Gold Nanoparticles Supported on Ceria Nanoparticles Modulate Leukocyte–Endothelium Cell Interactions and Inflammation in Type 2 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Sample Collection
2.2. Biochemical Parameters
2.3. Leukocyte Isolation
2.4. Isolation of Human Endothelial Cells
2.5. Materials and Instrumentation
2.6. Evaluation of Leukocyte–Endothelium Interactions
2.7. Au/CeO2 Catalyst Preparation
2.8. Static Leukocyte–Endothelial Interaction Assay
2.9. Cellular Viability and Proliferation
2.10. Evaluation of Apoptosis
2.11. Measurement of Total ROS Production
2.12. Western Blotting
2.13. Statistical Analysis
3. Results and Discussion
3.1. Clinical and Endocrine Characteristics of the Study Subjects
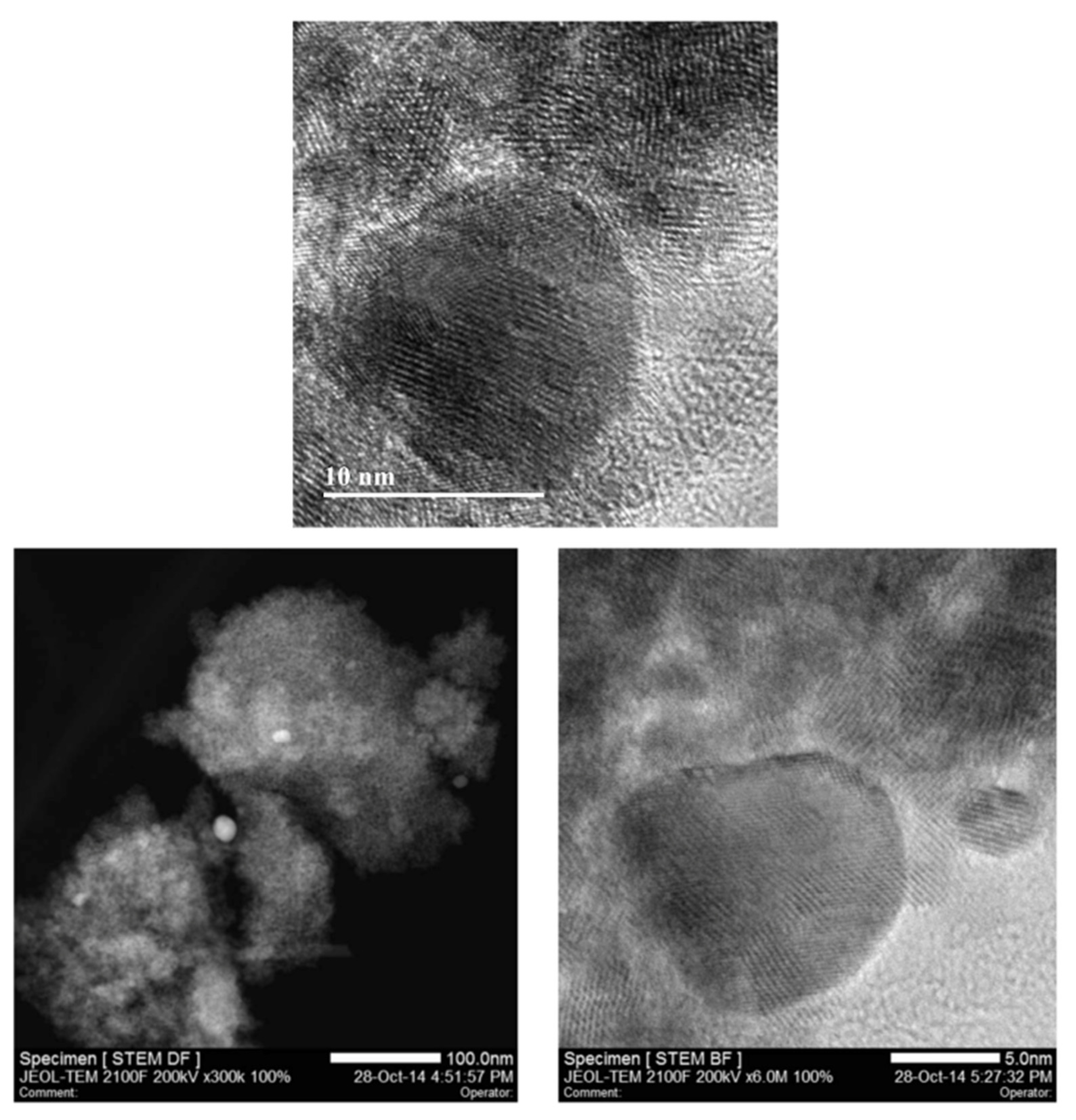
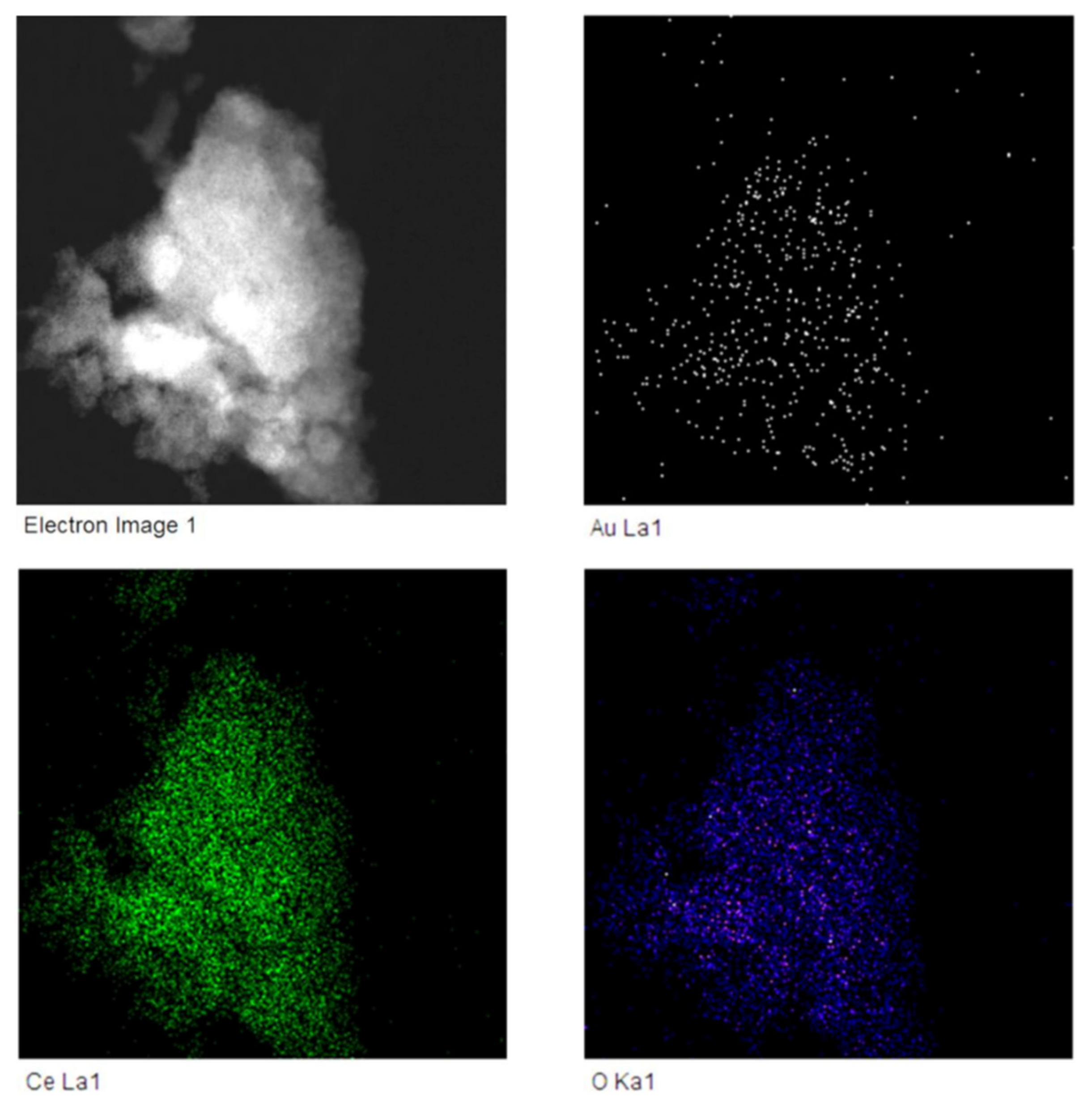
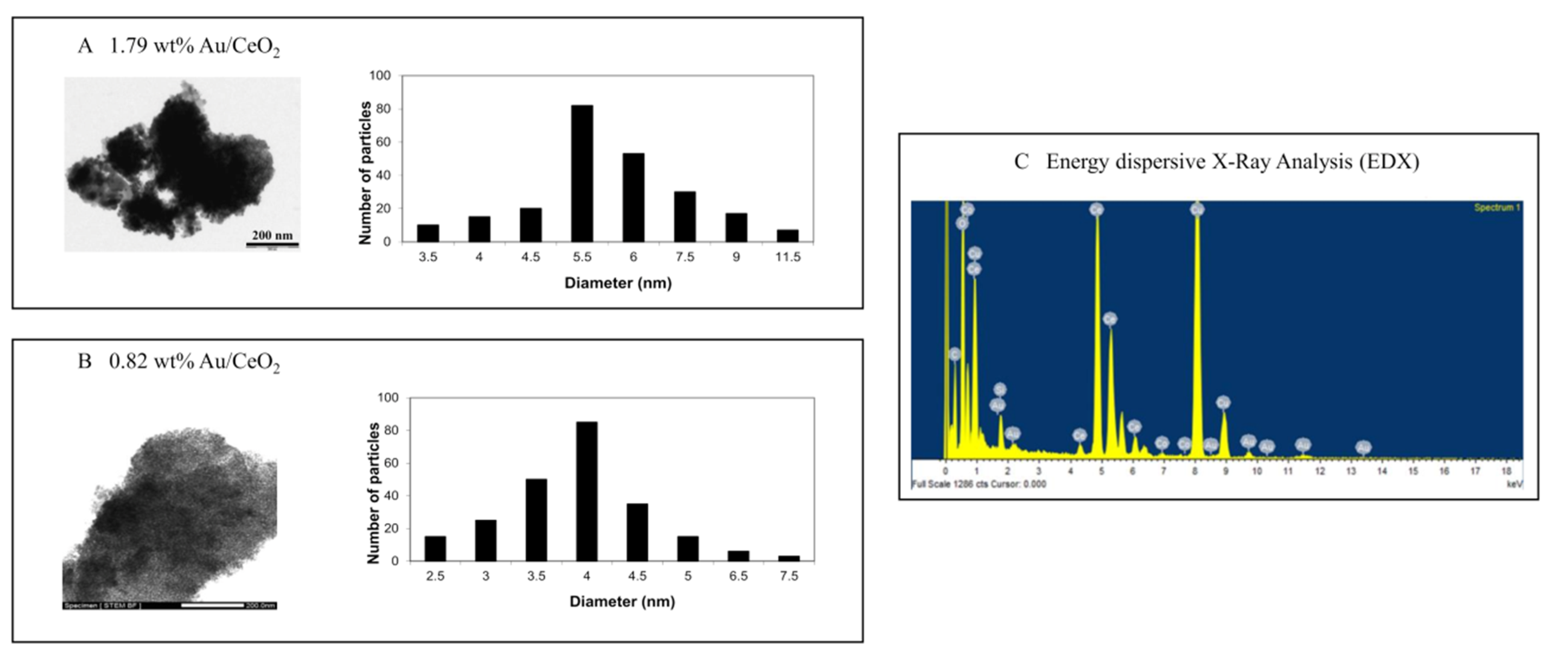
3.2. Characterisation of Nanoparticles
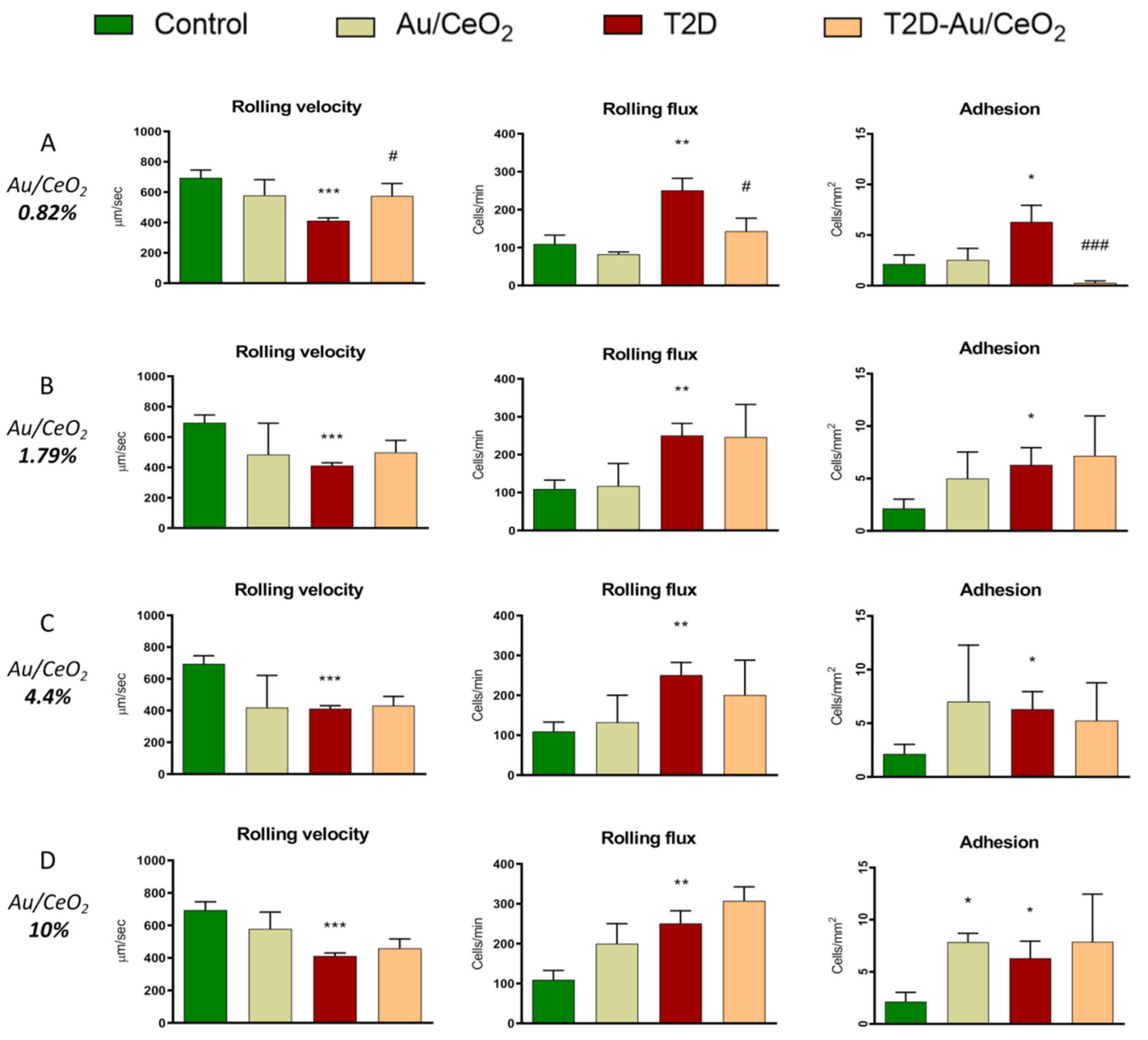
3.3. Leukocyte–Endothelium Interactions
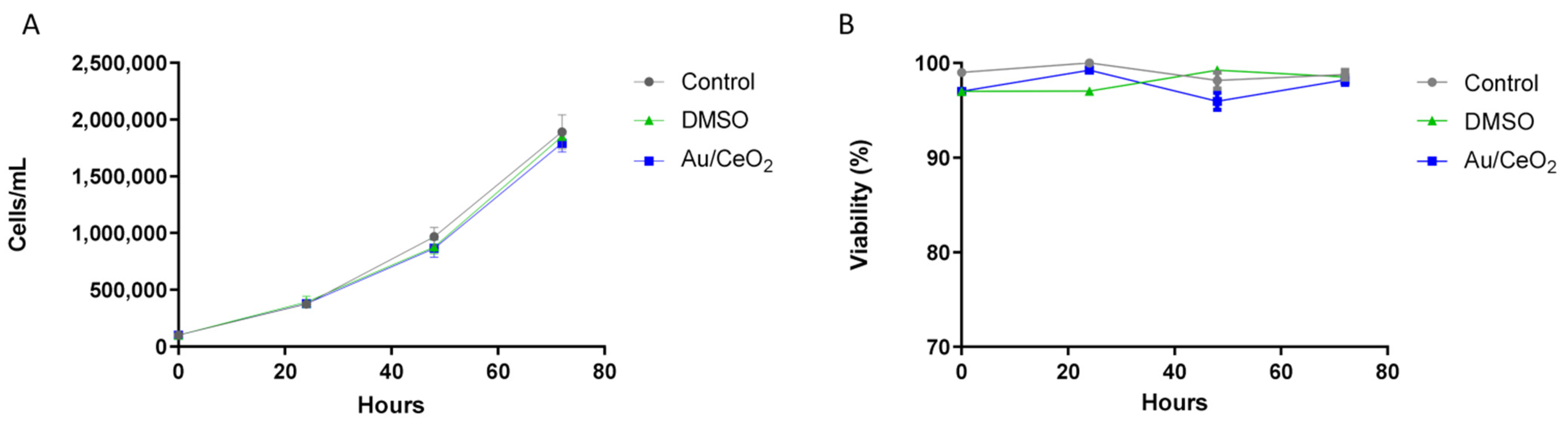
3.4. Biocompatibility of Au/CeO2
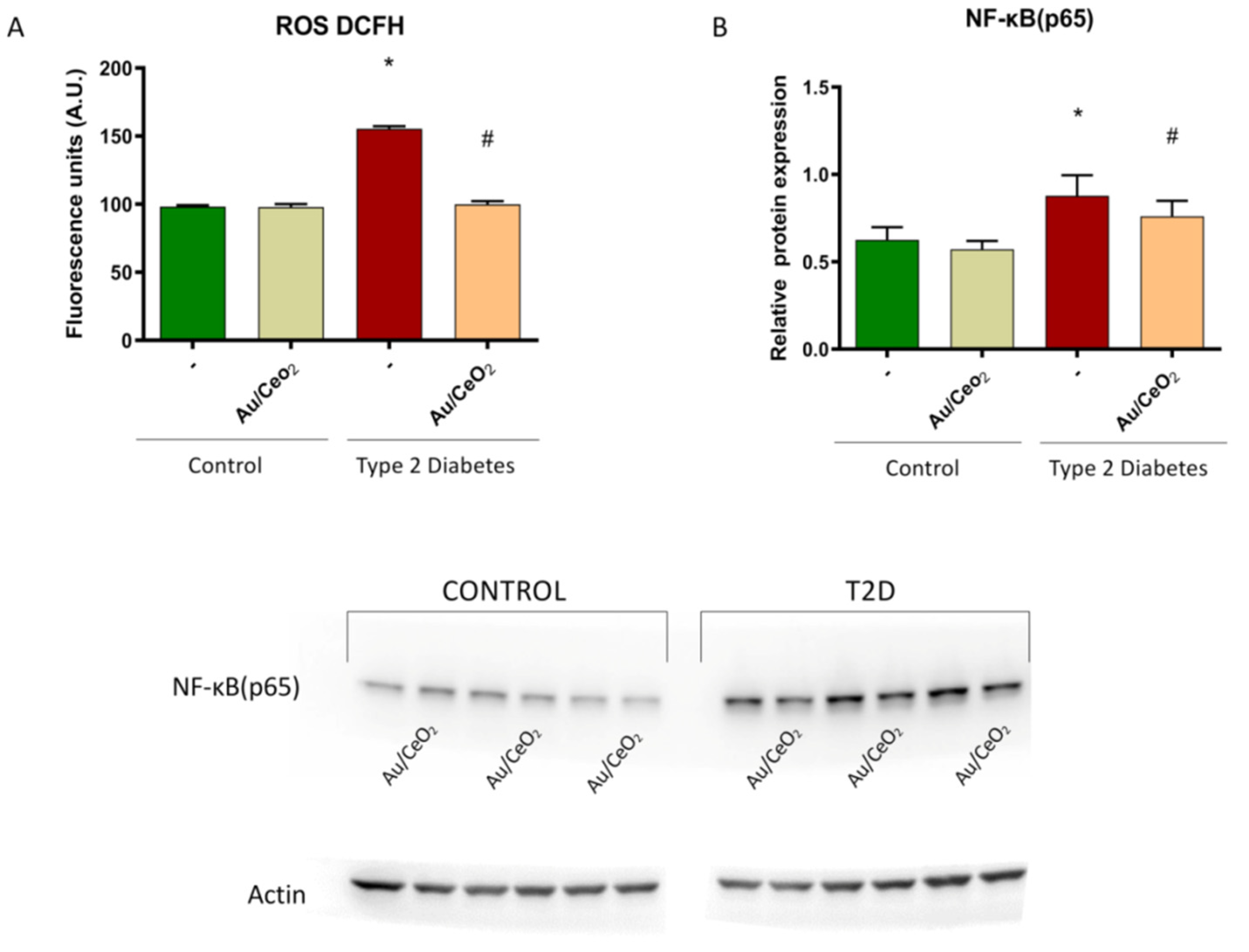
3.5. ROS Production and Levels of NF-κB (p65)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zimmet, P.; Alberti, K.G.M.M.; Shaw, J. Global and societal implications of the diabetes epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef]
- Engelmann, J.; Manuwald, U.; Rubach, C.; Kugler, J.; Birkenfeld, A.L.; Hanefeld, M.; Rothe, U. Determinants of mortality in patients with type 2 diabetes: A review. Rev. Endocr. Metab. Disord. 2016, 17, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Katsiki, N.; Purrello, F.; Tsioufis, C.; Mikhailidis, D.P. Cardiovascular disease prevention strategies for type 2 diabetes mellitus. Expert Opin. Pharmacother. 2017, 18, 1243–1260. [Google Scholar] [CrossRef] [PubMed]
