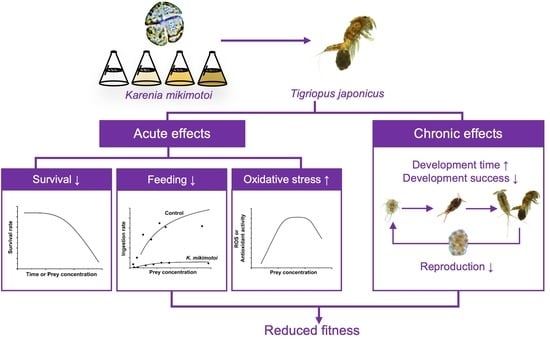
Reduced Fitness and Elevated Oxidative Stress in the Marine Copepod Tigriopus japonicus Exposed to the Toxic Dinoflagellate Karenia mikimotoi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal and Copepod Cultures
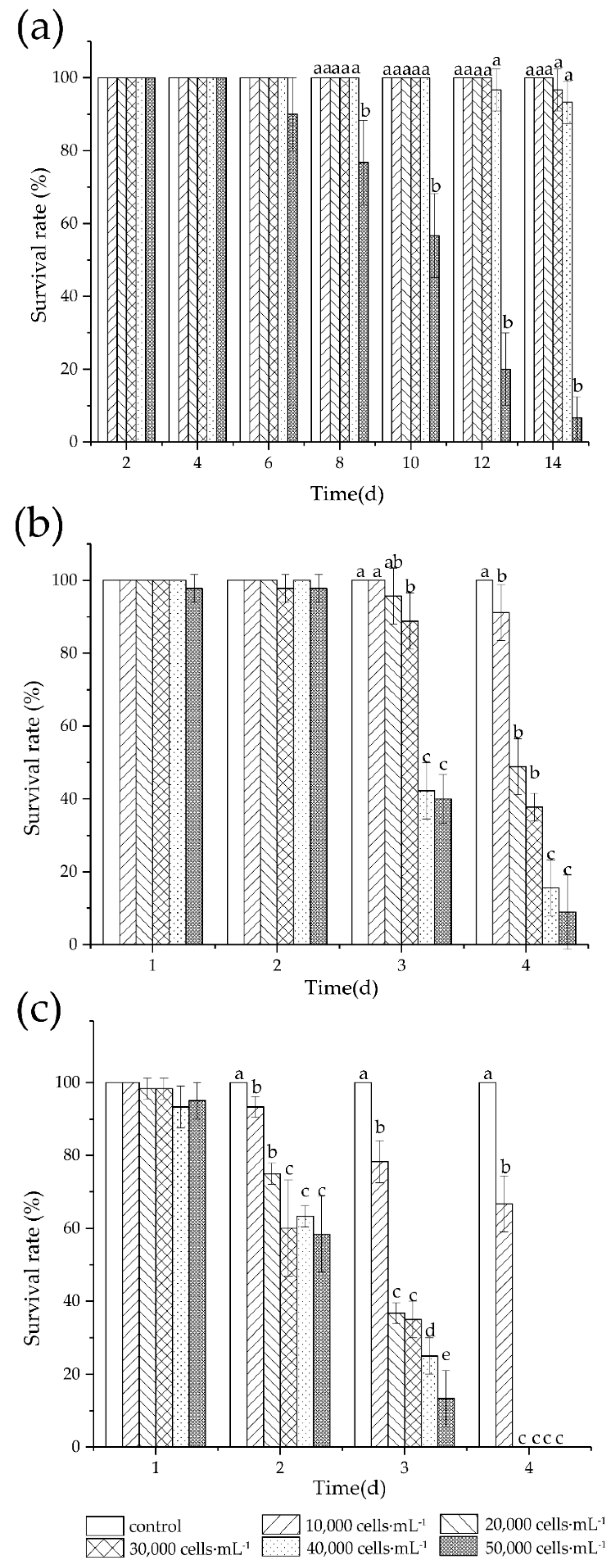
2.2. Survival
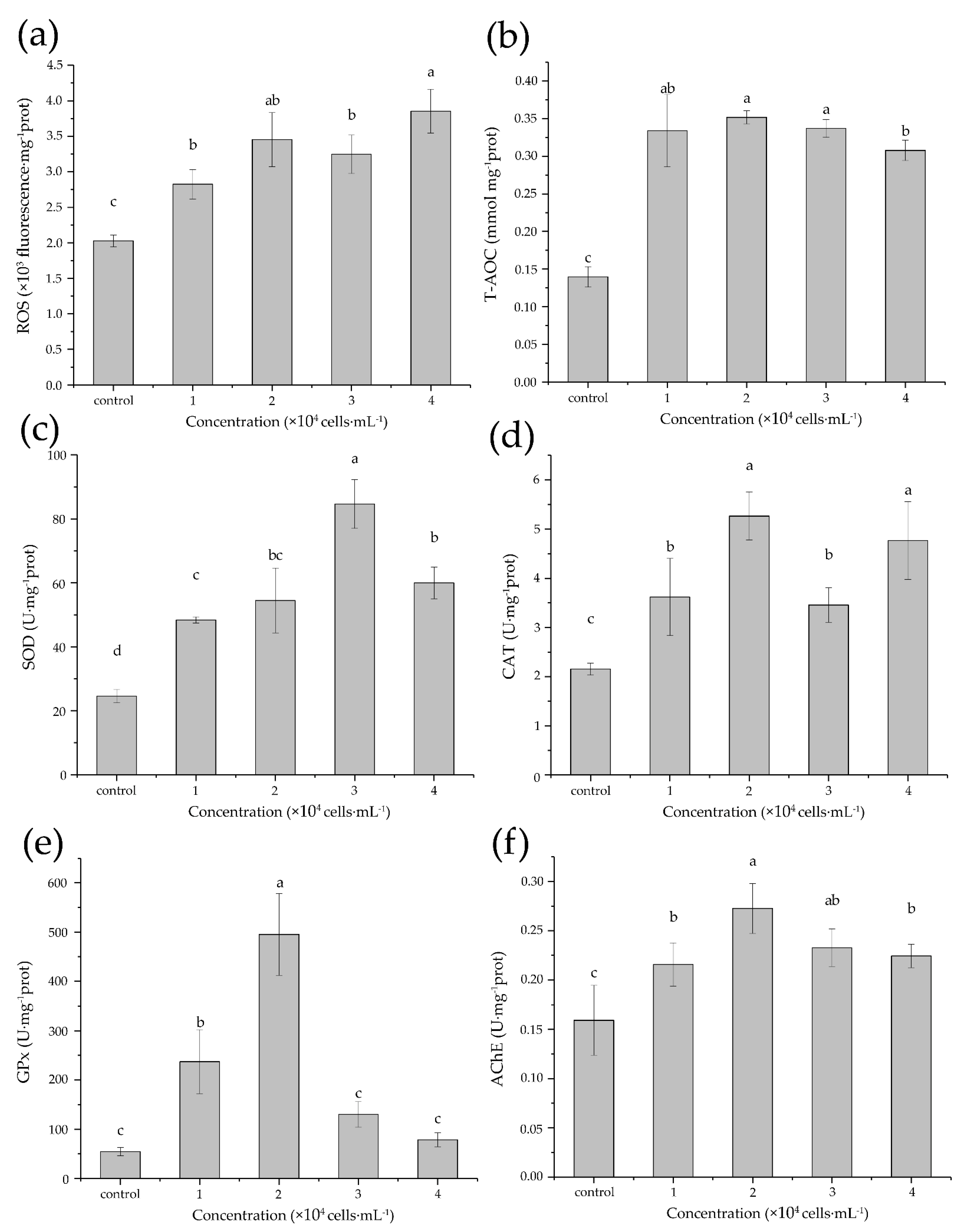
2.3. Biochemical Assays
2.4. Feeding
2.5. Chronic Test
2.6. Statistical Analysis
3. Results
3.1. Survival
3.2. Feeding
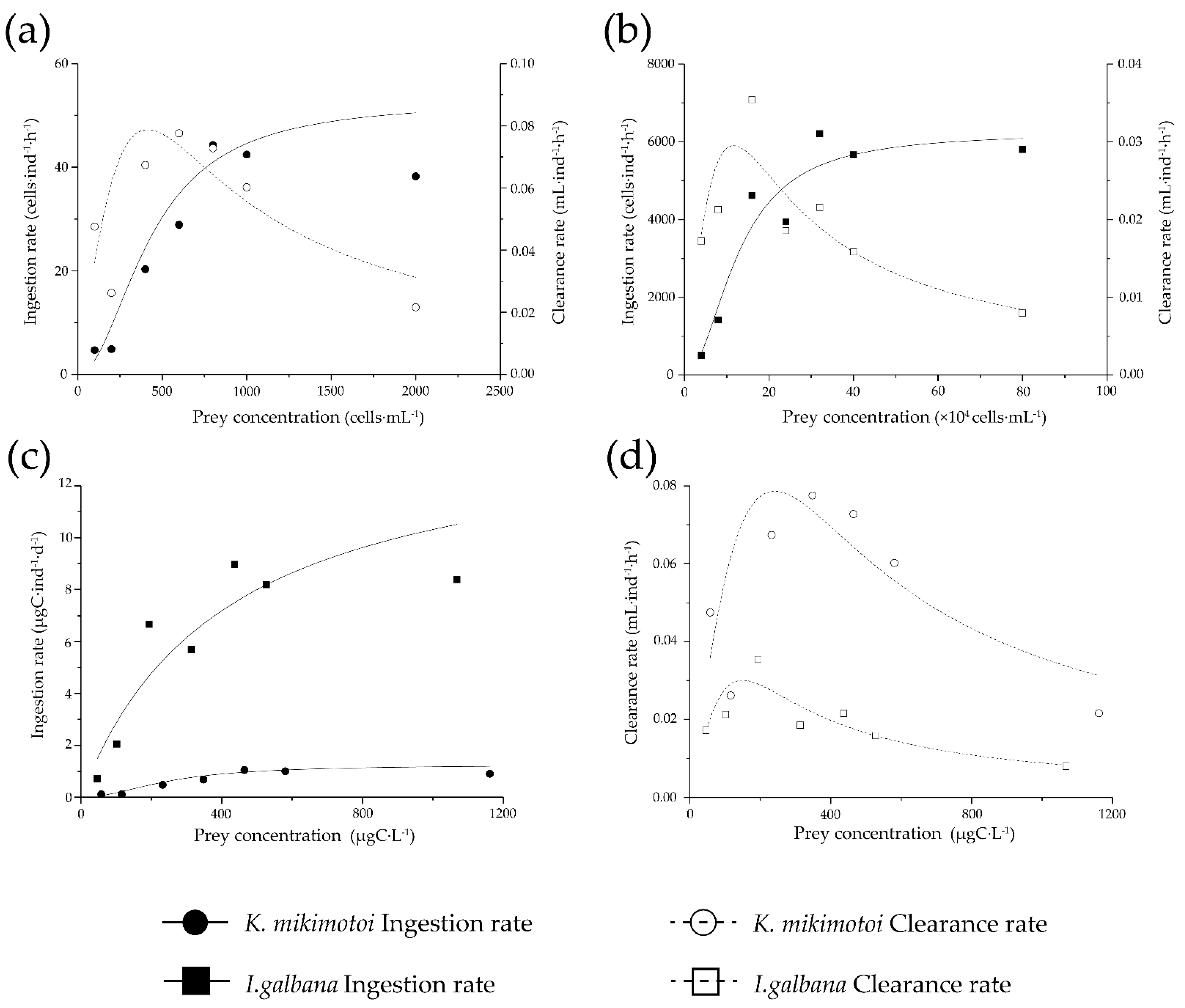
| Clearance Rate | Ingestion Rate | |||||||
|---|---|---|---|---|---|---|---|---|
| Carbon Based | Cell Based | |||||||
| Prey Species | Fmax (mL·ind−1·h−1) | R2 | Imax (ìgC·ind−1·d−1) | Km (ìgC·L−1) | R2 | Imax (cell·ind−1·d−1) | Km (mL·L−1) | R2 |
| K. mikimotoi | 0.078 | 0.79 | 1.25 | 250.32 | 0.94 | 52.85 | 430.95 | 0.94 |
| I. galbana | 0.035 a | 0.95 | 8.92 | 155.08 | 0.99 | 6240.17 | 127,351.46 | 0.99 |
3.3. Acute Biochemical Responses
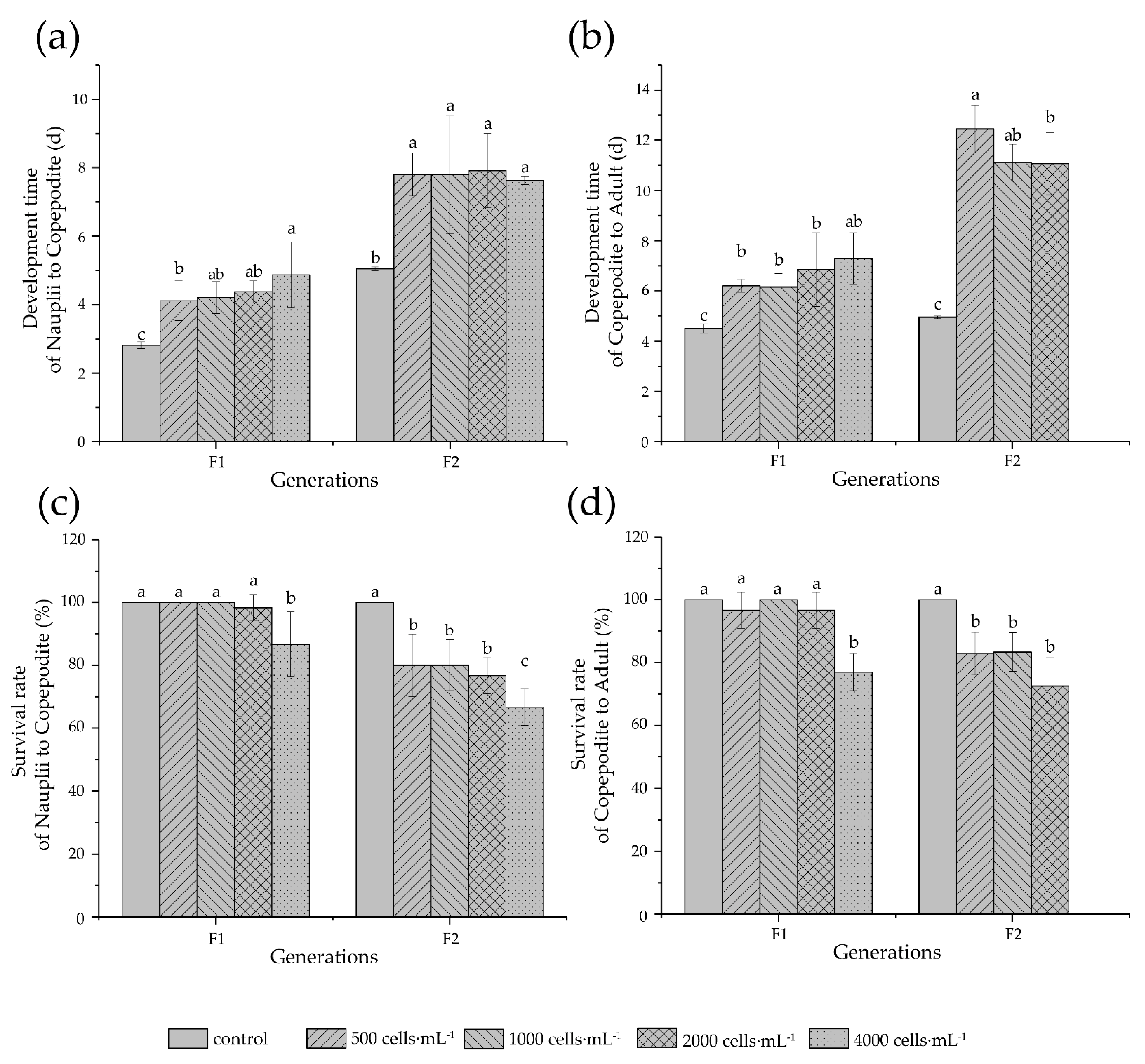
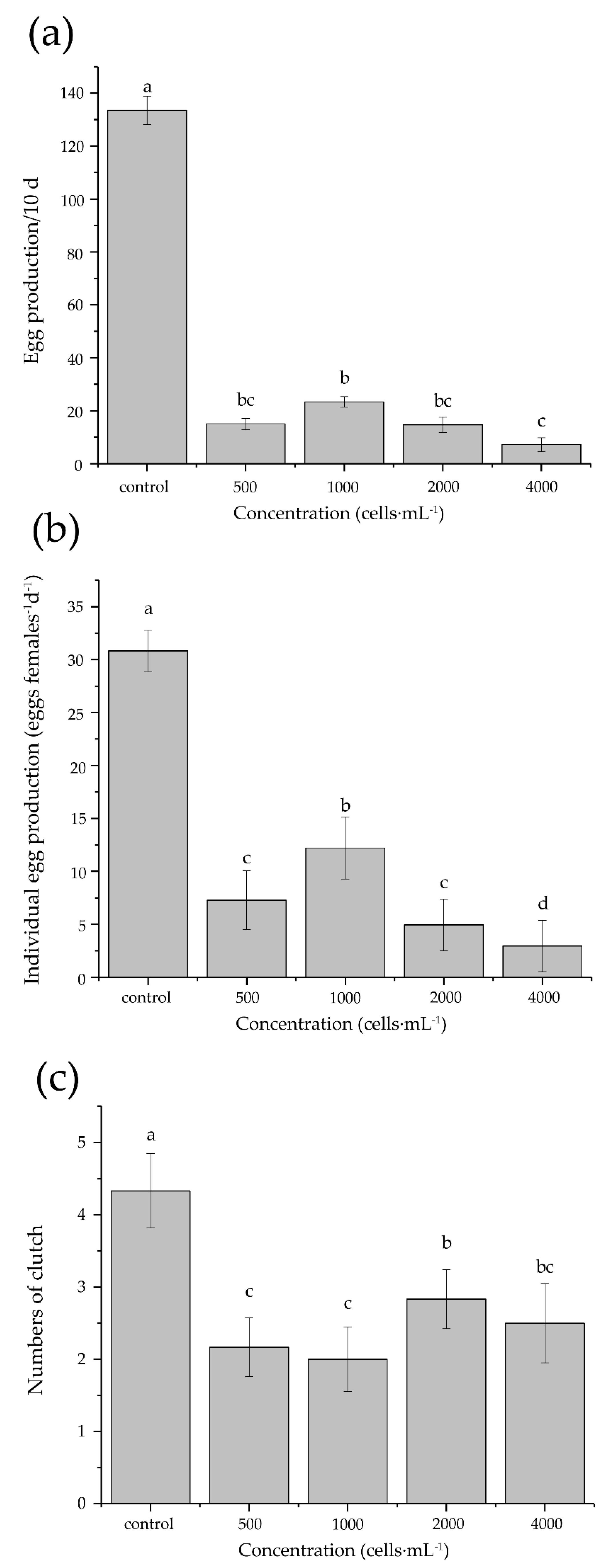
3.4. Development and Reproduction
4. Discussion
4.1. Survival
4.2. Feeding
4.3. Oxidative Stress
4.4. Development and Reproduction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matsuyama, Y.; Shumway, S. Impacts of Harmful Algal Blooms on Shellfisheries Aquaculture; Woodhead Publishing: Sawston, UK, 2009; pp. 580–609. [Google Scholar]
- Landsberg, J.H. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 2002, 10, 113–390. [Google Scholar] [CrossRef]
- Smayda, T.J. Harmful algal blooms: Their ecophysiology and general relevance to phytoplankton blooms in the sea. Limnol. Oceanogr. 1997, 42, 1137–1153. [Google Scholar] [CrossRef]
- Li, X.; Yan, T.; Yu, R.; Zhou, M. A review of Karenia mikimotoi: Bloom events, physiology, toxicity and toxic mechanism. Harmful Algae 2019, 90, 101702. [Google Scholar] [CrossRef]
- Gentien, P. Bloom dynamics and ecophysiology of the Gymnodinium mikimotoi species complex. In Physiological Ecology of Harmful Algal Blooms; Anderson, D.M., Cembella, A.D., Hallegraeff, G.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 155–173. [Google Scholar]
- Brand, L.E.; Campbell, L.; Bresnan, E. Karenia: The biology and ecology of a toxic genus. Harmful Algae 2012, 14, 156–178. [Google Scholar] [CrossRef]
- Mooney, B.D.; Nichols, P.D.; Salas, M.F.D.; Hallegraeff, G.M. Lipid, fatty acid, and sterol composition of eight species of Kareniaceae (Dinophyta): Chemotaxonomy and putative lipid phycotoxins. J. Phycol. 2007, 43, 101–111. [Google Scholar] [CrossRef]
- Neely, T.; Campbell, L. A modified assay to determine hemolytic toxin variability among Karenia clones isolated from the Gulf of Mexico. Harmful Algae 2006, 5, 592–598. [Google Scholar] [CrossRef]
- Satake, M.; Shoji, M.; Oshima, Y.; Naoki, H.; Fujita, T.; Yasumoto, T. Gymnocin-A, a cytotoxic polyether from the notorious red tide dinoflagellate, Gymnodinium mikimotoi. Tetrahedron Lett. 2002, 43, 5829–5832. [Google Scholar] [CrossRef]
