at day 7 compared to untreated mice. | [20] |
| Palanisamy (2011) | Guinea pigs were used | To establish the presence of oxidative stress conditions during experimental TB in guinea pigs and to determine whether antioxidant therapy could reverse the adverse effects of progressive inflammation, including lessening the bacterial burden and disease severity. | Guinea pigs of 9 Month of age were aerosolized using the Madison infection chamber with H37Rv M.tb with a conc. Of 106 CFU/mL, followed vaccination with or without BCG treated with or without NAC 400 mg/kg. | NAC conc. Of 400 mg/kg was used to treat infected mice. | Daily administration of NAC resulted in nearly one log reduction in the number of bacilli in the spleen on day 30. No significant differences in the numbers of bacilli were observed between control and NAC-treated groups on days 30 and 60 in lungs and peribronchial lymph node. An increase in whole blood GSH was seen in NAC-treated animals compared to the mock-treated control group on day 60 of infection. | [28] |
| Lin (2020) | Cell culture and Experimental animal model (ICR mice) | Animal experiments were performed to investigate the role of NAC in antagonizing the effects of Mycolic Acid in the induction of apoptosis and autophagy. | NAC on Mycolic Acid; ICR mice were used to evaluate the lung injury. | The intranasal NAC dose used in the studies is not mentioned in the study. | NAC inhibited the expression of the TNF-α and caspase-9 genes and reduced the translation of apoptotic proteins. NAC reduced the secretion of IL-6 significantly; also, NAC attenuated apoptosis and autophagy in response to incubation with Mycolic acid. | [21] |
| Vilchèze (2021) | Cell culture and experimental animal model (CBA/J mice) | Assessing the function of NAC in vitro, in boosting activity with various combinations of first- and second-line TB drugs against drug-susceptible and multidrug-resistant M. tuberculosis strains. | Adjunctive activity of NAC combined with first- or second-line TB drugs in cultures of M. tb, in M. tb-infected macrophage and in M. tb-infected mice. | NAC, 0.5 or 1 g/kg; was used orally to treat infected CBA/J mice. | NAC enhanced the killing of M. tb by first- and second-line TB drugs in vitro. | [18] |
| Author and Publication Time | Country | No. of Subjects (Mean, Age) | Clinical Criteria (Aim of the Study) | Length of Research (Months) | Intervention (Methods Overview) | Results/Outcome | Reference |
|---|---|---|---|---|---|---|---|
| Baniasadi (2010) | IRAN | 60 (60) | Protective effect of NAC against anti-TB drug-induced hepatotoxicity. | Over 2 weeks | NAC (600 mg, orally, BID | NAC protects against anti-TB drug-induced hepatotoxicity. | [29] |
| Moosa (2021) | SOUTH AFRICA | 102 (38) | Assessing whether i.v NAC hastens liver recovery in hospitalized adult patients with anti-tuberculosis drug induced liver injury (AT-DILI). | Not reported | NAC dosage was as per Acetaminophen toxicity dosage 150 mg/kg over 1 h, 50 mg/kg over 4 h, and 100 mg/kg over 16 h | NAC did not shorten time to ALT < 100 U/L in subjects with AT-DILI, but significantly reduced length of hospital stay. [nausea and vomiting, anaphylaxis, pain at drip site.] | [30] |
| Safe (2020) | BRAZIL | 39 (≥18) | Impact of adjunctive NAC treatment on host immune response and redox homeostasis in population of hospitalized patients with HIV-associated TB. | 16 months | NAC 600 mg BID for 8 weeks | RIPENAC group had elevated plasma levels of GSH compared to RIPE group at the same time-point. | [31] |
| Safe (2020) | BRAZIL | 39 (≥18) | Testing the hypothesis that NAC is safe, well tolerated and secondarily efficacious as adjunctive anti-TB therapy in hospitalized individuals with HIV-TB. | 16 months | NAC 600 mg bid for 8 weeks | The use of NAC in the HIV/TB population seems promising in terms of safety, and mycobacterial clearance results indicate that RIPE plus NAC regimen is suitable for a larger phase III trial. | [19] |
| Mahakalkar (2017) | INDIA 67(18–60) | Effect of NAC (add-on to DOTS Category I regimen) on sputum conversion, radiological improvement, and GSH peroxidase; weight and immunological response compared to placebo. | 18 Months | Standard anti-TB treatment with or without NAC600 mg daily | Adjunctive NAC increased GSH peroxidase levels in TB patients. GSH increase might reduce ROS, TNF-α production. The combination of NAC effects on both the pathogen and the host might be required to observe Early sputum negativity also Radiological improvement (87.5% was achieved by NAC group compared to 33.33% in placebo). | [17] | |
| Fox (2020) | USA | 1 (30) | Reversal of ALF due to DILI in a patient receiving anti-tubercular agents for active TB. NAC be considered for patients with anti-TB-associated DILI. | Not reported | I.V. NAC dosage for acute acetaminophen was followed by infusions of 50 mg/kg over 4 h and 100 mg/kg over 16 h, as well as 100 mg/kg as a continuous for a period of 48 h until 2 additional bags had been infused. | Oral NAC reported with nausea and vomiting NAC use can be considered for patients with anti-TB therapy-associated DILI. | [32] |