- Szendroedi, J.; Phielix, E.; Roden, M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2011, 8, 92–103. [Google Scholar] [CrossRef]
- Yazıcı, D.; Sezer, H. Insulin Resistance, Obesity and Lipotoxicity. Adv. Exp. Med. Biol. 2017, 960, 277–304. [Google Scholar] [CrossRef]
- Rocha, M.; Apostolova, N.; Diaz-Rua, R.; Muntane, J.; Victor, V.M. Mitochondria and T2D: Role of Autophagy, ER Stress, and Inflammasome. Trends Endocrinol. Metab. 2020, 31, 725–741. [Google Scholar] [CrossRef]
- Barzilay, J.I.; Abraham, L.; Heckbert, S.R.; Cushman, M.; Kuller, L.H.; Resnick, H.E.; Tracy, R.P. The Relation of Markers of Inflammation to the Development of Glucose Disorders in the Elderly. Diabetes 2001, 50, 2384–2389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, B.B.; Schmidt, M.I.; Pankow, J.S.; Ballantyne, C.M.; Couper, D.; Vigo, A.; Hoogeveen, R.; Folsom, A.R.; Heiss, G. Low-Grade Systemic Inflammation and the Development of Type 2 Diabetes. Diabetes 2003, 52, 1799–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, B.; Hu, X.; Zhang, H.; Huang, J.; Liu, J.; Hu, J.; Li, W.; Huang, L. Nuclear NF-κB p65 in Peripheral Blood Mononuclear Cells Correlates with Urinary MCP-1, RANTES and the Severity of Type 2 Diabetic Nephropathy. PLoS ONE 2014, 9, e99633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tousoulis, D.; Papageorgiou, N.; Androulakis, E.; Siasos, G.; Latsios, G.; Tentolouris, K.; Stefanadis, C. Diabetes mellitus-associated vascular impairment: Novel circulating biomarkers and therapeutic approaches. J. Am. Coll. Cardiol. 2013, 62, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Fan, C.-Q.; Dong, H.; Wang, S.-M.; Yang, X.-C.; Yang, S.-M. Current applications and future prospects of nanomaterials in tumor therapy. Int. J. Nanomed. 2017, 12, 1815–1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín, R.; Menchón, C.; Apostolova, N.; Víctor, V.M.; Álvaro, M.; Herance, J.R.; García, H. Nano-Jewels in Biology. Gold and Platinum on Diamond Nanoparticles as Antioxidant Systems Against Cellular Oxidative Stress. ACS Nano 2010, 4, 6957–6965. [Google Scholar] [CrossRef]