- Satake, M.; Tanaka, Y.; Ishikura, Y.; Oshima, Y.; Naoki, H.; Yasumoto, T. Gymnocin-B with the largest contiguous polyether rings from the red tide dinoflagellate, Karenia (formerly Gymnodinium) mikimotoi. Tetrahedron Lett. 2005, 46, 3537–3540. [Google Scholar] [CrossRef]
- Cho, K.; Ueno, M.; Liang, Y.; Kim, D.; Oda, T. Generation of Reactive Oxygen Species (ROS) by Harmful Algal Bloom (HAB)-Forming Phytoplankton and Their Potential Impact on Surrounding Living Organisms. Antioxidants 2022, 11, 206. [Google Scholar] [CrossRef]
- Kim, D.; Wencheng, L.; Matsuyama, Y.; Cho, K.; Yamasaki, Y.; Takeshita, S.; Yamaguchi, K.; Oda, T. Extremely high level of reactive oxygen species (ROS) production in a newly isolated strain of the dinoflagellate Karenia mikimotoi. Eur. J. Phycol. 2019, 54, 632–640. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Kim, D.I.; Matsuyama, Y.; Oda, T.; Honjo, T. Production of superoxide anion and hydrogen peroxide by the red tide dinoflagellate Karenia mikimotoi. J. Biosci. Bioeng. 2004, 97, 212–215. [Google Scholar] [CrossRef]
- Lin, J.; Yan, T.; Zhang, Q.; Wang, Y.; Liu, Q.; Zhou, M. Effects of Karenia mikimotoi blooms on antioxidant enzymes in gastropod abalone, Haliotis discus hannai. Mar. Sci. 2016, 40, 17–22. [Google Scholar]
- Niu, X.; Xu, S.; Yang, Q.; Xu, X.; Zheng, M.; Li, X.; Guan, W. Toxic effects of the dinoflagellate Karenia mikimotoi on zebrafish (Danio rerio) larval behavior. Harmful Algae 2021, 103, 101996. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.P.; Song, X.X.; Zhang, Y.; Zhu, J.A.; Shen, H.H.; Yu, Z.M. Assessing the effect of modified clay on the toxicity of Karenia mikimotoi using marine medaka (Oryzias melastigma) as a model organism. Toxics 2022, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.F.; Yan, T.; Qing-Chun, Z.; Xiao-Dong, L.I.; Zhou, M.J. Acute effect of four typical bloom forming algae on abalone Haliotis discus hannai and its antioxidant enzymes system. Mar. Environ. Sci. 2018, 37, 207–214. [Google Scholar]
- Zheng, J.; Mao, X.; Ye, M.; Li, H.; Liu, J.; Yang, W. Allelopathy and underlying mechanism of Karenia mikimotoi on the diatom Thalassiosira pseudonana under laboratory condition. Algal Res. 2021, 54, 102229. [Google Scholar] [CrossRef]
- Dang, L.X.; Li, Y.; Liu, F.; Zhang, Y.; Yang, W.D.; Li, H.Y.; Liu, J.S. Chemical response of the toxic dinoflagellate Karenia mikimotoi against grazing by three species of zooplankton. J. Eukaryot. Microbiol. 2015, 62, 470–480. [Google Scholar] [CrossRef]