| Author and Publication Time | Objectives | NAC Dose | Results/Outcome | Reference |
|---|---|---|---|---|
| Atkuri (2007) | Summarizes the biochemical and pharmacological aspects of NAC that make it a “wise choice” to treat cysteine/GSH deficiencies. | NAC ≥ 600 mg/day | Compared to the placebo group, a small fraction of individuals to whom oral NAC was administered experienced nausea, vomiting, and heartburn. | [33] |
| Kranzer (2015) | Provision of evidence for the safety and oto- protective effect of NAC when co-administered with aminoglycoside in MDR-TB. | NAC (600 mg, orally, twice daily Co-administered with aminoglycoside | NAC reduced ototoxicity in 146 patients with end-stage renal failure receiving aminoglycosides, while 83 studies reported with an increased mild adverse events. | [34] |
| Mokhtari (2017) | The paper presents a review on various applications of NAC in treatment of several diseases. | Use of NAC in the treatment of several diseases | NAC is a safe and well-tolerated supplementary drug without any considerable side effects. | [35] |
| Dawit (2020) | NAC-attenuate hearing loss in MDR-TB | NAC (600 mg, orally, twice daily | NAC appears to have various beneficial effects on TB treatment. | [36] |
| Young (2020) | The review discusses promising pre-clinical candidates and forerunning compounds at advanced stages of clinical investigation in TB host-directed therapeutic (HDT) efficacy trials. | TB preventative therapy. HDTs could enhance anti-mycobacterial properties of lung phagocytes, which would prevent infection. For TB contacts and LTBI, we host strengthening preventative strategies, including vitamin supplementation, NAC, and BCG re-vaccination. | Applicability of HDTs to MDR-TB, TB treatment shortening, TB/HIV, and TB-derived lung diseases, although highlighted in some studies, have not been considered for all HDTs. | [37] |
| Schwalfenberg. (2021) | Review the clinical usefulness of NAC as treatment or adjunctive therapy in a number of medical conditions. | NAC 1200 mg/day | NAC appears to be well tolerated with minimal side effects when used as a supplement or in treatment of various disorders. | [38] |
| Tenório (2021) | Overview of the medicinal effects and applications of NAC to human health based on current therapeutic evidence. | NAC 1200 mg/day | There is a need to clarifying adequate dosages and treatment protocols. | [39] |
| Risk Ratio | Risk Ratio | ||||
|---|---|---|---|---|---|
| Study or Subgroup | Log [Risk Ratio] | SE | Weight | IV, Random, 95% CI | IV, Random, 95% CI |
| Mhakalkar (2017) [17] | 0.086 | 0.06 | 97.3% | 1.09 [0.97, 1.23] | 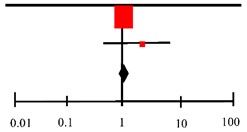 |
| Safe (2020) [19] | 0.3 | 0.36 | 2.7% | 1.35 [0.67, 2.73] | |
| Total (95%CI) | 100.0% | 1.10 [0.98, 1.23] | |||
| Heterogeneity: Tau2 = 0.00; Chi2 = 0.34, df = 1 (p < 0.56); I2 = 0% | |||||
| Test for overall effect: Z = 1.55 (p = 0.12) | Favors [Experimental] Favors [Control] | ||||
| Risk Difference | Risk Difference | ||||
|---|---|---|---|---|---|
| Study or Subgroup | Risk Difference | SE | Weight | IV, Random, 95% CI | IV, Random, 95% CI |
| Baniasadi (2010) [29] | −0.38 | 0.08 | 34.8% | −0.38 [−0.54–0.22] |  |
| Moosa (2020) [30] | 0.14 | 0.09 | 34.2% | 0.14 [−0.04, 0.32] | |
| Safe (2020) [31] | 0.16 | 0.14 | 31.0% | 0.16 [−0.11, 0.43] | |
| Total (95% CI) | 100.0% | −0.03 [−0.42, 0.35] | |||
| Heterogeneity: Tau 2 = 0.10; Chi2 = 22.81, df = 2 (p < 0.0001); I2 = 91% | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mapamba, D.A.; Sauli, E.; Mrema, L.; Lalashowi, J.; Magombola, D.; Buza, J.; Olomi, W.; Wallis, R.S.; Ntinginya, N.E. Impact of N-Acetyl Cysteine (NAC) on Tuberculosis (TB) Patients—A Systematic Review. Antioxidants 2022, 11, 2298. https://doi.org/10.3390/antiox11112298
Mapamba DA, Sauli E, Mrema L, Lalashowi J, Magombola D, Buza J, Olomi W, Wallis RS, Ntinginya NE. Impact of N-Acetyl Cysteine (NAC) on Tuberculosis (TB) Patients—A Systematic Review. Antioxidants. 2022; 11(11):2298. https://doi.org/10.3390/antiox11112298
Chicago/Turabian StyleMapamba, Daniel Adon, Elingarami Sauli, Lucy Mrema, Julieth Lalashowi, David Magombola, Joram Buza, Willyhelmina Olomi, Robert S. Wallis, and Nyanda Elias Ntinginya. 2022. "Impact of N-Acetyl Cysteine (NAC) on Tuberculosis (TB) Patients—A Systematic Review" Antioxidants 11, no. 11: 2298. https://doi.org/10.3390/antiox11112298
APA StyleMapamba, D. A., Sauli, E., Mrema, L., Lalashowi, J., Magombola, D., Buza, J., Olomi, W., Wallis, R. S., & Ntinginya, N. E. (2022). Impact of N-Acetyl Cysteine (NAC) on Tuberculosis (TB) Patients—A Systematic Review. Antioxidants, 11(11), 2298. https://doi.org/10.3390/antiox11112298