- Diab, M.S.; Dkhil, M.A.; Bauomy, A.A.; Al-Quraishy, S. Antioxidant and hepatoprotective role of gold nanoparticles against murine hepatic schistosomiasis. Int. J. Nanomed. 2015, 10, 7467–7475. [Google Scholar] [CrossRef] [Green Version]
- Charbgoo, F.; Bin Ahmad, M.; Darroudi, M. Cerium oxide nanoparticles: Green synthesis and biological applications. Int. J. Nanomed. 2017, 12, 1401–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esch, F.; Fabris, S.; Zhou, L.; Montini, T.; Africh, C.; Fornasiero, P.; Comelli, G.; Rosei, R. Electron Localization Determines Defect Formation on Ceria Substrates. Science 2005, 309, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Lazar, S.; Freitag, B.; Egoavil, R.; Verbeeck, J.; Put, S.; Strauven, Y.; Van Tendeloo, G. High resolution mapping of surface reduction in ceria nanoparticles. Nanoscale 2011, 3, 3385–3390. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.C.; Johnson, M.E.; Walker, M.L.; Riley, K.R.; Sims, C.M. Antioxidant Cerium Oxide Nanoparticles in Biology and Medicine. Antioxidants 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herance, J.R.; García, H.; Gutiérrez-Carcedo, P.; Navalón, S.; Pineda-Lucena, A.; Palomino-Schätzlein, M. A translational approach to assess the metabolomic impact of stabilized gold nanoparticles by NMR spectroscopy. Analyst 2018, 144, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, S.; Vallabani, N.V.S.; Shutthanandan, V.; Bowden, M.; Karakoti, A.S.; Singh, S. Gold core/ceria shell-based redox active nanozyme mimicking the biological multienzyme complex phenomenon. J. Colloid Interface Sci. 2018, 513, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C.W. Determining the Size and Shape Dependence of Gold Nanoparticle Uptake into Mammalian Cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef]