- Zou, Y.; Kim, D.; Yagi, M.; Yamasaki, Y.; Kurita, J.; Iida, T.; Matsuyama, Y.; Yamaguchi, K.; Oda, T. Application of LDH-release assay to cellular-level evaluation of the toxic potential of harmful algal species. Biosci. Biotechnol. Biochem. 2013, 77, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Turner, J.T. Planktonic marine copepods and harmful algae. Harmful Algae 2014, 32, 81–93. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kang, H.; Kim, M.; Wang, M.; Kim, J.H.; Jeong, C.; Lee, J. Effects of ocean acidification on life parameters and antioxidant system in the marine copepod Tigriopus japonicus. Aquat. Toxicol. 2019, 212, 186–193. [Google Scholar] [CrossRef]
- Li, H.; Shi, L.; Wang, D.; Wang, M. Impacts of mercury exposure on life history traits of Tigriopus japonicus: Multigeneration effects and recovery from pollution. Aquat. Toxicol. 2015, 166, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Yi, A.X.; Han, J.; Lee, J.S.; Leung, K.M. Ecotoxicity of triphenyltin on the marine copepod Tigriopus japonicus at various biological organisations: From molecular to population-level effects. Ecotoxicology 2014, 23, 1314–1325. [Google Scholar] [CrossRef]
- Raisuddin, S.; Kwok, K.W.H.; Leung, K.M.Y.; Schlenk, D.; Lee, J. The copepod Tigriopus: A promising marine model organism for ecotoxicology and environmental genomics. Aquat. Toxicol. 2007, 83, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, G.; Zeng, C.; Wu, L. Comparative study on the effects of two diatoms as diets on planktonic calanoid and benthic harpacticoid copepods. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2018, 329, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xu, J.; Zeng, C.; Jia, Q.; Wu, L.; Li, S. Pelagic microalgae as suitable diets for the benthic harpacticoid copepod Tigriopus japonicus. Hydrobiologia 2015, 762, 81–88. [Google Scholar] [CrossRef]
- Gentien, P.; Lunven, M.; Lazure, P.; Youenou, A.; Crassous, M.P. Motility and autotoxicity in Karenia mikimotoi (Dinophyceae). Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 1937–1946. [Google Scholar] [CrossRef] [Green Version]
- Uye, S.; Takamatsu, K. Feeding interactions between planktonic copepods and red-tide flagellates from Japanese coastal waters. Mar. Ecol. Prog. Ser. 1990, 59, 97–107. [Google Scholar] [CrossRef]
- Gill, C.; Harris, R.P. Behavioural responses of the copepods Calanus helgolandicus and Temora longicornis to dinoflagellate diets. J. Mar. Biol. Assoc. UK 1987, 67, 785–801. [Google Scholar] [CrossRef]
- Lin, J.; Song, J.; Yan, T.; Zhang, Q.; Zhou, M. Large-scale dinoflagellate bloom species Prorocentrum donghaiense and Karenia mikimotoi reduce the survival and reproduction of copepod Calanus sinicus. J. Mar. Biol. Assoc. UK 2015, 95, 1071–1079. [Google Scholar] [CrossRef]
- Li, X.; Yan, T.; Lin, J.; Yu, R.; Zhou, M. Detrimental impacts of the dinoflagellate Karenia mikimotoi in Fujian coastal waters on typical marine organisms. Harmful Algae 2017, 61, 1–12. [Google Scholar] [CrossRef]