- Menchón, C.; Martín, R.; Apostolova, N.; Victor, V.M.; Álvaro, M.; Herance, J.R.; García, H. Gold Nanoparticles Supported on Nanoparticulate Ceria as a Powerful Agent against Intracellular Oxidative Stress. Small 2012, 8, 1895–1903. [Google Scholar] [CrossRef]
- Versiani, A.F.; Andrade, L.M.; Martins, E.M.; Scalzo, S.; Geraldo, J.M.; Chaves, C.R.; Ferreira, D.C.; Ladeira, M.; Guatimosim, S.; Ladeira, L.O.; et al. Gold nanoparticles and their applications in biomedicine. Future Virol. 2016, 11, 293–309. [Google Scholar] [CrossRef]
- Mahmoudi, F.; Mahmoudi, F.; Gollo, K.H.; Amini, M.M. Novel Gold Nanoparticles: Green Synthesis with Eryngium thyrsoideum Boiss Extract, Characterization, and In Vivo Investigations on Inflammatory Gene Expression and Biochemical Parameters in Type 2 Diabetic Rats. Biol. Trace Element Res. 2021, 200, 2223–2232. [Google Scholar] [CrossRef]
- Ponnanikajamideen, M.; Rajeshkumar, S.; Vanaja, M.; Annadurai, G. In Vivo Type 2 Diabetes and Wound-Healing Effects of Antioxidant Gold Nanoparticles Synthesized Using the Insulin Plant Chamaecostus cuspidatus in Albino Rats. Can. J. Diabetes 2019, 43, 82–89.e6. [Google Scholar] [CrossRef] [PubMed]
- Alenazi, F.; Saleem, M.; Khaja, A.S.S.; Zafar, M.; Alharbi, M.S.; Al Hagbani, T.; Ashraf, J.M.; Qamar, M.; Rafi, Z.; Ahmad, S. Metformin encapsulated gold nanoparticles (MTF-GNPs): A promising antiglycation agent. Cell Biochem. Funct. 2022. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Wang, C.H. ER stress in adipocytes and insulin resistance: Mechanisms and significance (Review). Mol. Med. Rep. 2014, 10, 2234–2240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozcan, U.; Cao, Q.; Yilmaz, E.; Lee, A.