- Schultz, M.; Kiorboe, T. Active prey selection in two pelagic copepods feeding on potentially toxic and non-toxic dinoflagellates. J. Plankton Res. 2009, 31, 553–561. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.Y.; Kiorboe, T. Toxic dinoflagellates produce true grazer deterrents. Ecology 2018, 99, 2240–2249. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.; Hargraves, P.E. Stichochrysis immobilis is a diatom, not a chrysophyte. Phycologia 1993, 32, 234–236. [Google Scholar] [CrossRef]
- Pamplona, R.; Costantini, D. Molecular and structural antioxidant defenses against oxidative stress in animals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, 843–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorokhova, E.; El-Shehawy, R. Antioxidant responses in copepods are driven primarily by food intake, not by toxin-producing cyanobacteria in the diet. Aquat. Physiol. 2022, 12, 805646. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, G. Oxidative damage effects in the copepod Tigriopus japonicus Mori experimentally exposed to nickel. Ecotoxicology 2010, 19, 273–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frost, B.W. Effects of size and concentration of food particles on the feeding behavior of the marine planktonic copepod Calanus pacificus. Limnol. Oceanogr. 1972, 17, 805–815. [Google Scholar] [CrossRef] [Green Version]
- Menden-DEUER, S.; Lessard, E.J. Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol. Oceanogr. 2000, 45, 569–579. [Google Scholar] [CrossRef] [Green Version]
- Sotka, E.E.; McCarty, A.; Monroe, E.A.; Oakman, N.; Van Dolah, F.M. Benthic herbivores are not deterred by breve-toxins produced by the red tide dinoflagellate Karenia brevis. J. Chem. Ecol. 2009, 31, 851–859. [Google Scholar] [CrossRef]
- Taylor, R.L.; Caldwell, G.S.; Dunstan, H.J.; Bentley, M.G. Short-term impacts of polyunsaturated aldehyde- producing diatom on the harpacticoid copepod, Tisbe holothuriae. J. Exp. Mar. Biol. Ecol. 2007, 341, 60–69. [Google Scholar] [CrossRef]
- Han, J.; Park, J.S.; Park, Y.; Lee, J.; Shin, H.H.; Lee, K. Effects of paralytic shellfish poisoning toxin-producing dinoflagellate Gymnodinium catenatum on the marine copepod Tigriopus japonicus. Mar. Pollut. Bull. 2021, 163, 111937. [Google Scholar] [CrossRef]
- Hong, H.; Wang, J.; Shi, D. Effects of salinity on the chronic toxicity of 4-methylbenzylidene camphor (4-MBC) in the marine copepod Tigriopus japonicus. Aquat. Toxicol. 2021, 232, 105742. [Google Scholar] [CrossRef] [PubMed]
- Kwok, K.W.H.; Leung, K.M.Y. Toxicity of antifouling biocides to the intertidal harpacticoid copepod Tigriopus japonicus (Crustacea, Copepoda): Effects of temperature and salinity. Mar. Pollut. Bull. 2005, 51, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Carpenter, E.J. Blooms of the dinoflagellate Gyrodinium aureolum in a Long Island estuary: Box model analysis of bloom maintenance. Mar. Biol. 1985, 89, 83–93. [Google Scholar] [CrossRef]