-H.; Iwakoshi, N.N.; Özdelen, E.; Tuncman, G.; Görgün, C.; Glimcher, L.H.; Hotamisligil, G.S. Endoplasmic Reticulum Stress Links Obesity, Insulin Action, and Type 2 Diabetes. Science 2004, 306, 457–461. [Google Scholar] [CrossRef] [Green Version]
- Yakimovich, N.O.; Ezhevskii, A.A.; Guseinov, D.V.; Smirnova, L.A.; Gracheva, T.A.; Klychkov, K.S. Antioxidant properties of gold nanoparticles studied by ESR spectroscopy. Bull. Acad. Sci. USSR Div. Chem. Sci. 2008, 57, 520–523. [Google Scholar] [CrossRef]
- Barath ManiKanth, S.; Kalishwaralal, K.; Sriram, M.; Pandian, S.R.K.; Youn, H.-S.; Eom, S.; Gurunathan, S. Anti-oxidant effect of gold nanoparticles restrains hyperglycemic conditions in diabetic mice. J. Nanobiotechnol. 2010, 8, 16. [Google Scholar] [CrossRef]
- Hernandez-Mijares, A.; Rocha, M.; Rovira-Llopis, S.; Bañuls, C.; Bellod, L.; de Pablo, C.; Alvarez, A.; Roldan-Torres, I.; Sola-Izquierdo, E.; Victor, V.M. Human Leukocyte/Endothelial Cell Interactions and Mitochondrial Dysfunction in Type 2 Diabetic Patients and Their Association With Silent Myocardial Ischemia. Diabetes Care 2013, 36, 1695–1702. [Google Scholar] [CrossRef] [Green Version]
- Kiely, J.-M.; Luscinskas, F.W.; Gimbrone, M.A., Jr. Leukocyte-Endothelial Monolayer Adhesion Assay (Static Conditions). Methods Mol. Biol. 1999, 96, 131–136. [Google Scholar] [CrossRef]
- Niu, J.; Azfer, A.; Wang, K.; Wang, X.; Kolattukudy, P.E. Cardiac-Targeted Expression of Soluble Fas Attenuates Doxorubicin-Induced Cardiotoxicity in Mice. J. Pharmacol. Exp. Ther. 2008, 328, 740–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakhlband, A.; Eskandani, M.; Omidi, Y.; Saeedi, N.; Ghaffari, S.; Barar, J.; Garjani, A. Combating atherosclerosis with targeted nanomedicines: Recent advances and future prospective. BioImpacts 2018, 8, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Varlamova, E.G.; Gudkov, S.V.; Plotnikov, E.Y.; Turovsky, E.A. Size-Dependent Cytoprotective Effects of Selenium Nanoparticles during Oxygen-Glucose Deprivation in Brain Cortical Cells. Int. J. Mol. Sci. 2022, 23, 7464. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-H.; Babu, K.S.; Anandkumar, M.; Tsai, T.; Kao, T.; Inbaraj, B.S. Cytotoxicity and antibacterial activity of gold-supported cerium oxide nanoparticles. Int. J. Nanomed. 2014, 9, 5515–5531. [Google Scholar] [CrossRef] [Green Version]
- Hirst, S.M.; Karakoti, A.S.; Tyler, R.D.; Sriranganathan, N.; Seal, S.; Reilly, C.M. Anti-inflammatory Properties of Cerium Oxide Nanoparticles. Small 2009, 5, 2848–2856. [Google Scholar] [CrossRef]
- Gojova, A.; Lee, J.-T.; Jung, H.S.; Guo, B.; Barakat, A.I.; Kennedy, I.M. Effect of cerium oxide nanoparticles on inflammation in vascular endothelial cells. Inhal. Toxicol. 2009, 21 (Suppl. S1), 123–130. [Google Scholar] [CrossRef] [Green Version]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [Green Version]
- Contreras, C.; Sanchez, A. Endothelial Dysfunction, Obesity and Insulin Resistance. Curr. Vasc. Pharmacol. 2014, 12, 412–426. [Google Scholar] [CrossRef]
- Gutiérrez-Carcedo, P.; Navalón, S.; Simó, R.; Setoain, X.; Aparicio-Gómez, C.; Abasolo, I.; Victor, V.M.; García, H.; Herance, J.R. Alteration of the Mitochondrial Effects of Ceria Nanoparticles by Gold: An Approach for the Mitochondrial Modulation of Cells Based on Nanomedicine. Nanomaterials 2020, 10, 744. [Google Scholar] [CrossRef] [PubMed]
- Mezzano, S.; Aros, C.; Droguett, A.; Burgos, M.E.; Ardiles, L.; Flores, C.; Schneider, H.; Ruiz-Ortega, M.; Egido, J. NF-B activation and overexpression of regulated genes in human diabetic nephropathy. Nephrol. Dial. Transplant. 2004, 19, 2505–2512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajpai, A.; Tilley, D.G. The Role of Leukocytes in Diabetic Cardiomyopathy. Front. Physiol. 2018, 9, 1547. [Google Scholar] [CrossRef]
- Al-Shwaheen, A.; Aljabali, A.A.; Alomari, G.; Al Zoubi, M.; Alshaer, W.; Al-Trad, B.; Tambuwala, M.M. Molecular and cellular effects of gold nanoparticles treatment in experimental diabetic myopathy. Heliyon 2022, 8, e10358. [Google Scholar] [CrossRef]
- Escribano-Lopez, I.; Diaz-Morales, N.; Rovira-Llopis, S.; de Marañon, A.M.; Orden, S.; Alvarez, A.; Bañuls, C.; Rocha, M.; Murphy, M.P.; Hernandez-Mijares, A.; et al. The mitochondria-targeted antioxidant MitoQ modulates oxidative stress, inflammation and leukocyte-endothelium interactions in leukocytes isolated from type 2 diabetic patients. Redox Biol. 2016, 10, 200–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.; Rashid, A.; Jang, M.; Kim, Y.; Won, H.; Lee, J.; Woo, J.-T.; Kim, Y.S.; Murphy, M.P.; Ali, L.; et al. Mitochondria-targeted Antioxidants Protect Pancreatic β-cells against Oxidative Stress and Improve Insulin Secretion in Glucotoxicity and Glucolipotoxicity. Cell. Physiol. Biochem. 2011, 28, 873–886. [Google Scholar] [CrossRef]



| Control | T2D | p-Value | BMI Adjusted p-Value | |
|---|---|---|---|---|
| n | 57 | 51 | ||
| Age (years) | 53.7 ± 7.7 | 57.10 ± 10.3 | NS | |
| Weight (kg) | 72.8 ± 18.0 | 84.7 ± 16.4 | <0.001 | |
| BMI (kg/m2) | 25.7 ± 4.14 | 30.6 ± 5.48 | <0.001 | |
| Waist (cm) | 87.7 ± 12.7 | 103.5± 11.7 | <0.001 | |
| Glucose (mg/dL) | 94.1 ± 9.4 | 153.7± 45.0 *** | <0.001 | <0.001 |
| Insulin (μL/mL) | 7.19± 2.62 | 25.9± 15.7 *** | <0.001 | <0.001 |
| HOMA-IR | 1.67 ± 0.70 | 9.74 ± 6.87 *** | <0.001 | <0.001 |
| HbA1c (%) | 5.31 ± 0.34 | 7.14 ± 1.16 *** | <0.001 | <0.001 |
| TC (mg/dL) | 203.7 ± 34.5 | 158.7 ± 39.1 *** | <0.001 | <0.001 |
| HDL-c (mg/dL) | 57.2 ± 19.7 | 43.5 ± 9.85 ** | <0.001 | <0.003 |