- Amado, L.L.; Monserrat, J.M. Oxidative stress generation by microcystins in aquatic animals: Why and how. Environ. Int. 2010, 36, 226–235. [Google Scholar] [CrossRef]
- Hinojosa, M.G.; Gutiérrez-Praena, D.; Prieto, A.I.; Guzmán-Guillén, R.; Jos, A.; Cameán, A.M. Neurotoxicity induced by microcystins and cylindrospermopsin: A review. Sci. Total Environ. 2019, 668, 547–565. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P. Redefining oxidative stress. Antioxid. Redox Signal. 2006, 8, 1865–1879. [Google Scholar] [CrossRef]
- Yasumoto, T.; Underdal, T.; Aune, T.; Hormazabal, V.; Skulberg, O.M.; Oshima, Y. Screening for hemolytic and ichthyotoxic components of Chrysochromulina polylepis and Gyrodinium aureolum from Norwegian coastal waters. In Toxic Mar. Phytoplankton; Graneli, E., Sundstrom, B., Edler, L., Anderson, D.M., Eds.; Elsevier: New York, NY, USA, 1990; pp. 436–440. [Google Scholar]
- Marshall, J.; de Salas, M.; Oda, T.; Hallegraeff, G. Superoxide production by marine microalgae I. Survey of 37 Species From 6 Classes. Mar. Biol. 2005, 147, 533–540. [Google Scholar] [CrossRef]
- Kim, D.; Li, W.; Matsuyama, Y.; Matsuo, A.; Yagi, M.; Cho, K.; Yamasaki, Y.; Takeshita, S.; Yamaguchi, K.; Oda, T. Strain-dependent lethal effects on abalone and haemolytic activities of the dinoflagellate Karenia mikimotoi. Aquaculture 2020, 520, 734953. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J.; Sun, T.; Liu, C.; Sun, Y.; Wang, Y. Using the marine rotifer Brachionus plicatilis as an endpoint to evaluate whether ROS-dependent hemolytic toxicity Is involved in the allelopathy induced by Karenia mikimotoi. Toxins 2018, 10, 439. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.N.; Nam, S.E.; Shin, Y.K.; Rhee, J.S. The dinoflagellate Alexandrium affine acutely induces significant modulations on innate immunity, hepatic function, and antioxidant defense system in the gill and liver tissues of red seabream. Aquat. Toxicol. 2021, 240, 105985. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Ong, C.N. Role of oxidative stress and mitochondrial changes in cyanobacteria-induced apoptosis and hepatotoxicity. FEMS Microbiol. Lett. 2003, 220, 1–7. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Chen, Y.; Zhu, J. Influence of intracellular Ca2+, mitochondriamembrane potential, reactive oxygen species, and intracellular ATP on the mechanism of microcystin-LR induced apoptosis in Carassius auratus lymphocytes in vitro. Environ. Toxicol. 2007, 22, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Song, L.; Liu, J. Responses of antioxidant systems in the hepatocytes of common carp (Cyprinus carpio L.) to the toxicity of microcystin-LR. Toxicon 2003, 42, 85–89. [Google Scholar] [CrossRef]
- Colón, R.; Wheater, M.; Joyce, E.; Ste. Marie, E.; Hondal, R.; Rein, K. The marine neurotoxin brevetoxin (PbTx-2) inhibits Karenia brevis and Mammalian Thioredoxin reductases by targeting different residues. J. Nat. Prod. 2021, 84, 2961–2970. [Google Scholar] [CrossRef]
- Chen, W.; Tuladhar, A.; Rolle, S.; Lai, Y.; Rodriguez del Rey, F.; Zavala, C.E.; Rein, K.S. Brevetoxin-2, is a unique inhi bi tor of the C-terminal redox center of mammalian thioredoxin reductase-1. Toxicol. Appl. Pharmacol. 2017, 329, 58–66. [Google Scholar] [CrossRef]
- Tuladhar, A.; Hondal, R.J.; Colon, R.; Hernandez, E.L.; Rein, K.S. Effectors of thioredoxin reductase: Brevetoxins and manumycin-A. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 217, 76–86. [Google Scholar] [CrossRef]
- Matsuzawa, A. Thioredoxin and redox signaling: Roles of the thioredoxin system in control of cell fate. Arch. Biochem. Biophys. 2017, 617, 101–105. [Google Scholar] [CrossRef]
- Sasaki, M.; Tsukano, C.; Tachibana, K. Studies toward the total synthesis of gymnocin A, a cytotoxic polyether: A highly con vergent entry to the F-N ring frag- ment. Org. Lett. 2007, 4, 1747–1750. [Google Scholar] [CrossRef]
- Tanaka, Y.; Satake, M.; Yotsu-Yamashita, M. Gymnocin-A carboxylic acid and gymnocin-A2, cytotoxic polyethers from the red tide dinoflagellate Karenia mikimotoi. Heterocycles 2013, 87, 2037–2046. [Google Scholar] [CrossRef]
- Seo, J.S.; Lee, K.; Rhee, J.; Hwang, D.; Lee, Y.; Park, H.G.; Ahn, I.; Lee, J. Environmental stressors (salinity, heavy metals, H2O2) modulate expression of glutathione reductase (GR) gene from the intertidal copepod Tigriopus japonicus. Aquat. Toxicol. 2006, 80, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, S.; Wang, G.; Guo, D.; Xing, K.; Zhang, S. Biochemical responses of the copepod Centropages tenuiremis to CO2-driven acidified seawater. Water Sci. Technol. 2012, 65, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Pavagadhi, S.; Gong, Z.; Hande, M.P.; Dionysiou, D.D.; de la Cruz, A.A.; Balasubramanian, R. Biochemical response of diverse organs in adult Danio rerio (zebrafish) exposed to sub-lethal concentrations of microcystin-LR and microcystin-RR: A balneation study. Aquat. Toxicol. 2012, 109, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gorokhova, E.; Löf, M.; Reutgard, M.; Lindström, M.; Sundelin, B. Exposure to contaminants exacerbates oxidative stress in amphipod Monoporeia affinis subjected to fluctuating hypoxia. Aquat. Toxicol. 2013, 127, 46–53. [Google Scholar] [CrossRef]
- Forget, J.; Beliaeff, B.; Bocquene, G. Acetylcholinesterase activity in copepods (Tigriopus brevicornis) from the Vilaine River estuary, France, as a biomarker of neurotoxic contaminants. Aquat. Toxicol. 2003, 62, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Yan, T.; Zhang, Q.; Yu, R.; Zhou, M. Inhibition to crucial enzymes in the lethal effects of the dinoflagellate Karenia mikimotoi on the rotifer Brachionus plicatilis. Mar. Environ. Res. 2020, 157, 104866. [Google Scholar] [CrossRef]