| LDL-c (mg/dL) | 126.5 ± 29.5 | 87.0 ± 31.7 *** | <0.001 | <0.001 |
| TG (mg/dL) | 90.5 (62.8–150.5) | 132 (92.8–164.75) ** | <0.009 | NS |
| Hs-CRP (mg/L) | 1.17 (0.44–2.17) | 2.45 (1.22–5.39) | <0.001 | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Pozo, P.; Canet, F.; Grirrane, A.; Lopez-Domenech, S.; Herance, J.R.; Apostolova, N.; Luna-Marco, C.; Rovira-Llopis, S.; Marti, M.; Morillas, C.; et al. Gold Nanoparticles Supported on Ceria Nanoparticles Modulate Leukocyte–Endothelium Cell Interactions and Inflammation in Type 2 Diabetes. Antioxidants 2022, 11, 2297. https://doi.org/10.3390/antiox11112297
Díaz-Pozo P, Canet F, Grirrane A, Lopez-Domenech S, Herance JR, Apostolova N, Luna-Marco C, Rovira-Llopis S, Marti M, Morillas C, et al. Gold Nanoparticles Supported on Ceria Nanoparticles Modulate Leukocyte–Endothelium Cell Interactions and Inflammation in Type 2 Diabetes. Antioxidants. 2022; 11(11):2297. https://doi.org/10.3390/antiox11112297
Chicago/Turabian StyleDíaz-Pozo, Pedro, Francisco Canet, Abdessamad Grirrane, Sandra Lopez-Domenech, José Raul Herance, Nadezda Apostolova, Clara Luna-Marco, Susana Rovira-Llopis, Miguel Marti, Carlos Morillas, and et al. 2022. "Gold Nanoparticles Supported on Ceria Nanoparticles Modulate Leukocyte–Endothelium Cell Interactions and Inflammation in Type 2 Diabetes" Antioxidants 11, no. 11: 2297. https://doi.org/10.3390/antiox11112297
APA StyleDíaz-Pozo, P., Canet, F., Grirrane, A., Lopez-Domenech, S., Herance, J. R., Apostolova, N., Luna-Marco, C., Rovira-Llopis, S., Marti, M., Morillas, C., Rocha, M., Garcia, H., & Victor, V. M. (2022). Gold Nanoparticles Supported on Ceria Nanoparticles Modulate Leukocyte–Endothelium Cell Interactions and Inflammation in Type 2 Diabetes. Antioxidants, 11(11), 2297. https://doi.org/10.3390/antiox11112297