- Qian, H.; Liu, G.; Lu, T.; Sun, L. Developmental neurotoxicity of Microcystis aeruginosa in the early life stages of zebrafish. Ecotox. Environ. Safe 2018, 151, 35–41. [Google Scholar] [CrossRef]
- Kimura, K.; Okuda, S.; Nakayama, K.; Shikata, T.; Takahashi, F.; Yamaguchi, H.; Skamoto, S.; Yamaguchi, M.; Tomaru, Y. RNA sequencing revealed numerous polyketide synthase genes in the harmful dinoflagellate Karenia mikimotoi. PLoS ONE 2015, 10, e142731. [Google Scholar] [CrossRef] [Green Version]
- Soreq, H.; Seidman, S. Acetylcholinesterase—New roles for an old actor. Nat. Rev. Neurosci. 2001, 4, 294–302. [Google Scholar] [CrossRef]
- Azzam, E.I. Exposure to low level environmental agents: The induction of hormesis. Mutat. Res. 2011, 726, 89–90. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Lonsdale, D.J.; Gobler, C.J. Density-dependent nutritional value of the dinoflagellate Cochlodinium polykrikoides to the copepod Acartia tonsa. Limnol. Oceanogr. 2010, 55, 1643–1652. [Google Scholar] [CrossRef]
- Vehmaa, A.; Hogfors, H.; Gorokhova, E.; Brutemark, A.; Holmborn, T.; Engstrom-Ost, J. Projected marine climate change: Effects on copepod oxidative status and reproduction. Ecol. Evol. 2013, 3, 4548–4557. [Google Scholar] [CrossRef] [PubMed]
- Jager, T.; Crommentuijn, T.; Van Gestel, C.A.M.; Kooijman, S.A.L.M. Simultaneous modeling of multiple end points in life-cycle toxicity tests. Environ. Sci. Technol. 2004, 38, 2894–2900. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, R.W.; Escher, B.I.; Schwarzenbach, R.P. Acute toxicity of triorganotin compounds: Different specific effects on the energy metabolism and role of pH. Environ. Toxicol. Chem. 2002, 21, 1191–1197. [Google Scholar] [CrossRef]
- Joy, K. Sexual Biology and Reproduction in Crustaceans. Proc. Indian Natl. Sci. Acad. 2017, 83, 231–232. [Google Scholar] [CrossRef]
- Voordouw, M.J.; Anholt, B.R. Environmental sex determination in a splash pool copepod. Biol. J. Linn. Soc. 2002, 76, 511–520. [Google Scholar] [CrossRef]
- Gusmao, L.F.M.; McKinnon, A.D. Sex ratios, intersexuality and sex change in copepods. J. Plankton Res. 2009, 31, 1101–1117. [Google Scholar] [CrossRef] [Green Version]
- Avery, D.E.; Altland, K.K.; Dam, H.G. Sex-related differential mortality of a marine copepod exposed to a toxic dinoflagellate. Limnol. Oceanogr. 2008, 53, 2627–2635. [Google Scholar] [CrossRef]
| Copepod | K. mikimotoi Strain or Source | Adverse Effects | References |
|---|---|---|---|
| Acartia omorii | Japanese coastal waters | Survival, feeding, egg production | [29] |
| Calanus helgolandicus | - | Feeding, egg production | [30] |
| C. sinicus | East China Sea | Survival, reproduction | [31] |
| C. sinicus | East China Sea | Survival | [32] |
| Pseudocalanus elongatus | K-260, Oslofjorden | Feeding | [33] |
| Pseudodiaptomus marinus | Japanese coastal waters | Survival, feeding, egg production | [29] |
| Pseudodiaptomus annandalei | South China Sea | Survival | [19] |
| Temora longicornis | - | Feeding, egg production | [30] |
| T. longicornis | K-260, Oslofjorden, Norway | Feeding | [33,34] |
| T. longicornis | CCMP429, Sutton Harbor, UK | Feeding | [34] |
| Tigriopus japonicus | Coast of Qingdao, China | Survival, feeding, biochemical response, development and reproduction | this study |
| Algae | Cell Size (μm) | Cell Volume (μm3) | Carbon Content (×10−6 µg·C·cell−1) |
|---|---|---|---|
| K. mikimotoi | 26.9 | 10,146.6 | 3368.4 |
| I. galbana | 5.1 | 35.3 | 8.05 |
| Biochemical Parameter | F | df | p |
|---|---|---|---|
| ROS | 20.02 | 4 | 0.000 |
| T-AOC | 39.90 | 4 | 0.000 |
| SOD | 47.21 | 4 | 0.000 |
| CAT | 13.79 | 4 | 0.000 |
| GPx | 40.31 | 4 | 0.000 |
| AChE | 8.68 | 4 | 0.003 |
| ROS | T-AOC | SOD | CAT | GPx | AChE | |
|---|---|---|---|---|---|---|
| ROS | 1.000 | |||||
| T-AOC | 0.705 ** | 1.000 | ||||
| SOD | 0.676 ** | 0.701 ** | 1.000 | |||
| CAT | 0.766 ** | 0.733 ** | 0.356 | 1.000 | 1.000 | |
| GPx | 0.256 | 0.528 * | 0.058 | 0.569 * | 1.000 | |
| AChE | 0.679 ** | 0.721 ** | 0.701 ** | 0.723 ** | 0.700 ** | 1.000 |
| Response Variable | Treatment | Generation | Treatment × Generation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F | df | p | F | df | p | F | df | p | |
| Nauplii to copepodite evelopment time | 16.7 | 4 | 0 | 220.6 | 1 | 0 | 3.8 | 4 | 0.156 |
| Copepodite to adult development time | 43.7 | 4 | 0 | 187.9 | 1 | 0 | 19.9 | 3 | 0 |
| Nauplii to copepodite development success rate | 19.5 | 4 | 0 | 84.5 | 1 | 0 | 5.5 | 4 | 0.002 |
| Copepodite to adult development success rate | 16.8 | 4 | 0 | 38.8 | 1 | 0 | 5.3 | 3 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Wang, J.; Zhuang, Y.; Yu, W.; Liu, G. Reduced Fitness and Elevated Oxidative Stress in the Marine Copepod Tigriopus japonicus Exposed to the Toxic Dinoflagellate Karenia mikimotoi. Antioxidants 2022, 11, 2299. https://doi.org/10.3390/antiox11112299
Chen H, Wang J, Zhuang Y, Yu W, Liu G. Reduced Fitness and Elevated Oxidative Stress in the Marine Copepod Tigriopus japonicus Exposed to the Toxic Dinoflagellate Karenia mikimotoi. Antioxidants. 2022; 11(11):2299. https://doi.org/10.3390/antiox11112299
Chicago/Turabian StyleChen, Hongju, Jing Wang, Yunyun Zhuang, Wenzhuo Yu, and Guangxing Liu. 2022. "Reduced Fitness and Elevated Oxidative Stress in the Marine Copepod Tigriopus japonicus Exposed to the Toxic Dinoflagellate Karenia mikimotoi" Antioxidants 11, no. 11: 2299. https://doi.org/10.3390/antiox11112299
APA StyleChen, H., Wang, J., Zhuang, Y., Yu, W., & Liu, G. (2022). Reduced Fitness and Elevated Oxidative Stress in the Marine Copepod Tigriopus japonicus Exposed to the Toxic Dinoflagellate Karenia mikimotoi. Antioxidants, 11(11), 2299. https://doi.org/10.3390/antiox11112299